Abstract
The nitric oxide (NO) cascade and endothelial NO synthase (eNOS) are best known for their role in endothelium-mediated relaxation of vascular smooth muscle. Activation of eNOS by certain inflammatory stimuli and enhanced NO release have also been shown to promote increased microvascular permeability. However, it is not entirely clear why activation of eNOS by certain vasodilatory agents, like acetylcholine, does not affect microvascular permeability, whereas activation of eNOS by other inflammatory agents that increase permeability, like platelet-activating factor, does not cause vasodilation. In this review, we discuss the evidence demonstrating the role of eNOS in the elevation of microvascular permeability. We also examine the relative importance of eNOS phosphorylation and localization in its function to promote elevated microvascular permeability as well as emerging topics with regard to eNOS and microvascular permeability regulation.
Keywords: Endothelial nitric oxide synthase, Microvascular permeability, Inflammation, Protein traffic
1. Introduction
Inflammation is an important component of host defence; however, excessive or uncontrolled inflammation can disrupt normal tissue homeostasis. Traumatic injury can produce a massive, systemic inflammatory response that complicates resuscitation and patient outcomes.1 In addition, smaller, chronic tissue insults that occur during disease formation, such as long-term hyperglycaemia, ischaemic injuries, elevated oxidative stress, etc., can alter the expression of inflammatory mediators and receptors, as well as intracellular signal transduction pathways and structural components in the microvascular endothelium, facilitating exaggerated inflammatory responses or a chronic inflammatory state.2,3
Inflammatory processes are characterized by an increase in microvascular permeability (hyperpermeability) to macromolecules. The extravasation of macromolecules is a normal ongoing process that occurs predominantly at post-capillary venules, and plays an important role in formation of lymph and immune function. However, excessive leakage of macromolecules into tissues causes oedema, which can disrupt homeostasis and cause tissue dysfunction. Several inflammatory mediators can rapidly increase microvascular permeability to macromolecules across the venular endothelium. The mechanisms by which these inflammatory stimuli elicit hyperpermeability of the endothelium have been an area of intense investigation. In this Spotlight, we will review evidence obtained about cellular regulation of enhanced microvascular permeability obtained in isolated, cultured endothelial cells (EC) as well as in the complex cellular environment of the in vivo microcirculation. Application of the knowledge acquired in the in vitro setting to the in vivo interactions is fundamental to understand the integrated regulation of microvascular transport and its functional alterations in vascular disease.
Nitric oxide (NO) is an established important signalling regulator of cardiovascular function,4 but recognition of the significance of its role in the control of microvascular permeability has developed at a slower rate. The first experimental evidence for the involvement of NO in transport of macromolecules in intact microvessels was published in the early 1990s and caused immediate controversy.5–8 Studies performed in the intestinal microvasculature reported that NO serves to maintain a tight microvascular barrier; inasmuch as inhibition of nitric oxide synthases (NOS) with L-arginine analogs increased leakage of macromolecules in the intestine.5,9 However, evidence in other tissues, in isolated venules, single capillaries and in EC monolayers indicated that the activity of endothelial nitric oxide synthase (eNOS) increases microvascular permeability to macromolecules in response to inflammatory agents.6–8,10–14 These initial opposite results may have been due to species differences or to different targeted mechanisms operating under the specific experimental conditions. In both cases, the experimental evidence was derived from the ability of L-arginine analogs to block NOS or from the reliance on NO donors to mimic endogenous NO actions. Definitive evidence to settle this initial controversy was obtained in eNOS knockout mice. Studies performed in these molecularly engineered animals contributed unequivocally to establish that eNOS-derived NO causes hyperpermeability in response to pro-inflammatory agents such as platelet-activating factor (PAF) and vascular endothelial growth factor (VEGF).15,16
PAF and VEGF are agents that have proven useful in studying microvascular endothelial permeability in vivo and in vitro. Administration of PAF rapidly and significantly increases permeability in the mouse cremaster and mesenteric microvasculature as well as in the hamster cheek pouch.12,16,17 Administration of PAF to organs of eNOS knockout mice fails to mount a robust hyperpermeability response. Interestingly, the data in eNOS knockout mice show that baseline permeability is not affected by the presence or absence of eNOS. Thus, the eNOS-associated signalling cascade becomes important mainly in the response to inflammatory challenges inasmuch as administration of VEGF also fails the initiate a hyperpermeability response in these genetically altered animals.15
The specificity of eNOS-derived NO in the hyperpermeability process is supported by the observation that administration of VEGF or PAF to inducible NOS (iNOS) knockout mice elicits hyperpermeability responses that are not significantly different from control normal mice.15,16 In addition, VEGF-induced NO production is nearly abolished in mice lacking eNOS,15 and PAF-induced NO release is largely inhibited in single-perfused microvessels treated with a fusion protein containing the eNOS-inhibitory binding domain of caveolin-1.18 Moreover, in single-perfused microvessels, the PAF-induced NO production is calcium-sensitive, a feature attributed to eNOS activity but not iNOS.18 The fundamental importance of eNOS-derived NO for the onset of hyperpermeability has also been confirmed in EC in culture. Specific knockdown of eNOS expression using eNOS-targeting siRNA abolishes the ability of coronary venular EC to increase permeability to macromolecules in response to PAF.19 Although these studies demonstrate that eNOS-derived NO is of greater relevance in the immediate regulation of microvascular permeability, iNOS-derived NO may play a more significant role in events taking longer periods of time such as the changes in permeability associated with systemic inflammation or sustained angiogenesis.15,20
