Abstract
OBJECTIVES
Vascular alpha2B adrenergic receptors (α2B-ARs) mediate vasoconstriction and contribute to peripheral regulation of vascular tone. In vitro, a common 301-303 deletion in the α2B-AR gene, ADRA2B, results in loss of α2B-AR desensitization. We examined the hypothesis that ADRA2B del301-303 or other common ADRA2B variants alter vascular desensitization in vivo.
METHODS
We measured sensitivity to a highly selective α2-AR agonist, dexmedetomidine, (0.01–1000 ng/min) in the dorsal hand vein in 41 healthy subjects. To induce desensitization a dose of dexmedetomidine that resulted in submaximal constriction was infused for 180 minutes and dorsal hand vein responses measured. Desensitization was defined as the ratio between the area-under-the-effect curve for each individual’s response, and the hypothetical area-under-the-effect curve assuming that the initial response had been maintained for 180 minutes (ratio below 1 reflecting desensitization). The relationship between six ADRA2B variants (1 promoter, 3 coding, and 2 in the 3′ UTR) with an allele frequency > 5% and desensitization was determined.
RESULTS
Forty-one subjects (22 men, 21 whites, age 18-45 years) were studied. The ADRA2B 301-303 deletion allele (ins/del and del/del, n=18) was associated with resistance to desensitization [1.01 (IQR 0.90-1.06)] compared to ins/ins homozygous subjects (n=23) [0.91 (IQR 0.73-0.99)], p=0.026. In addition, the −98 GG, 1182 CC, and 1776 CC genotypes were associated with significantly less desensitization than GC or CC, and CA or AA genotypes, respectively.
CONCLUSION
Common ADRA2B variants contribute to the interindividual variability in vascular desensitization to an α2-AR agonist in vivo.
Keywords: Adrenergic receptor agonists; Alpha 2B adrenergic receptor; Genetic variability; Human; Receptor, adrenergic alpha; Vasoconstriction; Receptor desensitization
INTRODUCTION
Alpha-adrenergic receptors (α-ARs) are G-protein coupled receptors (GPCRs) that contribute to the regulation of cardiovascular responses. Central nervous system α2-ARs decrease sympathetic output, and vascular α2-ARs mediate vasoconstriction post-synaptically and contribute to the regulation of vascular tone [1]. In the forearm vasculature of healthy young men, postsynaptic α2-ARs contributed more to the basal vascular tone than did α1-ARs [2]. Also, activation of α2-ARs, but not α1–ARs, induced vasoconstriction in normal coronary arteries [3].
Intravenous infusion of an α2–AR agonist, dexmedetomidine, causes vasoconstriction [4], and a transient increase in blood pressure [4, 5]. We and others have shown that the activation of α2-ARs in the hand vein causes pronounced venoconstriction [6]. Thus, vascular α2-ARs play a significant role in the regulation of vascular tone in both arterial and venous vasculature.
There are three α2-AR subtypes: A, B, and C. Studies performed in genetically engineered mice indicate that α2B–ARs mediate direct vasoconstriction in response to agonist [7-9]. In mice lacking the α2B–AR, the peripheral hypertensive action of an α2–AR agonist was absent [8]. Although there is little information about the localization and function of α2B-AR in the human dorsal hand vein, all three receptor subtypes are present in the vasculature [10] and ADRA2B mRNA is expressed in the human saphenous vein [11]. Thus, it is likely that all subtypes are found on the human dorsal hand vein, but the animal studies suggest that the α2B-AR subtype is key to α2-AR-mediated vasoconstriction. However, the lack of α2-AR subtype specific agonists and antagonists for use in humans has precluded definition of the specific role of the α2B-AR subtype in vivo.
