Abstract
Hyperferritinemia occurs in Gaucher disease but its clinical spectrum or its association with systemic iron overload and HFE mutations is not known. In 114 patients with type 1 Gaucher disease, we determined serum ferritin, transferrin saturation and HFE genotype. The results were correlated with the extent of hepatosplenomegaly, overall Gaucher disease severity score index, and response to enzyme replacement therapy. In a subset of patients with radiological and/or laboratory evidence of systemic iron overload, liver biopsy was performed. There was a mean 3.7-fold elevation of serum ferritin over the upper limit of normal (ULN). Prior splenectomy was associated with most severe hyperferritinemia compared to patients with intact spleen (6.53 × ULN vs. 2.69 × ULN, P=0.003). HFE genotyping revealed two patients homozygous for H63D mutation and 30% of patients heterozygote carriers of H63D mutation; no patients harbored C282Y mutation; there was no correlation of ferritin with HFE genotype. Ferritin level correlated with liver volume (Pearson correlation coefficient = 0.254, p=0.035) and it was negatively correlated with hemoglobin (r = −0.315, p=0.004); there was no relationship with other indicators of Gaucher disease activity. Enzyme replacement therapy (ERT) resulted in amelioration of hyperferritinemia: 707±898 ng/ml vs. 301±310 ng/ml (p=0.001), transferrin saturation remained normal. Three patients were suspected of clinical iron overload, confirmed on liver biopsy. Iron accumulation was variably noted in hepatocytes and Kupffer cells. There is a high prevalence of hyperferritinemia in type 1 Gaucher disease that is associated with indicators of disease severity, reversed by ERT and is not related to HFE mutations.
Keywords: Gaucher Disease, Hemochromatosis, Ferritin, Iron Overload, Hepcidin, HFE, Enzyme Replacement Therapy, Splenectomy, chitotriosidase, CCL18, biomarkers
Introduction
In Gaucher disease (GD), deficiency of lysosomal glucocerebrosidase due to mutations in GBA1 gene, leads to accumulation of glucocerebroside in lysosomes of mononuclear phagocytes [1]. Non-neuronopathic Type 1 GD (GD1) is the most common lysosomal storage disease affecting ~ 1 in 40,000 newborns but its frequency in Ashkenazi Jews is as high as ~1 in 850. Lysosomal accumulation of glucocerebroside in mononuclear phagocytes leads to a complex phenotype of hepatosplenomegaly, anemia and thrombocytopenia, a diverse pattern of bone involvement and, less commonly, lung involvement. In addition unusual manifestations of type 1 GD are recognized, i.e., increased risk of cancers, Parkinsonian syndrome and pulmonary hypertension [2]. In contrast to the non-neuronopathic type 1 GD, the rare neuronopathic forms (types 2 and 3) exhibit variable severity of neurologic involvement [3]. The pathways from accumulation of glucocerebroside in the lysosomes of tissue macrophages to the complex phenotype of GD are not understood. However, pro-inflammatory cytokines and the adaptive immune system appear to be involved [4, 5].
Serum ferritin was found to be elevated in type 1 Gaucher disease in two studies reported almost 2 decades ago in small number of patients [6, 7]. Clinically, hyperferritinemia appears to be a frequent finding in GD and it may lead to an erroneous diagnosis of hemochromatosis, before the correct diagnosis is made [8]. However, the full clinical spectrum of hyperferritinemia or its association with HFE gene mutations is not known and whether it could be used as a biomarker of GD activity or its association with systemic iron overload is not known. We addressed these questions in an observational study of 114 patients with type 1 GD.
Methods
We performed a retrospective chart review of patients with diagnosis of type 1 Gaucher disease (GD1) followed for up to 10 years at Yale’s National Gaucher Disease Treatment Center. A database of GD patients followed for up to 10 years was created. For this study, the dataset was limited to patients who had a measurement of serum ferritin and transferrin saturation and received imiglucerase enzyme replacement therapy (ERT). A total of 114 patients were identified. Patient demographics are summarized in Table I. Patients had confirmed diagnosis of type 1 Gaucher disease based on low (i.e., <10% of normal) leukocyte acid β-glucosidase activity. GBA1 genotyping was performed as described previously [9]. In 62 patients, both pretreatment and post-treatment serum ferritin and transferrin saturation were available. In this group, 49 had intact spleens while 13 had undergone therapeutic splenectomy. Effect of ERT in serum ferritin was evaluated in this subset of patients, stratified for spleen status. For analysis of results, the most current laboratory data for each patient in the database were used except in patients that received ERT. In these patients, lab data used was the most current prior to the initiation of ERT. For the 62 patients with paired data, post-ERT results were database results that were closest in time to after 3 years of receiving ERT.
Table I.