2. Mechanisms of signal transduction in hyperpermeability
In vivo evidence indicates that the signalling pathways for several inflammatory agents involve the activation of protein kinase C (PKC) and eNOS.10,14,21–23 These reactions are presumably followed by synthesis of cGMP. Experiments in isolated microvessels and EC support the concept that stimulation of NOS and production of cGMP leads to changes in permeability to small and large solutes.24,25
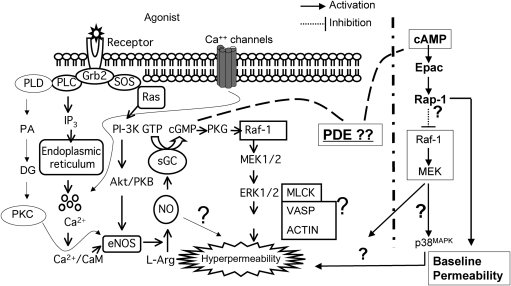
A simplified scheme of our understanding of the regulatory signalling in the control of microvascular permeability is shown in Figure 1. The diagram in Figure 1 is based both on experiments performed in vivo and in vitro. Briefly, the interactions of pro-inflammatory agonists with their specific receptors, lead to activation of phospholipases (PL) A2, C, and D, as well as Ca2+-channels. PLA2 (not shown) exerts a dual action. Through the lipoxygenase pathway, PLA2 activation leads to formation of leucotrienes, particularly C4, which activate NOS and elevate permeability. Through the cyclooxygenases pathway, PLA2 activates thromboxane synthase and is mainly responsible for the vasoconstrictor effect of PAF in the hamster cheek pouch.26 PLC is an important phospholipase that serves as a catalyst for the synthesis of inositol triphoshpate and diacylglycerol (DAG). The role of PLCγ in microvascular permeability has been evaluated with specific inhibitors. Indeed, inhibition of PLC reduces the impact of pro-inflammatory agonists on microvascular transport in isolated venules and in vivo13,27 suggesting a role for this phospholipase in the control of basal permeability. Importantly, the activity of PLC leads to activation of PKC, an important element in the signal transduction associated with the regulation of microvascular permeability.23
Figure 1.
Simplified scheme of pro-inflammatory signal transduction pathways leading to eNOS activation and endothelial hyperpermeability. Binding of pro-inflammatory agonists to their receptors activates a myriad of intracellular second messengers, leading to release of calcium from intracellular stores, PKC activation, Akt/PKB activation, and ERK-1/2 activation. The overall result is a functional state favouring changes in phosphorylation state of eNOS and elevated production of NO and increased permeability of the microvascular barrier. Several elements of the overall signalling paradigm remain elusive, such as the downstream effectors linking eNOS-derived NO to changes in the cytoskeleton and intercellular junctions that produce the hyperpermeable state, the precise role of the balance between cGMP and cAMP [via phosphodiesterases (PDE)] in permeability regulation. The mechanisms involved in the restoration of basal permeability and/or promotion of enhanced barrier integrity are represented by the Epac/Rap1 pathway.
2.1. Protein kinase C
The standard methods to study the impact of PKC in vivo consist of applying exogenous phorbol esters, such as phorbol 12-myristate 13-acetate (PMA) or phorbol ester dibutyrate (PDBu), or exogenous DAG or DAG analogs. These agents stimulate conventional and novel PKCs to translocate from the cytoplasm to the cell membrane and become activated.28,29 Another standard method is to inhibit the catalytic or the regulatory domains of PKC. PKCα, PKCβ, PKCδ, and PKCμ have been documented to influence endothelial permeability.29–31
The role of PKC as a biochemical pathway for PAF has been evaluated in vivo using inhibitors of the catalytic (sphingosine, iso H-7) and regulatory (calphostin C) domains of the enzyme. These experiments demonstrate that inhibition of PKC leads to a reduction in agonist-induced hyperpermeability.23,32 In addition, stimulation of PKC with phorbol esters (PDBu at 10−7, 10−6, and 10−5 M and PMA at 10−6 M and 5 × 10−7 M) increases microvascular transport of macromolecules in a dose-dependent fashion. Further evidence for PKC as a signalling pathway in the in vivo regulation of microvascular transport is derived from the ability of calphostin C to inhibit PDBu-induced macromolecular extravasation.23 Pharmacologic inhibition of PKC reduces the impact of agonists on the hyperpermeability induced by bradykinin and VEGF in vivo and in vitro.11,13,21–23,32 Importantly, inhibition of NOS blocks PDBu-stimulated hyperpermeability to FITC-dextran in the hamster cheek pouch,11 which demonstrates PKC-NO interactions in the promotion of hyperpermeability.
There are controversial reports on the role of PKC in the regulation of eNOS. PKC increases phosphorylation of eNOS on threonine-495 in EC, resulting in decreased eNOS activity.33 It has also been reported that inhibition of PKC promotes increased release of nitrogen oxides in bovine aortic EC.34 The functional outcomes of these changes in phosphorylation obtained in cultured or isolated cells are unknown. In contrast, it is clear that activation of PKC in vivo promotes hyperpermeability.11,23 PKC may activate, via phosphorylation, cytoskeletal proteins such as myosin light chain kinase, caldesmon and vimentin35–37 as well as influence the reorganization of focal adhesions and intercellular junctions.21,30 On the basis of elegant studies using fluorescent resonance energy transfer, it has been suggested that an imbalance in the PKCβII/PKCδ expression and activity contributes to the endothelial hyperpermeability observed in diabetes in coronary venules.31 The preponderance of the evidence supports the concept that PKC increases macromolecular transport, and that the mechanism involves activation of eNOS.
