Abstract
The heart, perhaps more than any other organ, is exquisitely sensitive to increases in microvascular permeability and the accumulation of myocardial interstitial oedema fluid. Whereas some organs can cope with profound increases in the interstitial fluid volume or oedema formation without a compromise in function, heart function is significantly compromised with only a few percent increase in the interstitial fluid volume. This would be of little consequence if myocardial oedema were an uncommon pathology. On the contrary, myocardial oedema forms in response to many disease states as well as clinical interventions such as cardiopulmonary bypass and cardioplegic arrest common to many cardiothoracic surgical procedures. The heart's inability to function effectively in the presence of myocardial oedema is further confounded by the perplexing fact that the resolution of myocardial oedema does not restore normal cardiac function. We will attempt to provide some insight as to how microvascular permeability and myocardial oedema formation compromise cardiac function and discuss the acute changes that might take place in the myocardium to perpetuate compromised cardiac function following oedema resolution. We will also discuss compensatory changes in the interstitial matrix of the heart in response to chronic myocardial oedema and the role they play to optimize myocardial function during chronic oedemagenic disease.
Keywords: Myocardial oedema, Oedemagenic gain, Interstitial compliance, Lymphatics
1. Basic principles governing myocardial microvascular permeability
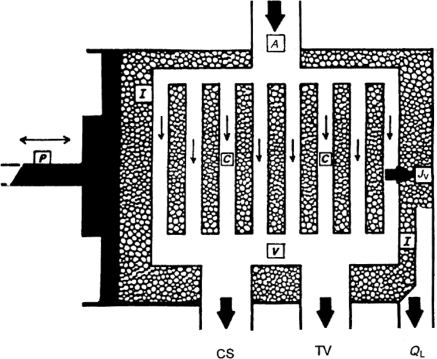
Figure 1 depicts the schematic representation of the functional components that determine myocardial fluid balance. The arterial input (A) to the left ventricular myocardium branches into a dense microvascular exchange vessel network (C) which, in turn, drains into several low-resistance outflow pathways. The coronary sinus (CS) drains into the right atrium and handles ∼75% of the total myocardial venous outflow under normal conditions. The remaining venous flow exits via thebesian veins (TV). I denotes the myocardial interstitial space, whereas P and JV represent the compressive force of the heart contractions and transmicrovascular fluid filtration rate, respectively. Myocardial fluid balance is determined by the fluid filtration rate (JV) out of the coronary microvascular exchange vessels into the cardiac interstitium (I) and its removal rate from the interstitium via myocardial lymphatic vessels (QL). Under normal conditions, the rate at which fluid enters the cardiac interstitium (JV) is equal to the myocardial lymph flow rate (QL), and, thus, myocardial water content remains relatively constant.
Figure 1.
The functional components that affect myocardial fluid balance. A, coronary arteries; V, coronary veins; CS, coronary sinus; TV, thebesian veins; I, myocardial interstitium; C, microvascular exchange vessels (capillaries); P, compressive force in the ventricular wall due to cardiac contractions; JV, transmicrovascular fluid flow rate; QL, lymph flow rate. (Modified from Laine and Granger.9)
The fluid filtration rate across a microvascular membrane, originally described by the Starling hypothesis, is a result of an imbalance between two competing pressures—hydrostatic and colloid osmotic forces. The forces that determine the fluid filtration rate (JV) out of the coronary microvasculature exchange vessels and into the interstitium can be summarized by the Starling–Landis equation [Eq. (1)],1,2
| 1 |
where Pc is the microvascular hydrostatic pressure at end-diastole, Pi is the interstitial hydrostatic pressure at end-diastole, Πc is the plasma colloid osmotic pressure, and Πi is the interstitial colloid osmotic pressure. The difference between Pc and Pi tends to force fluid into the interstitium. Whereas, the difference between Πc and Πi tends to retain or draw fluid in the opposite direction, from the interstitium into the microvessels.
Pc is difficult to determine in the heart, even in experimental preparations. Because microvascular exchange in the myocardium probably takes place primarily on the venular side of the capillary bed,3 investigators have used the average of coronary sinus wedge pressure and coronary sinus pressure as an estimate of Pc.4 Alternatively, Pc has been estimated by determining both coronary venular cross-sectional area and blood flow under direct visualization.5 Both techniques result in similar end-diastolic microvascular hydrostatic pressures of 20–30 mmHg. Πc represents the osmotic pressure generated by the plasma proteins (molecular weight >30 000 Da) that do not easily pass through the microvascular exchange vessel membrane. Direct measurement of Πc typically involves the use of an artificial membrane with pore sizes smaller than plasma proteins, whereas the microvascular exchange vessel membrane probably consists of pores of various sizes. Because the artificial membrane does not reproduce the microvascular exchange vessel membrane precisely, many investigators measure the protein concentration and calculate Πc.6,7 Normal range of Πc is 21–24 mmHg in man and 17–19 mmHg in dogs.6,8,9 On the other hand, the interstitial colloid osmotic pressure, Πi, cannot be measured directly but is thought to be similar to that measured in myocardial lymph. Normal Πi is 14 mmHg in the dog, which results in a colloid osmotic pressure gradient across the coronary microvascular of only 3–5 mmHg.8,9
The transmicrovascular fluid flow rate is determined by the microvascular filtration coefficient (Kf) in response to the effective microvascular driving pressure resulting from hydrostatic and colloid osmotic pressure gradient differences. The value of Kf depends on myocardial microvascular surface area and hydraulic conductivity. Compared with other organs, the density of microvascular exchange vessels in the myocardium is high, resulting in a larger microvascular exchange vessel surface area.3,10 In addition, a larger effective microvascular pore size produces a large Kf. Taken together, myocardial fluid flow is at least an order of magnitude greater on a per gram basis than in other organs such as the lung or skeletal muscle.11–14
The difficulty in measuring changes in the weight of a beating heart has limited Kf measurement using conventional gravimetric techniques. Although conventional gravimetric techniques could be applied to a non-beating heart, no direct measurement of myocardial microvascular Kf has been reported. However, myocardial microvascular Kf has been indirectly estimated using tracer–indicator-dilution techniques to be ∼0.35 mL min−1 mmHg−1 100 g−1.10,15
The reflection coefficient (σ), having a value between 0 and 1, characterizes the relative permeability of the microvascular exchange vessels to plasma proteins. It describes the fraction of protein molecules that are ‘reflected’ away from the microvascular membrane and thus modulates the contribution of the colloid osmotic pressure to the effective microvascular driving pressure.16 Estimation of the reflection coefficient for total plasma proteins or individual proteins is typically made utilizing a ‘protein washdown’ technique.9,17 Protein washdown, the decrease in interstitial protein concentration following an increase in the microvascular filtration rate (JV), is usually accomplished by elevating venous pressure. Determining the reflection coefficient with the washdown technique requires a several-fold increase in the microvascular filtration rate to ensure that the lymph-to-plasma protein concentration ratio is filtration-independent. Elevation of microvascular pressure by partial venous occlusion, within the physiological range, has not been reported to alter microvascular permeability, which may be seen following chemical or physical disruption of the microvasculature. However, because of the higher magnitude of the myocardial microvascular filtration rate, the application of this technique in the heart is limited.9,17 In the normal myocardium, absent any permeability disturbances, σ has been estimated to be in the range from 0.51 to 0.67 for total plasma protein and from 0.41 to 0.59 for albumin.9,17,18 Laine19 reported a direct relationship between (dP/dt)max and myocardial microvascular permeability, and therefore suggested the use of a change in (dP/dt)max as an index of changes in myocardial microvascular permeability, allowing permeability measurements in chronic animals on a long-term survival basis.
