Abstract
Ischaemia and reperfusion (I/R) elicits an acute inflammatory response that is characterized by the recruitment of inflammatory cells, oxidative stress, and endothelial barrier failure. Over the past three decades, much progress has been made in our understanding of the mechanisms that underlie the inflammatory response and microvascular dysfunction associated with I/R. This review is focused on the role of leucocytes (neutrophils and T-lymphocytes) and platelets, and their activation products, as mediators of I/R-induced endothelial barrier failure. The contributions of cytokines, chemokines, and oxidative stress to I/R-induced barrier dysfunction are also discussed. It concludes with an analysis of how risk factors for cardiovascular disease, i.e. hypertension, diabetes, hypercholesterolaemia, and obesity, influence the vascular permeability response to I/R. Areas of uncertainty and controversy in this field of investigation are also identified.
Keywords: Inflammation, Leucocyte-endothelial cell adhesion, Oxidative stress, Platelets, Cardiovascular risk factors
1. Introduction
Endothelial barrier dysfunction is a well-recognized response of the microvasculature to different pathological conditions that are associated with inflammation. The increased vascular permeability can lead to excess filtration of fluid and proteins, resulting in interstitial oedema and impairment of organ function. The hyperpermeability state generally does not result from overt endothelial cell injury or detachment of endothelial cells from the vessel wall. Instead, the response reflects more subtle changes in the fine structure of the endothelial monolayer, such as a widening of the endothelial paracellular junctions that results from the dissociation of junctional proteins and/or cytoskeletal contraction. The endothelial hyperpermeability that accompanies inflammation has been attributed to a variety of soluble mediators, released from resident and/or circulating inflammatory cells, which engage with specific endothelial cell receptors and consequently open the paracellular pathways for fluid and solute exchange. Other physicochemical factors and processes such as shear rate and the adhesion and transendothelial migration of leucocytes have also been implicated in the endothelial barrier dysfunction associated with inflammation.1–4
Ischaemia and reperfusion (I/R) has been implicated in a variety of clinical conditions including thrombolytic therapy for stroke and myocardial infarction, organ transplantation, and the multiple organ dysfunction syndrome.2 Reperfusion of previously ischaemic tissue, while essential for the prevention of irreversible tissue injury, elicits a response in the microvasculature which is very similar to inflammation, i.e. it results in an increased production of reactive oxygen species (ROS) and soluble inflammatory mediators, enhanced adhesion of leucocytes and platelets to vascular endothelium and an increased microvascular permeability. These vascular responses, coupled to the injury inflicted on non-vascular cells, result in the phenomenon of reperfusion injury, which can lead to impaired organ function.5 Although a variety of mediators and mechanisms have been implicated in reperfusion injury, a large and growing body of evidence supports a role for specific blood cell populations in the accompanying microvascular dysfunction. This review is focused on the contribution of leucocytes and platelets to the endothelial barrier failure that is associated with I/R. Some attention is also devoted to the influence of cardiovascular risk factors, which predispose tissues to ischaemic tissue injury, on the endothelial barrier responses to I/R.
2. Leucocytes and platelets are recruited into post-ischaemic microvessels
Different approaches have been used to demonstrate that a variety of blood cell populations adhere in the vasculature and emigrate into the perivascular compartment of tissues exposed to I/R (Table 1). Most attention has been devoted to analysing the infiltration of leucocytes, particularly neutrophils, into post-ischaemic tissues. In some vascular beds (e.g. gut and lung), neutrophils are observed to adhere in post-capillary venules within minutes after reperfusion, whereas hours to days are required to detect significant neutrophil recruitment in other organs (e.g. brain). Lymphocytes have also been detected in a variety of post-ischaemic organs, including lung, intestine, brain, kidney, and liver. This response generally reflects the accumulation of CD4+ and/or CD8+ T-lymphocytes; however, other mononuclear leucocyte populations have been detected, including B-lymphocytes, monocytes, natural killer cells, and Foxp3+ regulatory T-cells, which are known to blunt immune responses. The accumulation of non-neutrophilic leucocyte populations is typically detected by histopathology after days or weeks of reperfusion, however the trafficking of these blood cell populations has been detected as early as 30 min (gut) to 24 h (brain) after reperfusion. The time dependency of the population-specific recruitment of leucocytes is evident in cerebral microvessels, for which it is estimated that neutrophils account for the majority (∼85%) of the total adherent leucocytes detected at 4 h following reperfusion, whereas neutrophils account for only about 20% of total adherent leucocytes (which have more than doubled) at 24 h after reperfusion.2,6
Table 1.
Ischaemia–reperfusion elicits the recruitment of different blood cell populations and endothelial barrier dysfunction in different tissues
| Neutrophils | T-lymphocytes | Monocytes | Platelets | Endothelial barrier dysfunction | |
|---|---|---|---|---|---|
| Heart | 76 | 77 | 78 | 79 | 80,81 |
| Intestine | 82 | 41 | 83 | 84 | 38 |
| Lung | 85 | 86 | 87 | — | 88,89 |
| Brain | 90 | 91 | 92 | 9 | 93 |
| Skeletal muscle | 94 | — | 95 | — | 96 |
| Kidney | 97 | 98 | 99 | 7 | 100 |
| Liver | 101 | 102 | 103 | 104 | 105 |
| Testis | 106 | — | — | — | — |
| Retina | 107 | — | 108 | 109 | — |
All numbers are relevant references.
The recruitment of leucocytes in post-ischaemic microvessels is often accompanied by the accumulation of platelets (Table 1). Whereas the kinetics of platelet accumulation can differ between regional vascular beds, typically the recruitment of platelets either parallels or precedes that observed for leucocytes. For example, significant platelet accumulation is observed as early as 30–45 min after reperfusion in kidney,7 liver,8 and brain.9 The temporal relationship between leucocyte and platelet accumulation in post-ischaemic tissues has lead to the proposal that the two recruitment processes are interdependent. This assertion is supported by evidence that platelet accumulation in post-ischaemic venules is dependent on leucocyte adhesion as well as studies showing that leucocyte adhesion after I/R requires the presence of platelet-associated P-selectin. It is estimated that following I/R approximately 25% of the platelets bind directly to venular endothelium, whereas the remaining 75% of the adherent platelets are attached to leucocytes (primarily neutrophils) that are bound to the vessel wall.10 Direct visualization of the recruitment processes by intravital microscopy reveals that the two blood populations do bind to one another on the vessel wall and that interference with the binding of one cell population (e.g. leucocytes) to vessel wall reduces binding of the other (e.g. platelets). The significance of these cell–cell interactions relative to I/R injury remains unclear. However, it may allow for leucocytes to inflict more damage after I/R because of the increased capacity of neutrophils with attached platelets to produce superoxide11 and platelet-activating factor12 than either cell is capable of producing alone. In addition to neutrophil–platelet interactions, it has been demonstrated that mice that are genetically deficient in either CD4+ or CD8+ T-lymphocytes exhibit a significantly blunted platelet recruitment response to I/R.13 It is not clear whether this effect reflects a direct interaction between platelets and T-cells or whether the latter cells exert their effects indirectly by limiting the recruitment of adherent neutrophils in post-ischaemic microvessels.