Prolonged exposure of GPCRs to agonist is often associated with attenuated signal transduction by the stimulated receptor as a result of desensitization [12]. The extent of desensitization varies substantially among the α2-AR subtypes [13-15]. In vitro studies have shown that α2B-ARs are rapidly and extensively internalized following short-term (≈30 minutes) exposure to agonist in parallel with protein kinase C-mediated phosphorylation of the third intracellular loop of the α2B-AR [13-15]. In vivo, agonist-induced desensitization of vascular adrenergic receptors may be an adaptive mechanism to a chronic increase in sympathetic tone, for example as is present in congestive heart failure. Thus, interindividual variability in α2B-AR receptor desensitization, some of which is determined by genetic factors, may have important clinical consequences.
There is known genetic variability in the third intracellular loop of the α2B-AR in the putative G-protein binding area; a deletion variant (ADRA2B del301-303) encodes a receptor that manifests loss of short-term agonist-induced desensitization in vitro [16]. The ADRA2B del301-303 variant is relatively common, with a minor allele frequency of 31% in Caucasians and 12% in African Americans [16]. We have previously resequenced the ADRA2B gene and identified additional common variants; none of these, including the del301-303 variant, contributed to variability in vasoconstrictor response to short-term agonist infusion [17, 18]. However, since the major effect of the ADRA2B del301-303 variant in vitro is loss of desensitization after prolonged exposure to agonist, we sought to define the effect of ADRA2B genetic variability on desensitization of vascular responses in vivo. Specifically, we examined the hypothesis that common ADRA2B variants are associated with altered vascular desensitization in vivo in response to prolonged exposure to the highly selective α2-AR agonist, dexmedetomidine.
METHODS
The Institutional Review Board of Vanderbilt University Medical Center approved the study and subjects gave written informed consent. Caucasians and African-Americans of either sex were eligible for the study if they were 18 to 45 years of age and had no clinically significant abnormalities according to history, physical examination, and laboratory testing.
Forty-one subjects were studied at the Clinical Research Center at Vanderbilt University. Ethnicity and family history of hypertension were determined by self-report. Subjects took no medications for at least 2 weeks, and abstained from alcohol and caffeine for 5 days before the study. Each subject received 5 days of a diet containing sodium 150 mmol/day, potassium 70 mmol/day, and calcium 600 mmol/day. All studies were performed in the same temperature-controlled room after an overnight fast.
Measurement of Vascular Responses
A dexmedetomidine (Precedex, Abbott Laboratories, Chicago, IL, USA) dose-response study was performed on the morning of the 5th day of diet. The dose-response to dexmedetomidine in these subjects has previously been reported [17]. Venous responses were measured in a dorsal hand vein by use of a linear variable differential transformer (LVDT) (Schaevitz, model 100 MHR, Hampton, VA, USA) as previously described [19, 20]. This instrument, when mounted on the hand, measures and records changes in the diameter of the vein. Subjects rested on a comfortable bed and remained supine throughout the study. The subject’s arm was placed on a support sloping upward. A 23-gauge needle was inserted into a suitable dorsal hand vein, and an infusion of normal saline was administered for 30 minutes.
Desensitization studies were performed on a separate occasion, following a second 5 day period of an identical diet. Preliminary studies showed that maximum venoconstriction was obtained within 10 minutes. The dexmedetomidine dose that had caused at least 50% venoconstriction in the previous dose-response study was infused for three hours using a Harvard syringe pump (Harvard Apparatus, Holliston, Ma.), and responses measured using the LVDT after 10 minutes and then every 30 minutes for 3 hours.
Heart rate was monitored continuously with a bedside cardiac monitor, and blood pressure was measured in the arm opposite the side receiving the hand vein infusion, using a semiautomated device (Dinamap MPS, Johnson and Johnson Medical, Tampa, Fla.).