Myocardial microvascular permeability plays a critical role in determining microvascular fluid filtration and, thus, in myocardial fluid balance and oedema formation. The glycocalyx is a negatively charged layer of proteoglycans, glycosaminoglycans, and absorbed plasma proteins coating the luminal surface of the microvascular exchange vessel endothelium.20 Disrupting the glycocalyx or neutralizing its negative charge has been reported to alter microvascular permeability.21 These findings suggest that by limiting the passage of macromolecules, the glycocalyx contributes to the endothelial barrier function, which may be affected by circulating inflammatory agents via their effect on the glycocalyx. Acute and chronic microvascular permeability changes have been reported in various pathophysical conditions, including arterial hypertension22 and hypoproteinaemia,11 and may be responsible for myocardial oedema and myocardial dysfunction associated with these conditions. Increase in microvascular permeability and cardiac dysfunction has been reported in both experimental and clinical sepsis.23 Increased myocardial microvascular permeability and hypoperfusion have been reported as major contributors to endotoxin-induced myocardial dysfunction.24 Furthermore, the finding that sepsis-induced myocardial oedema was associated with a loss of negatively charged molecules that are part of the microvascular exchange barrier25 suggests the possible role of an altered glycocalyx in sepsis-induced increased microvascular permeability.
2. Unique aspects of myocardial microvascular fluid balance
Under normal conditions, the myocardial interstitial fluid pressure varies between ∼15 (at end-diastole) and 120 mmHg (at end-systole).9 The heart is the only organ in the body with such large cyclical variation in the interstitial fluid pressure. Although many organs have negative interstitial fluid pressures, the interstitial fluid pressure in the heart could not be shown to be negative at any time during the cardiac cycle.9,26 As myocardial oedema accumulates in the heart, myocardial end-diastolic interstitial fluid pressure increases.26
During systole, both capillaries and lymphatic vessels are crimped, minimizing blood flow and thus transmicrovascular fluid flow from the coronary vasculature into the myocardial interstitium (Figure 1). Since changes in microvascular permeability lead to myocardial oedema formation and increased myocardial interstitial fluid pressure, the important concept of myocardial interstitial compliance must be introduced. Compliance is a structural property of a system which relates changes in the interstitial fluid volume to changes in the interstitial fluid pressure [Eq. (2)], and is the inverse of elastance.
| 2 |
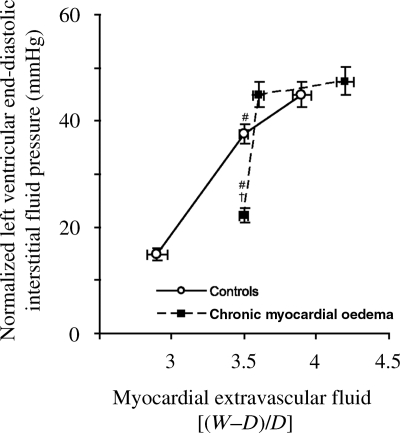
As microvascular permeability increases and oedema begins to form in the parenchyma of an organ, knowledge of interstitial compliance allows the estimation of the interstitial fluid pressure. This is important since the interstitial fluid pressure can alter cardiac function and modulate the transmicrovascular fluid flow rate or oedema formation. Interstitial compliance is of particular importance in discussions of myocardial microvascular permeability and oedema formation because interstitial compliance of the myocardium has been reported to undergo profound changes in conditions characterized by the presence of myocardial oedema (Figure 2). These changes in compliance have a significant impact on myocardial function, allowing the heart to compensate in chronic pathological states that would normally cause the heart to fail. Understanding the relationship between the myocardial interstitial fluid pressure and the myocardial interstitial fluid volume (i.e. interstitial compliance) may thus provide an opportunity to predict a patient's susceptibility to myocardial oedema formation (i.e. the oedemagenic gain), thus directing potential therapeutic interventions.27
Figure 2.
Normalized left ventricular end-diastolic interstitial fluid pressure plotted as a function of myocardial extravascular fluid [(wet weight – dry weight)/dry weight], indicating the degree of myocardial oedema. The inverse of the slope of the relationship between myocardial interstitial fluid pressure and extravascular fluid is myocardial interstitial compliance.45 (Desai et al.,45 used with permission.)
Transmicrovascular fluid flow can only occur in the myocardium during diastole when blood flows through the coronary microvascular exchange vessels and while the myocardial interstitial fluid pressure is relatively low. The length of time the heart is in diastole during a cardiac cycle can vary with several factors, such as heart rate.
As with other organs, the primary mechanism for the removal of interstitial oedema from the myocardial interstitium is lymph flow. The rate of myocardial contraction, the force of contraction, and the heart rate have significant impact on myocardial lymph flow rate, thus oedema resolution. Other organs such as the intestine also rely on rhythmic contractions of the whole organ to propel lymph from the interstitium to the central venous circulation. Unlike most organs where lymphatics demonstrate auto-rhythmicity, such contractions are not evident in the heart or intestine since organ contractions serve this purpose. When organ contractions are compromised, lymph flow decreases and organ oedema and malfunction ensue. This is demonstrated by compromised function in the heart and ileus in the intestine. Not only do the intestinal lymphatics lack auto-rhythmicity to remove oedema fluid, the small lymphatics also lack valves to ensure unidirectional lymph flow since peristalsis tends to move gastrointestinal luminal contents and lymph in a single direction. Because of the nature of myocardial contractions, however, valves do exist in the cardiac lymphatics to ensure unidirectional lymph flow away from the heart.
Of particular interest are changes in myocardial microvascular permeability and fluid balance associated with cardiopulmonary bypass used in a variety of surgical procedures including coronary artery bypass and open-heart procedures. As mentioned earlier, myocardial contractions are necessary to generate lymph flow and limit myocardial oedema.28 While on the cardiopulmonary bypass pump, the heart is arrested in diastole, causing several problems including (i) a lack of contractions to remove oedema fluid via the lymphatic system,28 (ii) a state of ‘full time’ diastole, increasing the time available for transmicrovascular fluid flow,8,9,29 (iii) activation of multiple humoral and cellular mediators, increasing microvascular permeability and thus microvascular fluid filtration,30–32 and (iv) a near-zero colloid osmotic pressure caused by crystalloid solutions commonly used to arrest the heart and perfuse the coronary vasculature.28,29,33 We should also point out that some circulating protein (albumin) is necessary to maintain normal microvascular permeability.11 Although controlled experimental data have not been reported, the lack of plasma colloids in cardioplegic solutions could lead to compromised myocardial microvascular permeability. It is well-documented that patients undergoing cardiopulmonary bypass have myocardial oedema post-operatively, some to a point that their chests must remain open until myocardial oedema resolves and their hearts will again fit within the chest cavity. Based on the nature of cardiopulmonary bypass, the presence of oedema in these patients should be no surprise.