3. Endothelial barrier dysfunction in post-ischaemic tissues
Evidence for impaired endothelial barrier function following I/R has been reported for a variety of tissues (Table 1). A number of different experimental approaches have been used to assess barrier function after I/R, including estimates of the osmotic reflection coefficient for plasma proteins using a lymphatic flux analysis,14 single-vessel hydraulic conductivity (Lp),15 electron microscopic evaluation of horseradish peroxidase (HRP) leakage,16 and the leakage of Evans Blue dye17 or fluorescently labelled macromolecules.18 Although the findings generated from these studies are generally interpreted as reflecting changes in vascular permeability, caution should be given to the possibility that, under some circumstances, the demonstration of increased solute extravasation may not reflect a corresponding change in the restrictive properties of the endothelial barrier to solutes but rather increased diffusive and/or convective fluxes of the solute across a normal barrier.
The increased microvascular permeability induced by I/R appears to reflect the cumulative toll of the barrier changes that occur during both the ischaemic and reperfusion phases. For example, exposure of the small bowel to 1 h of ischaemia results in a doubling of vascular permeability to plasma proteins, while the same period of ischaemia following by reperfusion results in a five-fold increase.5 Similarly, the hydraulic conductivity of mesenteric venules is increased over three-fold above baseline by 45 min of ischaemia, whereas a six- to eight-fold increase is observed after reperfusion.19 The results of several studies (summarized below) indicate that the ischaemia and reperfusion components of the barrier dysfunction caused by I/R are distinct and involve different mechanisms and that the reperfusion phase is not merely a delayed manifestation of endothelial ‘injury’ that is incurred during the ischaemic period.20 However, the relative contributions of reperfusion to the overall barrier dysfunction induced by I/R is likely to diminish as the duration of the ischaemic period increases. The notion that there are distinct contributions of ischaemia and reperfusion to the barrier dysfunction is also supported by in vitro studies demonstrating that endothelial cell monolayers exposed to hypoxia exhibit a 50% increase in albumin permeability, while the same duration of hypoxia followed by reoxygenation yields a 2.3-fold increase.21
Direct visualization of protein extravasation after reperfusion of an ischaemic tissue reveals a very rapid permeability response that is evident within minutes after reperfusion. Electron microscopic evaluation of HRP leakage across mesenteric venules after I/R is consistent with impaired endothelial barrier function as early as 30 min after reperfusion, and the HRP leakage appears to preferentially occur across endothelial junctions that exhibit significant leucocyte emigration.16 Rapid impairment of the endothelial barrier after I/R is also supported by measurements of Lp in single mesenteric venules, which exhibit large increases (above that induced by ischaemia alone) within 60 min of reperfusion.19 This study also revealed a biphasic water permeability response to I/R, with an initial transient increase (six-fold above baseline) observed 1 h after reperfusion and a second, more sustained increase (eight-fold) in Lp noted at 3 h after reperfusion. Although some adherent leucocytes were noted in the venules during the initial minutes of reperfusion, a larger population of adherent leucocytes was detected during the second phase of the increased Lp after I/R. On the basis of the overlap in kinetics of leucocyte recruitment with the second phase changes in Lp and other more direct evidence, the authors proposed that adherent leucocytes were largely responsible for the second, larger increase in Lp, while a different mechanism underlies the initial increase in Lp after I/R.19
4. Mediators of I/R-induced blood cell recruitment and endothelial barrier dysfunction
A number of different cell populations are activated when ischaemic tissues are reperfused with well-oxygenated blood. Cells comprising the wall of blood vessels (e.g. endothelial cells) as well as cells residing in the perivascular compartment (e.g. mast cells, macrophages) show signs of activation following I/R. Endothelial cells assume an inflammatory phenotype following activation, which is characterized by an enhanced production of ROS, release of inflammatory cytokines (e.g. IL-8) and an increased expression of adhesion molecules that bind leucocytes and platelets. The oxidative stress experienced by post-ischaemic endothelial cells appears to underlie the increased production and expression of adhesion molecules via activation of the nuclear transcription factor NFκB.22 ROS (superoxide and hydrogen peroxide) can also promote inflammation and endothelial barrier dysfunction by (i) eliciting the production of platelet-activating factor, via phospholipase activation; (ii) promoting the activation and deposition of complement on the endothelial cell surface; (iii) mobilizing the stored pool of P-selectin to the endothelial cell surface, where it mediates leucocyte rolling; (iv) inactivating nitric oxide (NO), and eliciting the activation of perivascular cells, such as mast cells.1–5
There are several potential sources of ROS in endothelial cells, including xanthine oxidase, NADPH oxidase, mitochondria, and uncoupled endothelial nitric oxide synthase (eNOS). Of these, xanthine oxidase and NADPH oxidase have received the most attention. Many of the phenotypic changes in endothelial cell function that have been described from in vivo experiments have been recapitulated in monolayers of cultured endothelial cells exposed to hypoxia and reoxygenation, including the activation of xanthine oxidase, increased ROS production, NFκB activation, increased adhesion molecule expression, and impaired barrier function.23 Pharmacological inhibition of xanthine oxidase or treatment with superoxide dismutase blocks the reoxygenation-induced barrier dysfunction in vitro.21 In vivo studies of I/R-induced microvascular protein and water permeability also implicate xanthine oxidase-derived ROS in the impaired barrier function.19,24 However, since xanthine oxidase inhibition or ROS scavenging has also been shown to largely prevent the recruitment of adherent leucocytes, it is not entirely clear from these observations whether the ROS directly alter barrier function or do so indirectly by limiting the adhesion of leucocytes to endothelial cells.2,5
Although less attention has been devoted to NADPH oxidase as a source of the ROS that mediates leucocyte recruitment and impaired barrier function following I/R, there is evidence to support a role for this enzyme in post-ischaemic lung and brain.25,26 Mouse lungs exposed to I/R exhibit enhanced neutrophil infiltration, lipid peroxidation (a consequence of enhanced ROS production), and increased vascular permeability. However, in wild-type (WT) mice treated with the NADPH oxidase inhibitor apocynin or mice genetically deficient in p47phox, a subunit of the NADPH oxidase protein complex, the lung responses to I/R are significantly attenuated.25 Bone marrow chimeras produced by the transplantation of bone marrow from p47phox deficient into WT recipients (or vice versa) reveal that bone marrow-derived cells, rather than endothelial cells, mediate the I/R-induced, NADPH oxidase-dependent responses in the lung vasculature.16 Brain I/R also results in impaired endothelial barrier function that is dependent on NADPH oxidase, as evidenced by experiments showing reduced blood–brain barrier (BBB) dysfunction after focal ischaemia stroke and reperfusion in NADPH oxidase (gp91phox) deficient mice and in WT mice treated with apocynin.26 Although bone marrow chimeras were not employed to distinguish between the contribution of blood cell and endothelial cell NADPH oxidase, it was shown that monolayers of cultured brain endothelial cells exposed to hydrogen peroxide exhibited a blunted permeability response when treated with apocynin.26
An important pathophysiological consequence of increased superoxide production in vascular endothelium subjected to I/R is inactivation of NO. Physiological levels of NO play an important role in preventing cell–cell interactions such as leucocyte–endothelial cell adhesion as well as the binding of platelets with each other and to other cells (endothelial cells, leucocytes).27 NO donors have been shown to blunt the recruitment of adherent leucocytes, reduce the formation of platelet–leucocyte aggregates, and diminish albumin leakage in post-ischaemic venules.28 Similar protection against I/R-induced inflammation and endothelial barrier function has been noted in mice that genetically overexpress eNOS.29 Treatment of non-ischaemic tissues with NOS inhibitors, on the other hand, can reproduce most of the microvascular alterations induced by I/R, including enhanced leucocyte recruitment and increased vascular permeability.30 However, the magnitude of the responses observed in post-ischaemic tissues treated with an NOS inhibitor does not differ from those observed with either treatment alone, suggesting that NO production is largely inhibited or most of the NO is inactivated in post-ischaemic microvessels. Although these studies suggest that NO normally protects against endothelial barrier failure, there is also evidence indicating that NO directly diminishes barrier function when leucocytes are not present. The incongruent permeability responses to NO can be explained by measurements of Lp in NOS inhibitor-treated mesenteric venules perfused with or without blood-borne constituents. In the absence of blood-borne constituents, an ∼50% reduction in Lp is noted, whereas Lp increases by >75% in vessels perfused by blood during exposure to the NOS inhibitor.31