Analysis of hand-vein responses to dexmedetomidine
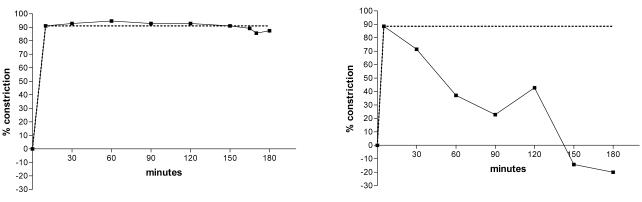
Venoconstriction was expressed as the percentage reduction in vein diameter from maximal dilation, defined as the average of three stable baseline measurements of hand vein diameter. Initial venoconstriction was measured 10 minutes after starting the dexmedetomidine infusion, and then every 30 minutes for 3 hours. The percent venoconstriction was plotted over time and the area-under-the-effect curve (AUC) calculated using the trapezoidal rule (AUCobserved). To measure desensitization of vascular response, the individual AUCobserved was normalized to the AUC of a hypothetical curve describing no change in venoconstriction over 180 minutes (AUCno desensitization), i.e. as if the initial venoconstriction at 10 minutes was maintained over 180 minutes. The ratio between AUCobserved and AUCno desensitization was calculated and termed nAUC (see examples in Figure 1).
Figure 1.
Representative individual dorsal hand vein responses to 3 hour infusion of dexmedetomidine. The dotted line represents the theoretical response of no desensitization i.e., nAUC=1.0. Figure 1A shows an individual with stable venoconstriction and almost no desensitization (nAUC=0.98). Figure 1B shows an individual with decreased venoconstriction in response to the same dose of dexmedetomidine over time (i.e., desensitization). Negative constriction indicates venodilation beyond baseline (nAUC=0.44).
Genotyping
We previously sequenced the ADRA2B gene and identified variants in these subjects 13. Six variants were observed in more than 5% of the 41 subjects studied: variants at mRNA positions −98 (rs3111873), 36 (rs9333567), 901-909 (the del301-303 polymorphism, rs4066772), 1182 (rs2229169), 1776 (rs4907299) and 2855. A reliable genotype result was not available for position 36 in 1 subject.
Data and Statistical analyses
Continuous variables were expressed as median and interquartile range (IQR) or mean and 95% confidence interval (95% CI). Preliminary analysis suggested that for all polymorphisms homo- and heterozygous carrier status of the variant alleles had similar functional effects (Table 2); we therefore assumed a dominant mode of inheritance for the functional effects of the polymorphisms and compared the homozygous “wild-type” genotype with hetero- and homozygous carriers of the respective variant allele. Comparisons of nAUC among genotype groups were performed by Mann Whitney test (SPSS version 13, SPSS Inc., Chicago, IL, USA).
Table 2.
Desensitization (nAUC*) and ADRA2B variants
| mRNA position (Region) |
Genotype | N | nAUC*(Median, IQR) | P value† |
|---|---|---|---|---|
| −98 (promoter), 901-909 (301-303del), (Coding) †† |
GG, ins/ins | 23 | 0.91 (0.73 to 0.99) | 0.026 |
|
|
||||
| GC, ins/del | 11 | 1.01 (0.91 to 1.09) | ||
| CC, del/del | 7 | 0.98 (0.90 to 1.09) | ||
|
| ||||
| 36 (Coding) | AA | 35 | 0.98 (0.86 to 1.03) | 0.228 |
|
|
||||
| AG | 5 | 0.85 (0.71 to 1.01) | ||
|
| ||||
| 1182 (Coding) | CC | 22 | 0.91 (0.73 to 0.99) | 0.011 |
|
|
||||
| CA | 11 | 1.01 (0.91 to 1.05) | ||
| AA | 8 | 1.01 (0.90 to 1.11) | ||
|
| ||||
| 1776 (3′-URT) | CC | 22 | 0.91(0.82 to 0.99) | |
|
|
||||
| CA | 14 | 1.01 (0.86 to 1.06) | 0.016 | |
| AA | 5 | 1.03 (0.94 to 1.15) | ||
|
| ||||
| 2855 (3′-URT) | AA | 35 | 0.98 (0.85 to 1.03) | 0.627 |
|
|
||||
| AG | 6 | 0.96 (0.81 to 1.00) | ||
nAUC, Area under the curve (median, IQR) for dexmedetomidine dorsal hand vein response over 180 minutes normalized to the initial response
P values represent comparisons of nAUC between subjects homozygous for the major allele and carriers of at least one variant allele (heterozygous + homozygous).
Variants in mRNA positions −98 and 901-909 (del301-303) that are in complete linkage disequilibrium [13] were analyzed together.
