Abstract
Mechanisms underlying the triggers and maintenance of atrial fibrillation(AF) are not fully understood. One potential unproven mechanism is that gastroesophageal reflux disease(GERD) where acid reflux induces local and systemic inflammation may increase in triggered activity in the myocardium and pulmonary veins and increase AF risk. A self-report questionnaire was mailed to a random sample of 5288 Olmsted County residents aged 25-74 years to assess the presence and frequency of GERD from 1988-1994. Long-term risk of AF over a period of 11.4 ± 5.0 years was determined through review of clinical evaluations and the electrocardiogram database in those without prior AF. The average age was 53±17 years and 2571(49%) were male. Of these patients, 741 developed AF[cumulative probability of AF was 20%(95% CI 17-22%) at 18 years]. Age[HR 1.09(95% CI 1.08-1.10),p<0.001], male gender[HR 1.81(95% CI 1.53-2.14),p<0.001], hypertension[HR 1.36(95% CI 1.14-1.61),p=0.0006), and heart failure[HR 1.74(95% CI 1.16-2.60),p=0.007) were independently associated with the risk of AF. The presence of any GERD was not associated with risk of AF[HR 0.81(95% CI 0.68-0.96),p=0.014] after adjustment for other risk factors. Frequency of GERD did not significantly impact risk of AF, although patients with more frequent GERD had a slightly higher AF risk. Esophagitis increased risk of AF [HR = 1.94(95% CI 1.35-2.78),p<0.001], but the association did not persist when accounting for other risk factors(p=0.72). In conclusion, in this large population-based study of patients surveyed for GERD, we did not find an association with presence or frequency of symptoms and AF. Patients with esophagitis were more likely to develop AF, although this association requires further study.
Introduction
New risk factors or risk “markers” for AF continue to be reported. Broadly these risk factors include systemic inflammation1, obesity and sleep apnea2, alcohol3,4, and specific genetic mutations5-7. Environmental factors also play a key role in certain situations. In one study of patients who developed lone AF, the environment triggers were variable but distinct and included: sleeping (44%), exercise (36%), alcohol use (36%), and eating (34%).8 Although the role of sleeping2 and alcohol intake3,4 have been previously established, less is known about mechanisms underlying the association of AF and the gastrointestinal tract. One potential explanation is that gastroesophageal reflux disease (GERD) underlies the association of eating and AF. A study of 3 patients showed that AF onset was associated with a pH drop during 24 hour intraesophageal pH monitoring.9 It is reasonable to surmise that this association exists due to the proximity of the esophagus, left atrium, and pulmonary veins. Focal inflammation of the esophagus may inflame the myocardial and pulmonary vein tissues and increase the risk of triggered atrial activity. Also, it is conceivable that systemic effects from cytokine release and impaired esophageal contractility associated with GERD could potentially increase the risk of AF.10 Therefore, to examine this potential association we undertook a large population-based study that surveyed the presence and frequency of GERD and long-term risk of AF.
Methods
Olmsted County has a population of nearly 120,000 people based upon the United States census in 2005. Nearly 80% of the population resides within 5 miles of the city of Rochester. The health care is predominantly provided by two groups: Mayo Medical Center and the Olmsted Medical Center. Within these two health systems medical diagnoses and surgical procedures are indexed when made as outpatients, emergency room visits, nursing home care, hospital admissions, and death certificates.11 This database allows investigation of the impact of diseases on a population over time. Using this database a random sample of the population was obtained with ages from 25-74 years between 1988 and 1994. Patients were excluded from the estimation of the AF risk if they had a preexisting diagnosis of AF. This database was used to abstract the general patient demographics as contained in Table 1. The diagnoses were determined by the attending physician and not based upon strict criteria.
Table 1.