Mast cells residing in the perivascular space also appear to contribute to the leucocyte–endothelial cell adhesion and impaired endothelial barrier function elicited by I/R.1 Approximately 30% of mast cells surrounding post-capillary venules degranulate following I/R.28 Mast cell degranulation is generally accompanied by the production and release of superoxide, amines (histamine, serotonin), and cytokines (e.g. TNF-alpha, IL-1), all of which can promote the recruitment of leucocytes and diminish endothelial barrier function. A role for mast cell degranulation in I/R-induced microvascular dysfunction is supported by reports describing an attenuating influence of mast cell stabilizing drugs or a genetic deficiency of mast cells on leucocyte recruitment and vascular permeability after I/R in the intestine and brain, but not in skeletal muscle.1,32,33 For example, the impaired BBB function and neutrophil recruitment that occur in brain following middle cerebral artery occlusion and reperfusion is significantly blunted in WT rats treatment with a mast cell stabilizer (cromoglycate) and in mast cell deficient rats.33
Macrophages that reside in the perivascular compartment have also been implicated in the leucocyte recruitment and impaired barrier function induced by I/R. Activated macrophages produce and release a variety of cytokines, chemokines as well as reactive oxygen and nitrogen species that can increase endothelial adhesivity to blood cells and reduce endothelial barrier function. Macrophage depletion studies in lung and intestine indicate less accumulation of inflammatory mediators (cytokines, chemokines) and neutrophils after I/R compared with untreated animals.34–36 These anti-inflammatory actions of macrophage depletion are also associated with a significant reduction in I/R-induced vascular permeability.34,36
5. Evidence implicating blood cells in I/R-induced endothelial barrier failure (Figure 1)
Figure 1.
Mechanisms of endothelial barrier failure following ischaemia–reperfusion (I/R). The recruitment of leucocytes and platelets into post-capillary venules is enhanced by I/R. The products of leucocyte, platelet, and endothelial cell activation ultimately lead to a widening of the inter-endothelial junctions secondary to dissociation of junctional proteins and/or cytoskeletal contraction. Potential mediators of the inflammatory response and barrier failure include interleukin-12 (IL-12), interferon-γ (IFN-γ), superoxide anion (O2−), hydrogen peroxide (H2O2) tumour necrosis factor-α (TNF-α), interleukin-1 (IL-1), histamine, interleukin-6 (IL-6), and macrophage inflammatory protein-1α (MIP-1α).
5.1. Neutrophils
There are several lines of evidence suggesting that neutrophil accumulation in post-ischaemic microvessels is a cause rather than a consequence of I/R-induced endothelial barrier failure. In some tissues (e.g. mesentery, brain), a strong positive correlation exists between the magnitude of the I/R-induced increase in vascular permeability and the number of adherent and/or emigrated neutrophils in/around post-capillary venules.28,37 Direct evidence for the involvement of neutrophils is provided by studies demonstrating that animals rendered neutropenic exhibit a blunted vascular permeability response to I/R.38–40 Similarly, it has been demonstrated that immune blockade of adhesion molecules (P-selectin, ICAM-1, CD11/CD18) that mediate the rolling, firm adherence and/or emigration of neutrophils also diminish the endothelial barrier failure associated with I/R.38,40
5.2. T-lymphocytes
CD4+ and CD8+ T-lymphocytes are recruited into post-capillary venules of the small intestine in WT mice following I/R and this recruitment of T-cells is associated with an increased vascular permeability to albumin.41 The pathophysiological relevance of the T-cell recruitment is evidenced by the observation that immunodeficient SCID mice (which lack T-cells) exhibit a significantly blunted vascular permeability response to I/R, but the permeability response can be restored to WT levels when SCID mice are reconstituted with T-cells from WT mice.41 A similar role for T-lymphocytes as a mediator of I/R-induced endothelial barrier failure has been reported in the renal vasculature, where WT mice, but not mice deficient in CD3+ T-cells, exhibit an increased vascular permeability, and adoptive transfer of WT T-cells into the mutant mice restores the permeability response.42 Immunoblockade of CD4+, but not CD8+, T-cells has been shown to reduce the lung vascular permeability response to I/R, although the barrier compromising effect of the CD4+ T-cells may have resulted from their modulating influence on neutrophil recruitment.17
5.3. Platelets
Less attention has been devoted to evaluating the contribution of platelet accumulation to I/R-induced endothelial barrier failure. The few reports that address this issue suggest that the presence of platelets either do not alter or improve endothelial barrier function. For example, one study of the post-ischaemic coronary vasculature indicates that platelets are not requisite for the barrier dysfunction, whereas another report describes an improved barrier function following the addition of platelets.43,44 A protective effect of platelets has also been reported for the lung.45 A limitation of all these studies is that the organs used to assess the permeability responses to I/R were perfused with artificial solutions, to which platelets are added. In situ, blood perfused tissues may yield a different contribution of platelets to the I/R-induced barrier dysfunction.
6. Blood cell-derived mediators of I/R-induced endothelial barrier failure
Leucocytes and platelets produce and release a variety of compounds that can diminish the barrier function of endothelial cells (Table 2). The contribution of these activation products to the blood cell-dependent endothelial barrier dysfunction elicited by I/R remains uncertain. However, there are a few studies that directly implicate blood cell-derived products in the vascular permeability responses to I/R. As mentioned above, bone marrow chimeras produced from NADPH oxidase knockout mice provide support for blood cell-derived superoxide as a mediator of the pulmonary endothelial barrier failure associated with I/R.25 Since both leucocytes and platelets can produce superoxide via NADPH oxidase, the specific cellular source cannot be identified from the chimera results; however, leucocytes are the more likely candidate because of their capacity to generate much more superoxide per cell.46
Table 2.
Activation products released by leucocytes and platelets that may impair endothelial barrier function
| Leucocytes | Platelets |
|---|---|
| Reactive oxygen species110 | Reactive oxygen species111 |
| Superoxide | Superoxide |
| Hydrogen peroxide | Hydrogen peroxide |
| Cytokines/chemokines112 | Cytokines/chemokines112–114 |
| Interleukins-1, -2, -6, -8, -12 | Interleukins-1, -7, -8 |
| Interferon-alpha, -gamma | RANTES (regulated upon activation, normal T-cell expressed and secreted) |
| Tumour necrosis factor-alpha, -beta | Tumour necrosis factor-beta |
| Transforming growth factor-beta | CD40 ligand |
| Monocyte chemotactic factor-1 | |
| Proteases115 | Growth factors113 |
| Cathepsin-G | Platelet-derived growth factor |
| Elastase | Transforming growth factor-beta |
| Collagenase | Vascular endothelial growth factor |
| Oxidases115 | Lipid mediators114,116 |
| Myeloperoxidase | Thromboxane A2 |
| 12-HETE | |
| Lipid mediators113 | Procoagulants113,117 |
| Leukotrienes B4, C4 | Thrombin |
| Platelet activating factor | Adenosine di- and tri-phosphates |
| Platelet factor-4 | |
| Miscellaneous115 | |
| Cationic proteins | |
| Histamine | |
| Vascular endothelial growth factor |
The roles of cytokines and chemokines to I/R-induced endothelial barrier failure have also been addressed, although few studies have directly assessed the contribution of blood cells to this response. TNF-α has been implicated in the I/R-induced endothelial barrier dysfunction observed in lung, skeletal muscle, and brain.47–49 A contribution of TNF-α to I/R-induced BBB dysfunction has been demonstrated using monolayers of cultured brain endothelial cells exposed to simulated flow cessation and reperfusion.50 The TNF-α-dependent permeability response was noted both in the presence and absence of leucocytes. However, more of the cytokine was generated in the presence of leucocytes and TNF-α immunoblockade exerted a more profound protection against the shear stress-dependent endothelial barrier dysfunction, suggesting a major role for leucocyte-derived TNF-α.