3. Significance of eNOS in the regulation of microvascular hyperpermeability
Several laboratories have contributed to paint the picture of how activation of eNOS proceeds. The process is complex and involves dissociation from caveolin-1, association with heat shock protein (HSP)-90, Ca2+-calmodulin binding, phosphorylation on serine-1177, and dephosphorylation of threonine-495.33,38–41 These steps are implicit in the initial reactions summarized in Figure 1. In particular, binding of eNOS to caveolin-1 through a consensus site in caveolae regulates the basal state of eNOS production of NO.42,43 Several agonists activate eNOS through multiple mechanisms such as phosphorylation or dephosphorylation of specific residues, interaction with different proteins, S-nitrosylation and specific subcellular localization.19,39,42,44–46 In this section the mechanisms of eNOS activation, NO production, and proposed mechanisms based on current experimental evidence will be discussed.
3.1. Phosphorylation of eNOS and NO production
The genes encoding for human and bovine endothelium eNOS have been characterized and their cDNA have been cloned.47–49 Although the gene sequence is highly conserved among species, the post-translational regulatory modalities may vary in different species.40,41 The regulation of eNOS by phosphorylation is supported by its deduced amino acid sequence. However, the extent to which phosphorylation or dephosphorylation of specific sites lead to activation or inactivation of eNOS activity has been the subject of debate, particularly in bovine arterial EC.34 When EC are stimulated by agonists such as VEGF, insulin-like growth factor, or elevated shear stress (which all can stimulate increased permeability of venules6,13,50), Akt phosphorylates eNOS on serine-1177, which increases eNOS activity.38,39 Bradykinin stimulates calmodulin-dependent kinase II to phosphorylate serine-1177 of eNOS as well.33 There are at least six phosphorylation sites identified to date that may play a role in the activity of eNOS, i.e. four sites are on serine (S116, S617, S635, S1179)51–53 one on threonine (T497)26 and at least one on tyrosine (Tyr83).52 The functional significance of each of these sites is undergoing systematic evaluation. It is of interest that the phosphorylation/dephosphorylation of these sites can be stimulated by a large number of agents through closely related pathways.53
The impact of each of the specific sites of eNOS phosphorylation on our knowledge of microvascular transport is difficult to evaluate. The majority of available molecular biology data comes from experiments designed to investigate the function of eNOS and of NO in control of blood flow and blood pressure, and performed in EC derived mainly from large vessels. Under these experimental conditions, investigators of the microcirculation are forced to extrapolate data from large vessel endothelia (not normally involved in blood-tissue transport) to post-capillary venular endothelium, where agonist-stimulated transport of macromolecules mainly occurs. In addition, the uncertainty of the extrapolation may be compounded by possible phenotypical changes undergone by EC grown in specifically designed and controlled culture media. In fact, the tissue culture situation may be at variance with the dynamic environment of EC in the microcirculation. Interestingly, acetylcholine (ACh), an agent that causes vasodilation but does not alter microvascular permeability,12 induces exactly the same changes in phosphorylation of eNOS as PAF and VEGF.54 These observations suggest that phosphorylation of eNOS per se is not a major determinant of the functional consequences or outcomes of eNOS-derived NO. This prompted our investigation as to whether ACh and PAF may cause differential translocation of eNOS.
3.2. Molecular movement of eNOS, location of eNOS and regulation of microvascular permeability
Endothelial nitric oxide is found mainly in the plasma membrane and in the Golgi complex in EC, but it distributes itself also in the cytosol. Thus, it is plausible that subcellular location of the enzyme contributes to determine its microvascular functions.
The ability of eNOS to translocate from plasma membrane to subcellular compartments is well documented in cultured EC, in non-vascular cells transfected with eNOS and in vivo after agonist stimulation using fractionation or extraction techniques and confocal microscopy.46,54–59 The movement of eNOS from plasma membrane to subcellular locations has been classically associated with de-palmitoylation of eNOS.40,41 Studies to determine the functional significance of eNOS traffic or internalization via caveolae have only recently been undertaken.19,46,60
Membrane-bound and cytosolic eNOS are able to release NO.51,61,62 Translocation of eNOS from the plasma membrane could be considered a mechanism to decrease the inhibitory association with caveolin-1 in order to activate the enzyme. However, elegant studies, using eNOS constructs designed to target it specific subcellular locations, have demonstrated that cell membrane-bound and Golgi-bound eNOS have the ability to release more basal NO than cytosolic eNOS.51,61,63 Cytosolic eNOS also appears to release less NO in response to agents that enhance inwardly directed movement of calcium, such as ionomycin.64 However, the location of the enzyme when it releases NO may be more important than the amount of NO released for development of extracellular significant functions, such as transport of macromolecules across the endothelium. The concept that eNOS must translocate to specific subcellular compartments to determine the NO-stimulated function is puzzling as one would assume that a highly diffusible gas would not need translocation of its releasing enzyme for appropriate activity. However, the experimental evidence supports the concept that precise location of eNOS at subcellular compartments is necessary to achieve the specific functionally efficacious concentration of NO.19,54 The existence of at least two intracellular arginine pools in EC has recently been reported,65 so it is conceivable that translocation may regulate access to substrates needed for NO production. In any case, this functional translocation may represent an adaptive and protective mechanism since NO is a highly reactive species and cells posses an abundance of scavengers of reactive species.
