Abstract
Aims
In this study, we investigated the mechanisms by which caveolin-1 (CAV) inhibits increases in permeability induced by platelet activating factor (PAF) and elucidated the relationship between the endothelial intracellular Ca2+ concentration ([Ca2+]i) and CAV in regulating endothelial nitric oxide synthase (eNOS) activity and microvessel permeability in intact microvessels.
Methods and results
Experiments were conducted in individually perfused mesenteric venules in Sprague–Dawley rats. Permeability was determined by measuring hydraulic conductivity (Lp). Endothelial [Ca2+]i and nitric oxide (NO) production were measured in fura-2- and DAF-2-loaded microvessels. Perfusion of the CAV scaffolding domain, AP-CAV, at 1 µM for 30 min did not affect PAF-induced increases in endothelial [Ca2+]i but significantly attenuated PAF-induced NO production from 143 ± 2 to 110 ± 3% of control fluorescence intensity (FI). The PAF-induced Lp increase was correlatively reduced from a mean peak value of 7.5 ± 0.9 to 1.9 ± 0.5 times that of the control. Increasing extracellular [Ca2+] that potentiated PAF-induced peak [Ca2+]i from 500 to 1225 nM augmented NO production to 193 ± 13% and further increased Lp to 17.3 ± 1.6 times the control value. More importantly, enhanced Ca2+ influx restored the reduced NO production and Lp by AP-CAV with NO FI at 149% and Lp at 7.7 ± 1.1 times the control value.
Conclusion
Our results indicate that eNOS inhibition and reduced NO production contribute to the inhibitory action of AP-CAV on PAF-induced increases in permeability. CAV and endothelial [Ca2+]i antagonistically regulate eNOS activity in intact microvessels, and the level of produced NO is the key determinant of the degree of permeability increases during inflammation.
Keywords: Caveolin-1, Endothelial [Ca2+]i, eNOS, Microvessel permeability, Nitric oxide
1. Introduction
Increases in microvessel permeability occur in a variety of cardiovascular diseases, often resulting in tissue oedema and organ dysfunction. A better understanding of the mechanisms that regulate microvascular permeability is crucial for defining the pathogenesis of many disease conditions and aiding in the development of novel therapeutic approaches.
Nitric oxide (NO), in addition to its role as a potent vasodilator regulating vascular tone, blood pressure, and local blood flow, has also been recognized as an important intrinsic modulator of microvessel permeability. Early studies in whole vascular beds reported that the inhibition of NOS increased basal levels of permeability and potentiated agonist-induced permeability increases, indicating that NO plays a protective role for vascular walls.1,2 In contrast, studies from our laboratory and others have demonstrated that pharmacological NOS inhibitors attenuated agonist-induced increases in microvessel permeability.3–6 Although this paradox remains to be resolved, recent evidence has suggested that the physiological functions of NO are modulated under inflammatory conditions. Whereas basal levels of NO are anti-inflammatory due to its roles in preventing leucocyte adhesion and platelet aggregation, inflammatory stimuli-induced NO exerts pathological effects, including increases in microvessel permeability.3,7–11 However, owing to the non-specificity of NOS inhibitors, previous studies have been unable to determine the NOS isoforms responsible for the increased microvessel permeability under inflammatory conditions. The important role of endothelial nitric oxide synthase (eNOS) activation during inflammation has only been recognized recently with the discovery of the scaffolding domain of caveolin-1 (CAV) that directly interacts with eNOS, and the use of Antennapedia homeodomain (AP) fusions to facilitate CAV uptake by living cells.7–9,11,12 The application of AP-CAV suppressed tissue oedema and vascular leakage in whole animals8,9 and prevented permeability increases in individually perfused microvessels.7,11 These in vivo studies implicate the involvement of eNOS activation under inflammatory conditions.
Endothelial NOS is markedly enriched in caveolae and co-localizes with CAV.10,13 Cell culture studies have shown that the over-expression of caveolin significantly attenuated eNOS activity and that purified calmodulin (CaM) reverses this inhibition, suggesting that CAV and Ca2+/CaM counteractively regulate eNOS activity.14,15 However, the direct measurement of NO with exogenously applied CAV in intact microvessels has not been explored, and the antagonistic relationship between CAV and Ca2+/CaM in regulating stimuli-induced eNOS activity in vivo remains unidentified. In addition, cell-culture studies reported that AP-CAV markedly reduced thrombin-induced Ca2+ influx,16 an essential signal for permeability increases.17,18 Considering the proximity of CAV to the signalling machinery regulating Ca2+ entry within caveolae,19 it is possible that the inhibitory role of AP-CAV in microvessel permeability is due to its inhibition on agonist-induced Ca2+ entry.