Baseline demographics of Olmsted County patients based upon presence of any gastroesophageal reflux disease symptoms
| GERD Symptoms | |||
|---|---|---|---|
| Variable | Yes (n=2577) |
No (n=2706) |
p value |
| Age (years) | 51 ± 17 | 55 ± 18 | 0.0001 |
| Men | 1316 (51%) | 1253 (46%) | 0.0006 |
| Hypertension | 592 (23%) | 649 (24%) | 0.399 |
| Diabetes Mellitus | 200 (8%) | 238 (9%) | 0.178 |
| Dyslipidemia | 478 (19%) | 454 (17%) | 0.097 |
| Coronary artery disease | 217 (8%) | 239 (9%) | 0.596 |
| Prior myocardial infarction | 133 (5%) | 155 (6%) | 0.396 |
| Congestive heart failure | 49 (2%) | 84 (3%) | 0.006 |
| Prior atrial fibrillation | 69 (3%) | 113 (4%) | 0.003 |
In order to assess the frequency of heartburn in the community, patients were sent a study questionnaire as previously reported.12 The gastroesophageal reflux questionnaire was designed as a self-report instrument. The following definitions were used to define GERD as previously reported12: 1) heartburn, a burning pain or discomfort behind the breast bone in the chest; 2) acid regurgitation, a bitter- or sourtasting fluid coming into the throat or mouth; 3) chest pain, any pain or discomfort felt inside the chest but no including heartburn or any pain that is primarily in the abdomen; 4) dysphagia (trouble swallowing), a feeling that food sticks in the throat or chest; 5) globus, a feeling as if there is a lump in the throat when not swallowing, 6) dyspepsia, an ache or pain occurring mainly in the upper abdomen and not including heartburn, chest pain, or pain with menstrual periods, 7) hoarseness, rough and harsh voice; 8) bronchitis, cough as often as 4 to 6 times a day on 4 or more days a week. Questions were also made to determine if the patient had asthma, heart disease, or pneumonia. Symptoms frequency was then measures using a scale, 1: none in past year, 2: less than once a month, 3: about once a month, 4: about once a week, 5: several times a week, 6: daily). For the purposes of this study we used a simplified scale of none, some (less than once a month or monthly), weekly, and daily. This study questionnaire was sent to 5288 random residents of Olmsted County from 1988-1994. They received reminder letters at 2, 4, and 7 weeks. Those who indicated at any point that they did not want to participate were not contacted further. Nonresponders were contacted by telephone to request their participation. The spectrum of response has been previously reported.12
In 2007 we then analyzed the records on the surveyed patients. AF was diagnosed from their medical records from both in- and out-patient evaluations, the electrocardiogram database, and International Classification of Diseases, Ninth Revision (ICD-9) codes.
Statistical Analysis
Continuous variables were reported as mean ± the standard deviation and categorical variables were summarized as percentages. Survival free of the end point of AF was estimated by the Kaplan-Meier method. These estimates were made using both the presence and frequency of GERD. Univariate associations of clinical variables with AF were assessed in a Cox proportional hazards model. Multivariate models were constructed using the stepwise selection technique with these Cox models.
Results
The average age was 53 ± 17 years and 2571 (49%) were male. Of these patients, 2577 (49%) reported GERD on the survey. One hundred and eighty two (3%) had a prior history of AF. These patients were excluded from the subsequent analysis that looked at impact of GERD on risk of AF.
Of the 2577 that reported GERD symptoms, the average age was 52 ± 17 years and 1316 (51%) were male. A comparison of the baseline variables based upon the report of GERD is listed in Table 1. Patients that reported GERD were younger and more often male. They were less likely to have prior AF or congestive heart failure. In Table 2, demographics are listed based upon frequency of GERD. Patients with daily GERD symptoms were older and more likely to have coronary artery disease and a prior myocardial infarction.
Table 2.