Many of these same issues arise during cardiac transplantation. An additional confounding factor during the removal of donor hearts is that the cardiac lymphatic vessels are frequently ‘tied off’. Upon re-implantation, the recipient lymphatic vessels normally re-anastomose and re-establish normal lymph flow. If, however, the lymphatics remain tied or otherwise occluded, oedema may develop along with the formation of a lymphocele or lymphatic blister. The accumulation of lymph may be eliminated by puncturing the lymphocele, allowing cardiac lymph to drain into the chest cavity to be removed with other plural effusions. The pericardial sack is typically not closed post-operatively during bypass and transplant procedures. However, if the pericardial sack is intact and the patients have increased microvascular hydrostatic pressures, microvascular permeability or compromised lymph flows, interstitial fluid, or lymph may exit the myocardial interstitium via the surface of the heart.34 These pericardial effusions may increase the pericardial pressure, causing cardiac tamponade, which can profoundly compromise cardiac function.
3. Myocardial oedema and cardiac function
Myocardial fluid homeostasis is determined by the balance between the fluid filtration rate from the coronary microvasculature exchange vessels into the interstitium and the lymphatic removal rate of fluid from the interstitium. Myocardial oedema, excess accumulation of fluid in the myocardial interstitium, develops when the microvascular fluid filtration rate is greater than the lymphatic fluid removal rate.1,9,17 The imbalance leading to myocardial oedema may be due to either increased fluid filtration or decreased lymphatic drainage. Increased microvascular hydrostatic pressure, decreased plasma colloid osmotic pressure, and increased microvascular permeability to water and proteins can lead to increased microvascular fluid filtration. Similarly, increased lymphatic outflow pressure and effective resistance to lymph flow (RL) can lead to decreased lymphatic drainage rate.35
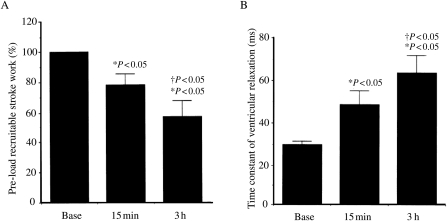
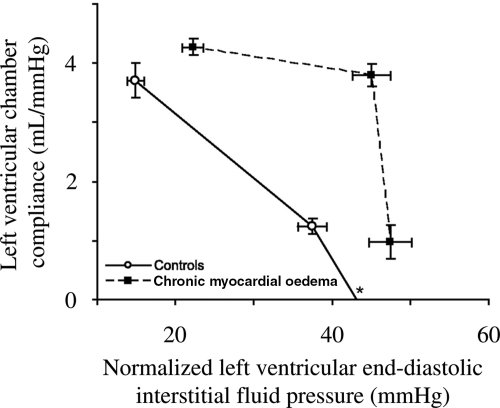
Several acute and chronic conditions including coronary sinus hypertension, pulmonary hypertension, arterial hypertension, myocardial infarction, cardiac transplantation, hypoproteinaemia, and cardioplegic arrest have been reported to cause myocardial oedema.6,28,29,36–41 Several pathophysiological consequences of myocardial oedema, including systolic and diastolic dysfunction, have been described in terms of (i) pre-load-recruitable stroke work, an index of myocardial contractility, (ii) the rate of left ventricular active relaxation, and (iii) ventricular diastolic stiffness. Pre-load recruitable stroke work is directly related to myocardial interstitial water content (Figure 3A) and decreases as a result of myocardial oedema induced by pulmonary hypertension,36 coronary sinus pressure elevation,39 cardiopulmonary bypass, and cold crystalloid28 and warm continuous blood cardioplegia.29 Several investigators have reported the causal relationship between myocardial oedema and impaired diastolic cardiac function. Myocardial oedema induced by pulmonary hypertension,36 coronary sinus pressure elevation,39 and hypoproteinaemia42 has been reported to impair isovolumic relaxation measured as an increase in the left ventricular relaxation time constant (Figure 3B). Studies have also shown that ventricular diastolic stiffness increases with oedema accumulation.42–44 One consequence of increased myocardial stiffness due to excess myocardial water content in acute oedema is decreased ventricular chamber compliance (Figure 4).45 Furthermore, a decrease in cardiac output by 40% for a given pre-load when myocardial water content was increased by 3.5% has been reported, due to coronary sinus pressure elevation.37
Figure 3.
(A) Effect of oedema on systolic function at baseline (n = 12), 15 min after pulmonary artery banding (n = 11), and 3 h after pulmonary artery banding (n = 8). Pre-load recruitable stroke work decreased significantly from baseline to 3 h. *P < 0.05 vs. baseline; †P < 0.05 vs. 15 min. (B) Effect of oedema on diastolic function at baseline, 15 min after pulmonary artery banding, and 3 h after pulmonary artery banding in 13 dogs. Rate of active relaxation slowed significantly.36 *P < 0.05 vs. baseline; †P < 0.05 vs. 15 min. (Davis et al.,36 used with permission.)
Figure 4.
Left ventricular chamber compliance plotted as a function of normalized left ventricular end-diastolic interstitial fluid pressure. The normalized value was calculated as the recorded myocardial left ventricular interstitial fluid pressure at end-diastole minus left ventricular end-diastolic chamber pressure. We placed boundary limits on the lower values of myocardial extravascular fluid to avoid negative or non-physiological values (*) of left ventricular chamber compliance.45 (Desai et al.,45 used with permission.)