This study aims to address these questions by investigating whether the inhibitory role of AP-CAV in platelet activating factor (PAF)-induced permeability increases is due to its inhibition of eNOS, and further elucidate the relationship between endothelial [Ca2+]i and CAV in regulating eNOS activity and microvessel permeability in intact microvessels. Experiments were designed to closely maintain in vivo physiology by using individually perfused venular microvessels in rat mesenteries. Permeability was determined by measuring hydraulic conductivity (Lp), and endothelial [Ca2+]i and NO were quantified in fura-2- and DAF-2-loaded microvessels, respectively. PAF was selected as a representative inflammatory mediator to increase microvessel permeability. The endothelial scaffolding domain of CAV level was modulated by perfusing vessels with AP-CAV. The competitive and antagonistic roles of endothelial [Ca2+]i and CAV in the regulation of eNOS activity and microvessel permeability were quantitatively evaluated when the relative magnitude of endothelial [Ca2+]i and CAV were modulated in PAF stimulated microvessels. Changes in microvessel Lp were measured under each experimental condition to elucidate the molecular basis of vascular function by correlating changes in microvessel permeability with the levels of second messengers in vivo.
2. Methods
2.1. Animal preparation
Experiments were carried out on female Sprague–Dawley rats (2–3 months old, 220–250 g, Hilltop Laboratory Animal, Scottdale, PA, USA). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All procedures and animal use were approved by the Animal Care and Use Committee at West Virginia University (06-0907). Surgery procedures and tissue preparation have been described.20 For details, see Supplementary material online.
2.2. Measurement of Lp in single perfused rat mesenteric microvessels
Modified Landis technique was used to measure the volume flux of water across the microvessel walls. The methods have been evaluated in detail.21,22 Briefly, a single microvessel was cannulated with a micropipette and perfused with albumin-Ringer solution (control) containing red blood cells as markers under certain hydrostatic pressure (range 40–60 cmH2O). Lp was calculated as the slope of the relationship between the initial water flow per unit area of microvessel wall and the pressure difference across the microvessel wall. In each experiment, the baseline Lp and the Lp after application of testing solutions were measured in the same vessel and the changes in Lp were expressed as the ratio of Lptest/Lpcontrol. See Supplementary material online for details.
2.3. Measurements of endothelial [Ca2+]i
Endothelial [Ca2+]i was measured in individually perfused microvessels using the fluorescent Ca2+ indicator fura 2-AM. A Nikon Diaphod 300 microscope equipped with a Nikon photometer was used for the measurements. In each experiment, the vessel was perfused with albumin-Ringer solution containing 10 µM fura 2-AM for 45 min, followed by albumin-Ringer perfusion for 10 min before collecting the fluorescence intensity (FI) from a segment of the vessel. At the end of each experiment, the microvessel was superfused with a modified Ringer solution (5 mM Mn2+ without Ca2+), while perfused with the same solution containing ionomycin (10 µM) to bleach the Ca2+-sensitive form of fura 2. The remaining background FI was subtracted from the signals. The ratios of the two FIfura values were converted to Ca2+ concentrations using an in vitro calibration curve.17 See Supplementary material online for details.
2.4. Fluorescence imaging of endothelial NO production
Endothelial NO was quantified at cellular levels in individually perfused microvessels using fluorescence imaging and 4,5-diaminofluorescein diacetate (DAF-2 DA), a fluorescent indicator for NO. The experimental rigs were the same as those for the Ca2+ measurements, except that a cooled, charge-couple device camera (ORCA; Hamamatsu) was used for image acquisition. In each experiment, a venular microvessel was perfused with albumin-Ringer solution containing DAF-2 DA (5 µM) for 45 min, followed by albumin-Ringer perfusion for 10 min before acquiring the control images. Images under control conditions and after exposure to testing agents were acquired from the same group of endothelial cells in each vessel using identical instrument settings. At the end of each experiment, the NO donor, sodium nitroprusside (SNP, 50 mM), was applied to the superfusate to examine the loading status. Selected regions of interest (ROIs) along the vessel wall were used to measure the FIDAF. Each ROI covers the area of one endothelial cell, as indicated by the fluorescence outline upon the addition of SNP. The maximum stimulated FIDAF (at plateau level) was compared with the control FIDAF measured in the same ROI and expressed as (FI/FI0) × 100, in which FI is the FIDAF after stimulation and FI0 is the control FIDAF. The criterion for a responsive cell was a significant increase in FIDAF relative to the control in each ROI. The increase in FIDAF in each vessel was the mean of the FIDAF changes from all the responsive cells.20 NO production rate is derived from differential conversion of the cumulative DAF-2 FI. The details have been described elsewhere20 and in Supplementary material online.
2.5. Solutions and reagents
Mammalian Ringer solution17 was used for the experiments. The high Ca2+ solution has the same composition as that in normal Ringer solution except that CaCl2 was increased from 2 to 10 mM. All the perfusates contained BSA (10 mg/mL). AP-CAV, the fusion peptide of the CAV scaffolding domain with AP, the Antennapedia internalization sequence from Drosophila AP, and the AP-CAV-X, which is the fusion peptide of scrambled CAV with AP were synthesized by Tufts University.8,11 The stock solutions were prepared with 100% DMSO and each final concentration was achieved by >1:1000 dilution with albumin-Ringer solution. See Supplementary material online for details.