The variants at positions −98 and 901-909 (del301-303) are in complete linkage disequilibrium and are tag variants for a single haplotype [18], thus, these variants were grouped for analysis. Two of the remaining variants (at positions 1182 and 1776) are also in high linkage disequilibrium with the deletion variant [18]. In this study we performed single site analyses without haplotype analysis to prevent fragmentation of the cohort into small subgroups. Since this was an exploratory analysis, no adjustment for multiple comparisons was made. All tests were two-tailed, and P-values < 0.05 were considered significant.
RESULTS
Subject Characteristics
The median age of the 41 subjects studied was 24.5 (IQR 23 to 29) years, and body mass index (BMI) was 25.7 (IQR 22.6 to 28.7) kg/m2. Baseline systolic and diastolic blood pressure and heart rate are shown in Table 1.
Table 1.
Baseline characteristics of study subjects
| Characteristic | |
|---|---|
| Female / Male (n) | 19/22 |
| African American / Caucasian | 20/21 |
| Age (years) | 24.5 (23 - 29) |
| BMI (kg/m2) | 25.7 (22.6 - 28.7) |
| Systolic blood pressure (mmHg) | 110 (104 - 116) |
| Diastolic blood pressure (mmHg) | 62 (59 - 68) |
| Heart rate (beats per minute) | 61 (55 - 67) |
| Norepinephrine (pg/ml) | 155 (122 - 210) |
Data are shown as number of subjects (n) or median (interquartile range)
Hand-vein Response to 3 hours infusion of Dexmedetomidine
Based on the dose-finding study in day 1, the desensitizing dose of dexmedetomidine administered was 100 ng/min in 26 subjects and 50 ng/min in 15. The resulting median initial vasoconstriction was 83.0% (IQR 68.2 to 88.8%). The nAUC was <0.80 in 7 (16.8%) subjects. The two subjects with the lowest nAUC were studied twice. Their initial nAUCs were 0.35 and 0.44, and on the repeat study day were 0.25 and 0.24, respectively. In two subjects, nAUC was >1.2. In these subjects, vasoconstriction at 10 minutes of dexmedetomidine infusion was 60.7% and 63.4%, and increased at 30 minutes to 88% and 83%, respectively, suggesting that the maximal response had not been achieved after 10 minutes of dexmedetomidine infusion. There was no difference between Caucasians and African Americans in median nAUC [0.97 (0.80-1.05) and 0.97 (0.85-1.01), respectively, (p=0.774)].
The effect of six variants (1 in the promoter, 3 in the coding region and 2 in the 3′ UTR) with an allele frequency > 5% [13] on nAUC was determined (Table 2), and of these, 4 variants in linkage disequilibrium [positions −98/901-909 (the del301-303 variant), 1182, and 1776] were significantly associated with nAUC.
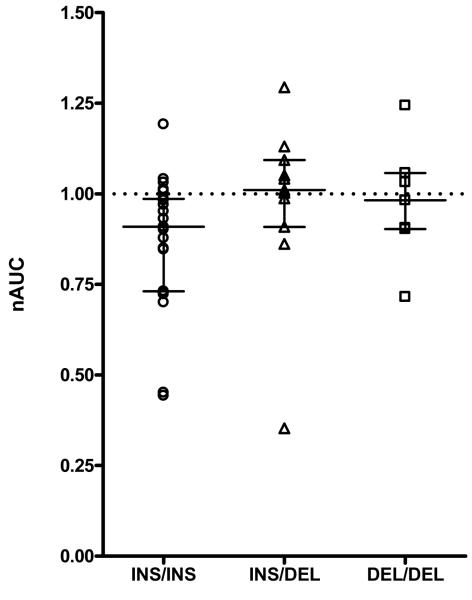
ADRA2B del301-303 genotype affected desensitization. Subjects without the deletion allele (ins/ins genotype n=23) had a significantly lower nAUC than carriers of one or two deletion alleles (ins/del and del/del subjects, n=18) [median 0.91 (IQR 0.73 to 0.99) vs 1.01 (IQR 0.91 to 1.06); p=0.026]. (Table 2; Figure 2). Heterozygous and homozygous carriers of the deletion variant did not differ in their responses (P=0.89) and did not show significant desensitization (Table 2, Figure 2).