Agonists that increase permeability, such as VEGF, PAF, and bradykinin, cause eNOS movement to the cytosol.19,44,46,54 In contrast, ACh—a vasodilating agent—induces movement of eNOS preferentially to the Golgi in vivo and in vitro and does not promote hyperpermeability in vivo and in vitro.12,54,55 The difference in microvascular site of action and functional characteristics of these agents has provided a reasonable approach to correlate in vitro and in vivo experiments. The seminal in vitro studies on this topic were carried out in ECV-304 cells transfected with eNOS-GFP (GFP, green fluorescent protein). ECV-304 cells, initially described as a HUVEC-derived cell line with lacks eNOS, were later found to be identical to T24/83 cells (urinary bladder carcinoma cell line) due to a cross-contamination of cultures.66 However, these cells have remained a useful tool to study certain protein–protein interactions, localization, and function of transfected eNOS.54,67 The initial observations were confirmed and advanced subsequently in human umbilical vein EC and coronary post-capillary venular EC.19 Both PAF and ACh caused separation between eNOS and caveolin-1 as ascertained by discontinuous sucrose gradient fractionation. However, ACh promoted preferential movement of eNOS to the Golgi complex, whereas PAF promoted preferential movement of eNOS to the cytoplasm, as indicated by confocal microscopy.54 Interestingly, the distribution of caveolin-1 is not significantly influenced either by PAF or ACh.54,55
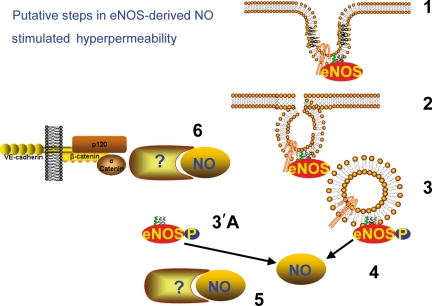
How does eNOS reach its target for development of hyperpermeability? The location of eNOS to the plasma membrane depends on N-myristoylation and palmitoylation reactions, which target it to the caveolae. In contrast, de-myristoylation and de-palmitoylation are processes associated with removal of eNOS from the membrane and its translocation to other subcellular compartments. Whether or not myristoylation and/or palmitoylation play a role in the translocation of eNOS was tested using a sophisticated, specific eNOS fusion protein (CD8-GFPeNOSmyr–) that transformed eNOS into a transmembrane protein with eNOS attached to the extracellular and transmembrane domain of the cell surface glycoprotein CD858, a feature that prevents translocation by de-palmitoylation. In addition, the construct includes a mutation at the G2A site of eNOS to prevent its myristoylation.58 The underlying assumption in the approach is that because of this specific transmembrane fusion protein eNOS would stay in the plasma membrane, would not translocate to the cytosol, and not contribute to PAF-stimulated hyperpermeability. In marked contrast to the expectations, PAF elicited a remarkable increase in permeability to FITC-dextran-70 in ECV-304 cells transfected with the CD8-GFPeNOSmyr-construct.19 Experiments utilizing biotinylation of surface proteins revealed that PAF internalized the CD8-GFPeNOSmyr–.19 A reasonable explanation for these experimental findings is the conclusion that eNOS is internalized via caveolae, since CD8-GFPeNOSmyr– is a transmembrane protein. This concept has been tested elegantly using constructs based on mutations of dynamin 2 and of the phosphorylation site for caveolin-146. These mutations prevent caveolar scission from the plasma membrane and thus anchor caveolae and eNOS to the plasma membrane. The evidence confirmed that PAF and VEGF stimulate eNOS internalization (endocytosis) via caveolae and that this step is fundamental to activate the eNOS–NO signalling cascade leading to induced hyperpermeability.19,46 The basic proposal for eNOS internalization as a mechanistic process in increased endothelial and microvascular permeability is displayed in Figure 2.
Figure 2.
Putative mechanisms by which eNOS-derived NO causes increased microvascular permeability. A logical sequence supported by experimental data is proposed. (1) During a non-inflammatory state, eNOS is associated with caveolin-1 (depicted as a hairpin) and localized primarily in caveolae at the plasma membrane. (2) Activation of EC by pro-inflammatory signals causes caveolae to pinch off the plasma membrane. Scission of caveolae from the membrane leads to eNOS internalization into the cell. (3) Within the cell, eNOS dissociates from caveolin-1 and may undergo phosphorylation. (3′A) It is also possible that eNOS may dissociate from the caveolar membranes. (4) The combination of activation of eNOS by calcium–calmodulin, dissociation of eNOS from caveolin-1 and eNOS phosphorylation significantly increases the production of NO by eNOS. (5) NO then can bind intracellular target(s) that remain to be identified, which produce signals that (6) alter the junctional proteins between EC and increase microvascular permeability.
3.3. The NO–cGMP axis
Soluble guanylyl cyclase (sGC) is the best described receptor of NO.68–72 Because of the low permeability of cyclic GMP through biological membranes, the assessment of the role of cGMP in the signalling cascade has been implemented using millimolar concentrations of its analog 8-Br-cGMP. Administration of 8-Br-cGMP increases permeability across EC monolayers (∼50% increase in permeability to FITC-Dextran 70 kDa) as well as in single microvessels (∼5-fold increase in hydraulic conductivity).25,73 The variation in reported magnitudes is probably due to (i) differences in baseline permeability between the two models, and (ii) differences in parameter measurement.74 The location of cGMP activity as downstream of eNOS-derived NO is supported by the observations that blocking sGC decreases the hyperpermeability induced by phorbol esters and by sodium nitroprusside.14 This concept is also corroborated by the observation that inhibition of PKG (cGMP-dependent protein kinase) causes a dramatic reduction in histamine-induced hyperpermeability in venules.14
The information in regards to the pathways downstream of cGMP–PKG leading to increases in permeability is very limited. PKG probably activates the ERK-1/2 MAP kinase pathway, inasmuch as pharmacological blockade of the ERK-1/2 MAP kinase pathway inhibits cGMP-induced hyperpermeability in HUVEC.25 In addition, PKG-induced increases in endothelial permeability may be the result of interactions of PKG with the PKA pathway. It is possible that feedback regulation through phosphodiesterases plays a significant role in modulating the relative concentrations of cGMP and cAMP, thus controlling the ability of these cyclic nucleotides to affect their specific targets. cGMP may activate phosphodiesterase 2 and induce degradation of cAMP; in turn, degradation of cAMP may reduce the barrier properties of the microvascular EC, and thus increase permeability to macromolecules.69,70,75 How the cGMP or cAMP signals reach the target junctional proteins is unknown.