2.6. Data analysis and statistics
All values are means ± SE. Paired t-test was used for paired data analysis. ANOVA was used to compare data between groups. A probability value of P < 0.05 was considered statistically significant. In summary figures, asterisk (*) indicates a significant increase from the negative or positive control, and dagger (†) indicates a significant decrease from the positive control.
3. Results
3.1. AP-CAV attenuates PAF-induced increases in Lp
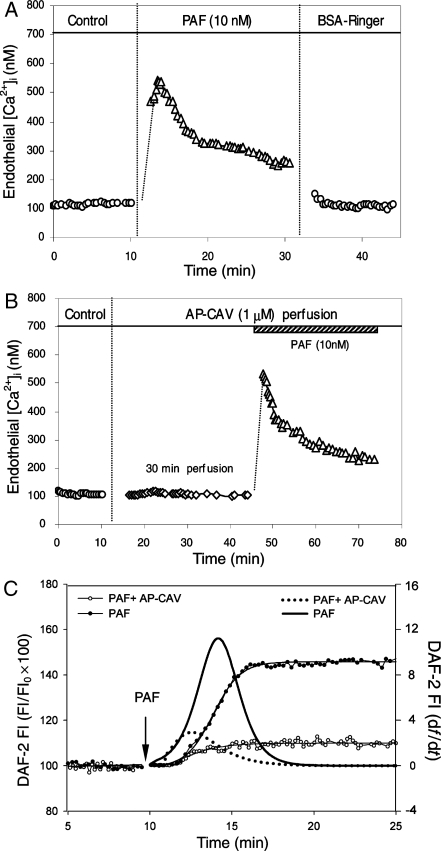
We first examined PAF-induced increases in Lp in the absence of AP-CAV application in six microvessels. The mean control Lp was 1.6 ± 0.2 × 10−7 cm s−1 cmH2O−1. A transient Lp increase was observed upon PAF (10 nM) addition. The peak Lp, occurred within 10 min of PAF perfusion, was 7.5 ± 0.9 times that of the control and then fell to 2.9 ± 0.3 times the control value in 20 min. Figure 1A shows a representative time course of PAF-induced Lp changes.
Figure 1.
AP-CAV attenuated PAF-induced Lp increases. (A) PAF induced a transient increase in Lp in the absence of AP-CAV. (B) PAF-induced Lp changes after AP-CAV (open symbols) or AP-CAV-X (filled symbols) perfusion from two individual experiments. Perfusion of 1 µM AP-CAV for 30 min abolished PAF-induced Lp increase and AP-CAP-X showed no effect.
Our previous study demonstrated that perfusion of mesenteric venules with AP-CAV at 10 µM for 2 h effectively attenuated PAF-induced increases in Lp.11 However, we do not know whether that is the minimum amount of AP-CAV needed to compete with PAF-induced increases in endothelial [Ca2+]i. To quantitatively evaluate the competing roles of CAV and endothelial [Ca2+]i in regulating microvessel permeability, we reduced the applied peptide concentration and perfusion time to identify the minimum amount of CAV required to inhibit PAF-induced eNOS activation and Lp increases. We first found that a 30 min perfusion with 0.1 µM AP-CAV had no effect on PAF-induced Lp increases (n = 2). Then we increased the AP-CAV concentration to 1 µM and kept the perfusion time at 30 min. The results from five microvessels showed that this combined concentration and perfusion time did not significantly affect the baseline Lp (1.5 ± 0.2 vs. 1.8 ± 0.4 × 10−7 cm s−1 cmH2O−1), but sufficiently attenuated PAF-induced peak Lp from 7.5 ± 0.9 to 1.9 ± 0.5 times that of the control (P < 0.01).
The specificity of the AP-CAV effect was examined in another four vessels using a scrambled CAV peptide, AP-CAV-X. Perfusion of AP-CAV-X (1 µM) for 30 min showed no effect on the baseline Lp (1.2 ± 0.1 vs. 1.3 ± 0.2 × 10−7 cm s−1 cmH2O−1) or PAF-induced increases in Lp (mean peak LpPAF/Lpcontrol was 9.2 ± 1.0). Figure 1B shows the comparison of PAF-induced increases in Lp with either AP-CAV or AP-CAV-X. Figure 5C summarizes the results.
Figure 5.