Figure 2.
nAUC of dorsal hand vein responses to 3 hour infusion of dexmedetomidine according to ADRA2B del301-303 genotype [wt/wt (n=23), wt/del (n=11), del/del (n=7) subjects. The horizontal line and bars represent median and IQR. The dotted horizontal line represents no desensitization.
Variants at two other positions were associated with desensitization. For the variant at position 1182, subjects homozygous for the C allele (n=22) had a significantly lower nAUC than carriers of the A allele (CA and AA subjects, n=19) [median 0.91 (IQR 0.82 to 0.99) vs 1.01 (IQR 0.91 to1.06)], p=0.011 (Table 2). Similarly, subjects homozygous for the C allele at position 1776 (n=22) had a significantly lower nAUC than carriers of the A allele (CA and AA subjects, n=19) [median 0.91 (IQR 0.82 to 0.99) vs 1.01 (IQR 0.91 to1.06)], p=0.016 (Table 2). The two other ADRA2B variants were not associated with nAUC (Table 2).
To explore the effect of ADRA2B variant alleles on nAUC in African Americans and in Caucasians, data were analyzed in each ethnic group separately. Among Caucasian subjects, ADRA2B ins301-303 homozygosity, and homozygosity for variants in high linkage disequilibrium with it (−98G, 1182C, and 1776C alleles), were significantly associated with a smaller nAUC (all p values <0.05). Among African Americans, the findings were directionally similar but not statistically significant.
DISCUSSION
The main finding of this study is that greater vascular desensitization in response to prolonged infusion of an α2-AR agonist in vivo is associated with homozygosity for the ADRA2B ins301-303 allele, and with three other variants in high linkage disequilibrium (the −98G, 1182C, and 1776C alleles) [18]. In contrast, heterozygous or homozygous carriers of variant alleles at these sites showed no significant desensitization. These findings were also observed in the Caucasian subgroup, but the differences were not statistically significant in African Americans, likely due to the lower frequency of these variant alleles in this ethnic group [16].
We previously directly addressed the hypothesis that the ADRA2B ins/del polymorphism at position 301-303 affected vascular response [17], and although there was substantial variability in sensitivity to dexmedetomidine, the ADRA2B del301-303 variant [17] and other common ADRA2B variants [18] did not contribute significantly to this variability. However, considering the in vitro observation that the effect of ADRA2B del301-303 variant is loss of desensitization after prolonged exposure to agonist, rather than altered initial vascular sensitivity, and given the potential clinical importance of altered desensitization, we set out to determine the effect of ADRA2B genetic variation on desensitization in vivo.
Agonist-induced desensitization of vascular adrenergic receptors may play an important role in the cardiovascular adaptation to prolonged stress, and loss of desensitization could affect cardiovascular outcomes. For example, altered adrenergic responses to chronic sympathetic stimulation have been suggested to play an important role in the pathogenesis of heart failure. Indeed, one of the cornerstones of the treatment of congestive heart failure, beta-blocker therapy, is suggested to reverse adrenergic desensitization. In the vasculature, loss of desensitization of α2-AR mediated vasoconstriction would be likely to result in more persistent vasoconstriction, and thus less response to vasodilators in the vascular beds affected.
Several clinical studies are concordant with this idea, suggesting greater risk for acute coronary events [21] and for pre-hospital fatal myocardial infarction and sudden cardiac death [22] among patients homozygous for the variant del301-303 allele compared to hetero- or homozygous carriers of the ins301-303 allele. Thus, loss of desensitization of α2-AR mediated vasoconstriction would be likely to result in more persistent vasoconstriction, and thus less response to vasodilators in the vascular beds affected. Indeed, an association has been noted between the ADRA2B del301-303 variant and cardiac events [21, 22].
