Abstract
Aims
The aim of this study was to determine direct effects and potential molecular mechanisms of HIV gp120, a viral envelope glycoprotein, on endothelial function.
Methods and results
Fresh porcine coronary artery rings and human coronary artery endothelial cells (HCAECs) were treated with recombinant HIV gp120 for 16 h with or without pretreatment with tumour necrosis factor-alpha (TNF-α) (8 h). With a myograph tension analysis, HIV gp120 with TNF-α pretreatment significantly decreased endothelium-dependent vasorelaxation in response to bradykinin in porcine coronary artery rings compared with untreated control vessels. In addition, HIV gp120 with TNF-α pretreatment significantly reduced endothelial nitric oxide synthase (eNOS) expression—both mRNA and protein levels—in porcine coronary artery rings and HCAECs compared with untreated controls. Furthermore, TNF-α pretreatment substantially increased intercellular adhesion molecule-1 (ICAM-1) expression in artery rings and HCAECs. Anti-gp120 or anti-ICAM-1 antibody significantly blocked these effects of HIV gp120. Silencing of ICAM-1 by siRNA oligonucleotides significantly blocked the effect of gp120 on eNOS downregulation in TNF-α-pretreated HCAECs.
Conclusion
HIV gp120 and TNF-α synergistically reduce eNOS expression and cause endothelial dysfunction in both porcine coronary arteries and HCAECs. ICAM-1 induced by TNF-α pretreatment may mediate HIV gp120-induced endothelial dysfunction, which suggests a novel molecular mechanism of HIV gp120–ICAM-1 interaction inducing endothelial dysfunction.
Keywords: HIV gp120, Endothelial dysfunction, eNOS, ICAM-1, TNF-α
1. Introduction
HIV infection is a major health problem worldwide. There are over 30 million people living with HIV in the world. In the USA, over 1 million people are currently infected with HIV, and about 40 000 new cases are diagnosed each year.1 The advent of the highly active antiretroviral therapy era in HIV medicine has dramatically slowed the rate of progression from HIV to AIDS, lengthened patients' lifespans and improved quality of life, concomitant with a decline in opportunistic infections. As HIV-infected patients are living longer, many HIV-related complications such as cardiovascular disease are becoming major problems in this population. Recently, several clinical studies have suggested HIV-infected patients suffer from unexpectedly high rates of vascular diseases such as vasculitis, coronary atherosclerosis, strokes, and pulmonary hypertension in the absence of traditional risk factors, suggesting an association between HIV infection and vascular pathology in AIDS patients.2,3 However, very little information is available regarding the specific mechanism whereby HIV infection produces endothelial cell dysfunction.
HIV envelope glycoprotein (HIV gp120) facilitates viral entry by interacting with HIV receptor CD40 and coreceptors CXCR4 or CCR5.4 Recently, HIV gp120 has been demonstrated to have a potential biological role in the regulation of cell proliferation, cell death, and gene expression in T lymphocytes, macrophages, and central nervous system cells.4,5 HIV infection causes profound functional alterations in the endothelium, resembling the subclinical inflammation in atherosclerosis.6 HIV gp120 can directly and indirectly induce breakdown of tight junctions between endothelial cells of the blood–brain barrier as well as cause apoptosis and death of endothelial cells, which may contribute to the HIV-associated complications.7,8
HIV infection is often associated with systemic inflammation.9,10 Levels of proinflammatory factors such as tumour necrosis factor-alpha and -beta (TNF-α and TNF-β) and interferon gamma are increased in the serum of AIDS patients and in macrophages isolated from HIV-infected patients;4,9,10 Serum intercellular adhesion molecule-1 (ICAM-1) levels correlate well with AIDS progression in HIV-infected patients.11,12 The presence of increased levels of proinflammatory cytokines may contribute to the increased ICAM-1 expression in HIV infection. However, it is unknown whether HIV proteins have any functional relationship with these cell surface adhesion molecules. It is believed that HIV may directly and/or indirectly cause endothelial dysfunction and atherogenesis in HIV-positive patients.13
Endothelium-derived nitric oxide (NO), synthesized by the endothelial nitric oxide synthase (eNOS), is a major mediator of endothelium-dependent vasorelaxation. It is also critically involved in the regulation of other protective properties of healthy endothelium.14 In the current study, we hypothesized that HIV gp120 induces endothelial dysfunction in cytokine-activated endothelial cells, and cellular adhesion molecules are involved in the effects of HIV gp120. We examined the effect of HIV gp120 combined with TNF-α pretreatment on eNOS expression and endothelium-dependent vasorelaxation, as well as ICAM-1 involvement, in porcine coronary arteries and HCAECs. This study may provide new insights into the mechanisms behind the interaction of HIV gp120 with endothelial cells, which may contribute to vascular lesion formation.
2. Methods
Detailed Materials and Methods are provided in Supplementary material online.
2.1. Isometric tension of porcine coronary arteries
The myograph system used in this study has been previously described.15 Briefly, fresh porcine right coronary artery rings were pretreated with TNF-α (2 ng/mL) for 8 h. The rings were then cultured in fresh DMEM with gp120 (1 µg/mL) and other molecules for 16 h. The rings were then subjected to myograph analysis in response to thromboxane A2 analogue U46619 (contraction), bradykinin (endothelium-dependant vasodilation), and sodium nitroprusside (SNP) (endothelium-independent vasodilation).