A summary of results demonstrating PAF-induced changes among endothelial [Ca2+]i, NO production, and microvessel Lp when the relative levels of endothelial CAV and [Ca2+]o varies. (A) PAF-induced changes in endothelial [Ca2+]i under either normal (2 mM, n = 4 for each group) or high (10 mM) [Ca2+]o, without (n = 6) or with (n = 7) AP-CAV (1 µM) application. (B) PAF-induced DAF-2 FI changes under either normal or high [Ca2+]o, in the absence or presence of AP-CAV (n = 5 for each group). (C) PAF-induced increases in Lp under normal [Ca2+]o (n = 6 for PAF alone; n = 2 for AP-CAV at 0.1 µM; n = 5 for AP-CAV at 1 µM; n = 4 for AP-CAV-X) or high [Ca2+]o (n = 5 for each group), in the absence or presence of AP-CAV. (D) The magnitude of PAF-induced increases in Lp, showing a linear correlation with the level of stimulated NO production under different experimental conditions.
3.2. AP-CAV inhibited PAF-induced NO production without affecting increased endothelial [Ca2+]i
We conducted two groups of experiments to investigate whether a reduction of Ca2+ influx contributes to AP-CAV-mediated inhibition on PAF-induced Lp increases. In the absence of AP-CAV, PAF transiently increased endothelial [Ca2+]i from a mean baseline of 112 ± 10 nM to a peak value of 500 ± 54 nM (n = 4). In another four microvessels, PAF-induced endothelial [Ca2+]i was measured after each vessel was perfused with AP-CAV (1 µM) for 30 min. The AP-CAV perfusion showed no effect on either baseline [Ca2+]i (94 ± 15 vs. 94 ± 8 nM) or PAF-induced increases in endothelial [Ca2+]i. The mean peak value upon PAF addition was 472 ± 34 nM, which was not significantly different from that measured in the absence of AP-CAV. Figure 2A and B shows a direct comparison of PAF-induced endothelial [Ca2+]i in the absence or presence of AP-CAV. Figure 5A summarizes the results.
Figure 2.
AP-CAV attenuated PAF-induced NO production without affecting endothelial [Ca2+]i. (A) PAF-induced transient increases in endothelial [Ca2+]i in the absence of AP-CAV. (B) PAF induced a similar magnitude increase in endothelial [Ca2+]i in a AP-CAV pre-perfused vessel, indicating that AP-CAV has no effect on PAF-induced Ca2+ influx. (C) PAF-induced NO production in the presence or absence of AP-CAV in two of the experiments. PAF-induced cumulative DAF-2 FI, an indication of increased NO production, in the presence (open circle) or absence (filled circle) of AP-CAV is shown as a function of time (left y-axis). The differential conversion of the cumulative FI (df/dt, right y-axis) represents the NO production rate (solid line: PAF alone; dotted line: with AP-CAV perfusion).
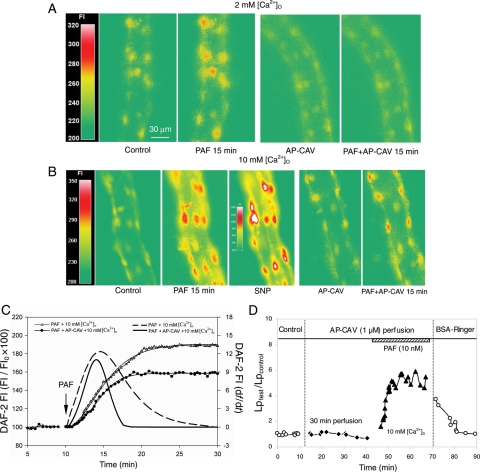
The effect of AP-CAV on PAF-induced NO production was examined in DAF-2-loaded vessels using fluorescence imaging. PAF-induced NO production in the absence of AP-CAV was quantified in 78 endothelial cells, (ROIs, 12–20 per vessel) in five microvessels, of which 83 ± 2% were responsive cells that showed significant increases in FIDAF relative to the control. The mean maximum FIDAF measured at plateau from all responsive cells was 143 ± 2% of the baseline. The effect of AP-CAV on PAF-induced NO production was examined in another five microvessels (total 54 ROIs, 9–13 per vessel). When PAF was applied to AP-CAV (1 µM) pre-perfused vessels, the number of responsive cells decreased to 34 ± 18% and the mean FIDAF of all responsive cells was only 110 ± 3% of the baseline level. Figure 2C shows the time-dependent cumulative FIDAF changes and the NO production rate in the presence or absence of AP-CAV. Figure 4A presents the images from two representative experiments. Figure 5B summarizes the results.
Figure 4.
AP-CAV and endothelial [Ca2+]i antagonistically regulate PAF-induced NO production and Lp increases. (A) Fluorescence images from two of the experiments showing the difference in PAF-induced NO production (DAF-2 FI changes relative to control) in the absence (left panel) or presence of applied AP-CAV (right panel). (B) Images from two of the experiments demonstrating the magnitude of the differences in NO production under normal and high [Ca2+]o. The left panel indicates that high [Ca2+]o potentiated PAF-induced NO production and the right panel shows that high [Ca2+]o partially restored AP-CAV-mediated NO reduction to a level comparable to that shown in the left panel of (A). The third image in the left panel shows further increases in FI after superfusion with NO donor sodium nitroprusside (SNP), which is the routine at end of each NO measurement to check the dye loading status. (C) The NO quantification from the images shown in (B) demonstrates that PAF stimulated cumulative DAF-2 FI and the calculated NO production rates as a function of time under high [Ca2+]o, with or without AP-CAV application. (D) Lp measurements showing that AP-CAV perfusion resulted in a moderate Lp increase under high [Ca2+]o, indicating that attenuated NO production by AP-CAV can override the high Ca2+ signal serving as a key determinant of Lp increases.