Additional indirect evidence supports the hypothesis that the del301-303 variant is associated with decreased desensitization, and therefore enhanced constriction and impaired vasodilation, in vivo. For example, the ADRA2B del301-303 variant was associated with decreased flow-mediated dilation in the brachial and carotid arteries [23] and with a blunted increase in coronary blood flow in response to the infusion of epinephrine [24].
The waning of vasoconstriction in response to dexmedetomidine is not a nonspecific effect. For example, continuous exposure to another venoconstrictor, the α1-AR agonist phenylephrine, results in stable constriction over prolonged periods of time. Indeed, because it is so stable, phenylephrine preconstriction is the background against which vasodilators are studied in the hand vein [25]. Thus, the relatively small magnitude of desensitization that occurred with dexmedetomidine reflects a true biological effect and not a nonspecific waning of vasoconstriction. Further evidence is provided by the reproducible desensitization that was demonstrated in the two individuals with the most marked responses.
In contrast to the minor degree of desensitization observed in vivo, exposure to agonist in vitro resulted in a 54% reduction in sensitivity to agonist in wild-type α2B-ARs and had no desensitizing effect in del301-303 variant receptors [16]. One potential explanation for the differences in magnitude of the effects of the del301-303 variant in vivo and in vitro could be that the desensitization response observed in vivo is the overall result of the contributions of α2B-ARs as well as other receptors and signaling mechanisms regulating vascular response. In order to minimize this possibility, we used a highly selective α2-AR agonist, dexmedetomidine, which has little effect at α1-ARs; [26] however, other α2-AR subtypes (α2A-ARs and α2C-ARs) may play a role in venoconstriction in the human hand vein.
In humans, intravenous infusion of dexmedetomidine induces an immediate increase in blood pressure mediated by vasoconstriction [5]. In genetically modified mice, the α2B-AR was shown to selectively mediate this increase in blood pressure following intravenous dexmedetomidine [27], indicating that this receptor subtype is the major α2-AR mediating peripheral vasoconstriction in this model [7-9]. However, all 3 receptor subtypes have been identified in human vasculature and may contribute to responses to agonist [10]. The absence of subtype-selective agonists and antagonists for use in humans precludes examination of that possibility.
Another potential explanation for the relatively small magnitude of desensitization after continuous exposure to an α2-AR agonist in our study is that at baseline, the α2B-AR Ins301-303 receptor is already substantially desensitized in the presence of physiological basal levels of sympathetic activity; thus additional pharmacological stimulation would result in only little additional desensitization. However, specific studies would be required to address this hypothesis.
The ADRA2B 301-303 deletion variant defines a haplotype (haplotype 3) that includes variants in positions −98, 1182, and 1776, all exhibiting high linkage disequilibrium [18]. Accordingly, stratifying the study population by genotypes at any of these positions resulted in similar subgroups and comparable genotype-phenotype associations. Since in vitro studies have shown that the 301-303 deletion results in loss of desensitization, it is probable that in our study the reduced desensitization in carriers of the deletion allele is attributable to that variant. However, contributions of any of the other variants cannot be ruled out.
The methods we used had some limitations. We used the dorsal hand vein model [19, 20] in order to avoid the prominent hemodynamic effects resulting from the systemic administration of an α2-AR agonist, i.e., the decrease in blood pressure mediated through the activation of central α2-ARs resulting in inhibition of sympathetic activity. Such systemic effects would act to confound measurement of direct peripheral agonist-induced vasoconstriction mediated by vascular α2-AR’s [5]. Using dexmedetomidine in the hand vein model, we have previously shown a robust α2-AR -mediated vasoconstriction, with no activation of systemic reflex responses [6]. Another potential limitation of our study is that responses in the venous and arterial vasculature may not be identical, and thus we cannot directly extrapolate our results to other vascular tissues. However, α2-AR-mediated venous response is important in itself, since the venous bed has major effects on cardiac filling and cardiac output, especially in patients with heart failure, a group in whom adrenergic activity is markedly increased.