Baseline demographics of Olmsted County patients based upon frequency of gastroesophageal reflux disease symptoms
| GERD Symptoms | |||||
|---|---|---|---|---|---|
| Variable | Daily (n=115) |
Weekly (n=653) |
Some (n=1809) |
None (n=2706) |
p value |
| Age (years) | 57 ± 16 | 53 ± 16 | 51 ± 17 | 54 ± 18 | 0.0001 |
| Men | 61 (53%) | 331 (51%) | 924 (51%) | 1253 (46%) | 0.007 |
| Hypertension | 30 (26%) | 171 (26%) | 391 (22%) | 649 (24%) | 0.072 |
| Diabetes Mellitus | 9 (8%) | 51 (8%) | 140 (8%) | 238 (9%) | 0.601 |
| Dyslipidemia | 27 (23%) | 136 (21%) | 315 (17%) | 454 (17%) | 0.033 |
| Coronary artery disease | 22 (19%) | 55 (8%) | 140 (8%) | 239 (9%) | 0.0004 |
| Prior myocardial infarction | 12 (10%) | 33 (5%) | 88 (5%) | 155 (6%) | 0.062 |
| Congestive heart failure | 1 (1%) | 10 (2%) | 38 (2%) | 84 (3%) | 0.030 |
| Prior atrial fibrillation | 3 (3%) | 14 (2%) | 52 (3%) | 113 (4%) | 0.021 |
| Esophagitis | 20 (17%) | 70 (11%) | 53 (3%) | 30 (1%) | 0.0001 |
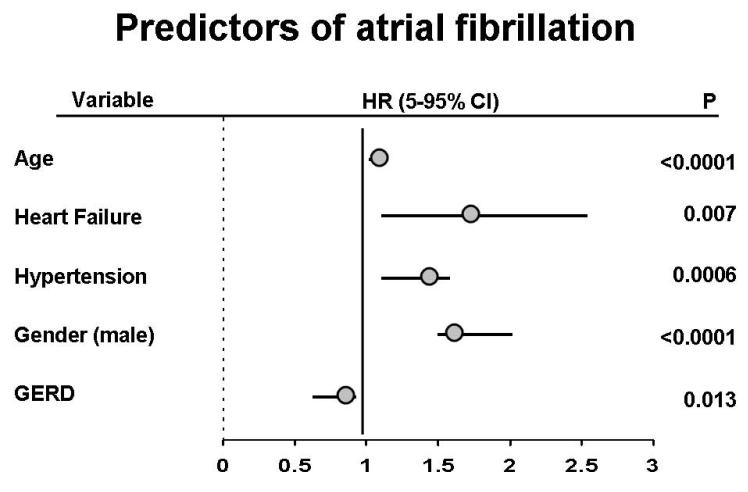
Of the 5288 patients initially surveyed, 741 (14%) developed AF over a follow up period of 11.4 ± 5.0 years. Of the baseline variables listed in Table 1, age, gender, hypertension, congestive heart failure, and esophagitis were associated with the risk of AF (Figure 1). There was a slight, but significant inverse association with reported GERD symptoms and risk of AF.
Figure 1.
Hazard ratios are displayed for each demographic factor associated with AF. The presence of any GERD symptoms was added to the model and slightly decreased the risk of AF [HR 0.81 (95% CI 0.68-0.96), p=0.013].
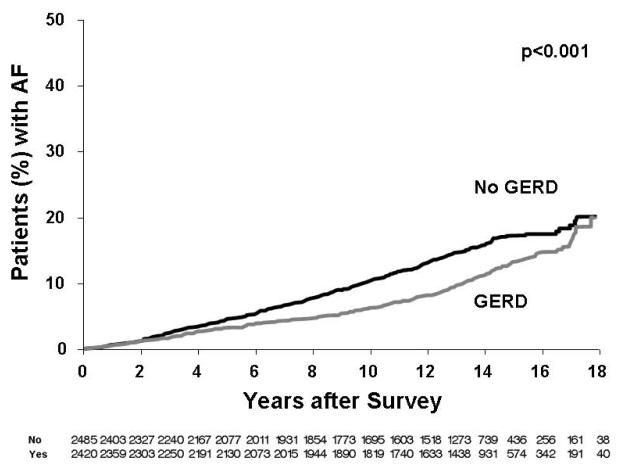
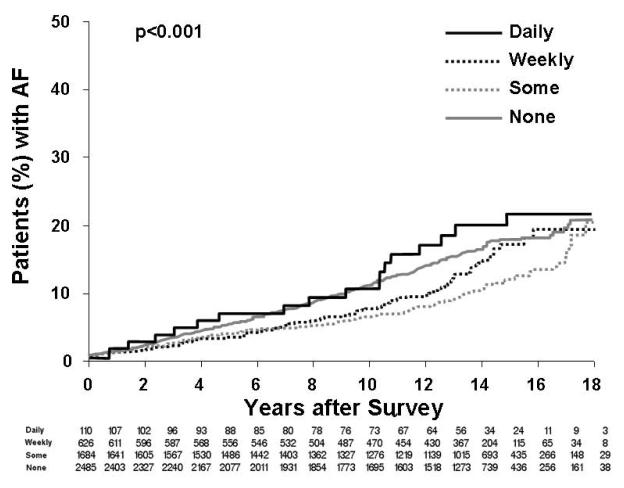
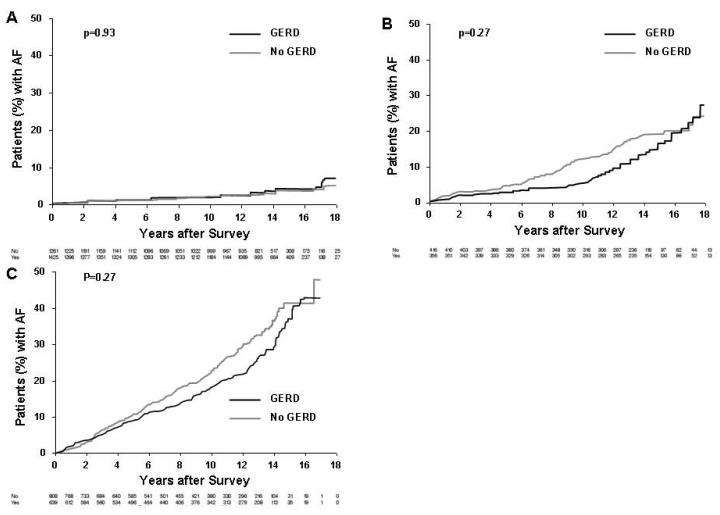