It has been demonstrated that both acute and chronic pulmonary hypertension are associated with the left ventricular myocardial oedema and dysfunction.36,37,46 Pulmonary hypertension, via the elevated central venous pressure, increases the coronary sinus pressure, and thus, the coronary microvascular pressure (Pc) which enhances microvascular fluid filtration into the cardiac interstitium.36,37,39 Furthermore, the elevated central venous pressure impedes fluid removal from the cardiac interstitium via the myocardial lymphatic vessels.36,37,46 Thus, pulmonary hypertension not only increases the rate of fluid entering the myocardial interstitium but also decreases the rate of oedema fluid leaving the myocardial interstitium. Moreover, chronic left ventricular oedema secondary to pulmonary hypertension has been shown to be accompanied by increased collagen types I and III and prolyl 4-hydroxylase mRNA levels as well as decreased collagenase activity, resulting in increased left ventricular collagen deposition.46
In the presence of myocardial oedema, several mechanisms perhaps working in concert, may account for the compromised cardiac function. As myocardial oedema accumulates within the interstitial spaces, the interstitial pressure rises, thus increasing the stiffness of the myocardium. This stiffness combined with the viscous effects of moving excess interstitial fluid on a beat-to-beat basis can compromise the heart's ability to contract efficiently.47 This is in agreement with data demonstrating increased cardiac energy requirements associated with the presence of oedema.48 Although interstitial collagen has great tensile strength, increases in the interstitial volume and pressure may displace the collagen fibres and potentially uncouple or break collagen struts loose from their anchoring points on fibroblasts.49,50 Because of the heart's reliance on a well-organized interstitial matrix around which to contract, a disruption in the collagen structure could also compromise function. As myocardial oedema accumulates within the interstitium, the diffusion distance for oxygen to the myocytes increases. This is of particular importance in an organ such as the heart, which operates near maximum oxygen extraction capacity at all times. This is supported by work that demonstrated increased coronary vascular resistance associated with myocardial oedema.51 Recent reports document that myocardial infarctions grow more rapidly in the hearts of subjects with chronic arterial hypertension.52 These reports speculate that increased diffusion distances for oxygen and relative ischaemia due to the presence of myocardial oedema may exacerbate the rapid infarction growth.53
Mechanisms that defend against myocardial oedema formation include an increase in lymph flow,9,29,37 an increase in the interstitial hydrostatic pressure,26 a decrease in the interstitial colloid osmotic pressure,9 and an increase in the flow of interstitial fluid across the epicardium.38,54 As myocardial oedema accumulates, the myocardial interstitial fluid pressure starts to increase. Increased lymph flow, which accompanies the increase in the myocardial interstitial fluid volume and pressure, moderates the myocardial oedema formation. Furthermore, the increase in the interstitial fluid pressure, in turn, moderates JV by decreasing the total transmicrovascular hydrostatic pressure gradient.55 Protein washdown, the decrease in protein concentration of interstitial fluid or lymph following an increase in JV, functions as a protective mechanism against oedema formation. This change, in turn, moderates JV by decreasing the total transmicrovascular colloid osmotic pressure gradient [Eq. (1)].55 Increased myocardial fluid volume and pressure, as well as decreased myocardial interstitial colloid osmotic pressure, have been reported to increase the flow of myocardial interstitial fluid across the epicardium.34
Although the formation of myocardial oedema has been investigated, the resolution of myocardial oedema has not been studied thoroughly, possibly due to the difficulty reversing the complex interventions used to induce myocardial oedema. Takoudes et al.56 have studied the resolution of myocardial oedema with cardioplegia by avoiding systemic haemodilution. They reported that myocardial oedema induced by crystalloid coronary perfusion resolved in the intact, working rat heart within 5 min of reperfusion. However, the relevance of the rat model to larger animals and humans is uncertain. Similar studies in a pig heart model reported that crystalloid-induced myocardial oedema and diastolic stiffness resolve after 45 min of reperfusion.57,58 Hsu et al.59 reported that after 90 min of hypothermic ischaemic arrest, perfusion-induced myocardial oedema in dog hearts did not resolve completely when the coronary arteries were perfused with hypertonic solutions. Allen et al.60 investigated the effect of cardiac contractility on the resolution of myocardial oedema and myocardial lymph flow. They concluded that organized myocardial contraction is the most important factor determining myocardial lymph flow and oedema resolution and suggested that enhancing cardiac contractility with inotropic drugs may hasten myocardial oedema resolution after cardioplegic arrest.28
4. Myocardial oedema and cardiac efficiency
Evidence suggests that acute myocardial oedema formation impairs cardiac function, in part, through reduced myocardial efficiency and further points to the perplexing fact that this impairment continues after the resolution of the oedema. A study reported by Geissler et al.61 demonstrated that cardiopulmonary bypass and cardioplegic arrest result in both reduced myocardial efficiency and increased myocardial water content. Warm blood cardioplegia and femoral artery-to-right atrium/vena cava bypass were initiated after baseline measurements and maintained for 60 min followed by a 30 min reperfusion period. In one of the two groups of dogs, the priming solution for the extracorporeal circuit included 3% dextran for the purpose of reducing oedema formation during bypass. In both groups, coronary blood flow and myocardial oxygen consumption were significantly greater than baseline 1 and 2 h following cessation of bypass, whereas stroke work and contractility [(dP/dt)max] were not different. Myocardial efficiency (the ratio of cardiac work to myocardial oxygen consumption) and cardiac index were reduced in both groups after bypass. Myocardial water content increased during bypass and returned to baseline by 1 h after the cessation of bypass in both groups. The presence of dextran in the priming solution moderated the myocardial oedema formation.
These results suggest the possibility of a relationship between myocardial oedema formation and myocardial efficiency, but fail to clarify that relationship for several reasons. The oedema that formed in both groups was only present in significant amounts during the period of arrest and bypass, and resolved rather quickly once bypass was terminated. During arrest, efficiency cannot be determined; therefore, the reported reduction in myocardial efficiency was observed after recovery from arrest and cessation of bypass at a time when the oedema had already resolved. In addition, cardioplegic arrest and cardiopulmonary bypass could be expected to have multiple effects on cardiac performance through mechanisms unrelated to interstitial fluid balance.
A study reported by Laine and Allen62 addressed the impact of myocardial oedema formation on myocardial efficiency more directly by using coronary sinus hypertension to induce oedema formation. Blood volume and anaesthetic depth were manipulated in an attempt to hold cardiac work constant. Myocardial oedema was induced in one group of dogs by partially occluding the coronary sinus by the inflation of a balloon-tipped catheter to maximize the coronary sinus pressure which would increase the coronary microvascular pressure and microvascular filtration.39 Elevation of coronary sinus pressure redirects coronary venous return through the thebesian veins, but does not induce ischaemia.63 Coronary sinus hypertension was maintained for 3 h. At the end of the experiment, myocardial water content was determined and expressed as the ratio of water weight to dry tissue weight.
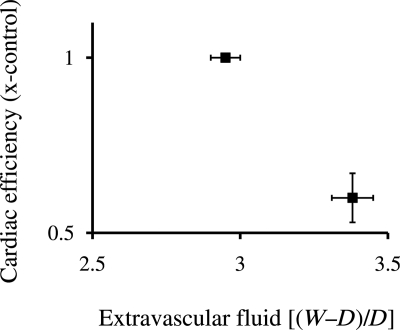
Compared with control, the coronary sinus hypertension group showed an increase in myocardial water content and a 40% decrease in myocardial efficiency. The efficiency change was the result of a significant increase in coronary blood flow with no change in arterial-coronary sinus O2 difference. In addition, there was no increase in myocardial lactate production. These results support the hypothesis that myocardial oedema impacts ventricular function, at least in part, by reducing myocardial efficiency (Figure 5). However, this study did not include an oedema recovery period and, thus, cannot explain the diminished efficiency after the resolution of oedema observed in Geissler's study.61
Figure 5.
Decreasing cardiac efficiency (x-control) plotted as a function of myocardial oedema. Myocardial oxygen extraction did not change, whereas coronary blood flow increased, leading to a significant rise in oxygen consumption for a constant level of cardiac work. (Composed from data in Laine and Allen.62)
Stewart et al.64 reported the results of a study which used a 3 h coronary sinus hypertension period to evaluate the consequences of oedema formation and resolution on cardiac function. Left ventricular pressure–volume loops generated during progressive vena caval occlusion were used to calculate pre-load-recruitable stroke work and the end-diastolic pressure–volume relationship expressed as ventricular chamber stiffness. Following the collection of baseline data, the coronary microvascular pressure was increased by the inflation of a balloon-tipped catheter in the coronary sinus. After 3 h, the balloon was deflated and the coronary sinus pressure was allowed to return to baseline followed by a 3 h recovery period.