Demographics of Patients and Baseline serum biomarker results comparing GD1 patients with intact spleen with splenectomized patients.
| All Patients | Intact Spleen Patients | Splenectomized Patients | p value* | |
|---|---|---|---|---|
| Number of Total Patients | 114 | 78 | 36 | |
| Gender, N (%) | 0.782 | |||
| Male | 56 (49) | 39 (50) | 17 (47) | |
| Female | 58 (51) | 39 (50) | 19 (53) | |
| Age at presentation | 0.0004 | |||
| Mean ± SD (yrs) | 40.4±19.9 | 36.0±19.6 | 50.0±17.1 | |
| Age at onset of first symptoms | 0.19 | |||
| Mean ± SD (yrs) | 22.1±17.9 | 23.7±17.7 | 19.0±18.0 | |
| Age at diagnosis | 0.18 | |||
| Mean ± SD (yrs) | 25.5±18.6 | 27.1±18.7 | 22.0±18.3 | |
| Current Age | <0.0001 | |||
| Mean ± SD (yrs) | 53.6±19.5 | 48.8±19.2 | 63.8±16.1 | |
| Genotype, N (%) | 0.0005 # | |||
| N370S/N370S | 60(53) | 50(64) | 10(28) | |
| N370S/84GG | 31(27) | 13(17) | 18(50) | |
| N370S/IVS2+1 | 3(3) | 2(3) | 1(3) | |
| N370S/L444P | 10(9) | 8(10) | 2(6) | |
| N370S/Other or unknown | 10(9) | 5(6) | 5(14) | |
| Ferritin Level × ULN (± SD) | 3.7 ±3.67 | 2.69±2.47 | 6.59±4.87 | 0.003 |
| Patient number | 111 | 75 | 36 | |
| Transferrin saturation% (± SD) | 28 ±11.4 | 25.3±8.7 | 35.7±14.3 | 0.002 |
| Patient number | 91 | 67 | 24 | |
| Chitotriosidase (± SD) | 8352±6424 | 7466±6859 | 10254±5665 | 0.106 |
| Patient number | 77 | 49 | 28 | |
| CCl 18 (± SD) | 406±419 | 413±474 | 389±268 | 0.861 |
| Patient number | 43 | 30 | 13 | |
p value compared patients with intact spleen vs. asplenic patients.
chi square compared N370S homozygous vs all compound heterozygotes
Patients had assays for chitotriosidase and CCL 18. The chitotriosidase assay was performed using 4-methylumbelliferyl-(4-deoxy)chitobiose as a substrate as described previously [10] Genotyping for chitotriosidase (CHIT 1) was performed as described previously [11]. Patients homozygous for CHIT 1 null allele were excluded. Patients that were heterozygous for 99 bp CHIT 1 gene polymorphism were normalized to wild-type homozygous (77/75 bp) by multiplying their chitotriosidase activity by a factor of 2 [12]. CCL 18 was determined by ELISA as previously described [13].
Liver and spleen volumes were determined by volumetric MRI, expressed as fold enlargement over expected size for body weight as previously described [14]. For these calculations, we used normal liver volume at 2.5% of the total body weight and normal spleen volume 0.2% body weight. Overall Gaucher disease severity score index (SSI) was calculated as described previously [15].
Differences in means among the subgroups of patients were determined by an independent samples two-tailed T-test for equality of means. The F-test (Levine’s test) for equality of variances was first run. Based on the results of the F-test, equal variances were either assumed or not assumed in the determination of the significance of the T-test. Two-tailed Pearson correlation tests were run to investigate correlations between biomarkers and liver volume. A paired T-test was performed to determine difference in ferritin pre- and post- enzyme replacement therapy. In our study we did not correct for multiple hypothesis testing as this was an exploratory study.
All patients received standard of care. Serum ferritin levels were expressed as fold-increase over the upper limit of normal. HFE genotyping for C282Y and H63D mutations was performed as previously described [16]. All patients underwent MRI of the abdomen for quantitative assessment of hepatosplenomegaly; we routinely check for evidence of iron accumulation in the liver, spleen and bone marrow. Patients were investigated for systemic iron overload with liver biopsy if transferrin saturation was >50 % and/or if there was suggestion of iron overload on MRI imaging. Quantitative determination of iron by atomic absorption spectrometry and grading of iron distribution were determined as described previously [17,18]. Three patients were identified who were suspected of systemic iron overload. All patients underwent GBA1 genotyping, HFE genotyping, serum iron panel, serum biomarkers and liver function tests.
The study was approved by the Human Investigation Committee of Yale School of Medicine.