Figure 2 displays the Kaplan Maier analysis examining the association of any reported GERD symptoms and AF. In this general analysis there was an inverse association of GERD symptoms and risk of AF (p<0.001). Next, Figure 3 displays the association of frequency of GERD symptoms and AF. Although those with daily symptoms had a higher risk of AF, it was of borderline significance in comparison to the other GERD-symptom groups [hazard ratio 1.54 (95% Confidence intervals: 0.96 -2.46), p=0.07] with no significance found when adjusting for other confounding variables (p=0.260). Finally, shown in Figure 4 is subgroup analysis based upon age. We did not find a clear association of risk with GERD symptoms in 3 age-based groups (<50 years, 50-65 years, >65 years). However, as expected the risk of AF in general was much greater in the older groups (Figure 4B, 4C) compared to the younger group (Figure 4A).
Figure 2.
Kaplan Maier analysis of the presence or absence of GERD symptoms and long-term risk of AF.
Figure 3.
Kaplan Maier analysis of the presence and frequency of GERD symptoms and long-term risk of AF. Daily GERD symptoms had the highest risk of AF [HR 1.30 (95% CI: 0.98-1.57), p=0.07].
Figure 4.
Kaplan Maier analysis of the presence or absence of GERD symptoms and long-term risk of AF based upon age. A includes patients less than 50 years of age. B includes patients from 50 to 65 years of age. C includes patients greater than 65 years of age. In general, the risk of AF increased with advancing age.
Finally, 173 patients had known esophagitis based upon ICD-9 codes when they were surveyed. We do not know if all of these underwent endoscopy. The general demographics of those patients with and without esophagitis are listed in Table 3. Patients with esophagitis were older, more likely to have coronary artery disease, a prior myocardial infarction, and AF. Of these patients, 45 (26%) developed AF as opposed to 696 (14%) in the group without esophagitis. There was an increased hazard of AF over time based upon the diagnosis of esophagitis [HR = 1.94(95% CI 1.35-2.78), p<0.001]. However, the hazard did not remain significant when accounting for age, sex, hypertension, and heart failure (p=0.72).
Table 3.
Baseline demographics of Olmsted County patients based upon presence of the diagnosis of esophagitis.
