Abstract
Purpose
In addition to regulating body weight, leptin is also recognized for its role in the regulation of immune function and inflammation. The purpose of this study was to investigate the effect of leptin on Prevotella (P.) intermedia lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production in differentiated THP-1 cells, a human monocytic cell line.
Methods
LPS from P. intermedia ATCC 25611 was prepared by the standard hot phenol-water method. THP-1 cells were incubated in the medium supplemented with phorbol myristate acetate to induce differentiation into macrophage-like cells. The amount of TNF-α and interleukin-8 secreted into the culture medium was determined by enzyme-linked immunosorbent assay (ELISA). TNF-α and Ob-R mRNA expression levels were determined by semi-quantitative reverse transcription-polymerase chain reaction analysis.
Results
Leptin enhanced P. intermedia LPS-induced TNF-α production in a dose-dependent manner. Leptin modulated P. intermedia LPS-induced TNF-α expression predominantly at the transcriptional level. Effect of leptin on P. intermedia LPS-induced TNF-α production was not mediated by the leptin receptor.
Conclusions
The ability of leptin to enhance P. intermedia LPS-induced TNF-α production may be important in the establishment of chronic lesion accompanied by osseous tissue destruction observed in inflammatory periodontal disease.
Keywords: Leptin, Lipopolysaccharide, Prevotella intermedia, Tumor necrosis factor-α
INTRODUCTION
Periodontal disease is a chronic inflammatory process accompanied by destruction of surrounding connective tissue and alveolar bone, and sometimes loss of teeth [1]. The primary causative agents of periodontal disease are particular gram-negative anaerobic bacteria that accumulate in the gingival sulcus. Prevotella (P.) intermedia is a major periodontal pathogen that is dominant in the periodontal pockets of patients with adult periodontitis [2]. This bacterium has also been frequently recovered from subgingival flora in patients with acute necrotizing ulcerative gingivitis [3] and pregnancy gingivitis [4].
Lipopolysaccharide (LPS) is a major constituent of the outer membrane of gram-negative bacteria, including P. intermedia. It has the ability to trigger a number of host cells, especially mononuclear phagocytes, to produce and release a wide variety of pharmacologically active mediators, including interleukin (IL)-1β, IL-6, IL-8, and, most importantly, tumor necrosis factor alpha (TNF-α) [5].
Leptin, the 16-kDa nonglycosylated protein encoded by the ob gene, is synthesized mainly by adipocytes and plays a crucial role in the homeostasis of body weight by regulating food intake and energy expenditure, through its action on the hypothalamus [6-8]. In addition to regulating body weight, leptin is also recognized for its role in the regulation of angiogenesis [9], hematopoiesis [10], reproduction [11], immune function [12], and most importantly, inflammation [13].
Leptin has been classified as a cytokine because it shows tertiary structure similar to the class of long-chain helical cytokines that includes IL-6, IL-11, and leukemia inhibitory factor [14]. The biological activities of leptin are mediated through the interaction with its specific cell surface-leptin receptor, Ob-R, which exists in multiple isoforms sharing the same extracellular domain but differing in the length of transmembrane coding regions [12,15]. Leptin and Ob-R have been identified in various tissues and organs including the hypothalamus, adipose tissue, and gastric and intestinal mucosa, as well as in oral mucosa and the acinar cells of salivary glands [15-18]. The presence of leptin and Ob-R in oral mucosa and salivary glands suggests that the activity of leptin is also of significance to diseases affecting the oral cavity. The purpose of this study was to investigate the effect of leptin on P. intermedia LPS-induced TNF-α production in differentiated THP-1 cells, a human monocytic cell line.
MATERIALS AND METHODS
Bacteria and culture conditions
P. intermedia ATCC 25611 was used throughout. It was grown anaerobically on the surface of enriched Trypticase soy agar containing 5% (v/v) sheep blood, or in GAM broth (Nissui, Tokyo, Japan) supplemented with 1 µg/mL menadione and 5 µg/mL hemin. Culture purity was assessed by gram staining and plating on solid medium.
LPS isolation
LPS was prepared from lyophilized P. intermedia ATCC 25611 cells by the standard hot phenol-water method [19], and subsequently purified by treatment with nuclease and proteinase K. The yield of LPS was about 0.26%. The protein content of the purified LPS, determined by the method of Markwell et al. [20], was less than 0.1%. Coomassie blue staining of overloaded sodium dodecyl sulfate (SDS)-polyacrylamide gels did not reveal any visible protein bands in the purified LPS, confirming the purity of the preparation (data not shown).