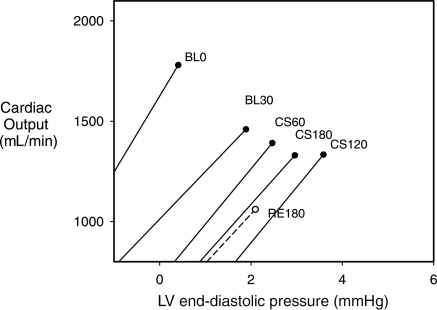
During the hypertension period, myocardial water content increased significantly, and then decreased by the end of the recovery period.64 Contractility, as measured by pre-load-recruitable stroke work, appeared to decline throughout the hypertension period, but did not reach statistical significance. However, it declined further during the recovery period and was statistically lower by the end of that period. Left ventricular chamber stiffness increased significantly from baseline by the end of the 3 h hypertension period. The cardiac response curves were composed by plotting cardiac output as a function of the end-diastolic pressure from data collected during progressive occlusion of the vena cava (Figure 6). This study strongly suggests that myocardial oedema formation decreases global cardiac function in large part by increasing ventricular chamber stiffness and that the diminished function does not immediately improve upon the resolution of the oedema.
Figure 6.
Cardiac function curves generated by vena caval occlusion. Cardiac function was determined as the cardiac output achieved for a given end-diastolic pressure. Data were collected at baseline (BL0), 30 min after BL0 (BL30), 1 h after coronary sinus balloon inflation (CS60), 2 h after inflation (CS 120), 3 h after inflation (CS180) and 3 h after balloon deflation (RE180). (Composed from data in Stewart.64)
5. Myocardial oedema detection
The last few decades have seen technological advances leading to better cardiovascular imaging techniques. The three commonly used modalities are echocardiography, computed tomography (CT), and magnetic resonance imaging. Despite the problems associated with 2D imaging, echocardiography remains the principle tool for cardiovascular functional and structural assessment due to wide availability, low cost, and real-time imaging.65 To characterize myocardial water content, ecocardiographic techniques rely on left ventricular mass and wall volume that increase with myocardial oedema formation. Since the increase in ventricular mass may not be specifically due to myocardial oedema, the use of echocardiography to characterize myocardial water content may be limited. However, recent advances in high-frequency ultrasound imaging techniques are proving to be capable of characterizing myocardial oedema. Based on previous observations of the increase in backscatter with myocardial ischaemia, Dent et al.66 reported the ability of high-frequency ultrasonic imaging techniques to characterize myocardial oedema. The authors have utilized the ability of high-frequency ultrasound to delineate mechanical properties of tissue and demonstrated the feasibility of characterizing myocardial water content by quantifying change in myocardial properties.
Powell et al.67 demonstrated the feasibility of detecting myocardial oedema using CT. CT has been demonstrated to be useful in cardiovascular functional and anatomical assessment; however, poor temporal and spatial resolution and motion artefacts have limited the use of CT in clinical settings to quantify myocardial oedema. Recent advancements in CT may change the picture. Mahnken et al.68 reported the ability of dual-source CT to detect myocardial oedema at par with other modalities in a porcine acute myocardial infarction model.
Magnetic resonance imaging is an alternative to echocardiography and CT for cardiovascular functional and anatomical assessment. Cardiovascular magnetic resonance (CMR) imaging has been reported to be as good as or better than other modalities but its application is limited due to high cost and it is not widely available as yet. Since Kiricuta et al.69 reported the correlation between tissue hydration and T1 (longitudinal) and T2 (transverse) relaxation times, magnetic resonance imaging has been used and has emerged as a dependable tool to quantify changes in myocardial water content. Higgins et al.70 demonstrated the linear relationship between T2 relaxation time and myocardial water content in acutely infarcted myocardium. Karolle et al.71 used lengthened T1 and T2 relaxation times following myocardial ischaemia and reperfusion to assess transmural distribution of myocardial oedema. Albers et al.72 demonstrated the feasibility of using CMR (T1-weighted) to detect myocardial oedema caused by crystalloid cardioplegic solutions. They reported the non-uniform three-dimensional distribution of myocardial oedema. T2-weighted CMR imaging has been used to assess myocardial oedema and area-at-risk associated with acute ischaemic injury. When used in combination with other CMR techniques, T2-weighted imaging has been reported to detect myocardial oedema associated with acute myocardial ischaemia even before irreversible injury.73 Recent technical advances have led to the development of more specific CMR algorithms focused on detecting myocardial water content and overcome limitations such as low signal-to-noise ratio, motion artefacts, etc. in the T2-weighted technique.74,75 With the promising outcome of several trials as well as several translational/research studies, CMR imaging will soon be available in emergency rooms specifically to help in diagnosis and prognosis along with other modalities.
6. Altering microvascular permeability and oedema formation initiates a cascade of events
Increasing the rate at which fluid exits the microvasculature and accumulates in the myocardial interstitium acutely compromises cardiac function, and the compromised cardiac function persists following oedema resolution. Myocardial interstitial compliance changes acutely,45 and that interstitial components including hyaluronan, which contribute to the mechanical properties of the ventricular wall, are removed due to increased fluid removal from the interstitium by the lymphatics. Altered myocyte volume and contractility, which have been reported to change in response to osmotic stress, may be responsible for cardiac dysfunction following hypothermic hyperkalaemic cardioplegia and metabolic inhibition (ischaemia)-induced myocardial oedema.76,77 Furthermore, along with altered myocyte calcium handling capacity, derangement in excitation–contraction coupling following myocyte swelling may also be involved in the contractile dysfunction following oedemagenic stress.78 However, change in myocyte volume and associated change in contractility with myocardial interstitial oedema (hydrostatic oedema) have not been reported. Recently, Uray et al.79 reported the inhibition of intestinal contractile activity with intestinal interstitial oedema via NF-κB activation. Although NF-κB and TNF-α have been reported to depress myocardial contractility,80,81 their role in myocardial interstitial oedema-associated contractile dysfunction has not been evaluated. The mechanism for persistent compromised cardiac function, during formation and following resolution of myocardial hydrostatic oedema remains unclear.
It is clear that chronic changes take place in the myocardial interstitial matrix, which improve the heart's ability to function normally in the presence of chronic myocardial oedema. Williams et al.82 reported a 60% increase in myocardial interstitial collagen in oedematous, non-infarcted human hearts at transplantation. These compensatory changes are critical to sustain life in disease states characterized by chronic myocardial oedema since these adaptations allow the chronically oedematous hearts with increased left ventricular chamber compliance to preserve function when challenged with acute oedema over a wide range of myocardial interstitial pressures.45
In conclusion, we should think of microvascular permeability and the interstitial matrix of all organs as a system which can undergo constant change in response to their environment. Microvascular permeability changes in response to vascular pressures, flows, and circulating factors both intrinsic and extrinsic. The myocardial microvascular–interstitial–lymphatic structure should be viewed as a complex system that senses and adapts to its environment in such a manner that optimizes organ function in the presence of pathological perturbations.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (R01 HL092916).