| Esophagitis | |||
|---|---|---|---|
| Variable | Yes (n=173) |
No (n=5115) |
p value |
| Age (years) | 64 ± 14 | 53 ±17 | <0.0001 |
| Men | 103 (60%) | 2468 (48%) | 0.0035 |
| Hypertension | 75 (43%) | 1168 (23%) | <0.0001 |
| Diabetes Mellitus | 26 (15%) | 412 (8%) | 0.0011 |
| Dyslipidemia | 56 (32%) | 876 (17%) | <0.0001 |
| Coronary artery disease | 42 (24%) | 414 (8%) | <0.0001 |
| Prior myocardial infarction | 28 (16%) | 260 (5%) | <0.0001 |
| Congestive heart failure | 7 (4%) | 126 (2%) | 0.191 |
| Renal failure | 3 (2%) | 38 (1%) | 0.144 |
| Prior atrial fibrillation | 14 (8%) | 168 (3%) | 0.0006 |
Discussion
In this large population-based study followed over a period more than a decade, we found an inverse relationship between presence of GERD and AF. Frequency of AF did impact this relationship, with patients that reported daily GERD symptoms having the highest risk of AF, although the risk differences did not reach statistical significance. This latter observation may have been due to confounding variables as these patients were more likely to be older and have prior coronary artery disease and esophagitis.
In general, the study results were contrary to what we had hypothesized. In fact, GERD symptoms as a whole resulted in AF less often. There are several potential explanations for this finding.
First, GERD patients that are symptomatic from a noncardiac disease may seek medical treatment more often than those without these symptoms. Repeat medical attention may also result in identification and treatment of traditional risk factors of AF such as hypertension, diabetes, and congestive heart failure.13-16 The observation that there were similar rates of these traditional risk factors in the GERD and no GERD groups makes this possibility less likely. Similarly, if patients with GERD presented more often to physicians then this action should also increase the likelihood of AF diagnosis, but the opposite was seen.
A second possibility is that the management of the disease may have secondary effects that reduce AF. For example, proton pump inhibitors are effective in the management of acid-related disorders. This class of medication reduces esophageal inflammation and extraesophageal manifestations of acid reflux such as noncardiac chest pain, asthma, and laryngitis.17-19 In a small study of patients with GERD with endoscopic findings of esophagitis and AF, proton pump inhibitors reduced the frequency and duration of palpitations. The mechanisms underlying the effect of this medication class on the arrhythmia are unknown, but the study suggests that these medications may play a role in the management of AF in select patients with GERD. Although this is a plausible explanation, the data set we presented did not include medication use information so we cannot test it within this population.
Third, in addition to traditional complications of GERD, injury to the distal esophagus may impair vagal nerve response, in particular nerve sensitization in the afferent pathways.20 This is important as vagal nerve mediated parasympathetic stimulation of the heart results in slowing of the sinus and ventricular rates and can increase atrial fibrillation inducibility.21,22 Subtle effects on vagal nerve function with GERD is a feasible possibility to explain the observed data on a population-based scale. Nonetheless, this hypothesis requires further study to confirm or refute its validity.
Fourth, a diagnosis of GERD is given to patients with symptoms suggestive of acid reflux. However, the amount of reflux and manifestations of reflux can be variable over time.23 For example, reflux of barium during radiographic evaluation is only positive in 25–75% of symptomatic patients and is falsely positive in up to 20% of normal controls.23-25 Also, the majority of patients with GERD will have a normal endoscopy26 a finding also suggested in our data be the low prevalence of esophagitis. For this reason esophagitis confirms the diagnosis of GERD, but lack of endoscopic findings does not exclude it.23 The discrepancy between symptoms and physical manifestations of injury in the distal esophagus may account for why there is no apparent risk of AF with GERD.
However, the finding that esophagitis was associated with AF support our hypothesis that local inflammation may increase risk of AF. Patients with daily GERD symptoms were more likely to have esophagitis which may account for some of the increased risk of AF in this group of patients. Unfortunately those with esophagitis had many other risk factors of AF and when taking these into account that increased hazard did not persist. Nonetheless, it is within this population that further study is needed.
One area that requires further study is the possible association of a hiatus hernia, GERD, and AF. A hiatus hernia has the potential to mechanically irritate the left atrium resulting in arrhythmia. Furthermore, the hernia may also increase reflux and result in severe symptoms and esophagitis.
The study strengths include a large random community sample which minimizes selection bias. The survey used was subjective tool to assess for GERD rather than a more objective tool such as endoscopic diagnosis. However, the questionnaire has been previously validated and the prevalence rates reported similar to those of other populations.12,27 AF diagnosis was based upon codes ICD-9 codes from hospital dismissal summaries, the electrocardiogram database, and review of the in and out-patient medical records. Although this is a common means to look for AF in a population-based study, subclinical or asymptomatic AF can be undetected. We do not have information on AF subtype. AF subtype risk may vary with GERD symptoms. Unfortunately we do not have data on use of medications to treat GERD. A study to look at use of these GERD medications, duration of therapy, and compliance to the therapy may provide insight into if these therapies may impact AF. Finally, not all patients had endoscopy and we do not have information on the relative number of patients that did.