2.2. Cell culture
Human coronary artery endothelial cells (HCAECs) were cultured with endothelial growth medium-2 (EGM-2) with growth supplements (SingleQuots) (BioWhittaker, Inc., Walkersville, MD, USA). The cells were pretreated with TNF-α (2 ng/mL) for 8 h. The cells were then cultured in fresh medium with HIV gp120 (1 µg/mL) or other molecules for 16 h. Serum starvation procedure was not used.
2.3. PCR
eNOS and ICAM-1 mRNA levels were analysed by conventional reverse transcription polymerase chain reaction (RT–PCR) and real-time RT–PCR.
2.4. Western blot
Porcine and human eNOS protein and human ICAM-1 protein levels were determined by western blot.
2.5. Immunohistochemistry
Immunoreactivity of eNOS and ICAM-1 in porcine coronary artery rings was determined by using the avidin–biotin complex immunoperoxidase procedure.15
2.6. Nitrite detection
The level of NO released from HCAECs was determined by measuring the accumulation of its stable degradation products, nitrite and nitrate (Griess reaction NO assay kit, Calbiochem).
2.7. Flow cytometry analysis
HCAECs were treated with or without TNF-α (2 ng/mL) for 8 h. Cell surface expression levels of ICAM-1, CD40, CXCR-5, CCR5, and DC-SIGN were determined by FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA) equipped with CellQuest software (Becton Dickinson).
2.8. Statistical analysis
All data are presented as the mean ± SEM. Sample size (n) refers to the number of independent experiments. Statistical analysis was completed by comparing multiple groups with a signal control group using an analysis of variance (ANOVA, one-way) followed by Bonferroni–Dunn's post hoc test (Minitab software, Sigma Breakthrough Technologies, Inc., San Marcos, TX, USA). A P-value less than 0.05 was considered statistically significant.
3. Results
3.1. HIV gp120 reduces endothelium-dependent vasorelaxation and eNOS expression in TNF-α-pretreated porcine coronary arteries and HCAECs
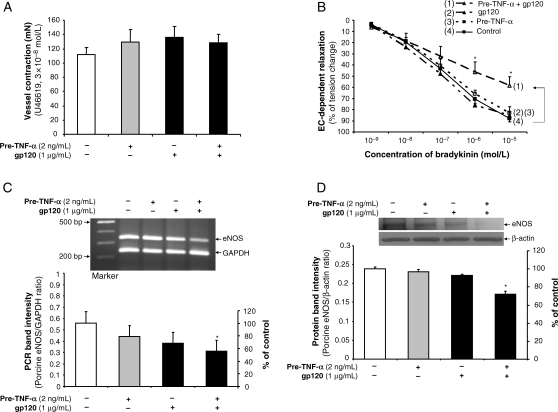
Porcine coronary artery rings were pre-incubated with or without TNF-α (2 ng/mL) for 8 h, and then treated with HIV gp120 (1 µg/mL) or vehicle for negative control (PBS) for another 16 h. Vasomotor function was studied using a well-characterized myograph system (n = 8). No significant difference was observed in all experimental groups in response to thromboxane A2 analogue U46619 (contraction) (Figure 1A) or SNP (endothelium-independent vasorelaxation) compared with controls (see Supplementary material online, Figure S1). However, in response to a series of increasing concentrations of bradykinin, endothelium-dependent vasorelaxation was significantly decreased in the vessel rings treated with HIV gp120 after pretreatment with TNF-α compared with control vessels, while a minimal effect was seen in rings treated with HIV gp120 alone or TNF-α alone compared with control vessels. Treatment with HIV gp120 after TNF-α pretreatment reduced vasorelaxation by 35% in response to bradykinin at 10−6 mol/L concentration compared with vessels in the control group (P < 0.05, ANOVA, Figure 1B).
Figure 1.
Effects of HIV gp120 and TNF-α on the vasomotor function and eNOS expression in porcine coronary arteries. Endothelium-intact porcine coronary artery rings were treated with TNF-α for 8 h and/or gp120 for 16 h. (A) Maximal contraction of the vessel rings in response to U46619 (3 × 10−8 mol/L) was analysed (n = 8). (B) Relaxation concentration response curves were generated by five cumulative additions of the endothelium-dependent vasodilator bradykinin (10−9, 10−8, 10−7, 10−6, and 10−5 mol/L) at 3 min intervals (n = 8, ANOVA). (C) eNOS mRNA levels were analysed by conventional RT–PCR (n = 7). (D) eNOS protein levels were analysed by western blot (n = 3). The bands were analysed and quantified by densitometry. The ratio of eNOS/GAPDH (mRNA) and ratio of eNOS/β-actin (protein) were evaluated. Results are means ± SEM. ANOVA test was used. *P < 0.05 vs. controls.