References
- 1.Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landis EM. Micro-injection studies of capillary permeability. II. The relation between capillary pressure and the rate at which fluid passes through the walls of single capillaries . Am J Physiol. 1927;82:217–238. [Google Scholar]
- 3.Wearn JT. The extent of the capillary bed of the heart. J Exp Med. 1928;47:273–290. doi: 10.1084/jem.47.2.273. doi:10.1084/jem.47.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyama T, Kikuchi Y, Kakiuchi Y, Nagashima C. An analysis of water movement between myocardial tissue and capillary blood during reactive hyperemia. Jpn J Physiol. 1979;29:1–13. doi: 10.2170/jjphysiol.29.1. [DOI] [PubMed] [Google Scholar]
- 5.Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circ Res. 1991;69:561–570. doi: 10.1161/01.res.69.3.561. [DOI] [PubMed] [Google Scholar]
- 6.Navar PD, Navar LG. Relationship between colloid osmotic pressure and plasma protein concentration in the dog. Am J Physiol Heart Circ Physiol. 1977;233:H295–H298. doi: 10.1152/ajpheart.1977.233.2.H295. [DOI] [PubMed] [Google Scholar]
- 7.Gabel JC, Drake RE. Plasma proteins and protein osmotic pressure. In: Staub NC, Taylor AE, editors. Edema. New York: Raven Press; 1984. [Google Scholar]
- 8.Mehlhorn U, Allen SJ, Davis KL, Geissler HJ, Warters RD, Rainer de Vivie E. Increasing the colloid osmotic pressure of cardiopulmonary bypass prime and normothermic blood cardioplegia minimizes myocardial oedema and prevents cardiac dysfunction. Cardiovasc Surg. 1998;6:274–281. doi: 10.1016/s0967-2109(97)00152-x. doi:10.1016/S0967-2109(97)00152-X. [DOI] [PubMed] [Google Scholar]
- 9.Laine GA, Granger HJ. Microvascular, interstitial, and lymphatic interactions in normal heart. Am J Physiol Heart Circ Physiol. 1985;249:H834–H842. doi: 10.1152/ajpheart.1985.249.4.H834. [DOI] [PubMed] [Google Scholar]
- 10.Michel CC. Fluid movements through capillary walls. In: Renken EM, Michel CC, editors. The Cardiovascular System: Microcirculation. Baltimore: Williams & Wilkins; 1984. [Google Scholar]
- 11.Mann GE. Alterations of myocardial capillary permeability by albumin in the isolated, perfused rabbit heart. J Physiol. 1981;319:311–323. doi: 10.1113/jphysiol.1981.sp013910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassingthwaighte JB, Yipintsoi T, Grabowski EF. Myocardial capillary permeability: hydrophilic solutes penetrate 100 A intercellular clefts. Bibl Anat. 1975;13:24–27. [PMC free article] [PubMed] [Google Scholar]
- 13.Laughlin MH, Diana JN. Myocardial transcapillary exchange in the hypertrophied heart of the dog. Am J Physiol. 1975;229:838–846. doi: 10.1152/ajplegacy.1975.229.3.838. [DOI] [PubMed] [Google Scholar]
- 14.Vargas F, Johnson JA. An estimate of reflection coefficients for rabbit heart capillaries. J Gen Physiol. 1964;47:667–677. doi: 10.1085/jgp.47.4.667. doi:10.1085/jgp.47.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chilian WM, Eastham CL, Layne SM, Marcus ML. Small vessel phenomena in the coronary microcirculation: phasic intramyocardial perfusion and coronary microvascular dynamics. Prog Cardiovasc Dis. 1988;31:17–38. doi: 10.1016/0033-0620(88)90009-6. doi:10.1016/0033-0620(88)90009-6. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AE, Granger DN. Exchange of macromolecules across the microcirculation. In: Renkin EM, Michel CC, editors. Handbook of Physiology. The Cardiovascular System Microcirculation. Bethesda, MD: American Physiological Society; 1984. [Google Scholar]
- 17.Mehlhorn U, Davis KL, Laine GA, Geissler HJ, Allen SJ. Myocardial fluid balance in acute hypertension. Microcirculation. 1996;3:371–378. doi: 10.3109/10739689609148309. doi:10.3109/10739689609148309. [DOI] [PubMed] [Google Scholar]
- 18.Pilati CF. Macromolecular transport in canine coronary microvasculature. Am J Physiol Heart Circ Physiol. 1990;258:H748–H753. doi: 10.1152/ajpheart.1990.258.3.H748. [DOI] [PubMed] [Google Scholar]
- 19.Laine GA. Change in (dP/dt)max as an index of myocardial microvascular permeability. Circ Res. 1987;61:203–208. doi: 10.1161/01.res.61.2.203. [DOI] [PubMed] [Google Scholar]
- 20.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. doi:10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 21.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. doi:10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 22.Laine GA. Microvascular changes in the heart during chronic arterial hypertension. Circ Res. 1988;62:953–960. doi: 10.1161/01.res.62.5.953. [DOI] [PubMed] [Google Scholar]
- 23.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. doi:10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 24.Chagnon F, Bentourkia M, Lecomte R, Lessard M, Lesur O. Endotoxin-induced heart dysfunction in rats: assessment of myocardial perfusion and permeability and the role of fluid resuscitation. Crit Care Med. 2006;34:127–133. doi: 10.1097/01.ccm.0000190622.02222.df. doi:10.1097/01.CCM.0000190622.02222.DF. [DOI] [PubMed] [Google Scholar]
- 25.Gotloib L, Shostak A, Galdi P, Jaichenko J, Fudin R. Loss of microvascular negative charges accompanied by interstitial edema in septic rats' heart. Circ Shock. 1992;36:45–56. [PubMed] [Google Scholar]
- 26.Stewart RH, Rohn DA, Mehlhorn U, Davis KL, Allen SJ, Laine GA. Regulation of microvascular filtration in the myocardium by interstitial fluid pressure. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1465–R1469. doi: 10.1152/ajpregu.1996.271.6.R1465. [DOI] [PubMed] [Google Scholar]
- 27.Dongaonkar RM, Quick CM, Stewart RH, Drake RE, Cox CS, Jr., Laine GA. Edemagenic gain and interstitial fluid volume regulation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R651–R659. doi: 10.1152/ajpregu.00354.2007. doi:10.1152/ajpregu.00354.2007. [DOI] [PubMed] [Google Scholar]
- 28.Mehlhorn U, Davis KL, Burke EJ, Adams D, Laine GA, Allen SJ. Impact of cardiopulmonary bypass and cardioplegic arrest on myocardial lymphatic function. Am J Physiol Heart Circ Physiol. 1995;268:H178–H183. doi: 10.1152/ajpheart.1995.268.1.H178. [DOI] [PubMed] [Google Scholar]
- 29.Mehlhorn U, Allen SJ, Adams DL, Davis KL, Gogola GR, de Vivie ER, et al. Normothermic continuous antegrade blood cardioplegia does not prevent myocardial edema and cardiac dysfunction. Circulation. 1995;92:1940–1946. doi: 10.1161/01.cir.92.7.1940. [DOI] [PubMed] [Google Scholar]