Both alpha (MIP-2, CINC) and beta (MIP-1, MCP-1, RANTES) chemokines have been implicated in the increased vascular permeability elicited by I/R. In the pulmonary microvasculature, immunoblockade of MIP-1, MIP-2, and CINC blunts the I/R-induced permeability response, whereas RANTES and MCP-1 antibodies do not afford protection.51,52 Antisense oligonucleotides as well as neutralizing antibodies directed against the chemokine CCL2 have been shown to blunt the BBB permeability response to focal cerebral I/R.53 This observation, coupled to studies demonstrating endothelial cells deficient in CCR2 (the receptor for CCL2) are resistant to I/R-induced BBB failure,54 suggest a major role for CCL2–CCR2 complex activation in the regulation of BBB function.
The role of blood cell-derived RANTES as a mediator of I/R-induced BBB dysfunction has been recently addressed.55 This chemokine, which promotes the directed migration of leucocytes into damaged or inflamed tissue, is produced by a variety of cells, including T-lymphocytes, platelets, endothelial cells, smooth muscle cells, and glial cells. The exaggerated leucocyte and platelet adhesion, and increased BBB permeability elicited in WT mice after focal cerebral I/R are significantly blunted in RANTES−/− mice. Similar attenuation of the I/R-induced responses are noted in chimeras produced by transplantation of bone marrow from RANTES−/− mice into WT recipients, suggesting that blood cells are the likely source of the chemokine. Since immunodeficient Rag-1−/− mice exhibit the same robust increase in plasma RANTES concentration as WT mice, T-lymphocytes are an unlikely source of the chemokine released following I/R. Platelets, which release RANTES upon activation, may be the major blood cell source of the chemokine.55
CD40/CD40 ligand (CD40L) signalling has been implicated in a variety of acute and chronic inflammatory conditions. Mice that are genetically deficient in either CD40 or CD40L exhibit significantly blunted blood cell (leucocyte and platelet) adhesion and vascular permeability responses to focal cerebral I/R.56 Although the cellular sources of CD40 and CD40L that mediate the altered BBB function were not directly addressed, it was proposed that the engagement of CD40 would promote the recruitment of activated adherent leucocytes, which release oxygen radicals, matrix etalloproteinase's, and/or other factors that increase BBB permeability. CD40/CD40L signalling has also been implicated in I/R-induced increases in pulmonary microvessel Lp, via a mechanism that involves T-lymphocytes.57 Since activated platelets shed large amounts of soluble CD40L (sCD40L) into plasma, and sCD40L retains biological function, it is possible that sCD40L contributes to both the local and distant organ vascular permeability responses elicited by I/R.2
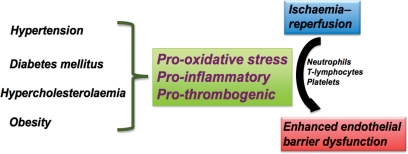
7. Modulating influence of risk factors for cardiovascular disease
Much of what is known about the pathobiology of I/R-induced microvascular dysfunction, inflammation, and tissue injury has been derived from studies on animals that are otherwise normal. However, epidemiological evidence in humans clearly demonstrates that the incidence of ischaemic tissue disease is largely determined by the presence of certain risk factors, including hypertension (HTN), hypercholesterolaemia (HCh), diabetes mellitus, obesity, and cigarette smoke, with combinations of risk factors (e.g. HTN & HCh) exerting a synergistic effect on the incidence of ischaemic disease. However, relatively little attention has been devoted to determining whether these risk factors (either alone or in combination) merely increase the likelihood that a tissue experiences an ischaemic episode (e.g. atherosclerosis-induced flow restriction) or whether the risk factors also render the microvasculature more sensitive to the deleterious effects of I/R, resulting in the recruitment of more inflammatory cells and platelets, and larger increases in vascular permeability following a given ischaemic stress. The latter possibility appears tenable in view of evidence that the risk factors induce a pro-oxidative, pro-inflammatory, and pro-thrombotic environment (Figure 2).
Figure 2.
Role of risk factors for cardiovascular disease in ischaemia/reperfusion (I/R)-induced endothelial barrier failure. Hypertension, diabetes mellitus, hypercholesterolaemia, and obesity induce a pro-oxidative, pro-inflammatory, and pro-thrombotic environment, mediated at least in part by blood cells (e.g. neutrophils, T-lymphocytes, and platelets), that exacerbates the I/R-induced endothelial barrier dysfunction.
7.1. Hypertension
Studies of the mesenteric microcirculation in normotensive (WKY) and hypertensive (SHR) rats after I/R have revealed no differences in the I/R-induced recruitment of leucocytes and platelets, whereas a more pronounced albumin extravasation occurs across venules in SHR.58 Retinal microvessels of SHR exhibit a larger leucocyte recruitment response in venules and more evidence of tissue damage after I/R than their normotensive counterparts.59 Transient ischaemia has been shown to increase BBB permeability in stroke-prone spontaneously hypertensive rats,60,61 however, it remains unclear whether this response is associated with (or linked to) an increased recruitment of blood cells into the cerebral microcirculation.
7.2. Hypercholesterolaemia
An exaggerated leucocyte recruitment response to I/R has been demonstrated in both genetic and diet-induced models of HCh. This phenomenon has been demonstrated in post-ischaemic mesenteric,62 skeletal muscle,63 and cerebral venules.64 Mesenteric and skeletal muscle microvessels also exhibit a more pronounced I/R-induced increase in vascular permeability with HCh. The risk factor-enhanced barrier failure appears to be directly linked to the leucocyte recruitment, since adhesion molecule-directed antibodies that significantly attenuate leucocyte recruitment also blunt the permeability response. A similar protective effect against the permeability response was noted when interventions were used (e.g. GPIIb/IIIa antagonist) to prevent the formation of homotypic or heterotypic platelet aggregates.62 Oxidized low-density lipoprotein (oxLDL), which has been implicated in the inflammatory responses associated with HCh, has also been shown to enhance I/R-induced leucocyte adhesion and emigration in mesenteric venules.65 However, this was not associated with a correspondingly larger vascular permeability response.