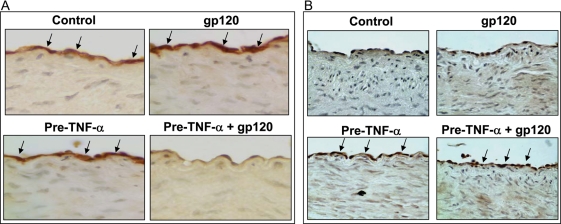
The eNOS mRNA and protein levels in the endothelial cells from these treated porcine artery rings were determined by conventional RT–PCR analysis and western blot, respectively (n = 3). The eNOS mRNA levels were significantly reduced by 36.7% in cells from rings treated with HIV gp120 after TNF-α pretreatment compared with controls (P < 0.05, Figure 1C). Western blot data showed that eNOS protein levels were likewise significantly decreased by 30% in cells treated with HIV gp120 after TNF-α pretreatment compared with controls (P < 0.05, Figure 1D). Minimal changes of eNOS expression in both mRNA and protein levels were detected in HIV gp120 alone or TNF-α pretreatment alone group compared with untreated controls (Figure 1C and D). Immunohistochemical staining also showed that immunoreactivity of eNOS was substantially reduced in the vessel rings treated with HIV gp120 after TNF-α pretreatment (Figure 2A).
Figure 2.
Effects of HIV gp120 and TNF-α on the immunoreactivity of eNOS and ICAM-1 in porcine coronary arteries. Porcine coronary artery rings were treated with TNF-α for 8 h and/or HIV gp120 for 16 h. Porcine coronary artery rings were fixed in formalin and embedded in paraffin. Immunostaining was performed. Brown colour represents positive staining of immunoreactivity. (A) eNOS immunoreactivity. (B) ICAM-1 immunoreactivity. Magnification ×400.
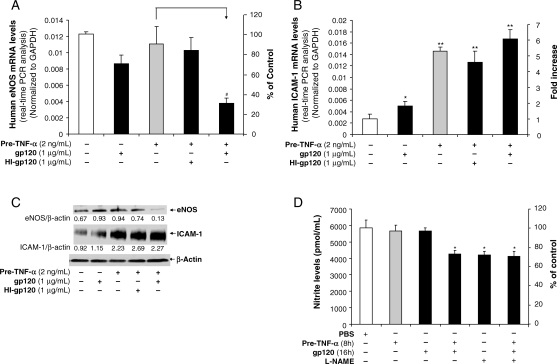
Consistent with the results in treated porcine artery rings, HIV gp120 treatment after TNF-α pretreatment in HCAECs substantially reduced eNOS mRNA and protein levels compared with the control group, whereas HIV gp120 treatment or TNF-α pretreatment alone had a minimal effect on eNOS mRNA and protein levels compared with controls (Figure 3A and C). Meanwhile, the NO levels in the supernatant of HCAEC cultures were determined by Griess reaction. A group of cells was also treated with NG-nitro-l-arginine methylester (l-NAME), which inhibits eNOS. Pretreatment with TNF-α or gp120 alone, without TNF-α pretreatment, did not affect NO levels in HCAECs compared with controls. However, treatment with gp120 after TNF-α pretreatment significantly reduced NO levels (P < 0.05). These NO levels were similar to those in cells treated with the l-NAME alone and in the cells treated with all three: pretreatment with TNF-α, then treatment with gp120 and l-NAME treatment (Figure 5D). Thus, these results indicate that gp120 reduces NO levels by inhibiting eNOS activities in TNF-α-pretreated HCAECs.
Figure 3.
Effects of HIV gp120 on eNOS expression and NO release in TNF-α-pretreated HCAECs. HCAECs were incubated in EGM-2 with TNF-α for 8 h and followed by HIV gp120 or heat-inactivated HIV gp120 for 16 h. (A) eNOS mRNA expression was examined by real-time RT–PCR. GAPDH was used as an internal control. n = 3. (B) ICAM-1 mRNA expression was examined by real-time RT–PCR. GAPDH was used as an internal control. n = 3. (C) eNOS and ICAM-1 protein levels were examined by western blot. β-Actin served as a loading control. The bands were analysed and quantified by densitometry. The ratio of eNOS/β-actin and ICAM-1/β-actin was calculated. n = 3. (D) NO levels released from HCAEC cultures were analysed by nitrite assay. Specific eNOS inhibitor l-NAME was used. n = 3. Error bar indicates SEM. ANOVA test was used. *P < 0.05 and **P < 0.01 vs. controls. #P < 0.05 vs. TNF-α pretreatment.
Figure 5.
Effects of HIV gp120, TNF-α, anti-gp120, and anti-ICAM-1 antibodies on eNOS expression in HCAECs. Cells were pretreated with TNF-α for 8 h and followed by HIV gp120, HIV gp120 plus anti-gp120 antibody or HIV gp120 plus anti-ICAM-1 antibody treatment for additional 16 h. (A) eNOS mRNA levels were examined by conventional RT–PCR and densitometry analyses (n = 4). (B) eNOS mRNA levels were determined by real-time RT–PCR analysis (n = 3). GAPDH was used for the loading control. (C) eNOS protein levels were examined by western blot and densitometry analyses (n = 3). (D) ICAM-1 mRNA levels were examined by conventional RT–PCR and densitometry analyses (n = 3). Results are means ± SEM. ANOVA test was used. *P < 0.05 vs. controls. #P < 0.05 vs. gp120 and TNF-α pretreatment.
Cell viability was studied with CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) kit (Promega, USA). HCAECs were cultured in complete EGM-2 with TNF-α pretreatment at 2 ng/mL for 8 h, the medium was replaced with fresh medium with gp120 (1 µg/mL), and the cells were cultured for additional 16 or 40 h. TNF-α pretreatment and/or gp120 treatment for 16 and 40 h did not affect cell viability (see Supplementary material online, Figure S2).