- 30.Dauber IM, Parsons PE, Welsh CH, Giclas PC, Whitman GJ, Wheeler GS, et al. Peripheral bypass-induced pulmonary and coronary vascular injury. Association with increased levels of tumor necrosis factor. Circulation. 1993;88:726–735. doi: 10.1161/01.cir.88.2.726. [DOI] [PubMed] [Google Scholar]
- 31.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–692. doi: 10.1378/chest.112.3.676. doi:10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 32.Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304:497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- 33.Laks H, Standeven J, Blair O, Hahn J, Jellinek M, Willman VL. The effects of cardiopulmonary bypass with crystalloid and colloid hemodilution on myocardial extravascular water. J Thorac Cardiovasc Surg. 1977;73:129–138. [PubMed] [Google Scholar]
- 34.Stewart RH, Rohn DA, Allen SJ, Laine GA. Basic determinants of epicardial transudation. Am J Physiol Heart Circ Physiol. 1997;273:H1408–H1414. doi: 10.1152/ajpheart.1997.273.3.H1408. [DOI] [PubMed] [Google Scholar]
- 35.Drake RE, Allen SJ, Katz J, Gabel JC, Laine GA. Equivalent circuit technique for lymph flow studies. Am J Physiol Heart Circ Physiol. 1986;251:H1090–H1094. doi: 10.1152/ajpheart.1986.251.5.H1090. [DOI] [PubMed] [Google Scholar]
- 36.Davis KL, Mehlhorn U, Laine GA, Allen SJ. Myocardial edema, left ventricular function, and pulmonary hypertension. J Appl Physiol. 1995;78:132–137. doi: 10.1152/jappl.1995.78.1.132. doi:10.1063/1.360663. [DOI] [PubMed] [Google Scholar]
- 37.Laine GA, Allen SJ. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ Res. 1991;68:1713–1721. doi: 10.1161/01.res.68.6.1713. [DOI] [PubMed] [Google Scholar]
- 38.Miller AJ, Pick R, Johnson PJ. The rates of formation of cardiac lymph and pericardial fluid after the production of myocardial venous congestion in dogs. Lymphology. 1972;5:156–160. [PubMed] [Google Scholar]
- 39.Pratt JW, Schertel ER, Schaefer SL, Esham KE, McClure DE, Heck CF, et al. Acute transient coronary sinus hypertension impairs left ventricular function and induces myocardial edema. Am J Physiol Heart Circ Physiol. 1996;271:H834–H841. doi: 10.1152/ajpheart.1996.271.3.H834. : [DOI] [PubMed] [Google Scholar]
- 40.Waldenstrom A, Martinussen HJ, Gerdin B, Hallgren R. Accumulation of hyaluronan and tissue edema in experimental myocardial infarction. J Clin Invest. 1991;88:1622–1628. doi: 10.1172/JCI115475. doi:10.1172/JCI115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139:682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto M, McClure DE, Schertel ER, Andrews PJ, Jones GA, Pratt JW, et al. Effects of hypoproteinemia-induced myocardial edema on left ventricular function. Am J Physiol Heart Circ Physiol. 1998;274:H937–H944. doi: 10.1152/ajpheart.1998.274.3.H937. [DOI] [PubMed] [Google Scholar]
- 43.Pogatsa G, Dubecz E, Gabor G. The role of myocardial edema in the left ventricular diastolic stiffness. Basic Res Cardiol. 1976;71:263–269. doi: 10.1007/BF01906451. doi:10.1007/BF01906451. [DOI] [PubMed] [Google Scholar]
- 44.Weng ZC, Nicolosi AC, Detwiler PW, Hsu DT, Schierman SW, Goldstein AH, et al. Effects of crystalloid, blood, and University of Wisconsin perfusates on weight, water content, and left ventricular compliance in an edema-prone, isolated porcine heart model. J Thorac Cardiovasc Surg. 1992;103:504–513. [PubMed] [Google Scholar]
- 45.Desai KV, Laine GA, Stewart RH, Cox CS, Jr., Quick CM, Allen SJ, et al. Mechanics of the left ventricular myocardial interstitium: effects of acute and chronic myocardial edema. Am J Physiol Heart Circ Physiol. 2008;294:H2428–H2434. doi: 10.1152/ajpheart.00860.2007. doi:10.1152/ajpheart.00860.2007. [DOI] [PubMed] [Google Scholar]
- 46.Davis KL, Laine GA, Geissler HJ, Mehlhorn U, Brennan M, Allen SJ. Effects of myocardial edema on the development of myocardial interstitial fibrosis. Microcirculation. 2000;7:269–280. doi:10.1111/j.1549-8719.2000.tb00127.x. [PubMed] [Google Scholar]
- 47.Weng ZC, Schierman SW, Goldstein AH, Nicolosi AC, Spotnitz HM. Effects of perfusate tonicity on myocardial edema and compliance. (Abstract) Circulation. 1988;78(Suppl. II):70. [Google Scholar]
- 48.Kahles H, Mezger VA, Hellige G, Spieckermann PG, Bretschneider HJ. The influence of myocardial edema formation on the energy consumption of the heart during aerobiosis and hypoxia. Basic Res Cardiol. 1982;77:158–169. doi: 10.1007/BF01908169. doi:10.1007/BF01908169. [DOI] [PubMed] [Google Scholar]
- 49.Capasso JM, Robinson TF, Anversa P. Alterations in collagen cross-linking impair myocardial contractility in the mouse heart. Circ Res. 1989;65:1657–1664. doi: 10.1161/01.res.65.6.1657. [DOI] [PubMed] [Google Scholar]
- 50.Weber K, Pick R, Moe G, Zucker I, Armstrong P. Collagen and the failing canine ventricle. (Abstract) Circulation. 1989;80(Suppl. II):504. [Google Scholar]
- 51.Rubboli A, Sobotka PA, Euler DE. Effect of acute edema on left ventricular function and coronary vascular resistance in the isolated rat heart. Am J Physiol Heart Circ Physiol. 1994;267:H1054–H1061. doi: 10.1152/ajpheart.1994.267.3.H1054. [DOI] [PubMed] [Google Scholar]
- 52.Dellsperger KC, Clothier JL, Hartnett JA, Haun LM, Marcus ML. Acceleration of the wavefront of myocardial necrosis by chronic hypertension and left ventricular hypertrophy in dogs. Circ Res. 1988;63:87–96. doi: 10.1161/01.res.63.1.87. [DOI] [PubMed] [Google Scholar]
- 53.Ziegler WH, Goresky CA. Transcapillary exchange in the working left ventricle of the dog. Circ Res. 1971;29:181–207. doi: 10.1161/01.res.29.2.181. [DOI] [PubMed] [Google Scholar]
- 54.Miller AJ, Pick R, Johnson PJ. The production of acute pericardial effusion: the effects of various degrees of interference with venous blood and lymph drainage from the heart muscle in the dog. Am J Cardiol. 1971;28:463–466. doi: 10.1016/0002-9149(71)90011-7. doi:10.1016/0002-9149(71)90011-7. [DOI] [PubMed] [Google Scholar]
- 55.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Takoudes TG, Amirhamzeh MM, Hsu DT, Wise BR, Odeh SO, Spotnitz HM. Time course of perfusion-induced myocardial edema resolution in rats. J Surg Res. 1994;57:641–646. doi: 10.1006/jsre.1994.1194. doi:10.1006/jsre.1994.1194. [DOI] [PubMed] [Google Scholar]