7.3. Diabetes
Increased vascular permeability and inflammation are characteristic features of the microvascular responses to diabetes mellitus. A variety of factors have been implicated in the endothelial barrier dysfunction associated with diabetes, including hyperglycaemia, vascular endothelial growth factor, and oxidative stress.66 Diabetic animals exposed to I/R exhibit an exaggerated vascular permeability response that is accompanied by accelerated ROS production and more adherent leucocytes in post-capillary venules.67,68 Whereas leucocytes have been implicated as a source of ROS in the post-ischaemic microvasculature of diabetic animals,69 hyperglycaemia-induced overproduction of ROS by mitochondrial electron transport chain may also contribute to the exaggerated permeability response to I/R.70 PAF and LTB4 have been implicated in the exaggerated leucocyte recruitment,67,71 which involves CD11/CD18-ICAM-1 adhesive interactions (firm adhesion) and P-selectin (rolling).72 Immunoblockade of these adhesion molecules blunts the I/R-induced, diabetes-enhanced endothelial barrier dysfunction, and oxidative stress,67,72 suggesting a critical role for the adherent leucocytes. NO donating compounds are equally effective in blunting the diabetes-enhanced I/R-induced microvascular dysfunction.73
7.4. Obesity
The expanded pool of activated adipocytes that accompanies obesity appears to elicit a systemic inflammatory response that is characterized by endothelial cell dysfunction, oxidative stress, and the activation of circulating immune cells. It has been reported that the brain inflammation and BBB dysfunction elicited by sepsis are exacerbated in obese mice compared with their lean counterparts.74 A similar effect of obesity has been noted following focal cerebral I/R,75 where leptin-deficient obese mice exhibit larger increases in leucocyte and platelet adhesion, BBB permeability, water content, and infarct volume compared with lean mice. The post-ischaemic obese mice also exhibited higher plasma levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) than lean mice. Immunoneutralization of MCP-1, but not IL-6, was shown to reduce infarct volume in the obese mice.
8. Conclusions
The endothelial barrier failure elicited by I/R is accompanied by oxidative stress and the recruitment of leucocytes and platelets. The blood cells that are recruited into the post-ischaemic microvasculature appear to contribute to both the endothelial barrier dysfunction and enhanced production of ROS, via mechanisms that require adhesive interactions between the blood cells and vascular endothelium. The available evidence suggests that products of blood cell activation, including ROS, cytokines, and chemokines play a major role in mediating the adhesion-dependent increase in vascular permeability caused by I/R. Whether these agents act directly on endothelial cells or do so indirectly by activating perivascular cells such as mast cells and macrophages remain unclear. Despite these uncertainties about the nature of the involvement of different cell populations and molecular mediators in the I/R-induced endothelial barrier dysfunction, there is mounting evidence that the well-established risk factors for cardiovascular disease amplify the inflammatory and oxidative responses elicited by I/R, with a corresponding exacerbation of the barrier failure. Drugs that target blood cell-derived factors may provide a novel therapeutic strategy for restoring endothelial barrier function in clinical conditions associated with permeability oedema.
Conflict of interest: none declared.
Funding
The authors are supported by a grant from the National Heart Lung and Blood Institute (HL26441) of the National Institutes of Health.
References
- 1.Kubes P, Granger DN. Leukocyte-endothelial cell interactions evoked by mast cells. Cardiovasc Res. 1996;32:699–708. [PubMed] [Google Scholar]
- 2.Carden DL, Granger DN. Pathophysiology of ischaemia–reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. doi:10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. doi:10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost. 2008;6:1453–1460. doi: 10.1111/j.1538-7836.2008.03087.x. doi:10.1111/j.1538-7836.2008.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger DN. Role of xanthine oxidase and granulocytes in ischemia–reperfusion injury. Am J Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2009 doi: 10.1007/s12017-009-8074-1. doi:10.1007/s12017-009-8074-1. Published online ahead of print 6 July 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chintala MS, Bernardino V, Chiu PJ. Cyclic GMP but not cyclic AMP prevents renal platelet accumulation after ischemia–reperfusion in anesthetized ratss. J Pharmacol Exp Ther. 1994;271:1203–1208. [PubMed] [Google Scholar]
- 8.Nakano Y, Kondo T, Matsuo R, Hashimoto I, Kawasaki T, Kohno K, et al. Platelet dynamics in the early phase of postischemic liver in vivo. J Surg Res. 2008;149:192–198. doi: 10.1016/j.jss.2007.09.016. doi:10.1016/j.jss.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Jafar JJ, Menoni R, Feinberg H, LeBreton G, Crowell RM. Selective platelet deposition during focal cerebral ischemia in cats. Stroke. 1989;20:664–667. doi: 10.1161/01.str.20.5.664. [DOI] [PubMed] [Google Scholar]
- 10.Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirculation. 2005;12:275–285. doi: 10.1080/10739680590925691. doi:10.1080/10739680590925691. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Sugimura K, Hasegawa K, Yoshida K, Suzuki A, Ishizuka K, et al. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301–1306. doi: 10.1080/003655201317097164. doi:10.1080/003655201317097164. [DOI] [PubMed] [Google Scholar]
- 12.Herd CM, Page CP. Pulmonary immune cells in health and disease: platelets. Eur Respir J. 1994;7:1145–1160. [PubMed] [Google Scholar]
- 13.Osman M, Russell J, Granger DN. Lymphocyte-derived interferon-gamma mediates ischemia–reperfusion-induced leukocyte and platelet adhesion in intestinal microcirculation. Am J Physiol Gastrointest Liver Physiol. 2009;296:G659–G663. doi: 10.1152/ajpgi.90495.2008. doi:10.1152/ajpgi.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilati CF. Macromolecular transport in canine coronary microvasculature. Am J Physiol. 1990;258:H748–H753. doi: 10.1152/ajpheart.1990.258.3.H748. [DOI] [PubMed] [Google Scholar]
- 15.Victorino GP, Ramirez RM, Chong TJ, Curran B, Sadjadi J. Ischemia–reperfusion injury in rats affects hydraulic conductivity in two phases that are temporally and mechanistically separate. Am J Physiol Heart Circ Physiol. 2008;295:H2164–H2171. doi: 10.1152/ajpheart.00419.2008. doi:10.1152/ajpheart.00419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver MG, Specian RD, Perry MA, Granger DN. Morphologic assessment of leukocyte-endothelial cell interactions in mesenteric venules subjected to ischemia and reperfusion. Inflammation. 1991;15:331–346. doi: 10.1007/BF00917350. doi:10.1007/BF00917350. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. doi:10.1016/j.jtcvs.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khandoga AG, Khandoga A, Anders HJ, Krombach F. Postischemic vascular permeability requires both TLR-2 and TLR-4, but only TLR-2 mediates the transendothelial migration of leukocytes. Shock. 2009;31:592–598. doi: 10.1097/SHK.0b013e318193c859. [DOI] [PubMed] [Google Scholar]