The in vivo and in vitro evidence indicates an important role for cGMP in the signalling leading to hyperpermeability to macromolecules. However, it is important to note that we are lacking experimental evidence that clearly documents an enhancement in cGMP mass or activity in direct relationship to endothelial hyperpermeability. A recent PhD Thesis reported an increase in tissue cGMP in the hamster cheek pouch in association with PAF-induced hyperpermeability; however, the cellular source of cGMP was not identified.76 Other indirect evidence comes from studies using HSP90 inhibitors. HSP90 serves as a chaperone to that binds to both eNOS and sGC and facilitates their interaction, stabilizing sGC and enhancing cGMP production. Application of HSP90 inhibitors to cultured EC monolayers caused an enhancement of barrier function and was protective against barrier dysfunction caused by various oedemagenic agents.77,78
3.4. S-nitrosylation (nitrosation)
In addition to modulating the sGC–cGMP–PKG pathway, NO may influence biological processes by causing S-nitrosylation of proteins. The significance of S-nitrosylation as a post-translational modification that regulates enzymatic activity was reported in enzyme biology several years ago, but recognition of its contributions to the control of eNOS has emerged relatively recently.44 There is evidence that S-nitrosylation inhibits eNOS activity; and this inhibition is independent of eNOS phosphorylation and may contribute to its return to basal state following enzyme activation.79 S-nitrosylation also decreases NO-sensitivity of downstream sGC.71,80 However, the impact of S-nitrosylation of eNOS or sGC on endothelial permeability has not yet been investigated. The concept that S-nitrosylation of proteins depends more on the amount of NO produced by eNOS than on eNOS subcellular location has been advanced recently81 but requires further exploration as opposing views have been proposed.53
3.5. Mechanisms that restore baseline microvascular permeability
Inflammatory processes are characterized by an increase in microvascular hyperpermeability to macromolecules. The regulation of this process involves factors controlling proteins that form intercellular adhesions. We have pointed out some of the mechanisms by which eNOS-derived NO contributes to the onset of hyperpermeability. Important remaining questions, addressed in depth elsewhere on this Spotlight issue, are: how does hyperpermeability return to baseline? Is this merely a passive phenomenon or does it involve regulation by cellular signalling? The notion that microvascular permeability is regulated by counterbalancing signalling mechanisms to increase or restore permeability is one that is simple and attractive.
It is well established that baseline permeability is regulated by a constellation of factors such that reduction of microvascular permeability below baseline has been rarely reported.14,25,82,83 Phospholipase C has been reported to impact baseline permeability to albumin in coronary venules,14 whereas inhibition of ERK-1/2 reduces baseline permeability to FITC-dextran 70 in human umbilical vein EC.25 Activation of Epac (exchange protein activated by cAMP) leads to reduced baseline in endothelial permeability.69 Plasmapheresis appears to be the only reported experimental intervention that causes a fall in baseline permeability in vivo.82 Blockade of PLC does not change basal permeability in the hamster cheek pouch.27 The failure to recapitulate in vivo the results obtained under ex vivo conditions points to the need to reconcile the differences that exist in terms of environment, interactions among different cells, etc. when working in the better controlled in vitro or ex vivo situations relative to the more complex conditions that operate in vivo. However, recent communications confirm that stimulation of the Epac–Rap1 pathways contribute to enhance the microvascular endothelial barrier properties in vivo.84,85
4. Final comments
The evidence in the literature demonstrates that (i) baseline permeability is not compromised by deletion of the gene encoding for eNOS; (ii) eNOS is an integral element of the signalling pathway for the microvascular hyperpermeability response to pro-inflammatory agonists, such as PAF and VEGF; (iii) eNOS-derived NO is essential for the onset of hyperpermeability inasmuch as NO produced by other NOS-isozymes in eNOS knockout mice does not restore the ability of striated muscle microvasculature to produce a robust hyperpermeability in response to PAF; (iv) eNOS translocates from the plasma membrane to cytosol via caveolae in response to pro-inflammatory stimuli; (v) location of eNOS in the cytosol is fundamental for hyperpermeability. In regards to eNOS internalization via caveolae, we speculate that (a) caveolae may possess a necessary target recognizing molecule that allows eNOS to efficaciously promote the appropriate protein–protein signalling interactions in the intracellular environment; (b) this traffic may serve to deliver the appropriate NO concentration to achieve the correct stimulation of sGC or of an unidentified protein that represents the link to the cell junctions.