3.3. Increasing extracellular [Ca2+] potentiated PAF-induced increases in endothelial [Ca2+]i, NO production, and microvessel Lp
To evaluate whether eNOS activity positively correlates with the magnitude of endothelial [Ca2+]i, we investigated the effect of enhanced [Ca2+]i on NO production and microvessel Lp. Enhanced [Ca2+]i was achieved by increasing Ca2+ influx with high Ca2+ (10 mM) Ringer solution. When PAF was applied to vessels with high Ca2+ solutions, endothelial [Ca2+]i increased from a mean baseline of 120 ± 10 nM to a peak of 1225 ± 129 nM (n = 6), a remarkable increase from 500 ± 54 nM measured with normal Ringer solution (2 mM [Ca2+]o, P < 0.01). Figure 3A shows different [Ca2+]o-induced increases in endothelial [Ca2+]i upon PAF stimulation. Figure 5A summarizes the results.
Figure 3.
Increasing extracellular Ca2+, [Ca2+]o, potentiated PAF-induced increases in endothelial [Ca2+]i, NO production, and microvessel permeability. (A) Endothelial [Ca2+]i measurements from two of the experiments showing that increasing [Ca2+]o potentiates PAF-induced increases in endothelial [Ca2+]i. (B) High [Ca2+]o further increased PAF-induced NO production, as indicated by DAF-2 cumulative FI curves (open triangle vs. filled circle) and NO production rate (dashed line vs. solid line). (D) Lp measurements from two of the experiments showing a potentiated Lp increase relative to that measured with normal [Ca2+]o, which correlated with high magnitude increases in endothelial [Ca2+]i and NO production.
The effect of enhanced endothelial [Ca2+]i on eNOS activity was examined in five microvessels. When PAF was applied to each vessel with high Ca2+ solutions, 100% of the endothelial cells, a total of 62 ROIs at 9–20 per vessel, showed significant increases in FIDAF, and the mean maximum FIDAF from all the cells was 193 ± 12% relative to the control level. This was significantly higher than PAF-induced NO under normal [Ca2+]o (83% responsive cells with mean FIDAF at 143%, P < 0.01). Figure 3B shows the time-dependent FIDAF changes and the calculated NO production rate from two of the experiments under each condition. Figure 5B presents the summarized results.
The correlated changes in microvessel permeability with potentiated endothelial [Ca2+]i and NO production were examined in five vessels. PAF-induced peak Lp with high [Ca2+]o was 17.3 ± 1.6 times that of the control, a significant augmentation from that measured with normal [Ca2+]o (7.5-fold, P < 0.01). Figure 3C shows the comparisons of the Lp increases from two of the experiments. Figure 5C summarizes the Lp results.
3.4. Enhanced endothelial [Ca2+]i antagonistically competed with AP-CAV and restored the reduced NO production and microvessel permeability mediated by AP-CAV
Cultured cell studies have reported the reciprocal regulation of eNOS by Ca2+/CaM and caveolin.14 This study investigated the antagonistic roles of CAV and endothelial [Ca2+]i in eNOS activation and Lp increases in intact microvessels. NO was measured in five vessels in which AP-CAV was applied and PAF-induced endothelial [Ca2+]i was potentiated by high Ca2+ solutions. Figure 4B shows representative images of two individual experiments. After AP-CAV (1 µM) perfusion for 30 min, the PAF-induced mean maximum FIDAF with high [Ca2+]o was 149 ± 8% of the baseline, with responsive cells being 95 ± 5%, (total 52 ROIs, 8–13 per vessel). This is significantly lower than that measured in the absence of AP-CAV (FIDAF at 193 ± 12% with 100% responsive cells, P < 0.01), but higher than that measured with PAF under normal [Ca2+]o in the presence of AP-CAV (FIDAF at 110 ± 3%, P < 0.01). Figure 4C shows the differences in PAF-induced NO under these two experimental conditions. Figure 5B presents the summarized results.