- 19.Victorino GP, Chong TJ, Cripps MW, Ereso AQ, Cureton E, Curran B, et al. The effect of hypoxia, reoxygenation, ischemia, and reperfusion on hydraulic permeability in rat mesenteric venules. Shock. 2009;31:317–321. doi: 10.1097/SHK.0b013e318183376c. doi:10.1097/SHK.0b013e318183376c. [DOI] [PubMed] [Google Scholar]
- 20.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- 21.Inauen W, Payne DK, Kvietys PR, Granger DN. Hypoxia/reoxygenation increases the permeability of endothelial cell monolayers: role of oxygen radicals. Free Radic Biol Med. 1990;9:219–223. doi: 10.1016/0891-5849(90)90031-d. doi:10.1016/0891-5849(90)90031-D. [DOI] [PubMed] [Google Scholar]
- 22.Cooper D, Stokes KY, Tailor A, Granger DN. Oxidative stress promotes blood cell–endothelial cell interactions in the microcirculation. Cardiovasc Toxicol. 2002;2:165–180. doi: 10.1007/s12012-002-0002-7. doi:10.1007/s12012-002-0002-7. [DOI] [PubMed] [Google Scholar]
- 23.Kvietys PR, Granger DN. Endothelial cell monolayers as a tool for studying microvascular pathophysiology. Am J Physiol. 1997;273:G1189–G1199. doi: 10.1152/ajpgi.1997.273.6.G1189. [DOI] [PubMed] [Google Scholar]
- 24.Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 25.Yang Z, Sharma AK, Marshall M, Kron IL, Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia–reperfusion injury. Am J Respir Cell Mol Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. doi:10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, et al. NADPH oxidase plays a central role in blood–brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. doi:10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 27.Walter U, Gambaryan S. cGMP and cGMP-dependent protein kinase in platelets and blood cells. Handb Exp Pharmacol. 2009;191:533–548. doi: 10.1007/978-3-540-68964-5_23. doi:10.1007/978-3-540-68964-5_23. [DOI] [PubMed] [Google Scholar]
- 28.Kurose I, Wolf R, Grisham MB, Granger DN. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ Res. 1994;74:376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki M, Kawashima S, Hirase T, Yamashita T, Namiki M, Inoue N, et al. Overexpression of endothelial nitric oxide synthase in endothelial cells is protective against ischemia–reperfusion injury in mouse skeletal muscle. Am J Pathol. 2002;160:1335–1344. doi: 10.1016/s0002-9440(10)62560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, et al. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- 31.Rumbaut RE, Wang J, Huxley VH. Differential effects of L-NAME on rat venular hydraulic conductivity. Am J Physiol Heart Circ Physiol. 2000;279:H2017–H2023. doi: 10.1152/ajpheart.2000.279.4.H2017. [DOI] [PubMed] [Google Scholar]
- 32.Kanwar S, Hickey MJ, Kubes P. Postischemic inflammation: a role for mast cells in intestine but not in skeletal muscle. Am J Physiol. 1998;275:G212–G218. doi: 10.1152/ajpgi.1998.275.2.G212. [DOI] [PubMed] [Google Scholar]
- 33.Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. doi:10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- 34.Naidu BV, Krishnadasan B, Farivar AS, Woolley SM, Thomas R, Van Rooijen N, et al. Early activation of the alveolar macrophage is critical to the development of lung ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 2003;126:200–207. doi: 10.1016/s0022-5223(03)00390-8. doi:10.1016/S0022-5223(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Lui VC, Rooijen NV, Tam PK. Depletion of intestinal resident macrophages prevents ischaemia reperfusion injury in gut. Gut. 2004;53:1772–1780. doi: 10.1136/gut.2003.034868. doi:10.1136/gut.2003.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia–reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1018–L1026. doi: 10.1152/ajplung.00086.2006. doi:10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 37.Nagai M, Terao S, Yilmaz G, Yilmaz CE, Esmon CT, Watanabe E, et al. Roles of inflammation and the activated protein c pathway in the brain edema associated with cerebral venous sinus thrombosis. Stroke. 2009;41:147–152. doi: 10.1161/STROKEAHA.109.562983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia–reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 39.Eppinger MJ, Jones ML, Deeb GM, Bolling SF, Ward PA. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J Surg Res. 1995;58:713–718. doi: 10.1006/jsre.1995.1112. doi:10.1006/jsre.1995.1112. [DOI] [PubMed] [Google Scholar]
- 40.Carden DL, Smith JK, Korthuis RJ. Neutrophil-mediated microvascular dysfunction in postischemic canine skeletal muscle. Role of granulocyte adherence. Circ Res. 1990;66:1436–1444. doi: 10.1161/01.res.66.5.1436. [DOI] [PubMed] [Google Scholar]
- 41.Shigematsu T, Wolf RE, Granger DN. T-lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia–reperfusion. Microcirculation. 2002;9:99–109. doi: 10.1038/sj/mn/7800126. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Chien CC, Grigoryev DN, Gandolfo MT, Colvin RB, Rabb H. Effect of T cells on vascular permeability in early ischemic acute kidney injury in mice. Microvasc Res. 2009;77:340–347. doi: 10.1016/j.mvr.2009.01.011. doi:10.1016/j.mvr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds JM, McDonagh PF. Platelets do not modulate leukocyte-mediated coronary microvascular damage during early reperfusion. Am J Physiol. 1994;266:H171–H181. doi: 10.1152/ajpheart.1994.266.1.H171. [DOI] [PubMed] [Google Scholar]
- 44.Heindl B, Zahler S, Welsch U, Becker BF. Disparate effects of adhesion and degranulation of platelets on myocardial and coronary function in postischaemic hearts. Cardiovasc Res. 1998;38:383–394. doi: 10.1016/s0008-6363(98)00032-7. doi:10.1016/S0008-6363(98)00032-7. [DOI] [PubMed] [Google Scholar]
- 45.Zamora CA, Baron D, Heffner JE. Washed human platelets prevent ischemia–reperfusion edema in isolated rabbit lungs. J Appl Physiol. 1991;70:1075–1084. doi: 10.1152/jappl.1991.70.3.1075. [DOI] [PubMed] [Google Scholar]
- 46.Stokes KY, Russell JM, Jennings MH, Alexander JS, Granger DN. Platelet-associated NAD(P)H oxidase contributes to the thrombogenic phenotype induced by hypercholesterolemia. Free Radic Biol Med. 2007;43:22–30. doi: 10.1016/j.freeradbiomed.2007.02.027. doi:10.1016/j.freeradbiomed.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khimenko PL, Bagby GJ, Fuseler J, Taylor AE. Tumor necrosis factor-alpha in ischemia and reperfusion injury in rat lungs. J Appl Physiol. 1998;85:2005–2011. doi: 10.1152/jappl.1998.85.6.2005. [DOI] [PubMed] [Google Scholar]
- 48.Gaines GC, Welborn MB, 3rd, Moldawer LL, Huber TS, Harward TR, Seeger JM. Attenuation of skeletal muscle ischemia/reperfusion injury by inhibition of tumor necrosis factor. J Vasc Surg. 1999;29:370–376. doi: 10.1016/s0741-5214(99)70390-3. doi:10.1016/S0741-5214(99)70390-3. [DOI] [PubMed] [Google Scholar]
- 49.Yang GY, Gong C, Qin Z, Liu XH, Lorris Betz A. Tumor necrosis factor alpha expression produces increased blood–brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res Mol Brain Res. 1999;69:135–143. doi: 10.1016/s0169-328x(99)00007-8. doi:10.1016/S0169-328X(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 50.Krizanac-Bengez L, Mayberg MR, Cunningham E, Hossain M, Ponnampalam S, Parkinson FE, et al. Loss of shear stress induces leukocyte-mediated cytokine release and blood–brain barrier failure in dynamic in vitro blood–brain barrier model. J Cell Physiol. 2006;206:68–77. doi: 10.1002/jcp.20429. doi:10.1002/jcp.20429. [DOI] [PubMed] [Google Scholar]