- 57.Dean DA, Amirhamzeh MM, Jia CX, Cabreriza SE, Rabkin DG, Sciacca R, et al. Reversal of iatrogenic myocardial edema and related abnormalities of diastolic properties in the pig left ventricle. J Thorac Cardiovasc Surg. 1998;115:1209–1214. doi: 10.1016/S0022-5223(98)70423-4. doi:10.1016/S0022-5223(98)70423-4. [DOI] [PubMed] [Google Scholar]
- 58.Amirhamzeh MM, Dean DA, Jia CX, Cabreriza SE, Starr JP, Sardo MJ, et al. Iatrogenic myocardial edema: increased diastolic compliance and time course of resolution in vivo. Ann Thorac Surg. 1996;62:737–743. doi: 10.1016/s0003-4975(96)00391-8. doi:10.1016/S0003-4975(96)00391-8. [DOI] [PubMed] [Google Scholar]
- 59.Hsu DT, Weng ZC, Nicolosi AC, Detwiler PW, Sciacca R, Spotnitz HM. Quantitative effects of myocardial edema on the left ventricular pressure–volume relation. Influence of cardioplegia osmolarity over two hours of ischemic arrest . J Thorac Cardiovasc Surg. 1993;106:651–657. [PubMed] [Google Scholar]
- 60.Allen SJ, Geissler HJ, Davis KL, Gogola GR, Warters RD, de Vivie ER, et al. Augmenting cardiac contractility hastens myocardial edema resolution after cardiopulmonary bypass and cardioplegic arrest. Anesth Analg. 1997;85:987–992. doi: 10.1097/00000539-199711000-00006. doi:10.1097/00000539-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Geissler H, Allen S, Davis K, Sauer H, Laine G, Kuhn-Regnier F, et al. Impact of cardiopulmonary bypass and cardioplegic arrest on myocardial efficiency. Critical Care. 1999;3:P21. Abstract ( doi:10.1186/cc332) [Google Scholar]
- 62.Laine GA, Allen SJ. Increased cardiac energy consumption accompanies myocardial interstitial edema. FASEB J. 1992;6:A2038. (Abstract) [Google Scholar]
- 63.Schertel ER, Pratt JW, Robitaille P-M, Ying AJ, Heck CF, Myerowitz PD. Coronary venous hypertension and myocardial edema do not alter ventricular high energy phosphate metabolism. FASEB J. 1996;10:A319. (Abstract) [Google Scholar]
- 64.Stewart RH, Rohn DA, Fischer UM, Cox CS, Jr, Allen SJ, Criscione JC, et al. Alterations in cardiac and vascular function curves following resolution of acute myocardial edema. FASEB J. 2002;16:A1128. (Abstract) [Google Scholar]
- 65.Liang DH. Advances in echocardiography. Semin Thorac Cardiovasc Surg. 2008;20:374–379. doi: 10.1053/j.semtcvs.2008.11.010. doi:10.1053/j.semtcvs.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Dent CL, Scott MJ, Wickline SA, Hall CS. High-frequency ultrasound for quantitative characterization of myocardial edema. Ultrasound Med Biol. 2000;26:375–384. doi: 10.1016/s0301-5629(99)00144-1. doi:10.1016/S0301-5629(99)00144-1. [DOI] [PubMed] [Google Scholar]
- 67.Powell WJ, Jr, Wittenberg J, Maturi RA, Dinsmore RE, Miller SW. Detection of edema associated with myocardial ischemia by computerized tomography in isolated, arrested canine hearts. Circulation. 1977;55:99–108. doi: 10.1161/01.cir.55.1.99. [DOI] [PubMed] [Google Scholar]
- 68.Mahnken AH, Bruners P, Bornikoel CM, Kramer N, Guenther RW. Assessment of myocardial edema by computed tomography in myocardial infarction. JACC Cardiovasc Imaging. 2009;2:1167–1174. doi: 10.1016/j.jcmg.2009.05.014. doi:10.1016/j.jcmg.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 69.Kiricuta IC, Jr, Simplaceanu V. Tissue water content and nuclear magnetic resonance in normal and tumor tissues. Cancer Res. 1975;35:1164–1167. [PubMed] [Google Scholar]
- 70.Higgins CB, Herfkens R, Lipton MJ, Sievers R, Sheldon P, Kaufman L, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52:184–188. doi: 10.1016/0002-9149(83)90093-0. doi:10.1016/0002-9149(83)90093-0. [DOI] [PubMed] [Google Scholar]
- 71.Karolle BL, Carlson RE, Aisen AM, Buda AJ. Transmural distribution of myocardial edema by NMR relaxometry following myocardial ischemia and reperfusion. Am Heart J. 1991;122:655–664. doi: 10.1016/0002-8703(91)90508-f. doi:10.1016/0002-8703(91)90508-F. [DOI] [PubMed] [Google Scholar]
- 72.Albers J, Schroeder A, de Simone R, Mockel R, Vahl CF, Hagl S. 3D evaluation of myocardial edema: experimental study on 22 pigs using magnetic resonance and tissue analysis. Thorac Cardiovasc Surg. 2001;49:199–203. doi: 10.1055/s-2001-16100. doi:10.1055/s-2001-16100. [DOI] [PubMed] [Google Scholar]
- 73.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194–1201. doi: 10.1016/j.jacc.2008.10.065. doi:10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 74.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. doi:10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. doi:10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaffer RF, Baumgarten CM, Damiano RJ., Jr. Prevention of cellular edema directly caused by hypothermic cardioplegia: studies in isolated human and rabbit atrial myocytes. J Thorac Cardiovasc Surg. 1998;115:1189–1195. doi: 10.1016/S0022-5223(98)70420-9. doi:10.1016/S0022-5223(98)70420-9. [DOI] [PubMed] [Google Scholar]
- 77.Starr JP, Jia CX, Amirhamzeh MM, Rabkin DG, Hart JP, Hsu DT, et al. Coronary perfusate composition influences diastolic properties, myocardial water content, and histologic characteristics of the rat left ventricle. Ann Thorac Surg. 1999;68:925–930. doi: 10.1016/s0003-4975(99)00688-8. doi:10.1016/S0003-4975(99)00688-8. [DOI] [PubMed] [Google Scholar]
- 78.Li GR, Zhang M, Satin LS, Baumgarten CM. Biphasic effects of cell volume on excitation–contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1270–H1277. doi: 10.1152/ajpheart.00946.2001. [DOI] [PubMed] [Google Scholar]
- 79.Uray KS, Wright Z, Kislitsyna K, Xue H, Cox CS., Jr. Nuclear factor-kappaB activation by edema inhibits intestinal contractile activity. Crit Care Med. 2010;38:861–870. doi: 10.1097/CCM.0b013e3181ce4aaa. doi:10.1097/CCM.0b013e3181ce4aaa. [DOI] [PubMed] [Google Scholar]
- 80.Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303–2312. doi: 10.1172/JCI116834. doi:10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeMeules JE, Pigula FA, Mueller M, Raymond SJ, Gamelli RL. Tumor necrosis factor and cardiac function. J Trauma. 1992;32:686–692. doi: 10.1097/00005373-199206000-00003. doi:10.1097/00005373-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Williams J, Laine GA, Hanley E, Eberlein D, Sweeney M. Myocardial interstitial changes in humans with congestive cardiomyopathy. Anesth Analg. 1990;70:S435. (Abstract) [Google Scholar]