3.2. HIV gp120-induced decrease in endothelium-dependent vasorelaxation and eNOS expression requires enhanced ICAM-1 expression in TNF-α-treated porcine coronary arteries and HCAECs
To study potential mechanisms for the effect of TNF-α pretreatment on HIV gp120-induced eNOS downregulation, we examined ICAM-1 mRNA levels and protein levels by using conventional RT–PCR or quantitative real-time RT–PCR and western blot and immunohistochemistry, respectively. Immunoreactivity of ICAM-1 was substantially increased in TNF-α-pretreated vessel rings compared with the control and HIV gp120 only treatment groups (Figure 2B). In HCAECs, pretreatment with TNF-α substantially increased ICAM-1 mRNA levels by about 5-fold (Figure 3B) compared with controls. Meanwhile, HIV gp120 increased ICAM-1 mRNA levels by 80% compared with controls (Figure 3B). Western blot also showed a large increase of ICAM-1 protein in the groups with TNF-α pretreatment. HIV gp120 alone increased ICAM-1 expression; however, this effect was relatively weak compared with that seen after treatment with TNF-α (Figure 3B and C). Heat-inactivated HIV gp120 had no effect on eNOS mRNA and protein levels (Figure 3A and C).
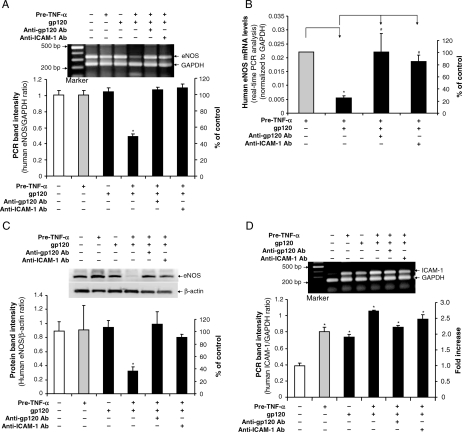
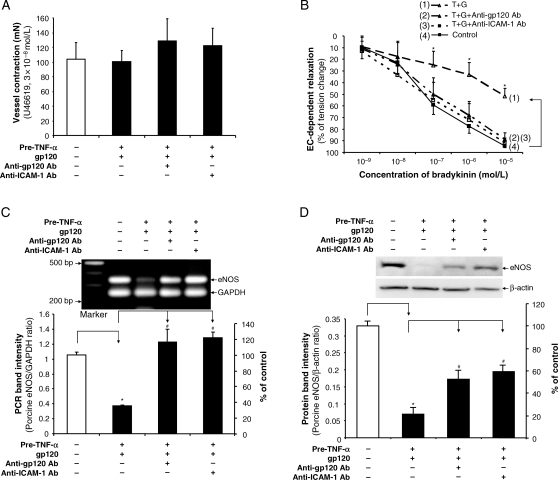
To study the specificity of HIV gp120 action on endothelial cells, porcine vessel rings were pretreated with TNF-α for 8 h, and then HIV gp120 plus anti-gp120 antibody (pre-mixed for 1 h) was added to the vessel ring cultures for additional 16 h. To investigate whether enhanced ICAM-1 expression could be involved in HIV gp120-induced endothelial dysfunction, porcine vessel rings were pretreated with TNF-α for 8 h, and then ICAM-1 neutralizing antibody for 1 h before treatment with HIV gp120 for an additional 16 h. Vasomotor reactivity was again studied as described above. Again, in response to U46619, the maximal contraction of the vessel rings was not significantly different in all three groups compared with controls (Figure 4A). Similarly, in response to SNP, the treatment groups did not significantly differ with respect to smooth muscle cell-related (endothelial-independent) vasorelaxation (see Supplementary material online, Figure S3). In response to bradykinin at both 10−6 and 10−5 mol/L, however, the endothelium-dependent relaxation of vessel rings was again significantly reduced in the group pretreated with TNF-α, then HIV gp120 compared with controls (P < 0.05), whereas both anti-gp120 and anti-ICAM-1 antibodies could effectively block this effect of HIV gp120 in TNF-α-pretreated vessels (Figure 4B).
Figure 4.
Blocking effects of anti-gp120 and anti-ICAM-1 antibodies on HIV gp120-induced decrease in vasomotor function and eNOS expression in TNF-α-pretreated porcine coronary arteries. Porcine coronary artery rings were treated with TNF-α for 8 h, and followed by HIV gp120 alone, HIV gp120 plus anti-gp120 antibody or HIV gp120 plus anti-ICAM-1 antibody treatment for additional 16 h. Vehicle (PBS) treatment served as a negative control. (A) Maximal contraction of the vessel rings in response to thromboxane A2 analogue U46619 (3 × 10−8 mol/L) n = 4. (B) Relaxation concentration response curves were generated by five cumulative additions of the endothelium-dependent vasodilator bradykinin (10−9, 10−8, 10−7, 10−6, and 10−5 mol/L) at 3 min intervals (n = 4, ANOVA). (C) eNOS mRNA levels were examined by conventional RT–PCR (n = 4). (D) eNOS protein levels were determined by western blot (n = 3). The bands were analysed and quantified by densitometry. The ratio of eNOS/GAPDH (mRNA) and ratio of eNOS/β-actin (protein) were evaluated. Results are means ± SEM. ANOVA test was used. *P < 0.05 vs. controls. #P < 0.05 vs. gp120 and TNF-α pretreatment.