- 51.Farivar AS, Krishnadasan B, Naidu BV, Woolley SM, Verrier ED, Mulligan MS. Alpha chemokines regulate direct lung ischemia–reperfusion injury. J Heart Lung Transplant. 2004;23:585–591. doi: 10.1016/S1053-2498(03)00300-0. doi:10.1016/S1053-2498(03)00300-0. [DOI] [PubMed] [Google Scholar]
- 52.Krishnadasan B, Farivar AS, Naidu BV, Woolley SM, Byrne K, Fraga CH, et al. Beta-chemokine function in experimental lung ischemia–reperfusion injury. Ann Thorac Surg. 2004;77:1056–1062. doi: 10.1016/S0003-4975(03)01600-X. doi:10.1016/S0003-4975(03)01600-X. [DOI] [PubMed] [Google Scholar]
- 53.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. doi:10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 54.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood–brain barrier permeability during ischemia–reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. doi: 10.1038/sj.jcbfm.9600229. doi:10.1038/sj.jcbfm.9600229. [DOI] [PubMed] [Google Scholar]
- 55.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia–reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. doi:10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. doi:10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- 57.Moore TM, Shirah WB, Khimenko PL, Paisley P, Lausch RN, Taylor AE. Involvement of CD40–CD40L signaling in postischemic lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1255–L1262. doi: 10.1152/ajplung.00016.2002. [DOI] [PubMed] [Google Scholar]
- 58.Kurose I, Wolf R, Cerwinka W, Granger DN. Microvascular responses to ischemia/reperfusion in normotensive and hypertensive rats. Hypertension. 1999;34:212–216. doi: 10.1161/01.hyp.34.2.212. [DOI] [PubMed] [Google Scholar]
- 59.Hirose F, Kiryu J, Miyamoto K, Nishijima K, Miyahara S, Katsuta H, et al. In vivo evaluation of retinal injury after transient ischemia in hypertensive rats. Hypertension. 2004;43:1098–1102. doi: 10.1161/01.HYP.0000123069.02156.8a. doi:10.1161/01.HYP.0000123069.02156.8a. [DOI] [PubMed] [Google Scholar]
- 60.Shima K, Ohashi K, Umezawa H, Chigasaki H, Okuyama S. Blood–brain barrier, cerebral blood flow, and brain edema in spontaneously hypertensive rats with chronic focal ischemia. Acta Neurochir Suppl (Wien) 1994;60:271–273. doi: 10.1007/978-3-7091-9334-1_72. [DOI] [PubMed] [Google Scholar]
- 61.Abrahám CS, Harada N, Deli MA, Niwa M. Transient forebrain ischemia increases the blood–brain barrier permeability for albumin in stroke-prone spontaneously hypertensive rats. Cell Mol Neurobiol. 2002;22:455–462. doi: 10.1023/A:1021067822435. doi:10.1023/A:1021067822435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurose I, Argenbright LW, Anderson DC, Tolley J, Miyasaka M, Harris N, et al. Reperfusion-induced leukocyte adhesion and vascular protein leakage in normal and hypercholesterolemic rats. Am J Physiol. 1997;273:H854–H860. doi: 10.1152/ajpheart.1997.273.2.H854. [DOI] [PubMed] [Google Scholar]
- 63.Mori N, Horie Y, Gerritsen ME, Granger DN. Ischemia–reperfusion induced microvascular responses in LDL-receptor −/− mice. Am J Physiol. 1999;276:H1647–H1654. doi: 10.1152/ajpheart.1999.276.5.H1647. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and P-selectin. Circ Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. doi:10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 65.Liao L, Harris NR, Granger DN. Oxidized low-density lipoproteins and microvascular responses to ischemia–reperfusion. Am J Physiol. 1996;271:H2508–H2514. doi: 10.1152/ajpheart.1996.271.6.H2508. [DOI] [PubMed] [Google Scholar]
- 66.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. doi:10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 67.Panés J, Kurose I, Rodriguez-Vaca D, Anderson DC, Miyasaka M, Tso P, et al. Diabetes exacerbates inflammatory responses to ischemia–reperfusion. Circulation. 1996;93:161–167. doi: 10.1161/01.cir.93.1.161. [DOI] [PubMed] [Google Scholar]
- 68.Bouskela E, Donyo KA. Effects of oral administration of purified micronized flavonoid fraction on increased microvascular permeability induced by various agents and on ischemia/reperfusion in diabetic hamsters. Int J Microcirc Clin Exp. 1995;15:293–300. doi: 10.1159/000179078. doi:10.1159/000179078. [DOI] [PubMed] [Google Scholar]
- 69.Salas A, Panes J, Elizalde JI, Granger DN, Pique JM. Reperfusion-induced oxidative stress in diabetes: cellular and enzymatic sources. J Leukoc Biol. 1999;66:59–66. doi: 10.1002/jlb.66.1.59. [DOI] [PubMed] [Google Scholar]
- 70.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2008;414:813–820. doi: 10.1038/414813a. doi:10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 71.Salas A, Panés J, Elizalde JI, Casadevall M, Anderson DC, Granger DN, et al. Mechanisms responsible for enhanced inflammatory response to ischemia–reperfusion in diabetes. Am J Physiol. 1998;275:H1773–H1781. doi: 10.1152/ajpheart.1998.275.5.H1773. [DOI] [PubMed] [Google Scholar]
- 72.Salas A, Panés J, Elizalde JI, Granger DN, Piqué JM. Reperfusion-induced oxidative stress in diabetes: cellular and enzymatic sources. J Leukoc Biol. 1999;66:59–66. doi: 10.1002/jlb.66.1.59. [DOI] [PubMed] [Google Scholar]
- 73.Salas A, Panés J, Rosenbloom CL, Elizalde JI, Anderson DC, Granger DN, et al. Differential effects of a nitric oxide donor on reperfusion-induced microvascular dysfunction in diabetic and non-diabetic rats. Diabetologia. 1999;42:1350–1358. doi: 10.1007/s001250051449. doi:10.1007/s001250051449. [DOI] [PubMed] [Google Scholar]
- 74.Vachharajani V, Russell JM, Scott KL, Conrad S, Stokes KY, Tallam L, et al. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–194. doi: 10.1080/10739680590904982. doi:10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- 75.Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–950. doi: 10.1161/STROKEAHA.107.494542. doi:10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- 76.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 77.Knight RJ, Dikman S, Liu H, Martinelli GP. Cold ischemic injury accelerates the progression to chronic rejection in a rat cardiac allograft model. Transplantation. 1997;64:1102–1107. doi: 10.1097/00007890-199710270-00003. doi:10.1097/00007890-199710270-00003. [DOI] [PubMed] [Google Scholar]
- 78.Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684–692. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- 79.Romson JL, Hook BG, Rigot VH, Schork MA, Swanson DP, Lucchesi BR. The effect of ibuprofen on accumulation of indium-111-labeled platelets and leukocytes in experimental myocardial infarction. Circulation. 1982;66:1002–1011. doi: 10.1161/01.cir.66.5.1002. [DOI] [PubMed] [Google Scholar]
- 80.Byrne JG, Smith WJ, Murphy MP, Couper GS, Appleyard RF, Cohn LH. Complete prevention of myocardial stunning, contracture, low-reflow, and edema after heart transplantation by blocking neutrophil adhesion molecules during reperfusion. J Thorac Cardiovasc Surg. 1992;104:1589–1596. [PubMed] [Google Scholar]