The results shown above demonstrate that AP-CAV has no effect on PAF-induced Ca2+ influx under normal [Ca2+]o. Because AP-CAV has been reported to play a role in Ca2+ influx in cultured cells,16 we further evaluated the effect of AP-CAV on Ca2+ influx under high [Ca2+]o conditions. The mean baseline endothelial [Ca2+]i of seven microvessels was 100 ± 7 nM. Perfusion of AP-CAV showed no effect on either the basal level (104 ± 8 nM) or PAF-induced endothelial [Ca2+]i. The mean peak value with high Ca2+ solution was 1263 ± 126 nM, a magnitude not significantly different from 1225 ± 129 nM measured under the same experimental conditions without AP-CAV. Our results further confirmed that the application of AP-CAV plays no role in agonist-induced Ca2+ influx. Figure 5A summarizes these results.
To examine whether the reduced NO mediated by AP-CAV can override the potentiated endothelial [Ca2+]i in determining the magnitude of microvessel permeability, Lp was measured when PAF and high Ca2+ solutions were applied to AP-CAV pre-perfused microvessels (n = 5). AP-CAV (1 µM) perfusion for 30 min did not significantly change the baseline Lp (2.0 ± 0.2 vs. 2.1 ± 0.2 × 10−7 cm s−1 cmH2O−1). However, when each vessel was exposed to high Ca2+ solution and PAF, the mean peak LpPAF/Lpcontrol was only 7.7 ± 1.1, which is a marked attenuation from the peak Lp measured with high [Ca2+]o in the absence of AP-CAV (17.3 ± 1.6 times the control value, P < 0.05). These results indicate that the reduced NO by AP-CAV determines the degree of the permeability increase. Figure 4D shows one of the experiments. Figure 5C summarized the results of Lp. A direct correlation between the level of endothelial NO production and the magnitude of Lp increases under four different experimental conditions is plotted in Figure 5D that illustrates a linear relationship.
4. Discussion
This study demonstrates the molecular mechanisms of eNOS regulation and NO-dependent changes in microvessel permeability in intact vessels. The important findings are that (i) CAV and endothelial [Ca2+]i antagonistically regulate eNOS activity and their overall balance determines the level of NO production in intact microvessels and (ii) eNOS activation downstream from Ca2+ signalling is essential for increases in microvessel permeability during acute inflammation. Our measurements of endothelial [Ca2+]i and NO under identical experimental conditions demonstrated that AP-CAV inhibits PAF-induced permeability increases via its direct inhibition of eNOS and NO production. By modulating the relative magnitude of intracellular CAV and [Ca2+]i, we provided the first quantitative evidence in intact microvessels that under stimulated conditions CAV and Ca2+/CaM complexes antagonistically regulate eNOS activity and NO production. Most importantly, the results indicate that eNOS activity is the key determinant of permeability increases under stimulated conditions.
Although the application of pharmacological NOS inhibitors has been shown to attenuate permeability increases induced by a variety of inflammatory mediators for more than a decade,3–5,20,23,24 the role of eNOS in regulating microvessel permeability has only been demonstrated recently with the use of a membrane-permeable, eNOS-specific binding domain of CAV (AP-CAV).7,8,11,12 Fluorescence images and electron-microscopic micrographs have illustrated that the AP-facilitated delivery of CAV through single vessel perfusion is specifically localized within the endothelial caveolae.11 The actions of AP appear to be mediated through its direct interaction with membrane phospholipids, which are receptor-independent and non-endocytotic, even though detailed mechanisms have not been well defined.25 The measurements of hydraulic conductivity in individually perfused microvessels, which enable the changes in permeability to be separated from haemodynamic factor-induced flux changes, demonstrated that AP-CAV in endothelial cells significantly attenuates PAF-induced increases in microvessel permeability.7,11 Our present study provides molecular mechanisms for CAV-mediated inhibition of permeability increases in intact microvessels.
It has been well established that agonist-induced Ca2+ influx is a necessary initial signal for permeability increases.17,26 Studies using genetic approaches showed that CAV is required for agonist-stimulated Ca2+ entry into vascular endothelial cells and may play regulatory roles for Ca2+ influx.13 Cell-culture studies reported that AP-CAV reduced thrombin and thapsigargin-induced Ca2+ influx by direct interaction with a transient receptor-potential cation channel in human pulmonary artery endothelial cells.16 Our results demonstrated that AP-CAV has no effect on PAF-induced increases in endothelial [Ca2+]i under both normal and high [Ca2+]o conditions, yet significantly reduces PAF-induced NO production, indicating that the inhibition of PAF-induced permeability by AP-CAV is due to its inhibition of eNOS activity rather than of Ca2+ influx. These results are consistent with our previous study using the NOS inhibitor L-NMMA.20 The discrepancies between our results and the cultured cell studies16 may reflect the differences between tissue culture and intact microvessels or differences in cell origins. It also should be noted that the CAV scaffolding domain may not simulate the diverse functions of endogenous CAV and that the role of AP-CAV that we observed may only represent its inhibitory domain on eNOS function in vascular endothelial cells.