Cell cultures
The human monocytic cell line THP-1 (American Type Culture Collection, Rockville, USA) was grown routinely in Nunc flasks in RPMI 1640 medium supplemented with 10% (v/v) heat inactivated FBS, 50 µM 2-mercaptoethanol, 1 mM sodium pyruvate, 25 mM HEPES, 100 U/mL of penicillin, and 100 µg/mL of streptomycin in a humidified chamber with 5% CO2/95% air at 37℃. The cells (5 × 105 cells/ml/well in 24-well culture plates) were incubated in the medium supplemented with 50 ng/mL of phorbol myristate acetate to induce differentiation into macrophage-like cells. The cells were allowed to differentiate and adhere to plastic for 72 hours and washed three times with medium. Various concentrations of P. intermedia LPS and leptin (R & D Systems, Minneapolis, USA) were then added and the cells were cultured for the indicated times, after which culture supernatants were collected and assayed for TNF-α.
Measurement of TNF-α and IL-8 production
The amount of TNF-α and IL-8 secreted into the culture medium was determined by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (OptEIA, BD Pharmingen, San Diego, USA) according to protocols recommended by the manufacturer. The sensitivity of the assay was 7.8 pg/mL, according to the manufacturer.
Reverse transcription-polymerase chain reaction (RT-PCR) and analysis of PCR products
Cells were plated in 100-mm tissue culture dishes, at a density of 5 × 106 cells per dish, and treated with various concentrations of P. intermedia LPS and leptin (R & D Systems, Minneapolis, USA) for the indicated periods of time. Following incubation, they were washed twice with phosphate buffered saline and collected by centrifugation. Total RNA was isolated with an RNeasy Mini Kit (Qiagen, Valencia, USA), according to the manufacturer's instructions. Synthesis of cDNA from the extracted RNA and subsequent amplification of the cDNA by RT-PCR were carried out with an AccuPower RT/PCR Premix kit (Bioneer, Seoul, Korea) and thermal cycler (GeneAmp PCR system 2400, PE Applied Biosystems, Foster City, USA). β-actin served as internal control. The number of cycles that ensured nonsaturating PCR conditions was established in preliminary experiments. Primer sequences and RT-PCR conditions are listed in Table 1. The PCR-amplified products were run on a 1.5% agarose gel containing ethidium bromide and visualized with UV light. The intensities of the PCR bands on gel photographs were quantified by densitometry.
Table 1.
Nucleotide sequences of probes used in RT-PCR assays and amplification conditions.
RT-PCR: reverse transcription-polymerase chain reaction, TNF: tumor necrosis factor.
Statistical analysis
Statistical analysis was performed using Student's paired t-test with P<0.05 considered statistically significant. Data are expressed as means±SD of three independent experiments.
RESULTS
Effect of leptin on production of TNF-α and IL-8
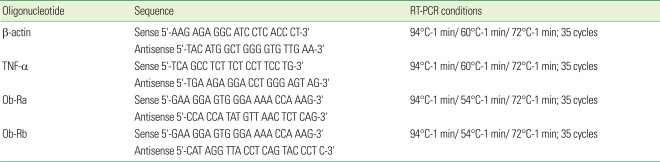
As shown in Fig. 1A, leptin induced a weak increase in TNF-α production in differentiated THP-1 cells, which became significant at 10 µg/mL. Moreover, P. intermedia LPS-induced TNF-α production was enhanced by leptin in a dose-dependent manner with a significant effect already seen at 0.01 µg/mL compared with LPS alone (Fig. 1B). Leptin at its concentration of 10 µg/mL elicited up to 3.25-fold increase in P. intermedia LPS-induced TNF-α production. Leptin increased IL-8 production without LPS with a significant effect at more than 1 µg/mL (Fig. 1A). However, P. intermedia LPS-induced IL-8 production was unaltered by leptin (Fig. 1B).
Figure 1.
Effect of leptin on the release of tumor necrosis factor (TNF)-α and interleukin (IL)-8 by differentiated THP-1 cells. Cells were incubated with increasing concentrations of leptin (0.01-10 µg/mL) in the absence (A) or presence (B) of Prevotella intermedia lipopolysaccharide (LPS) (0.01 µg/mL). Supernatants were removed after 24 hours and assayed for TNF-α and IL-8. The results are means±SD of three independent experiments.