Similarly, eNOS mRNA expression in rings pretreated with TNF-α, then HIV gp120 was markedly decreased compared with controls (P < 0.05, Figure 4C). In the presence of a neutralizing antibody against HIV gp120 or ICAM-1, the effect of HIV gp120 on eNOS mRNA downregulation in TNF-α-pretreated vessel rings was effectively blocked (Figure 4C). Western blot analysis also showed that the neutralizing antibody against HIV gp120 or ICAM-1 could partially block HIV gp120-induced decrease in eNOS protein levels in vessel rings pretreated with TNF-α (Figure 4D).
Specific effects of HIV gp120 on eNOS expression were confirmed in HCAECs. HCAECs were pretreated with TNF-α for 8 h, and then treated with HIV gp120 plus anti-gp120 antibody (10 µg/mL) or HIV gp120 plus anti-ICAM-1 antibody (10 µg/mL) for additional 16 h. eNOS expression was determined by real-time RT–PCR and western blot. Consistent with the results in treated porcine artery rings, anti-gp120 antibody or anti-ICAM-1 antibody effectively blocked the downregulation of eNOS mRNA and protein levels induced by HIV gp120 in TNF-α-pretreated HCAECs (Figure 5A–C). Meanwhile, adding anti-ICAM-1 or anti-HIV gp120 antibody had no effect on ICAM-1 mRNA levels compared with TNF-α pretreatment only (Figure 5D and Supplementary Material online Figure S4).
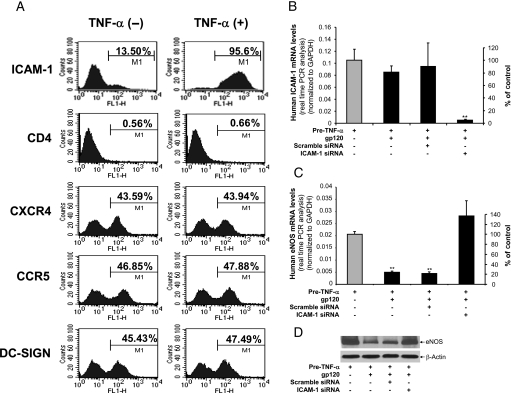
We also determined the potential role of HIV receptors or coreceptors including CD4, CXCR4, CCR5, and DC-SIGN in gp120-induced eNOS downregulation in TNF-α-pretreated HCACEs. Cells were treated with or without TNF-α (2 ng/mL) for 8 h, and the expression of ICAM-1 and HIV receptors or coreceptors was studied by flow cytometry analysis. HCAECs expressed ICAM-1, CXCR-4, CCR5, and DC-SIGN, but not CD4, and TNF-α treatment significantly increased ICAM-1 expression, but not CD4, CXCR-4, CCR-5, and DC-SIGN (Figure 6A). As the gp120-induced eNOS downregulation was only observed after TNF-α-pretreatment, and anti-ICAM-1 antibody effectively blocked this effect of gp120, ICAM-1 may mediate the action of gp120, while the HIV receptors and coreceptors mentioned above are less likely to be involved.
Figure 6.
Expression of ICAM-1 and HIV receptors/coreceptors and role of ICAM-1 silencing in HCAECs. (A) HCAECs were treated with or without TNF-α (2 ng/mL) for 8 h, and the expression of ICAM-1 and HIV receptors or coreceptors including CD4, CXCR4, CCR5, and DC-SIGN was studied by flow cytometry analysis. (B) HCAECs were transiently transfected with human ICAM-1 siRNA or scramble siRNA and pre-incubated with TNF-α (2 ng/mL) for 8 h, and then treated with or without HIV gp120 for 16 h. ICAM-1 mRNA levels were examined by real-time RT–PCR. GAPDH served as an internal control (n = 3). (C) eNOS mRNA levels were examined by real-time RT–PCR. GAPDH served as an internal control (n = 3). (D) eNOS protein levels were examined by western blot. β-Actin was served as a loading control. Error bar indicates SEM. ANOVA test was used. **P < 0.01 vs. controls.
3.3. Silence of ICAM-1 effectively blocks HIV gp120-induced eNOS downregulation in TNF-α-pretreated HCAECs
As TNF-α-induced ICAM-1 expression is directly involved in the effect of HIV gp120-induced eNOS downregulation, we tested whether silencing of ICAM-1 could block this effect of HIV gp120. ICAM-1 siRNA oligonucleotides were transiently transfected into HCAECs for 24 h before treatment of TNF-α for 8 h. The cells were then treated with HIV gp120 for additional 16 h. Scramble siRNA oligonucleotides were used for negative controls. Treatment with ICAM-1 siRNA, but not scramble siRNA, effectively blocked ICAM-1 expression induced by TNF-α (Figure 6B), thereby blocking HIV gp120-induced eNOS downregulation in TNF-α-pretreated HCAECs at both mRNA and protein levels (Figure 6C and D). Thus, the effect of HIV gp120 on eNOS downregulation is medicated by ICAM-1 in endothelial cells.