Results
A total of 114 patients with a diagnosis of GD1 were evaluated. The mean age was 53.6 years, a range of 2 to 85 years at time of first visit and male: female ratio was 49%:51%. The mean age at onset of GD1 symptoms was 22.1 years in all patients. Of the 114 patients, 36 (31.6%) had undergone prior therapeutic splenectomy. The most common GBA1 genotype was N370S/N370S (60/114, 53% of patients) and it was the most prevalent genotype among patients with intact spleen (50/78, 64%). However, among asplenic patients N370S/84GG was the more prevalent genotype, i.e., 50% (18/36) compared with 17% (13/78) among patients with intact spleen. By Fisher’s exact test, the difference in splenectomy rates between patients N370S homozygous vs. N370S/84GG is highly significant (p<0.0001). Compared to N370S homozygous patients, the relative risk of N370S/84GG patients having a prior splenectomy is 2.64 (95% confidence interval 1.89–3.68).
Only 4 patients gave a history of blood transfusions but this occurred >10 yrs before the study period and in each case the blood transfusion did not exceed 4 units. No patients were receiving regular iron supplementation. There were 5 patients in the study cohort with Hepatitis C. Only one of these five patients had severe iron overload. HFE genotyping revealed two patients homozygous for H63D mutation. One of these 2 patients had severe iron overload (patient 1 in Table II) while the other patient had isolated hyperferritinemia with normal transferrin saturation and no radiological evidence of iron overload. Among 36 patients heterozygous for H63D mutation, there was no correlation with ferritin levels (data not shown).
Table II.
Genetic and clinical characteristics of 3 patients with pathological iron overload
| ID # |
GBA1 Genotype | Age GD Diagnosis |
Age start ERT |
Age Dx Iron Overload |
Age at Splenectomy |
Ferritin µg/L |
Transferrin Sat % |
Biopsy-Liver iron (µg/g) |
Iron Distribution and Grade |
HFE Genotype | Comorbidity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hep | Kup | |||||||||||
| 1 | N370S/N370S | 42 | 42 | 42 | Intact spleen | 1839 | 39 | n/a | 3+ | 3+ | H63D/H63D | None |
| 2 | N370S/L444P | 11 | 55 | 53 | 11 | 3080 | 49 | 2723 | 2+ | 2+ | H63D/Wt | NHL Lung CA Hep C |
| 3 | N370S/L444P | 11 | 48 | 50 | 11 | 1470 | 79 | 7132 | 3–4+ | 2+ | Wt | none |
Iron Distribution and Grade(Scheuer, Williams et al. 1962; Deugnier, Turlin et al. 1993) Hep-hepatocyte Kup-Kupffer Cell NHL-Non-Hodgkin’s Lymphoma
Serum ferritin levels were elevated by a mean 3.7-fold elevation above the upper limit of normal of ferritin ranging from 65 µg/L to 6200µg/L. In contrast mean transferrin saturation was normal at 28%. Hyperferritinemia and transferrin saturation was related to spleen status: serum ferritin in asplenic patients was 6.53 × N ± 4.90 and transferrin saturation was 35.71% ± 14.36 compared to patients with intact spleen patients: 2.69 × N ± 2.48, p=0.003 and 25.32% ± 8.74, p=0.002 respectively. As expected, asplenic patients harbor higher platelet and WBC counts compared to those with intact spleens (187.48 ± 107.04 and 11.41 ± 3.80, respectively) compared to intact spleen patients (103.92 ± 60.84 and 4.53 ±1.60, respectively) p <0.0001 for both.
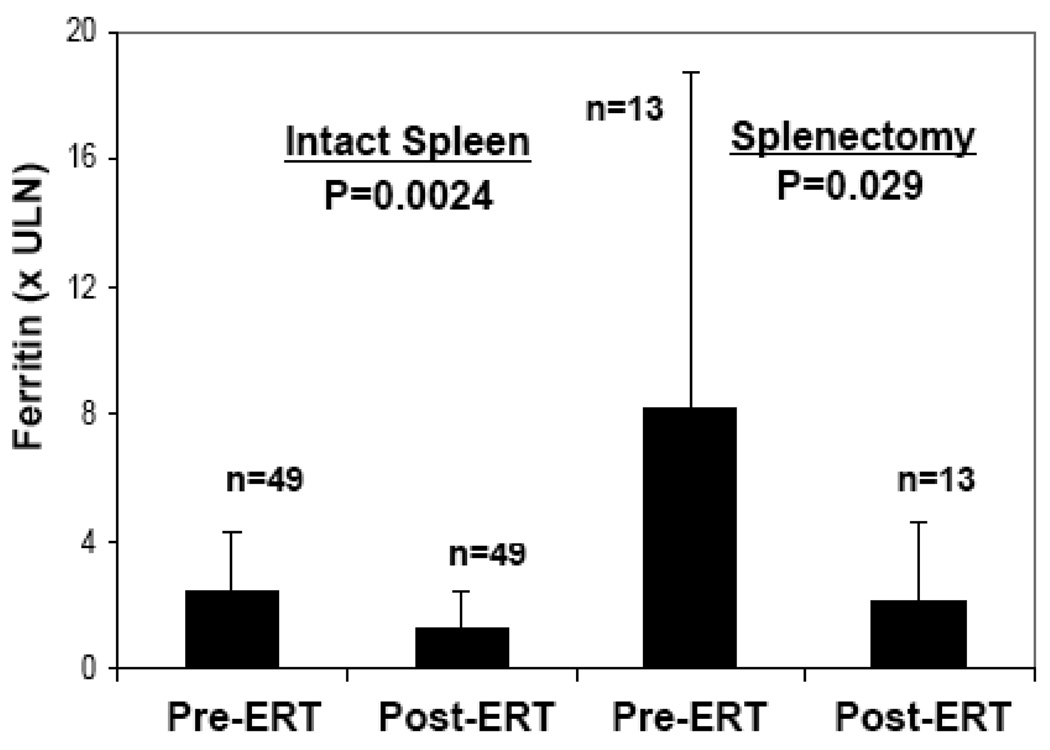