It is expected that, by combining physiologic and molecular biology approaches, future advancements in the knowledge and understanding of the mechanisms that regulate microvascular transport will lead to development of new pharmacologic agents and treatment modalities for conditions such as systemic inflammation, diabetes and ischaemia-reperfusion injury, in which management of the microvascular barrier is desirable for returning the afflicted tissues to an optimal functional state.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health Grants 5RO1 HL070634-07 and 5RO1 HL088479-02, 5P20 RR01876-07, and a grant from the American Heart Association.
References
- 1.Cioffi WG, Burleson DG, Pruitt BA., Jr Leukocyte responses to injury. Arch Surg. 1993;128:1260–1267. doi: 10.1001/archsurg.1993.01420230088014. [DOI] [PubMed] [Google Scholar]
- 2.Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertens. 2001;14:44S–54S. doi: 10.1016/s0895-7061(01)02069-6. doi:10.1016/S0895-7061(01)02069-6. [DOI] [PubMed] [Google Scholar]
- 3.Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, et al. Microvascular permeability in diabetes and insulin resistance. Microcirculation. 2007;14:363–373. doi: 10.1080/10739680701283091. doi:10.1080/10739680701283091. [DOI] [PubMed] [Google Scholar]
- 4.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. doi:10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 5.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol. 1992;263:H641–H646. doi: 10.1152/ajpheart.1992.263.2.H641. [DOI] [PubMed] [Google Scholar]
- 7.Mayhan WG. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5′-diphosphate and bradykinin. Inflammation. 1992;16:295–305. doi: 10.1007/BF00917622. doi:10.1007/BF00917622. [DOI] [PubMed] [Google Scholar]
- 8.Hughes SR, Williams TJ, Brain SD. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990;191:481–484. doi: 10.1016/0014-2999(90)94184-y. doi:10.1016/0014-2999(90)94184-Y. [DOI] [PubMed] [Google Scholar]
- 9.Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, et al. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- 10.Lal BK, Varma S, Pappas PJ, Hobson RW, 2nd, Durán WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res. 2001;62:252–262. doi: 10.1006/mvre.2001.2338. doi:10.1006/mvre.2001.2338. [DOI] [PubMed] [Google Scholar]
- 11.Ramírez MM, Kim DD, Durán WN. Protein kinase C modulates microvascular permeability through nitric oxide synthase. Am J Physiol. 1996;271:H1702–H1705. doi: 10.1152/ajpheart.1996.271.4.H1702. [DOI] [PubMed] [Google Scholar]
- 12.Ramírez MM, Quardt SM, Kim D, Oshiro H, Minnicozzi M, Durán WN. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc Res. 1995;50:223–234. doi: 10.1006/mvre.1995.1055. doi:10.1006/mvre.1995.1055. [DOI] [PubMed] [Google Scholar]
- 13.Wu HM, Yuan Y, Zawieja DC, Tinsley J, Granger HJ. Role of phospholipase C, protein kinase C, and calcium in VEGF-induced venular hyperpermeability. Am J Physiol. 1999;276:H535–H542. doi: 10.1152/ajpheart.1999.276.2.H535. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol. 1993;264:H1734–H1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- 15.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. doi:10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatakeyama T, Pappas PJ, Hobson RW, ii, Boric MP, Sessa WC, Durán WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol. 2006;574:275–281. doi: 10.1113/jphysiol.2006.108175. doi:10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon PK, Durán WN. Effect of platelet-activating factor on microvascular permselectivity: dose-response relations and pathways of action in the hamster cheek pouch microcirculation. Circ Res. 1988;62:732–740. doi: 10.1161/01.res.62.4.732. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, He P. Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc Res. 2010;87:340–347. doi: 10.1093/cvr/cvq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez FA, Kim DD, Durán RG, Meininger CJ, Durán WN. Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules. Am J Physiol Heart Circ Physiol. 2008;295:H1642–H1648. doi: 10.1152/ajpheart.00629.2008. doi:10.1152/ajpheart.00629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki Y, Deitch EA, Mishima S, Durán WN, Xu DZ. Endotoxin-induced mesenteric microvascular changes involve iNOS-derived nitric oxide: results from a study using iNOS knock out mice. Shock. 2000;13:397–403. doi: 10.1097/00024382-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Aramoto H, Breslin JW, Pappas PJ, Hobson RW, ii, Durán WN. Vascular endothelial growth factor stimulates differential signaling pathways in in vivo microcirculation. Am J Physiol Heart Circ Physiol. 2004;287:H1590–H1598. doi: 10.1152/ajpheart.00767.2003. doi:10.1152/ajpheart.00767.2003. [DOI] [PubMed] [Google Scholar]
- 22.Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol. 1997;273:H2442–H2451. doi: 10.1152/ajpheart.1997.273.5.H2442. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi I, Kim D, Hobson RW, ii, Durán WN. Platelet-activating factor modulates microvascular transport by stimulation of protein kinase C. Am J Physiol. 1994;266:H1214–H1220. doi: 10.1152/ajpheart.1994.266.3.H1214. [DOI] [PubMed] [Google Scholar]
- 24.Meyer DJ, Jr, Huxley VH. Capillary hydraulic conductivity is elevated by cGMP-dependent vasodilators. Circ Res. 1992;70:382–391. doi: 10.1161/01.res.70.2.382. [DOI] [PubMed] [Google Scholar]
- 25.Varma S, Breslin JW, Lal BK, Pappas PJ, Hobson RW, ii, Durán WN. p42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res. 2002;63:172–178. doi: 10.1006/mvre.2001.2381. doi:10.1006/mvre.2001.2381. [DOI] [PubMed] [Google Scholar]
- 26.Durán WN, Dillon PK. Acute microcirculatory effects of platelet-activating factor. J Lipid Mediat. 1990;2 Suppl:S215–S227. [PubMed] [Google Scholar]