- 81.Williams FM, Kus M, Tanda K, Williams TJ. Effect of duration of ischaemia on reduction of myocardial infarct size by inhibition of neutrophil accumulation using an anti-CD18 monoclonal antibody. Br J Pharmacol. 1994;111:1123–1128. doi: 10.1111/j.1476-5381.1994.tb14861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 83.Kalff JC, Cicalese L, Exner B, Schraut WH, Bauer AJ. Role of phagocytes in causing dysmotility after each stage of small bowel transplantation. Transplant Proc. 1998;30:2568. doi: 10.1016/s0041-1345(98)00729-5. doi:10.1016/S0041-1345(98)00729-5. [DOI] [PubMed] [Google Scholar]
- 84.Haglind E, Haglund U, Lundgren O, Stenberg B. Mucosal lesions of the small intestine after intestinal vascular obstruction in the rat. Acta Chir Scand. 1985;151:147–150. [PubMed] [Google Scholar]
- 85.Corris PA, Odom NJ, Jackson G, McGregor CG. Reimplantation injury after lung trsplantation in a rat model. J Heart Transplant. 1987;6:234–237. [PubMed] [Google Scholar]
- 86.Adoumie R, Serrick C, Giaid A, Shennib H. Early cellular events in the lung allograft. Ann Thorac Surg. 1992;54:1071–1076. doi: 10.1016/0003-4975(92)90072-c. [DOI] [PubMed] [Google Scholar]
- 87.Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Richter JA, et al. Significant leukocyte and platelet retention during pulmonary passage after declamping of the aorta in CABG patients. Eur J Med Res. 1999;4:178–182. [PubMed] [Google Scholar]
- 88.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. doi:10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma AK, Laubach VE, Ramos SI, Zhao Y, Stukenborg G, Linden J, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kochanek PM, Dutka AJ, Hallenbeck JM. Indomethacin, prostacyclin, and heparin improve postischemic cerebral blood flow without affecting early postischemic granulocyte accumulation. Stroke. 1987;18:634–637. doi: 10.1161/01.str.18.3.634. [DOI] [PubMed] [Google Scholar]
- 91.Benjelloun N, Renolleau S, Represa A, Ben-Ari Y, Charriaut-Marlangue C. Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal rat. Stroke. 1999;30:1916–1923. doi: 10.1161/01.str.30.9.1916. [DOI] [PubMed] [Google Scholar]
- 92.Clark WM, Lauten JD, Lessov N, Woodward W, Coull BM. The influence of antiadhesion therapies on leukocyte subset accumulation in central nervous system ischemia in rats. J Mol Neurosci. 1995;6:43–50. doi: 10.1007/BF02736758. doi:10.1007/BF02736758. [DOI] [PubMed] [Google Scholar]
- 93.Shiga Y, Onodera H, Kogure K, Yamasaki Y, Yashima Y, Syozuhara H, et al. Neutrophil as a mediator of ischemic edema formation in the brain. Neurosci Lett. 1991;125:110–112. doi: 10.1016/0304-3940(91)90003-c. doi:10.1016/0304-3940(91)90003-C. [DOI] [PubMed] [Google Scholar]
- 94.Smith JK, Grisham MB, Granger DN, Korthuis RJ. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol. 1989;256:H789–H793. doi: 10.1152/ajpheart.1989.256.3.H789. [DOI] [PubMed] [Google Scholar]
- 95.Skjeldal S, Torvik A, Grøgaard B, Nordsletten L, Lyberg T. Histological studies on postischemic rat skeletal muscles. With emphasis on the time of leukocyte invasion. Eur Surg Res. 1993;25:348–357. doi: 10.1159/000129300. doi:10.1159/000129300. [DOI] [PubMed] [Google Scholar]
- 96.Sirsjö A, Söderkvist P, Gustafsson U, Lewis DH, Nylander G. The relationship between blood flow, development of edema and leukocyte accumulation in post-ischemic rat skeletal muscle. Microcirc Endothelium Lymphatics. 1990;6:21–34. [PubMed] [Google Scholar]
- 97.Hellberg PO, Källskog OT, Ojteg G, Wolgast M. Peritubular capillary permeability and intravascular RBC aggregation after ischemia: effects of neutrophils. Am J Physiol. 1990;258:F1018–F1025. doi: 10.1152/ajprenal.1990.258.4.F1018. [DOI] [PubMed] [Google Scholar]
- 98.Ibrahim S, Jacobs F, Zukin Y, Enriquez D, Holt D, Baldwin W, 3rd, et al. Immunohistochemical manifestations of unilateral kidney ischemia. Clin Transplant. 1996;10:646–652. [PubMed] [Google Scholar]
- 99.Forbes JM, Leaker B, Hewitson TD, Becker GJ, Jones CL. Macrophage and myofibroblast involvement in ischemic acute renal failure is attenuated by endothelin receptor antagonists. Kidney Int. 1999;55:198–208. doi: 10.1046/j.1523-1755.1999.00253.x. doi:10.1046/j.1523-1755.1999.00253.x. [DOI] [PubMed] [Google Scholar]
- 100.Mizuno S, Nakamura T. Prevention of neutrophil extravasation by hepatocyte growth factor leads to attenuations of tubular apoptosis and renal dysfunction in mouse ischemic kidneys. Am J Pathol. 2005;166:1895–1905. doi: 10.1016/S0002-9440(10)62498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 102.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. doi:10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Okuaki Y, Miyazaki H, Zeniya M, Ishikawa T, Ohkawa Y, Tsuno S, et al. Splenectomy-reduced hepatic injury induced by ischemia/reperfusion in the rat. Liver. 1996;16:188–194. doi: 10.1111/j.1600-0676.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 104.Cywes R, Packham MA, Tietze L, Sanabria JR, Harvey PR, Phillips MJ, et al. Role of platelets in hepatic allograft preservation injury in the rat. Hepatology. 1993;18:635–647. doi:10.1002/hep.1840180324. [PubMed] [Google Scholar]
- 105.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. doi:10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 106.Celebi M, Paul AG. Blocking E-selectin inhibits ischaemia–reperfusion-induced neutrophil recruitment to the murine testis. Andrologia. 2008;40:235–239. doi: 10.1111/j.1439-0272.2008.00849.x. doi:10.1111/j.1439-0272.2008.00849.x. [DOI] [PubMed] [Google Scholar]
- 107.Szabo ME, Droy-Lefaix MT, Doly M, Braquet P. Free radical-mediated effects in reperfusion injury: a histologic study with superoxide dismutase and EGB 761 in rat retina. Ophthalmic Res. 1991;23:225–234. doi: 10.1159/000267107. doi:10.1159/000267107. [DOI] [PubMed] [Google Scholar]
- 108.Neufeld AH, Kawai S, Das S, Vora S, Gachie E, Connor JR, et al. Loss of retinal ganglion cells following retinal ischemia: the role of inducible nitric oxide synthase. Exp Eye Res. 2002;75:521–528. doi: 10.1006/exer.2002.2042. doi:10.1006/exer.2002.2042. [DOI] [PubMed] [Google Scholar]
- 109.Nishijima K, Kiryu J, Tsujikawa A, Honjo M, Nonaka A, Yamashiro K, et al. In vivo evaluation of platelet–endothelial interactions after transient retinal ischemia. Invest Ophthalmol Vis Sci. 2001;42:2102–2109. [PubMed] [Google Scholar]
- 110.Freitas M, Lima JL, Fernandes E. Optical probes for detection and quantification of neutrophils' oxidative burst. A review. Anal Chim Acta. 2009;649:8–23. doi: 10.1016/j.aca.2009.06.063. doi:10.1016/j.aca.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 111.Del Principe D, Frega G, Savini I, Catani MV, Rossi A, Avigliano L. The plasma membrane redox system in human platelet functions and platelet–leukocyte interactions. Thromb Haemost. 2009;101:284–289. [PubMed] [Google Scholar]
- 112.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. doi:10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. doi:10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 114.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. doi:10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 115.Boisseau MR. Leukocyte involvement in the signs and symptoms of chronic venous disease. Perspectives for therapy. Clin Hemorheol Microcirc. 2007;37:277–290. [PubMed] [Google Scholar]
- 116.Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3–11. [PubMed] [Google Scholar]
- 117.Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. doi:10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]