We performed a preliminary assessment of the candidacy of ferritin as a biomarker of Gaucher disease activity. There was weak correlation of ferritin with three indicators of disease severity but not with overall severity score index (SSI). First, liver volume was weakly correlated with ferritin levels: r = 0.254, p = 0.035. Secondly, there was a negative correlation between hemoglobin and ferritin levels: r= −0.315, p = 0.004. Thirdly, as described above, splenectomy, an indicator if disease severity, is associated with more marked hyperferritinemia compared to those with intact spleen. Next, we determined the effect of ERT on serum ferritin levels. Serum ferritin levels were compared at baseline and after mean 3.5±1.5 years after initiation of ERT. In 62 patients on whom data was available for pre-treatment and post treatment serum ferritin levels, there was marked reduction of ferritin levels from 707±898 to 301±310 ng/ml (p=0.001 by paired T test). This represents a reduction of serum ferritin levels from 3.7 × ULN to 1.5 × ULN. Pre-treatment and post-ERT serum ferritin levels were further examined after stratification by spleen status. The results are depicted in figure 1. ERT resulted in reduction of serum ferritin in asplenic patients as well as in patients with intact spleen but the effect was most marked in the latter: serum ferritin levels in asplenic patients fell from 1472 ±1593 to 416±363 ng/ml corresponding to 8.2 × ULN to 2.2 × ULN (p=0.029) while in patients with intact spleens serum ferritin levels fell from 504±436 to 271 ng/ml, i.e., 2.5 ×ULN to 1.3 ×ULN (p=0.0024).
Figure 1.
Effect of ERT on serum ferritin levels stratified for spleen status. The patients were treated with ERT for mean 3.5±1.5 yrs
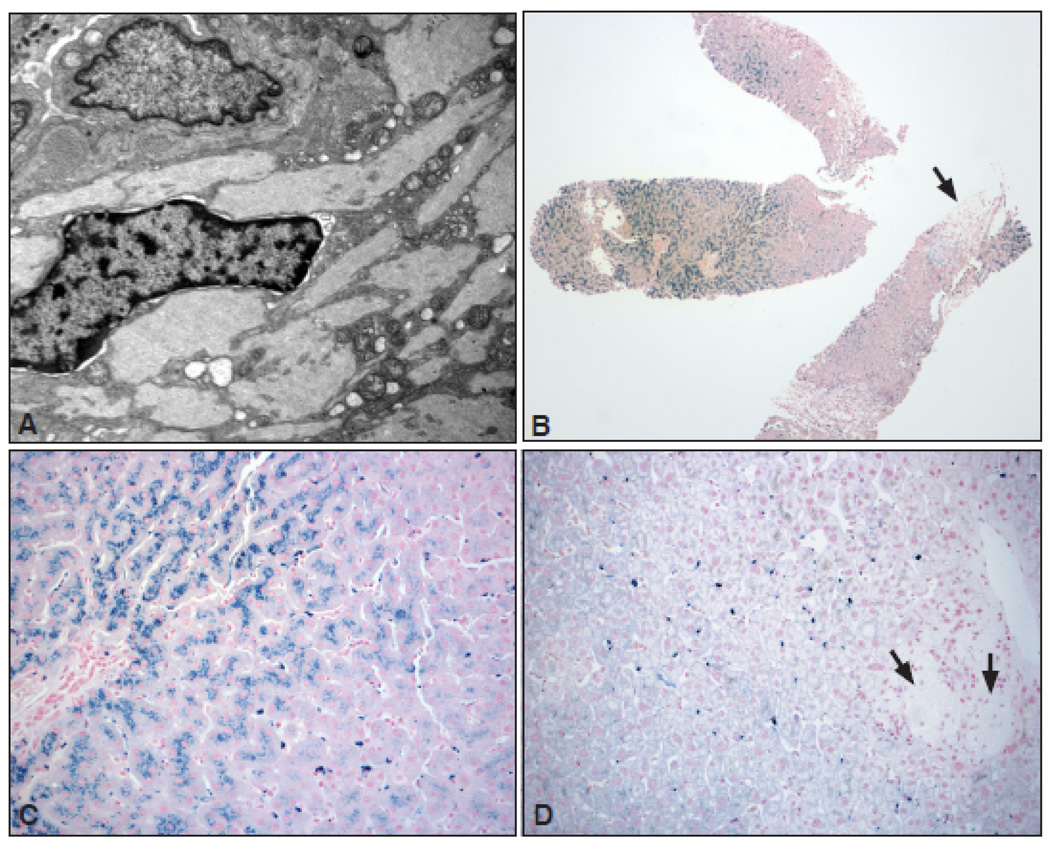
All patients underwent MRI of the abdomen as standard of care [14] for quantitative determination of extent of hepatosplenomegaly. During MRI we routinely assess for presence of iron overload in the liver, spleen and bone marrow. In our series of patients, we suspected 3 patients to have clinical iron overload on the basis of markedly elevated transferrin saturations and/or radiological evidence of iron overload (Table II). We performed percutaneous liver biopsy in these 3 patients for further evaluation of iron overload. These three patients demonstrated up to grade 3– 4 hepatocyte siderosis. The iron accumulation tended to occur variably in hepatocytes and Kupffer cells and to a lesser extent in Gaucher cells (figure 2). Quantitative determination of iron by atomic absorption spectrometry revealed pathological iron accumulation diagnostic of hemochromatosis. Representative liver biopsy and MRI are shown in figure 2 and supplementary online figure 3. After initial ERT and restoration of normal hemoglobin, these patients had modest reduction of ferritin levels however transferrin saturation remained markedly elevated indicating iron overload. Together with evidence of pathological iron accumulation in liver biopsy, these three patients underwent phlebotomy until their ferritin and transferrin saturation was restored to normal. In patient 1(Table II), phlebotomy reversed iron overload in the liver as well as the bone marrow as shown on MRI scans depicted in supplementary Figure 3 online. Interestingly this patient had striking skin pigmentation that reversed entirely after phlebotomy restored her iron indices to normal.