To check whether gp120 is able to induce TNF-α expression, HCACEs were pretreated with or without TNF-α (2 ng/mL) for 8 h, the medium was replaced with fresh medium including gp120 (1 µg/mL), and the cells were cultured for 16 h. The TNF-α mRNA levels were detected by real-time RT–PCR. Treatment with TNF-α and/or gp120 did not affect TNF-α mRNA expression, which was at very low levels, near background level (see Supplementary material online, Figure S5). Thus, it is not possible that TNF-α released from endothelial cells could play an autocrine action in eNOS downregulation.
4. Discussion
Current study demonstrates, for the first time, that HIV gp120 significantly reduces eNOS expression in both porcine coronary arteries and HCAECs through a unique mechanism involving ICAM-1. Anti-ICAM-1 antibody or ICAM-1 silencing effectively blocks HIV gp120-induced eNOS downregulation in TNF-α-pretreated vessels or cells. Functionally, HIV gp120 significantly decreases endothelium-dependent vasorelaxation of TNF-α-pretreated porcine coronary arteries. The specificity of these effects is confirmed by blocking experiments with anti-gp120 antibody and gp120 heat inactivation. Thus, HIV gp120 induces endothelial dysfunction, which may contribute to HIV-associated vascular diseases in HIV-infected patients.
NO, which is generated by eNOS, is a key regulator of vascular homeostasis. This involves a wide variety of regulatory mechanisms of the cardiovascular system including vascular tone, inhibition of smooth muscle cell proliferation, and inhibition of platelet adhesion and aggregation.14 A number of risk factors may inhibit eNOS activity and/or expression such as hypercholesterolaemia, hypertension, smoking, diabetes mellitus, hemocysteinaemia, and vascular inflammation.14 A body of evidence demonstrates that HIV infection and the associated inflammation correlate with a high incidence of cardiovascular disease.4,13 A more recent clinical study demonstrates that increased atherosclerosis with HIV infection can occur in the absence of antiretroviral therapy.16 However, the mechanisms of these deadly complications are not fully understood. HIV RNA sequences have been detected by in situ hybridization in the coronary vessels of HIV-infected patients who died from acute myocardial infraction.17 Meanwhile, HIV-1 transgenic rats displayed significantly less serum nitrite, aortic tissue NO, and impaired endothelium-dependent vasorelaxation than wild-type rats.18 In the current study, we found that HIV gp120 may impair endothelial function by reducing eNOS expression. Both fresh porcine coronary arteries and HCAECs were used in this study. Initially, we observed a minimal effect of HIV gp120 on eNOS expression in both porcine coronary arteries and HCAECs without TNF-α pretreatment. However, pretreatment with TNF-α significantly enhanced the effect of HIV gp120 on eNOS downregulation at both mRNA and protein levels in the vessels and cells.
Although other studies have shown that TNF-α can significantly decrease eNOS expression,19,20 in our experimental procedure, TNF-α pretreatment only minimally affects eNOS expression in both porcine coronary arteries and HCAECs. This discrepancy may exist for several reasons. In our study, a small concentration of TNF-α (2 ng/mL) was used for pretreatment of 8 h, and the vessels or cells were washed after TNF-α pretreatment and subsequently cultured with fresh medium without TNF-α for an additional 16 h. This procedure provides a useful model to test the effect of HIV gp120 on eNOS expression in cytokine-activated endothelial cells. Thus, our data demonstrate that HIV gp120 is able to reduce eNOS expression in TNF-α-activated endothelial cells; HIV gp120 and TNF-α have synergistic effects on inhibition of eNOS expression in endothelial cells.
We clearly demonstrate that endothelial dysfunction is associated with decreased eNOS expression levels. Bradykinin-mediated endothelium-dependent vasorelaxation was reduced by HIV gp120 in porcine coronary artery rings pretreated with TNF-α compared with control groups including PBS vehicle treatment, treatment with HIV gp120 alone, and TNF-α pretreatment alone. HIV gp120 after pretreatment with TNF-α had no effect on SNP-mediated endothelium-independent vasorelaxation. The specificity of the effect of HIV gp120 on endothelial dysfunction in both porcine coronary arteries and HCAECs was confirmed by blocking experiments using neutralizing anti-gp120 antibody and gp120 heat inactivation. Meanwhile, the phosphorylation of eNOS is one of the mechanisms by which eNOS activity is regulated.21 Although we did not study the eNOS phosphorylation in the current study, this interesting issue warrants future investigation.
Pretreatment with proinflammatory factor TNF-α may provoke certain cellular or molecular changes which, in turn, facilitate the effect of HIV gp120 on endothelial cells. Indeed, pretreatment with TNF-α for 8 h substantially increased ICAM-1 expression in both porcine coronary arteries and human endothelial cells. ICAM-1 is a ligand for LFA-1 (integrin), a receptor found on leucocytes. Under conditions of inflammation, leucocytes bind to endothelial cells via ICAM-1/LFA-1 and promote transmigration of leucocytes, initiating inflammatory diseases such as atherosclerosis.22 Increased levels of ICAM-1 in the circulation and local vascular lesions have been reported to occur in HIV infection.10,11 Our data show that HIV gp120 remarkably reduces eNOS expression and endothelium-dependent vasorelaxation under the conditions of TNF-α pretreatment and ICAM-1 upregulation. Consistent with our previous report,23 HIV gp120 alone also increased ICAM-1 expression in both porcine coronary artery rings and HCAECs. However, HIV gp120-induced ICAM-1 upregulation is much weaker than that induced by TNF-α, which may explain why HIV gp120 alone has a limited impact on eNOS expression and endothelium-dependent vasorelaxation in the current study. We have shown that ICAM-1 plays a critical role in mediating the effect of HIV gp120 on eNOS downregulation in TNF-α-pretreated cells; this is clearly demonstrated by the blocking experiments with anti-ICAM-1 monoclonal antibody and ICAM-1 silencing.