- 27.Kim DD, Ramírez MM, Durán WN. Platelet-activating factor modulates microvascular dynamics through phospholipase C in the hamster cheek pouch. Microvasc Res. 2000;59:7–13. doi: 10.1006/mvre.1999.2195. doi:10.1006/mvre.1999.2195. [DOI] [PubMed] [Google Scholar]
- 28.Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. doi:10.1016/S0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 29.Tinsley JH, Teasdale NR, Yuan SY. Involvement of PKCdelta and PKD in pulmonary microvascular endothelial cell hyperpermeability. Am J Physiol Cell Physiol. 2004;286:C105–C111. doi: 10.1152/ajpcell.00340.2003. doi:10.1152/ajpcell.00340.2003. [DOI] [PubMed] [Google Scholar]
- 30.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol. 2001;533:433–445. doi: 10.1111/j.1469-7793.2001.0433a.x. doi:10.1111/j.1469-7793.2001.0433a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudreault N, Perrin RM, Guo M, Clanton CP, Wu MH, Yuan SY. Counter regulatory effects of PKCbetaII and PKCdelta on coronary endothelial permeability. Arterioscler Thromb Vasc Biol. 2008;28:1527–1533. doi: 10.1161/ATVBAHA.108.166975. doi:10.1161/ATVBAHA.108.166975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray MA, Heistad DD, Mayhan WG. Role of protein kinase C in bradykinin-induced increases in microvascular permeability. Circ Res. 1991;68:1340–1348. doi: 10.1161/01.res.68.5.1340. [DOI] [PubMed] [Google Scholar]
- 33.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. doi:10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 34.Ohara Y, Sayegh HS, Yamin JJ, Harrison DG. Regulation of endothelial constitutive nitric oxide synthase by protein kinase C. Hypertension. 1995;25:415–420. doi: 10.1161/01.hyp.25.3.415. [DOI] [PubMed] [Google Scholar]
- 35.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. doi:10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 36.Moy AB, Blackwell K, Wang N, Haxhinasto K, Kasiske MK, Bodmer J, et al. Phorbol ester-mediated pulmonary artery endothelial barrier dysfunction through regulation of actin cytoskeletal mechanics. Am J Physiol Lung Cell Mol Physiol. 2004;287:L153–L167. doi: 10.1152/ajplung.00292.2003. doi:10.1152/ajplung.00292.2003. [DOI] [PubMed] [Google Scholar]
- 37.Stasek JE, Jr, Patterson CE, Garcia JG. Protein kinase C phosphorylates caldesmon77 and vimentin and enhances albumin permeability across cultured bovine pulmonary artery endothelial cell monolayers. J Cell Physiol. 1992;153:62–75. doi: 10.1002/jcp.1041530110. doi:10.1002/jcp.1041530110. [DOI] [PubMed] [Google Scholar]
- 38.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 39.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. doi:10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Garcia-Cardena G, Sessa WC. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization. Biochemistry. 1996;35:13277–13281. doi: 10.1021/bi961720e. doi:10.1021/bi961720e. [DOI] [PubMed] [Google Scholar]
- 42.Fulton D, Fontana J, Sowa G, Gratton JP, Lin M, Li KX, et al. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J Biol Chem. 2002;277:4277–4284. doi: 10.1074/jbc.M106302200. doi:10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. doi:10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 44.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. doi:10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 45.Scotland RS, Morales-Ruiz M, Chen Y, Yu J, Rudic RD, Fulton D, et al. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res. 2002;90:904–910. doi: 10.1161/01.res.0000016506.04193.96. doi:10.1161/01.RES.0000016506.04193.96. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, et al. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc Natl Acad Sci USA. 2009;106:6849–6853. doi: 10.1073/pnas.0812694106. doi:10.1073/pnas.0812694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA. 1992;89:6348–6352. doi: 10.1073/pnas.89.14.6348. doi:10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, et al. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. doi:10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D'Angelo DD, et al. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992;267:15274–15276. [PubMed] [Google Scholar]
- 50.Haurigot V, Villacampa P, Ribera A, Llombart C, Bosch A, Nacher V, et al. Increased intraocular insulin-like growth factor-I triggers blood-retinal barrier breakdown. J Biol Chem. 2009;284:22961–22969. doi: 10.1074/jbc.M109.014787. doi:10.1074/jbc.M109.014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem. 2006;281:1477–1488. doi: 10.1074/jbc.M505968200. doi:10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- 52.Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, et al. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. doi:10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- 53.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. doi:10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 54.Sánchez FA, Savalia NB, Durán RG, Lal BK, Boric MP, Durán WN. Functional significance of differential eNOS translocation. Am J Physiol Heart Circ Physiol. 2006;291:H1058–H1064. doi: 10.1152/ajpheart.00370.2006. doi:10.1152/ajpheart.00370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Figueroa XF, Gonzalez DR, Martinez AD, Durán WN, Boric MP. ACh-induced endothelial NO synthase translocation, NO release and vasodilatation in the hamster microcirculation in vivo. J Physiol. 2002;544:883–896. doi: 10.1113/jphysiol.2002.021972. doi:10.1113/jphysiol.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1999;96:2788–2793. doi: 10.1073/pnas.96.6.2788. doi:10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1993;90:6252–6256. doi: 10.1073/pnas.90.13.6252. doi:10.1073/pnas.90.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prabhakar P, Cheng V, Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J Biol Chem. 2000;275:19416–19421. doi: 10.1074/jbc.M001952200. doi:10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- 59.Cheng C, van Haperen R, de Waard M, van Damme LC, Tempel D, Hanemaaijer L, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. doi:10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 60.Maniatis NA, Brovkovych V, Allen SE, John TA, Shajahan AN, Tiruppathi C, et al. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ Res. 2006;99:870–877. doi: 10.1161/01.RES.0000245187.08026.47. doi:10.1161/01.RES.0000245187.08026.47. [DOI] [PubMed] [Google Scholar]