Figure 2.
A: Electron micrograph of a Gaucher cell showing abundant Gaucher bodies packed with typical tubular structures. No hemosiderin deposition is seen in this case (Magnification ×10,000). B: Low magnification view of a liver biopsy in a 50 year old patient (Patient 3) shows extensive siderosis, predominantly in the hepatocytes located in zones 1and 2 (Prussian blue stain). The arrow points to a collection of Gaucher cells that lack any staining for hemosiderin at this magnification (×40). C: Higher magnification of the same case showing extensive hemosiderin deposition in the hepatocytes of zones 1 and 2, and to a much lesser extent in the Kupffer cells (×200). D: Liver biopsy from a different case with hyperferritenemia and negative HFE mutation showing predominant deposition of hemosiderin in the Kupffer cells (Prussian blue stain) and very little in the hepatocytes. In addition, a faint blue blush is seen that likely represent staining for ferritin in hepatocytes. The arrows point to an area of Gaucher cell collection that is negative for any staining (×200).
It should be noted that 2 of the 3 patients with iron overload were asplenic, in keeping with finding in our study of presence of most marked hyperferritinemia in asplenic GD patients compared to those with intact spleen. Moreover, two of these three patients had co-morbidities that probably contributed somewhat to hyperferritinemia and iron overload. One patient was homozygous for H63D HFE mutation and another had chronic hepatitis C as well as a history of multiple cancers.
Discussion
We found an impressive elevation of serum ferritin in GD1 that correlated with some indicators of disease severity namely, hepatomegaly, and whether the patient had previously undergone splenectomy. In addition, serum ferritin was negatively correlated with hemoglobin level. Thus hyperferritinemia seemed to be more severe among anemic patients compared to patients with higher hemoglobin levels. However, overall disease severity score (SSI) did not correlate with ferritin. The majority of patients did not have evidence of pathological iron overload however, reflected by normal transferrin saturation and lack of evidence of iron accumulation in the viscera by MRI scanning. A small sub-set of patients (3/114) had severe iron overload indicated by persistently elevated transferrin saturation and ferritin together with evidence of pathological iron accumulation; this was confirmed by liver biopsy in these 3 patients. Two of these 3 patients were asplenic and in one patient, there was associated co-morbidity in the form of hepatitis C and history of malignancy. One patient was homozygous for H63D mutation in HFE gene. HFE C282Y was not found in our population, reflecting the predominantly Ashkenazi Jewish ancestry of our study population. ERT was associated with impressive reversal of hyperferritinemia, most marked among patients who had an intact spleen. A previous study reported amelioration of hyperferritinemia by ERT among patients that appeared to correlate with a satisfactory marrow response [19].