Endothelial NOS is a Ca2+/CaM-dependent enzyme, and increased [Ca2+]i has been shown to increase eNOS activity in cultured cells.14,15 This study quantitatively analysed this relationship in intact microvessels. Our previous study found that the agonist or superoxide-induced Ca2+ release from endothelial internal stores contributed about 30% of the initial rise in [Ca2+]i.17,27 About 70% of the initial peak are from Ca2+ influx through a passive conductance pathway, which could be potentiated by increasing extracellular [Ca2+].17,26 On the basis of these established methods, PAF-induced peak endothelial [Ca2+]i was potentiated from 500 to 1225 nM by increasing [Ca2+]o from 2 to 10 mM. Under these modified experimental conditions, PAF-stimulated NO markedly increased from 143 to 193% of the resting levels. These results demonstrate that the initial levels of stimulated endothelial [Ca2+]i positively correlate with the magnitude of NO production, indicating that the affinity of CaM for interacting with eNOS is directly related to the level of endothelial [Ca2+]i. Another interesting observation is that only 83% of the cells showed significant NO production upon PAF stimulation with normal [Ca2+]o, but 100% of the cells responded when [Ca2+]o was raised to 10 mM. Our previous study using Ca2+ imaging demonstrated variations in the magnitude of [Ca2+]i responses among endothelial cells in the same vessel segment.27 The heterogeneity of NO production with normal [Ca2+]o can therefore be the result of variations in PAF-induced [Ca2+]i among endothelial cells. Only under high [Ca2+]o conditions, when every endothelial [Ca2+]i reached the threshold for eNOS activation, did 100% of the cells show increased NO production. The eNOS among endothelial cells, however, may also have different activation thresholds in response to endothelial [Ca2+]i. Further simultaneous quantifications of endothelial [Ca2+]i and NO at the individual cell level in intact microvessels would provide detailed cellular mechanisms for this observation.
The potentiated endothelial [Ca2+]i-induced enhanced NO production also allowed us to further evaluate the relationship between endothelial [Ca2+]i and CAV in regulating eNOS activity and permeability in intact microvessels. The counteraction of Ca2+/CaM and CAV in regulating their common target enzyme eNOS has only been reported in cultured cells or purified proteins.14,15 This study demonstrates this mechanism for the first time in vivo. More importantly, this mechanism is correlated with quantitative measurements of microvessel permeability. Our results indicate that the perfusion of AP-CAV at 1 µM, not 0.1 µM, for 30 min provided the minimum amount of CAV required to override eNOS activation by PAF under normal [Ca2+]o. PAF-induced NO decreased from a mean of 143% FIDAF with 83% responsive cells in the absence of AP-CAV to 110% FIDAF with 34% responsive cells after AP-CAV perfusion. Under these experimental conditions, PAF-induced permeability increases showed a correlative reduction from 7.5 to 1.9 times of the control values. When PAF-induced endothelial [Ca2+]i was further potentiated with higher [Ca2+]o to 1225 nM, the reduced NO production by AP-CAV was restored from 110 to 149% of the resting levels, which made it comparable with that induced by PAF with normal [Ca2+]o without AP-CAV application. These results indicate that the applied AP-CAV antagonistically competes with the activated Ca2+/CaM in regulating eNOS activity and the overall balance between these two antagonistic regulatory proteins determines the level of NO production in stimulated intact microvessels.
Agonist-induced initial Ca2+ influx has been shown to correlate directly with the magnitude of permeability increases under most conditions.26,28 This study demonstrated that the magnitude of the changes in microvessel permeability are directly correlated with the level of NO rather than with endothelial [Ca2+]i (Figure 5D). This finding also suggests that NO regulates microvessel permeability downstream from endothelial [Ca2+]i. The direct regulation of cAMP or cGMP levels has also demonstrated a dissociation of Lp from increased endothelial [Ca2+]i in our previous studies.23 Taken together, these findings indicate that although an increase in endothelial [Ca2+]i is a necessary initial signal for increases in microvessel permeability, other regulatory mechanisms may modulate permeability downstream from Ca2+ entry.
PAF and other inflammatory mediator-induced increases in microvessel permeability have been recognized as the results of endothelial gap formation,18,29,30 which has been attributed to the contractile mechanism of actin–myosin interaction and/or the redistribution of adherens junctions between endothelial cells.30,31 This study indicates that CAV and endothelial [Ca2+]i counteractively regulate eNOS activity in intact microvessels and that eNOS activation is a critical step resulting in increased microvessel permeability during acute inflammation. The exact processes and signalling pathways between the increased intracellular NO and endothelial gap formation, however, remain to be identified. On the basis of the linear relationship between the magnitude of NO and Lp changes we observed at vessel level (Figure 5D), the variations of NO production among endothelial cells might directly associate with the heterogeneity of vascular leakages in vascular walls in response to inflammatory stimuli. Further simultaneous quantifications of endothelial [Ca2+]i, NO, and endothelial gap formation at the individual cell level in intact microvessels would provide detailed cellular mechanisms for permeability regulation. Furthermore, eNOS activity, in addition to being regulated by CAV and Ca2+/CaM, was also reported to be regulated by other proteins or via Ca2+-independent activation pathways, such as serine, threonine, or tyrosine phosphorylation. Although the regulation of eNOS by CAV and Ca2+/CaM under inflammatory conditions has been well documented by previous and the present studies, a heterogeneous picture of eNOS regulation and signalling is now emerging, and how these distinct pathways affect vascular functions remain to be investigated.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institute of Health Grant number HL56237 and HL084338 to P.H.