It is well known that ICAM-1 can induce a wide range of intracellular signalling events in the endothelium mediated by cytosolic free calcium, activation of the tyrosine kinase p60Src and/or the small GTPase RhoA and cytoskeletal rearrangements.22 For example, ICAM-1 can induce RhoA activation,24 inositol phosphate production and PLCγ phosphorylation, protein kinase C (PKC) activation, and Src tyrosine kinase phosphorylation.25 Meanwhile, eNOS expression and activities are highly regulated by several signal transduction pathways including kinase insert domain-containing receptor/foetal liver kinase-1 (KDR/Flk-1), PKC, phosphatidylinositol 3-kinase, Janus kinase 2, extracellular signal-regulated kinase 1/2 (ERK1/2), and Ca2+/calmodulin-dependent protein kinase II (CaMKII).26–28 Thus, it is possible that gp120 could directly interact with ICAM-1 and induce signal transduction pathways leading to eNOS downregulation and functional alternation. However, the detailed pathways by which HIV gp120 decreases eNOS expression through ICAM-1 involvement still remain unclear. When inflammation is present, increased TNF-α can induce ICAM-1 expression on endothelial cells, and leucocyte adhesion to endothelial cells is promoted through LFA-1 and ICAM-1 interaction; this may synergize with the intracellular signalling responses induced by ICAM-1, which may exacerbate responses to gp120, such as eNOS downregulation.
Several studies have shown that recombinant HIV gp120 induces apoptosis in a variety of human vascular endothelial cell types.7,29,30 However, the current study did not show any apoptosis or cell viability issues in HCAECs with current treatment molecules and conditions. This functional discrepancy regarding the cytotoxicity of gp120 between our results and previous publications is most likely due to the treatment conditions, although other contributing factors include cell types, gp120 types and concentrations and treatment times. We cultured HCAECs with EGM-2 with growth factor and serum supplements (SingleQuots). We did not treat cells with any serum starvation procedures. The complete growth medium with our treatment molecules (TNF-α or gp120) did not cause any cell death in HCAECs. However, in previous publications, serum starvation procedures or limited growth factors were used before and during treatment with gp120, resulting in endothelial apoptosis.
Several limitations are present in the current study. Some experiments showed variability in results like eNOS and ICAM-1 expression, which may be due to relatively small sample size, sensitivity of detection methods, experimental conditions, technique difficulties, and/or other unknown reasons. Soluble HIV gp120 is detectable in HIV infected individuals. Circulating gp120 may come from shedding of HIV particles and infected cells. For example, gp120 protein levels range from 12 to 92 ng/mL in the serum of AIDS patients.31 The current study used a recombinant gp120 (1 µg/mL), which is a higher concentration than that found in patient serum. Many in vitro studies use similar concentrations of gp120 as ours.7,29,30 In patients, gp120 can interact with endothelial cells for a long time and may induce endothelial dysfunction, even though gp120 levels are relatively low. However, the translation of our in vitro data to clinical applications requires further investigations using animal models and human trials.
In conclusion, HIV gp120 significantly induces eNOS expression and causes endothelial dysfunction in both porcine coronary arteries and HCAECs under the condition of TNF-α pretreatment. The expression of ICAM-1 is directly involved in HIV gp120-induced endothelial dysfunction, which suggests a novel mechanism whereby HIV gp120 affects the vascular system. In addition to HIV gp120, we have previously demonstrated a direct effect of other HIV viral components (Tat and Nef) on endothelial vasorelaxation in association with decreased eNOS expression.32,33 Thus, these HIV proteins themselves may contribute to the vascular complications in HIV-infected individuals. However, it is not known what the effect of HIV gp120 is on the expression or activity of prostacyclin endothelial relaxing factor. It is not known whether other proinflammatory mediators would also increase effect of gp120. On the basis of our data, we speculate that any cytokine which is able to increase ICAM-1 expression in endothelial cells would increase the effect of gp120. The current study showed gp120 reduces eNOS expression and endothelium-dependent vasorelaxation in TNF-α-pretreated porcine coronary arteries. Long-term endothelial dysfunction in the coronary system could contribute to coronary artery atherosclerosis. However, it is not known whether gp120 has similar effects on arterioles, which control tissue perfusion and blood pressure. Further studies to address these important issues are warranted.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work is partially supported by research grants from the National Institutes of Health (C.C. HL065916 and HL083471), and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas.
Supplementary Material
Acknowledgements
All authors would like to thank Dr Rongxin Zhang, Dr Hong Chai, Dr Hong Mu, Dr Hui Yang, Dr Hua-Kang Wu, and Dr Sarah M. Weakley for their technical assistance.
Conflict of interest: none declared.