The biomarkers of Gaucher disease activity in routine clinical practice include chitotriosidase and the chemokine, CCL18 [4]. These biomarkers are clearly superior to ferritin for which the correlation with indicators of disease severity was relatively weak compared to other biomarkers. However, composite biomarker panel including ferritin has the potential to reveal co-morbidity such as iron overload (or conversely iron deficiency). Interestingly asplenic patients did not have significantly different chitotriosidase or CCL 18 from patients with intact spleens. Pharmacokinetic studies with recombinant chitotriosidase indicate that there is nearly complete first pass extraction of the enzyme through the liver, in keeping with the concept that splenic Gaucher cells make virtually no contribution to peripheral circulating chitotriosidase levels [20]. Further evidence for this concept derives from anecdotal observations that following therapeutically indicated splenectomy in 3 GD1 patients, there was no reduction of serum chitotriosidase levels following splenectomy (P.K. Mistry, unpublished observations), as would have been expected if splenic Gaucher cells were contributing chitotriosidase to circulating serum levels.
The extent of hyperferritinemia is somewhat excessive to be accounted for solely by chronic inflammation that occurs in GD. It is interesting to consider potential mechanisms of commonly occurring hyperferritinemia in GD1 and of clinical iron overload in a small subset of patients. There have been studies on this topic since 1967 when Lee, Balcerak, and Westerman [21] examined tissue obtained from patients with GD1 under light and electron microscopy. There was increased amount of iron in Gaucher cells with no evidence of increased avidity between iron and glucocerebroside storage material. In radio-iron studies, there was avid take up of iron into the liver and marrow yet low incorporation into erythrocytes [22, 23].
Perhaps these findings can be better explained by current knowledge about body iron homeostasis. Maintenance is achieved by increasing or decreasing gastrointestinal tract absorption and parallel flux of iron from tissue macrophages that together contribute to the plasma iron pool. This maintenance is controlled through a circulating peptide, hepcidin, produced by the hepatocytes via a mechanism that is critically dependent upon normal HFE function, which is influenced by inflammatory cytokines. Circulating hepcidin exerts its effect by binding to membrane ferroportin, a cellular iron exporter [24]. Hepcidin is upregulated by multiple factors and several of these factors are involved in Gaucher disease as well. For example, cytokines which are known to increase transcription of the hepcidin gene are elevated in Gaucher disease, i.e., IL6 [5] and IL1-β [25]. Therefore, it would be interesting in a future study to determine the status of hepcidin in GD patients with high and low ferritin and change pre- and post-ERT. This could further elucidate hepcidin’s role in high ferritin level in patients with GD1. Interestingly, serum biomarkers of Gaucher disease, namely, chitotriosidase and CCL18 are also increased hemochromatosis complicating thalassemia, in which pseudo-Gaucher cells are known to occur [26].
We found asplenic GD1 patients had higher ferritin levels compared to those with intact spleen. It is unclear whether this phenomenon reflects more severe Gaucher disease known to be associated with asplenia [27] or whether splenectomy per se contributes to iron accumulation. To our knowledge there is no information on ferritin levels in healthy individuals who underwent splenectomy after trauma. However, in hemoglobinopathies such as sickle cell disease and thalassemia, asplenic patients may have worse hyperferritinemia and iron overload. Nevertheless, in this setting splenectomy is a marker of more severe disease reflecting greater requirement for blood transfusions. In fact, in a study examining iron overload in sickle cell disease, multiple logistic regression analysis, did not find splenectomy to be a independent predictor of liver iron [28]. It should be noted that while splenectomy is not current practice in GD1, according to the 2009 Gaucher Registry Annual Report shows that a significant number of currently treated patients worldwide have had prior splenectomy (1169 out of 5323, i.e., 22%) [29]. In the new generation of patients, splenectomy will be a rare event, and this may translate to disappearance of iron overload in a subset of patients.
There are several shortcomings of our study. This was a retrospective study. Available serum ferritin levels were obtained at variable lengths of time post ERT and not all patients had paired measurements. Therefore, it is not possible to establish a time course of reduction of ferritin on ERT. Three years post initiation of therapy was the intended data point used because visceral and hematological disease have shown response by this time point in other studies [30]. There tended to be greater hyperferritinemia among patients who had undergone prior therapeutic splenectomy. While, we have used prior splenectomy as an indicator of more severe disease, in some patients, splenectomy may have been performed due to misdiagnosis before Gaucher disease was suspected [8]. In contrast to previous studies, we did not find evidence of excessive iron accumulation in Gaucher cells. Most iron accumulation on liver biopsy was confined to the hepatocytes, similar to hereditary hemochromatosis. In our patients, we did not perform bone marrow biopsy because it was not clinically indicated. MRI is a current standard of care in managing patients with Gaucher disease. It helps further quantify visceral disease and bone marrow burden. This test and laboratory data were available at the time of this study and helped identify 3 patients with iron overload. After this study started, Ferriscan has become standard of care for non-invasive ascertainment of iron overload [31]. As this test has a higher sensitivity, lesser degree of iron overload in GD1 could be identified. Future studies should examine the use of this modality in Gaucher disease.