Effect of leptin on TNF-α mRNA expression
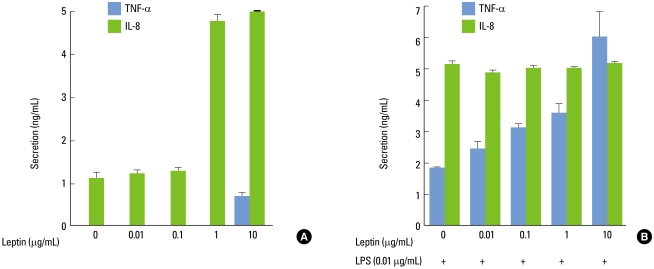
To investigate whether leptin modulates P. intermedia LPS-induced TNF-α production at the gene level, TNF-α mRNA levels were determined by semi-quantitative RT-PCR analysis. As shown in Fig. 2A, leptin induced a concentration-dependent increase in TNF-α mRNA expression in differentiated THP-1 cells, which became significant at 1 µg/mL. Moreover, leptin potentiated P. intermedia LPS-induced expression of TNF-α mRNA in a dose-dependent manner (Fig. 2B).
Figure 2.
Effect of leptin on the expression of tumor necrosis factor (TNF)-α mRNA in differentiated THP-1 cells. Cells were incubated with increasing concentrations of leptin (0.01-10 µg/mL) in the absence (A) or presence (B) of Prevotella intermedia lipopolysaccharide (LPS) (0.01 µg/mL). See MATERIALS AND METHODS for further details. The polymerase chain reaction bands on a gel photograph in one of two separate experiments yielding similar results are shown.
Effect on leptin receptor expression
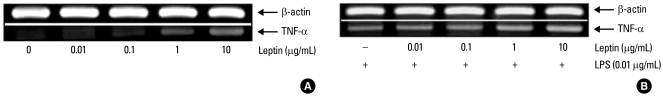
To verify whether the Ob-R is responsible for mediating the synergistic effect of leptin on P. intermedia LPS-induced TNF-α production in differentiated THP-1 cells, Ob-R mRNA expression levels were determined by semi-quantitative RT-PCR analysis. The long (Ob-Rb) and short (Ob-Ra) leptin receptors were expressed in untreated and leptin-treated cells, while upregulation of Ob-R by P. intermedia LPS stimulation or its potentiation by leptin was not confirmed (Fig. 3). As shown in Fig. 3, the levels of expression of the leptin receptors were similar in all samples.
Figure 3.
Effect of leptin on the expression of Ob-Ra and Ob-Rb mRNA in differentiated THP-1 cells. Cells were incubated with increasing concentrations of leptin (0.01-10 µg/mL) in the absence (A) or presence (B) of Prevotella intermedia lipopolysaccharide (LPS) (0.01 µg/mL). See MATERIALS AND METHODS for further details. The polymerase chain reaction bands on a gel photograph in one of two separate experiments yielding similar results are shown.
DISCUSSION
Because production of TNF-α has been recognized as a marker in a variety of human diseases associated with inflammation [21], the effect of leptin on P. intermedia LPS-induced TNF-α production in differentiated THP-1 cells, a human monocytic cell line, was studied. Macrophages are known to be the main producer of TNF-α and a dense infiltration of inflammatory cells, including macrophages, occurs in the gingival connective tissues of patients with periodontal disease [22].
Leptin levels are increased by inflammatory stimuli such as LPS and cytokines [23-25]. And, leptin enhances pro-inflammatory cytokine production and phagocytosis by macrophages [26]. The plasma levels of leptin have been reported to increase as periodontal disease progressed [27]. Conversely, it was shown that the leptin concentration is higher in healthy gingival tissue than in diseased tissue [28]. Further, the results of Karthikeyan and Pradeep [27] showed a strong negative correlation between the GCF leptin concentration and periodontal disease progression. Their results are in accordance with the study done by Johnson and Serio [29], who also showed that leptin concentration is correlated negatively with the probing pocket depth. The mechanism underlying the above findings is not known. Leptin may be used up as a substrate during inflammation.
The results of this study indicate that leptin potentiates P. intermedia LPS-induced TNF-α production in a dose-dependent manner. Leptin modulated P. intermedia LPS-induced TNF-α expression predominantly at the transcriptional level. There is evidence to suggest that TNF-α plays a central role in the pathogenesis of periodontal disease. TNF-α has been found at high levels in gingival crevicular fluids and in gingival tissues from periodontally diseased sites over those in healthy sites [30,31]. Moreover, it was shown that TNF-α has a strong potential to induce connective tissue degradation and alveolar bone resorption [32,33]. Furthermore, blockade of the activity of TNF-α was found to inhibit the inflammatory response and bone loss in a primate model of experimental periodontitis [34]. The ability of leptin to enhance P. intermedia LPS-induced TNF-α production may be important in the establishment of chronic lesion accompanied by osseous tissue destruction observed in inflammatory periodontal disease.