To our knowledge, the model reported is the first to measure desensitization of α2 AR mediated vascular response in vivo. However, since the study was exploratory, additional studies to confirm the findings in larger populations will be of interest. Also, given the complexity of the protocol, we only studied subjects once. However, the hand vein technique yields reproducible results when the same subjects are studied again [28]. In addition, we performed reproducibility studies in 2 subjects who had the lowest nAUCs and the results did not change materially.
Translational studies such as this one may not allow direct clinical extrapolation since they are proof-of-concept studies performed under carefully controlled conditions in healthy subjects, rather than in patients with diseases where therapy and co-morbidities may obscure the genetic signal. The studies act as a bridge between in vitro, cell-based data and clinical outcomes. Our study demonstrates a genotype-dependent variability in desensitization to an α2-AR selective agonist. Previous clinical studies have suggested that α2B-AR del301-303 homozygous genotype is associated with increased cardiovascular mortality and morbidity [21, 22]. Thus, our study provides a possible mechanistic explanation for these findings, and may suggest a population subgroup that might benefit from therapeutic strategies to compensate for loss of desensitization.
In summary, common ADRA2B variants are associated with the variability in vascular desensitization to an α2-AR agonist in vivo. In keeping with the in vitro findings, carriers of the ADRA2B del301-303 genotype (and three other variants in high linkage disequilibrium at position −98, 1182, and 1776 comprising haplotype 3) [18] are more resistant to desensitization. Further studies are necessary to characterize the functional consequences of ADRA2B genotypes and haplotypes in the regulation of vascular tone in normal physiology and disease states.
Acknowledgments
This study was supported in part by US Public Health Service grants PO1 HL56693, GM31304, UL1 RR024975 from NCRR/NIH, and a Pharmacogenetics Research Network Grant (U01 HL65962). Drs. Muszkat and Kurnik were recipients of a Merck Sharp & Dohme International Fellowship in Clinical Pharmacology.
Footnotes
This work was presented in part at the 2006 annual meeting of the American Society of Clinical Pharmacology and Therapeutics.
Conflict of interest: NONE
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Docherty JR. Subtypes of functional alpha1 and alpha2 adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- 2.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540(Pt 3):1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart D, Haude M, Gorge G, Liu F, Ge J, Grosse-Eggebrecht C, et al. Augmented alpha-adrenergic constriction of atherosclerotic human coronary arteries. Circulation. 1999;99:2090–7. doi: 10.1161/01.cir.99.16.2090. [DOI] [PubMed] [Google Scholar]
- 4.Talke P, Stapelfeldt C, Lobo E, Brown R, Scheinin M, Snapir A. Effect of alpha2B-adrenoceptor polymorphism on peripheral vasoconstriction in healthy volunteers. Anesthesiology. 2005;102:536–42. doi: 10.1097/00000542-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Muszkat M, Sofowora GG, Wood AJ, Stein CM. Alpha2-adrenergic receptor-induced vascular constriction in blacks and whites. Hypertension. 2004;43:31–5. doi: 10.1161/01.HYP.0000103694.30164.C7. [DOI] [PubMed] [Google Scholar]
- 7.Hein L, Altman DJ, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 8.Link RE, Desai K, Hein L, Stevens ME, Chruscinski A, Bernstein D, et al. Cradiovascular regulation in mice lacking alpha2-adrenergic receptor subtypes b and c. Science. 1996;273:803–5. doi: 10.1126/science.273.5276.803. [DOI] [PubMed] [Google Scholar]
- 9.MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- 10.Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–56. [PubMed] [Google Scholar]