In conclusion, our study has revealed a wide spectrum of hyperferritinemia in GD1 that appears to be related to some aspects of GD1 severity and it is reversed by ERT. A small subset of patients developed clinical iron overload. While, clinical iron overload occurs in only a small subset of patients (3/114 in our cohort), the incidence of hemochromatosis may to be significantly higher among GD1 patients compared to the general population, even in the absence of classic HFE mutations. Therefore, as previously recommended, among patients with GD1 and hyperferritinemia, those with elevated transferrin saturation and/or radiologic evidence of iron overload should be evaluated for concurrent hemochromatosis
Supplementary Material
Acknowledgements
We are grateful to our patients for their participation in these studies. PKM was supported by NIDDK K24DK066306 mid-career clinical investigator award. We thank Ruhua Yang for preparation of Figure 1.
Contributor Information
Philip Stein, Email: philip.stein@yale.edu.
Hannah Yu, Email: hy2281@columbia.edu.
Dhanpat Jain, Email: dhanpat.jain@yale.edu.
Pramod K. Mistry, Email: pramod.mistry@yale.edu.
References
- 1.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [PMID: 19094956] [DOI] [PubMed] [Google Scholar]
- 2.Cox TM, Schofield JP. Gaucher's disease: clinical features and natural history. Bailliere's Clin Haematol. 1997;10:659–687. doi: 10.1016/s0950-3536(97)80033-9. [PMID: 9497857] [DOI] [PubMed] [Google Scholar]
- 3.Sidransky E. Gaucher disease: complexity in a "simple" disorder. Mol Gen Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [PMID: 15464415] [DOI] [PubMed] [Google Scholar]
- 4.Aerts JM, Hollak CE, van Breemen M, et al. Identification and use of biomarkers in Gaucher disease and other lysosomal storage diseases. Acta Paediatr Suppl. 2005;94:43–46. doi: 10.1111/j.1651-2227.2005.tb02110.x. discussion 37-8. [PMID: 15895711] [DOI] [PubMed] [Google Scholar]
- 5.Allen MJ, Myer BJ, Khokher AM, et al. Pro-inflammatory cytokines and the pathogenesis of Gaucher's disease: increased release of interleukin-6 and interleukin-10. QJM. 1997;90:19–25. doi: 10.1093/qjmed/90.1.19. [PMID: 9093585] [DOI] [PubMed] [Google Scholar]
- 6.Morgan MA, Hoffbrand AV, Laulicht M, et al. Serum ferritin concentration in Gaucher's disease. Br Med J (Clin Res Ed) 1983;286:1864. doi: 10.1136/bmj.286.6381.1864. [PMID: 6407607] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutler E, Demina A, Laubscher K, et al. The clinical course of treated and untreated Gaucher disease. A study of 45 patients. Blood Cells Mol Dis. 1995;21:86–108. doi: 10.1006/bcmd.1995.0012. [ PMID: 8846048] [DOI] [PubMed] [Google Scholar]
- 8.Mistry PK, Sadan S, Yang R, et al. Consequences of diagnostic delays in type 1 Gaucher disease: the need for greater awareness among hematologists-oncologists and an opportunity for early diagnosis and intervention. Am J of Hematol. 2007;82:697–701. doi: 10.1002/ajh.20908. [PMID: 17492645] [DOI] [PubMed] [Google Scholar]
- 9.Taddei TH, Kacena KA, Yang M, et al. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. Am Journal Hematol. 2009;84:208–214. doi: 10.1002/ajh.21362. [PMID: 19260119] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilera B, Ghauharali-van der Vlugt K, Helmond MT, et al. Transglycosidase activity of chitotriosidase: improved enzymatic assay for the human macrophage chitanase. J Biol Chem. 2003;278:4091–4096. doi: 10.1074/jbc.M301804200. [PMID: 12890686] [DOI] [PubMed] [Google Scholar]
- 11.Schoonhoven A, Rudensky B, Elstein D, et al. Monitoring of Gaucher patients with a novel chitotriosidase assay. Clin Chim Acta. 2007;381:136–139. doi: 10.1016/j.cca.2007.02.042. [PMID:17408605] [DOI] [PubMed] [Google Scholar]
- 12.Bussink AP, van Eijk M, Renkema GH, et al. The biology of the Gaucher cell: the cradle of human chitinases. Int Rev Cytol. 2006;252:71–128. doi: 10.1016/S0074-7696(06)52001-7. [ PMID: 16984816] [DOI] [PubMed] [Google Scholar]