Acknowledgements
Supported by NIH Grant AR30582, The Rochester Epidemiology Project, Dr. Walter A. Rocca, PI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger PO, Wu CF, De La Cruz C, Jr., Weisse AB, Ahmed SS, Regan TJ. Arrhythmias and the “Holiday Heart”: alcohol-associated cardiac rhythm disorders. Am Heart J. 1978;95:555–562. doi: 10.1016/0002-8703(78)90296-x. [DOI] [PubMed] [Google Scholar]
- 4.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–1742. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 5.Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A, Bachinski LL, Roberts R. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 7.Ellinor PT, Shin JT, Moore RK, Yoerger DM, MacRae CA. Locus for atrial fibrillation maps to chromosome 6q14-16. Circulation. 2003;107:2880–2883. doi: 10.1161/01.CIR.0000077910.80718.49. [DOI] [PubMed] [Google Scholar]
- 8.Patton KK, Zacks ES, Chang JY, Shea MA, Ruskin JN, Macrae CA, Ellinor PT. Clinical subtypes of lone atrial fibrillation. Pacing Clin Electrophysiol. 2005;28:630–638. doi: 10.1111/j.1540-8159.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 9.Gerson LB, Friday K, Triadafilopoulos G. Potential relationship between gastroesophageal reflux disease and atrial arrhythmias. J Clin Gastroenterol. 2006;40:828–832. doi: 10.1097/01.mcg.0000225571.42890.a5. [DOI] [PubMed] [Google Scholar]
- 10.Rieder F, Cheng L, Harnett KM, Chak A, Cooper GS, Isenberg G, Ray M, Katz JA, Catanzaro A, O’Shea R, Post AB, Wong R, Sivak MV, McCormick T, Phillips M, West GA, Willis JE, Biancani P, Fiocchi C. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology. 2007;132:154–165. doi: 10.1053/j.gastro.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 13.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 15.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 16.Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI102–110. [PubMed] [Google Scholar]
- 17.Fass R, Fennerty MB, Ofman JJ, Gralnek IM, Johnson C, Camargo E, Sampliner RE. The clinical and economic value of a short course of omeprazole in patients with noncardiac chest pain. Gastroenterology. 1998;115:42–49. doi: 10.1016/s0016-5085(98)70363-4. [DOI] [PubMed] [Google Scholar]
- 18.Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med. 1996;100:395–405. doi: 10.1016/S0002-9343(97)89514-9. [DOI] [PubMed] [Google Scholar]
- 19.Wo JM, Grist WJ, Gussack G, Delgaudio JM, Waring JP. Empiric trial of high-dose omeprazole in patients with posterior laryngitis: a prospective study. Am J Gastroenterol. 1997;92:2160–2165. [PubMed] [Google Scholar]
- 20.Shaker R. Gastroesophageal reflux disease: beyond mucosal injury. J Clin Gastroenterol. 2007;41:S160–162. doi: 10.1097/MCG.0b013e318042d660. [DOI] [PubMed] [Google Scholar]
- 21.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 22.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 23.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 24.Ott DJ, Wu WC, Gelfand DW. Reflux esophagitis revisited: prospective analysis of radiologic accuracy. Gastrointest Radiol. 1981;6:1–7. doi: 10.1007/BF01890213. [DOI] [PubMed] [Google Scholar]
- 25.Sellar RJ, De Caestecker JS, Heading RC. Barium radiology: a sensitive test for gastro-oesophageal reflux. Clin Radiol. 1987;38:303–307. doi: 10.1016/s0009-9260(87)80077-6. [DOI] [PubMed] [Google Scholar]
- 26.Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol. 1997;32:965–973. doi: 10.3109/00365529709011211. [DOI] [PubMed] [Google Scholar]
- 27.Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Do distinct dyspepsia subgroups exist in the community? A population-based study. Am J Gastroenterol. 2007;102:1983–1989. doi: 10.1111/j.1572-0241.2007.01381.x. [DOI] [PubMed] [Google Scholar]