- 11.Chotani MA, Mitra S, Su BY, Flavahan S, Eid AH, Clark KR, Montague CR, Paris H, Handy DE, Flavahan NA. Regulation of alpha(2)-adrenoceptors in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H59–67. doi: 10.1152/ajpheart.00268.2003. [DOI] [PubMed] [Google Scholar]
- 12.Brunton Laurance L., editor. Goodman’s & Gilman’s The pharmacological basis of therapeutics. 11th ed. MCGRAW-HILL; pp. 31–32. Chapter 1. [Google Scholar]
- 13.Jewell-Motz EA, Liggett SB. An acidic motif within the third intracellular loop of the alpha2C2 adrenergic receptor is required for agonist-promoted phosphorylation and desensitization. Biochemistry. 1995 Sep 19;34:11946–53. doi: 10.1021/bi00037a036. [DOI] [PubMed] [Google Scholar]
- 14.Eason MG, Liggett SB. Subtype-selective desensitization of alpha 2-adrenergic receptors. Different mechanisms control short and long term agonist-promoted desensitization of alpha 2C10, alpha 2C4, and alpha 2C2. J Biol Chem. 1992;267:25473–9. [PubMed] [Google Scholar]
- 15.Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol Pharmacol. 1997;51:711–20. doi: 10.1124/mol.51.5.711. [DOI] [PubMed] [Google Scholar]
- 16.Small KM, Brown KM, Forbes SL, Liggett SB. Polymorphism deletion of three intracellular acidic residues of the alpha2B- adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276:4617–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- 17.Muszkat M, Sofowora GG, Xie HG, Wood AJJ, Stein CM. Alpha 2B – Adrenergic Receptor 301-303 Deletion Polymorphism and Vascular Alpha2 Adrenergic Receptor Response. Pharmacogenetics and Pharmacogenomics. 2005;15:23–28. doi: 10.1097/01213011-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Muszkat M, Kurnik D, Solus J, Sofowora GG, Xie HG, Jiang L, et al. Variation in the Alpha 2B –Adrenergic Receptor Gene (ADRA2B) and its relationship to vascular response in vivo. Pharmacogenetics and Pharmacogenomics. 2005;15:407–14. doi: 10.1097/01213011-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins--2. Br J Clin Pharmacol. 1994;38:289–305. doi: 10.1111/j.1365-2125.1994.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aellig WH. Clinical pharmacology, physiology and pathophysiology of superficial veins--1. Br J Clin Pharmacol. 1994;38:181–196. doi: 10.1111/j.1365-2125.1994.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snapir A, Heinonen P, Tuomainen TP, Alhopuro P, Karvonen MK, Lakka TA, et al. Insertion/deletion polymorphism in the alpha2B-adrenergic receptor gene is a novel genetic risk factor for acute coronary events. J Am Coll Cardiol. 2001;37:1516–22. doi: 10.1016/s0735-1097(01)01201-3. [DOI] [PubMed] [Google Scholar]
- 22.Snapir A, Mikkelsson J, Perola M, Penttila A, Scheinin M, Karhunen PJ. Variation in the alpha2B- adrenoceptor gene as a risk factor for prehospital fatal myocardial infarction and sudden cardiac death. J Am Coll Cardiol. 2003;41:190–4. doi: 10.1016/s0735-1097(02)02702-x. [DOI] [PubMed] [Google Scholar]
- 23.Heinonen P, Jartti L, Jarvisalo MJ, Pesonen U, Kaprios JA, Ronnemaa T, et al. Deletion polymorphism in the alpha2B-adrenergic receptor gene is associated with flow-mediated dilatation of the brachial artery. Clinical Science. 2002;103:517–524. doi: 10.1042/cs1030517. [DOI] [PubMed] [Google Scholar]
- 24.Snapir A, Koskenvuo J, Toikka J, Orho-Melander M, Hinkkas S, Saraste M, et al. Effects of common polymorphisms in the alpha1A-, alpha2B-, Beta1-, and Beta2-adrenoceptors on haemodynamic Responses to Adrenaline. Clinical Science. 2003;104:509–520. doi: 10.1042/CS20020299. [DOI] [PubMed] [Google Scholar]
- 25.Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, Wood AJ. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–5. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 26.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 27.Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, et al. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- 28.Schindler C, Grossmann M, Dobrev D, Francke K, Ravens U, Kirch W. Reproducibility of dorsal hand vein responses to phenylephrine and prostaglandin F2 alpha using the dorsal hand vein compliance method. J Clin Pharmacol. 2003;43:228–36. doi: 10.1177/0091270002251004. [DOI] [PubMed] [Google Scholar]