- 61.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, et al. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. doi:10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J, Hughes TE, Sessa WC. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J Cell Biol. 1997;137:1525–1535. doi: 10.1083/jcb.137.7.1525. doi:10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1015–1021. doi: 10.1161/01.ATV.0000216044.49494.c4. doi:10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]
- 64.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, et al. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. doi:10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 65.Simon A, Plies L, Habermeier A, Martine U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res. 2003;93:813–820. doi: 10.1161/01.RES.0000097761.19223.0D. doi:10.1161/01.RES.0000097761.19223.0D. [DOI] [PubMed] [Google Scholar]
- 66.Dirks WG, MacLeod RA, Drexler HG. ECV304 (endothelial) is really T24 (bladder carcinoma): cell line cross- contamination at source. In Vitro Cell Dev Biol Anim. 1999;35:558–559. doi: 10.1007/s11626-999-0091-8. doi:10.1007/s11626-999-0091-8. [DOI] [PubMed] [Google Scholar]
- 67.Sowa G, Liu J, Papapetropoulos A, Rex-Haffner M, Hughes TE, Sessa WC. Trafficking of endothelial nitric-oxide synthase in living cells. Quantitative evidence supporting the role of palmitoylation as a kinetic trapping mechanism limiting membrane diffusion. J Biol Chem. 1999;274:22524–22531. doi: 10.1074/jbc.274.32.22524. doi:10.1074/jbc.274.32.22524. [DOI] [PubMed] [Google Scholar]
- 68.Chang FJ, Lemme S, Sun Q, Sunahara RK, Beuve A. Nitric oxide-dependent allosteric inhibitory role of a second nucleotide binding site in soluble guanylyl cyclase. J Biol Chem. 2005;280:11513–11519. doi: 10.1074/jbc.M412203200. doi:10.1074/jbc.M412203200. [DOI] [PubMed] [Google Scholar]
- 69.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. doi:10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 70.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. doi:10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 71.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. doi:10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamothe M, Chang FJ, Balashova N, Shirokov R, Beuve A. Functional characterization of nitric oxide and YC-1 activation of soluble guanylyl cyclase: structural implication for the YC-1 binding site? Biochemistry. 2004;43:3039–3048. doi: 10.1021/bi0360051. doi:10.1021/bi0360051. [DOI] [PubMed] [Google Scholar]
- 73.He P, Zeng M, Curry FE. cGMP modulates basal and activated microvessel permeability independently of [Ca2+]i. Am J Physiol. 1998;274:H1865–H1874. doi: 10.1152/ajpheart.1998.274.6.H1865. [DOI] [PubMed] [Google Scholar]
- 74.Durán WN, Sánchez FA, Breslin JW. Microcirculatory Exchange Function. In: Tuma RF, Durán WN, Ley K, editors. Handbook of Physiology: Microcirculation. 2nd ed. San Diego, CA: Academic Press; 2008. pp. 81–124. [Google Scholar]
- 75.Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. doi:10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.González F. Participación del óxido nítrico en la hiperpermeabilidad a macromoléculas inducida por el factor activador de plaquetas. Acción del óxido nítrico sobre distintas vías de señalización. Santiago, Chile: Pontificia Universidad Católica de Chile; 2009. [Google Scholar]
- 77.Antonova G, Lichtenbeld H, Xia T, Chatterjee A, Dimitropoulou C, Catravas JD. Functional significance of hsp90 complexes with NOS and sGC in endothelial cells. Clin Hemorheol Microcirc. 2007;37:19–35. [PubMed] [Google Scholar]
- 78.Antonov A, Snead C, Gorshkov B, Antonova GN, Verin AD, Catravas JD. Heat shock protein 90 inhibitors protect and restore pulmonary endothelial barrier function. Am J Respir Cell Mol Biol. 2008;39:551–559. doi: 10.1165/rcmb.2007-0324OC. doi:10.1165/rcmb.2007-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. doi:10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 80.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Durán WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008;103:606–614. doi: 10.1161/CIRCRESAHA.108.175133. doi:10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qian J, Zhang Q, Church JE, Stepp DW, Rudic RD, Fulton DJ. The role of local production of endothelium-derived Nitric Oxide on cGMP signaling and S-nitrosylation. Am J Physiol Heart Circ Physiol. 2010;298:H112–H118. doi: 10.1152/ajpheart.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kramer GC, Harms BA, Bodai BI, Demling RH, Renkin EM. Mechanisms for redistribution of plasma protein following acute protein depletion. Am J Physiol. 1982;243:H803–H809. doi: 10.1152/ajpheart.1982.243.5.H803. [DOI] [PubMed] [Google Scholar]
- 83.Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. [PubMed] [Google Scholar]
- 84.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, et al. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol. 2008;294:H1188–H1196. doi: 10.1152/ajpheart.00937.2007. doi:10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- 85.Iwahashi T, Kim DD, Lal BK, Durán WN. Stimulation of Epac/Rap1 pathway deactivates hyperpermeability after its onset in PAF-induced acute inflammation in vivo. FASEB J. 2009;23:950.4. [Google Scholar]