Supplementary Material
References
- 1.Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, et al. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- 2.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 3.He P, Liu B, Curry FE. Effect of nitric oxide synthase inhibitors on endothelial [Ca2+]i and microvessel permeability. Am J Physiol. 1997;272:H176–H185. doi: 10.1152/ajpheart.1997.272.1.H176. [DOI] [PubMed] [Google Scholar]
- 4.Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol. 1996;271:H2735–H2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- 5.Mayhan WG. Nitric oxide accounts for histamine-induced increases in macromolecular extravasation. Am J Physiol. 1994;266:H2369–H2373. doi: 10.1152/ajpheart.1994.266.6.H2369. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol. 1993;264:H1734–H1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- 7.Bernatchez PN, Bauer PM, Yu J, Prendergast JS, He P, Sessa WC. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc Natl Acad Sci USA. 2005;102:761–766. doi: 10.1073/pnas.0407224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 9.Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, et al. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci USA. 2005;102:904–908. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L, Schwegler-Berry D, Castranova V, He P. Internalization of caveolin-1 scaffolding domain facilitated by Antennapedia homeodomain attenuates PAF-induced increase in microvessel permeability. Am J Physiol Heart Circ Physiol. 2004;286:H195–H201. doi: 10.1152/ajpheart.00667.2003. [DOI] [PubMed] [Google Scholar]
- 12.Hatakeyama T, Pappas PJ, Hobson RW, II, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol. 2006;574:275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282:16631–16643. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 14.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 15.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem. 1997;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–1183. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 17.He P, Zhang X, Curry FE. Ca2+ entry through conductive pathway modulates receptor-mediated increase in microvessel permeability. Am J Physiol. 1996;271:H2377–H2387. doi: 10.1152/ajpheart.1996.271.6.H2377. [DOI] [PubMed] [Google Scholar]
- 18.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 19.Isshiki M, Anderson RG. Function of caveolae in Ca2+ entry and Ca2+-dependent signal transduction. Traffic. 2003;4:717–723. doi: 10.1034/j.1600-0854.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol. 2005;288:H2869–H2877. doi: 10.1152/ajpheart.01080.2004. [DOI] [PubMed] [Google Scholar]
- 21.Kendall S, Michel CC. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol. 1995;80:359–372. doi: 10.1113/expphysiol.1995.sp003853. [DOI] [PubMed] [Google Scholar]
- 22.Curry PE, Huxley VH, Sarelius IH. Cardiovascular Physiology. Techniques in the Life Sciences. New York: Elsevier; 1983. Techniques in microcirculation: measurement of permeability, pressure and flow. [Google Scholar]
- 23.He P, Zeng M, Curry FE. cGMP modulates basal and activated microvessel permeability independently of [Ca2+]i. Am J Physiol. 1998;274:H1865–H1874. doi: 10.1152/ajpheart.1998.274.6.H1865. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez MM, Quardt SM, Kim D, Oshiro H, Minnicozzi M, Duran WN. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc Res. 1995;50:223–234. doi: 10.1006/mvre.1995.1055. [DOI] [PubMed] [Google Scholar]
- 25.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 26.He P, Curry FE. Depolarization modulates endothelial cell calcium influx and microvessel permeability. Am J Physiol. 1991;261:H1246–H1254. doi: 10.1152/ajpheart.1991.261.4.H1246. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Wen K, Yuan D, Ai L, He P. Calcium influx-dependent differential actions of superoxide and hydrogen peroxide on microvessel permeability. Am J Physiol Heart Circ Physiol. 2009;296:H1096–H1107. doi: 10.1152/ajpheart.01037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He P, Curry FE. Endothelial cell hyperpolarization increases [Ca2+]i and venular microvessel permeability. J Appl Physiol. 1994;76:2288–2297. doi: 10.1152/jappl.1994.76.6.2288. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y, Wen K, Zhou X, Schwegler-Berry D, Castranova V, He P. Three-dimensional localization and quantification of PAF-induced gap formation in intact venular microvessels. Am J Physiol Heart Circ Physiol. 2008;295:H898–H906. doi: 10.1152/ajpheart.00309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin–myosin contraction. Am J Physiol Heart Circ Physiol. 2003;285:H406–H417. doi: 10.1152/ajpheart.00021.2003. [DOI] [PubMed] [Google Scholar]
- 31.Wysolmerski RB, Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci USA. 1990;87:16–20. doi: 10.1073/pnas.87.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.