- 13.Boot RG, Verhoek M, de Fost M, et al. Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel Surrogate marker for assessing therapeutic intervention. Blood. 2004;103:33–39. doi: 10.1182/blood-2003-05-1612. [PMID: 12969956] [DOI] [PubMed] [Google Scholar]
- 14.Charrow J, Esplin JA, Gribble TJ, et al. Gaucher disease: recommendations on diagnosis, evaluation, and monitoring. Arch of Intern Med. 1998;158:1754–1760. doi: 10.1001/archinte.158.16.1754. [DOI] [PubMed] [Google Scholar]
- 15.Zimran A, Kay A, Gelbart T, et al. Gaucher disease. Clinical, laboratory, radiologic, and genetic features of 53 patients. Medicine (Baltimore) 1992;71:337–353. [ PMID 1435229] [PubMed] [Google Scholar]
- 16.Olynyk JK, Cullen DJ, Aquilia S, et al. A population- based study of the clinical expression of the hemochromatosis gene. N Engl J Med. 1999;341:718–724. doi: 10.1056/NEJM199909023411002. [PMID10471457] [DOI] [PubMed] [Google Scholar]
- 17.Scheuer PJ, Williams R, Muir AR. Hepatic pathology in relatives of patients with haemochromatosis. J Pathol and Bacteriol. 1962;84:53–64. [PubMed] [Google Scholar]
- 18.Deugnier YM, Turlin B, Powell LW, et al. Differentiation between heterozygotes and homozygotes in genetic hemochromatosis by means of a histological hepatic iron index: a study of 192 cases. Hepatology. 1993;17:30–34. [PMID: 8423039] [PubMed] [Google Scholar]
- 19.Poll LW, Koch JA, Willers R, et al. Correlation of bone marrow response with hematological, biochemical, and visceral responses to enzyme replacement therapy of nonneuronopathic (type 1) Gaucher disease in 30 adult patients. Blood Cells Mol Dis. 2002;28:209–220. doi: 10.1006/bcmd.2002.0511. [PMID: 12064917] [DOI] [PubMed] [Google Scholar]
- 20.Brinkman J, Wijburg FA, Hollak CE, et al. Plasma chitotriosidase and CCL18: early biochemical surrogate markers in type B Niemann-Pick disease. J Inherit Metab Dis. 2005;28:13. doi: 10.1007/s10545-005-4416-9. [PMID:15702402] [DOI] [PubMed] [Google Scholar]
- 21.Lee RE, Balcerzak SP, Westerman MP. Gaucher's disease. A morphologic study and measurements of iron metabolism. Am J Med. 1967;42:891–898. doi: 10.1016/0002-9343(67)90070-8. [PMID: 6067457] [DOI] [PubMed] [Google Scholar]
- 22.Lorber M. Adult-type Gaucher's disease: a secondary disorder of iron metabolism. Mt Sinai J Med. 1970;37:404–417. [PMID: 4194895] [PubMed] [Google Scholar]
- 23.Van Slyck EJ, Waldmann R, Rebuck JW. Letter: Unavailability of iron in Gaucher's cells. N Engl J Med. 1974;291:261. doi: 10.1056/nejm197408012910521. [ PMID: 4834760] [DOI] [PubMed] [Google Scholar]
- 24.Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol. 2009;4:489–515. doi: 10.1146/annurev.pathol.4.110807.092205. [PMID: 19400694] [DOI] [PubMed] [Google Scholar]
- 25.Mizukami H, Mi Y, Wada R, et al. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109(9):1215–1221. doi: 10.1172/JCI14530. [PMID 11994410] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimitriou E, Verhoek M, Altun S, et al. Elevated plasma chemokine CCL18/PARC in betathalassemia. Blood Cells Mol Dis. 2005;35:328–331. doi: 10.1016/j.bcmd.2005.07.006. [PMID: 16137900] [DOI] [PubMed] [Google Scholar]
- 27.Ashkenazi A, Zaizov R, Matoth Y. Effect of splenectomy on destructive bone changes in children with chronic (Type I) Gaucher disease. Eur J Ped. 1986;145:138–141. doi: 10.1007/BF00441877. [PMID: 3732318] [DOI] [PubMed] [Google Scholar]
- 28.Harmatz P, Butensky E, Quirolo K, et al. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76–79. [PMID: 10891433] [PubMed] [Google Scholar]
- 29.ICGG Annual Report. 2009 available at http://www.lsdregistry.net/gaucherregistry.
- 30.Weinreb NJ, Charrow J, Andersson HC, et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113:112–119. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- 31.St. Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver concentrations using proton magnetic resonance. Blood. 2005;105:855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.