To verify whether the mechanism behind the synergistic effect of leptin with P. intermedia LPS might be related to an increase in Ob-R expression, Ob-R mRNA expression levels were determined by semi-quantitative RT-PCR analysis. This study demonstrated the presence of the long and short leptin receptors in differentiated THP-1 cells. However, leptin receptors were not modulated by P. intermedia LPS or leptin. These results indicate that effect of leptin on P. intermedia LPS-induced TNF-α production is not mediated by the leptin receptor. The mechanism by which leptin potentiates P. intermedia LPS-induced TNF-α production remains to be elucidated.
ACKNOWLEDGEMENTS
This work was supported by the Financial Supporting Project of Long-Term Overseas Dispatch of Pusan National University's Tenure-Track Faculty, 2007.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 2.Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung CP, Nisengard RJ, Slots J, Genco RJ. Bacterial IgG and IgM antibody titers in acute necrotizing ulcerative gingivitis. J Periodontol. 1983;54:557–562. doi: 10.1902/jop.1983.54.9.557. [DOI] [PubMed] [Google Scholar]
- 4.Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980;15:111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 5.Morrison DC, Ryan JL. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 7.Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flier JS. The adipocyte: storage depot or node on the energy information superhighway? Cell. 1995;80:15–18. doi: 10.1016/0092-8674(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 9.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 11.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 12.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 13.Janik JE, Curti BD, Considine RV, Rager HC, Powers GC, Alvord WG, et al. Interleukin 1 alpha increases serum leptin concentrations in humans. J Clin Endocrinol Metab. 1997;82:3084–3086. doi: 10.1210/jcem.82.9.4214. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–36. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 15.Halaas JL, Friedman JM. Leptin and its receptor. J Endocrinol. 1997;155:215–216. doi: 10.1677/joe.0.1550215. [DOI] [PubMed] [Google Scholar]
- 16.Hill RA, Margetic S, Pegg GG, Gazzola C. Leptin: its pharmacokinetics and tissue distribution. Int J Obes Relat Metab Disord. 1998;22:765–770. doi: 10.1038/sj.ijo.0800656. [DOI] [PubMed] [Google Scholar]
- 17.Breidert M, Miehlke S, Glasow A, Orban Z, Stolte M, Ehninger G, et al. Leptin and its receptor in normal human gastric mucosa and in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1999;34:954–961. doi: 10.1080/003655299750025039. [DOI] [PubMed] [Google Scholar]
- 18.Groschl M, Rauh M, Wagner R, Neuhuber W, Metzler M, Tamguney G, et al. Identification of leptin in human saliva. J Clin Endocrinol Metab. 2001;86:5234–5239. doi: 10.1210/jcem.86.11.7998. [DOI] [PubMed] [Google Scholar]
- 19.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. In: Whistler RL, editor. Methods in carbohydrate chemistry. General polysaccharides. Vol. 5. New York: Academic Press; 1965. pp. 83–91. [Google Scholar]
- 20.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 21.Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28:500–510. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 22.Stoufi ED, Taubman MA, Ebersole JL, Smith DJ, Stashenko PP. Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. J Clin Immunol. 1987;7:235–245. doi: 10.1007/BF00915729. [DOI] [PubMed] [Google Scholar]
- 23.Arnalich F, Lopez J, Codoceo R, Jim nez M, Madero R, Montiel C. Relationship of plasma leptin to plasma cytokines and human survivalin sepsis and septic shock. J Infect Dis. 1999;180:908–911. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- 24.Finck BN, Johnson RW. Tumor necrosis factor (TNF)-alpha induces leptin production through the p55 TNF receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:R537–R543. doi: 10.1152/ajpregu.2000.278.2.R537. [DOI] [PubMed] [Google Scholar]
- 25.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 26.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 27.Karthikeyan BV, Pradeep AR. Gingival crevicular fluid and serum leptin: their relationship to periodontal health and disease. J Clin Periodontol. 2007;34:467–472. doi: 10.1111/j.1600-051X.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 28.Karthikeyan BV, Pradeep AR. Leptin levels in gingival crevicular fluid in periodontal health and disease. J Periodontal Res. 2007;42:300–304. doi: 10.1111/j.1600-0765.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RB, Serio FG. Leptin within healthy and diseased human gingiva. J Periodontol. 2001;72:1254–1257. doi: 10.1902/jop.2000.72.9.1254. [DOI] [PubMed] [Google Scholar]
- 30.Rossomando EF, Kennedy JE, Hadjimichael J. Tumour necrosis factor alpha in gingival crevicular fluid as a possible indicator of periodontal disease in humans. Arch Oral Biol. 1990;35:431–434. doi: 10.1016/0003-9969(90)90205-o. [DOI] [PubMed] [Google Scholar]
- 31.Stashenko P, Jandinski JJ, Fujiyoshi P, Rynar J, Socransky SS. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991;62:504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J Clin Invest. 1997;100:1557–1565. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]