References
- 1.World Health Organization. AIDS epidemic update. Geneva, Switzerland: 2007. pp. 1–60. [Google Scholar]
- 2.Mu H, Chai H, Lin PH, Yao Q, Chen C. Current update on HIV-associated vascular disease and endothelial dysfunction. World J Surg. 2007;31:632–643. doi: 10.1007/s00268-006-0730-0. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med. 2001;134:978–996. doi: 10.7326/0003-4819-134-10-200105150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Viviani B, Corsini E, Binaglia M, Galli CL, Marinovich M. Reactive oxygen species generated by glia is responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience. 2001;107:51–58. doi: 10.1016/s0306-4522(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 6.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Lüscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Ullrich CK, Groopman JE, Ganju RK. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood. 2000;96:1438–1442. [PubMed] [Google Scholar]
- 8.Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, et al. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- 9.Pober JS, Gimbrone MA, Jr, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, et al. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- 10.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 11.Greenwood AJ, Hughes J, Wallace G, Seed P, Stanford MR, Graham EM. Soluble intercellular adhesion molecule-1 (sICAM-1) and vascular cell adhesion molecule-1 (sVCAM-1) in patients with HIV/AIDS does not appear to correlate with cytomegalovirus retinitis. Int J STD AIDS. 1998;9:713–714. [PubMed] [Google Scholar]
- 12.Galea P, Vermot-Desroches C, Le Contel C, Wijdenes J, Chermann JC. Circulating cell adhesion molecules in HIV1-infected patients as indicator markers for AIDS progression. Res Immunol. 1997;148:109–117. doi: 10.1016/s0923-2494(97)82482-0. [DOI] [PubMed] [Google Scholar]
- 13.Barbaro G, Fisher SD, Lipshultz SE. Pathogenesis of HIV-associated cardiovascular complications. Lancet Infect Dis. 2001;1:115–124. doi: 10.1016/S1473-3099(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee A, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49:134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Chai H, Wang X, Jiang J, Jamaluddin MS, Liao D, et al. Soluble CD40 ligand induces endothelial dysfunction in human and porcine coronary artery endothelial cells. Blood. 2008;112:3205–3216. doi: 10.1182/blood-2008-03-143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsue RY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbaro G, Barbarini G, Pellicelli AM. HIV-associated coronary arteritis in a patient with fatal myocardial infarction. N Engl J Med. 2001;344:1799–1800. doi: 10.1056/NEJM200106073442316. [DOI] [PubMed] [Google Scholar]
- 18.Kline ER, Kleinhenz DJ, Liang B, Dikalov S, Guidot DM, Hart CM, et al. Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. Am J Physiol Heart Circ Physiol. 2008;294:H2792–H2804. doi: 10.1152/ajpheart.91447.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshizumi M, Perrella MA, Burnett JC, Jr, Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73:205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 20.Anderson HDI, Rahmutula D, Gardner DG. Tumor necrosis factor-{alpha} inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- 21.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J. Pharmacol Exp Ther. 2001;299:818–224. [PubMed] [Google Scholar]
- 22.van Buul JD, Kanters E, Hordijk PL. Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler Thromb Vasc Biol. 2007;27:1870–1876. doi: 10.1161/ATVBAHA.107.145821. [DOI] [PubMed] [Google Scholar]
- 23.Ren Z, Yao Q, Chen C. HIV-1 envelope glycoprotein 120 increases intercellular adhesion molecule-1 expression by human endothelial cells. Lab Invest. 2002;82:245–255. doi: 10.1038/labinvest.3780418. [DOI] [PubMed] [Google Scholar]
- 24.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, et al. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol. 2006;177:6440–6449. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- 26.Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and protein kinse C signaling pathway. J Biol Chem. 1999;274:33057–33063. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 27.Cieslik K, Abrams CS, Wu KK. Up-regulation of endothelial nitric-oxide synthase promoter by the phosphatidylinositol 3-kinase [gamma]/Janus kinase 2/MEK-1-dependent pathway. J Biol Chem. 2001;276:1211–1219. doi: 10.1074/jbc.M005305200. [DOI] [PubMed] [Google Scholar]
- 28.Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca2+/calmodulin-dependent protein kinase II/Janus Kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol. 2001;21:1571–1576. doi: 10.1161/hq1001.097028. [DOI] [PubMed] [Google Scholar]
- 29.Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333:1107–1115. doi: 10.1016/j.bbrc.2005.05.198. [DOI] [PubMed] [Google Scholar]
- 30.Yano M, Nakamuta S, Shiota M, Endo H, Kido H. Gatekeeper role of 14-3-3tau protein in HIV-1 gp120-mediated apoptosis of human endothelial cells by inactivation of Bad. AIDS. 2007;21:911–920. doi: 10.1097/QAD.0b013e32810539f3. [DOI] [PubMed] [Google Scholar]
- 31.Oh SK, Cruikshank WW, Raina J, Blanchard GC, Adler WH, Walker J, et al. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr. 1992;5:251–256. [PubMed] [Google Scholar]
- 32.Paladugu R, Fu W, Conklin BS, Lin PH, Lumsden AB, Yao Q, et al. HIV Tat protein causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2003;38:549–555. doi: 10.1016/s0741-5214(03)00770-5. [DOI] [PubMed] [Google Scholar]
- 33.Duffy P, Wang X, Lin PH, Yao Q, Chen C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res. 2009;156:257–264. doi: 10.1016/j.jss.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.