Abstract
Rationale
Erythropoietin (EPO) is often administered to cardiac patients with anemia, particularly from chronic kidney disease, and stimulation of erythropoiesis may stabilize left ventricular and renal function by recruiting protective effects beyond the correction of anemia.
Objective
We examined the hypothesis that EPO receptor (EpoR) ligand-binding, which activates endothelial nitric oxide synthase (eNOS), regulates the pro-survival program of mitochondrial biogenesis in the heart.
Methods and Results
We investigated the effects of EPO on mitochondrial biogenesis over 14 days in healthy mice. Mice expressing a mitochondrial GFP-reporter construct demonstrated sharp increases in myocardial mitochondrial density post-EPO by 3 days that peaked at 7 days and surpassed hepatic or renal effects and anteceded significant increases in blood hemoglobin content. Quantitatively, in wild-type (Wt) mice, Complex II activity, State 3 respiration and mtDNA copy number increased significantly; also resting energy expenditure and natural running speed improved with no evidence of an increase in LV mass index. Mechanistically, EPO activated cardiac mitochondrial biogenesis by enhancement of nuclear respiratory factor-1 (NRF-1), PGC-1α and mitochondrial transcription factor-A gene expression in Wt, but not in eNOS-/- or Akt1-/- mice. EpoR was required, as EpoR silencing in cardiomyocytes blocked EPO-mediated nuclear translocation of NRF-1.
Conclusions
These findings support a new physiological and protective role for EPO, acting through its cell surface receptor and eNOS-Akt1 signal transduction, in matching cardiac mitochondrial mass to the convective O2 transport capacity as erythrocyte mass expands.
Keywords: cardiac metabolism, erythropoietin, nitric oxide, Akt1/PKB, mitochondrial biogenesis
Introduction
The glycoprotein hormone EPO is secreted mainly by the kidneys during hypoxia and anemia to initiate hemoglobin production, regulate red blood cell (RBC) maturation, and increase RBC mass 1. EPO treatment reverses the anemia of chronic kidney disease and improves athletic endurance 2; moreover, EPO mitigates ischemic/hypoxic damage in the heart and the brain by activation of the EPO receptor (EpoR) 3, which appears to be widely expressed 4. Embryonic loss-of-function for EPO or EpoR results in early lethality 5, and EPO confers neuronal preconditioning effects 5, 6 and stimulates neurogenesis and recovery from stroke. In the cardiovascular system, EPO converts mature endothelial cells to an angiogenic phenotype 4, 7, fosters neovascularization, i.e. in diabetes 8, and prevents cardiomyocyte apoptosis 9, all by incompletely understood mechanisms.
EpoR signaling involves JAK2 recruitment to the receptor dimer, resulting in tyrosine phosphorylation and the formation of docking sites for SH2 domain-containing proteins such as STAT transcription factors and other regulators of cell proliferation and differentiation, including the PI-3 kinases (PI-3K) 10. In non-erythroid cells, EPO activates JAK/STAT and PI-3K/Akt and also endothelial nitric oxide synthase (eNOS) 9, 11. eNOS is involved in regulating mitochondrial biogenesis, which is fundamental for mitochondrial turnover and proliferation, e.g. during exercise, cell stress, energy deficits, calorie restriction, and disease mitigation, and is under calcium and redox control 12-15. Specialized nuclear transcription factors, such as NRF-1 and NRF-2, along with PGC-1 family co-activators are required for mitochondrial gene activation and for mtDNA replication and transcription. This program is also stimulated by Akt, which is known to be activated by EpoR as well as to activate eNOS. This background suggested the novel possibility that EPO induces mitochondrial biogenesis through EpoR-dependent activation of eNOS.
Materials and Methods
Mice
The studies were approved by the Institutional Animal Care and Use Committee and conducted in young male C57BL/6J, Akt1-/-, eNOS-/- and mitochondrial GFP reporter mice bred in our vivarium 16. Mice were injected subcutaneously with human recombinant EPO or an equal volume of 0.9% NaCl once daily for three consecutive days and tissues removed under general anesthesia at the indicated times.
Microscopy
Formalin-fixed and paraffin-embedded tissue blocks were sectioned (5μM), mounted on glass slides, and de-paraffinized. Laser-scanning confocal microscopy was performed on a Zeiss LSM 410 microscope and fluorescence imaging on a Nikon microscope through a 520 nm filter. The fluorescence signals were quantified using computer software.
Proteins
Cardiac proteins were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes and probed with primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). After application of the primary antibody, the membranes were washed and incubated with horseradish peroxidase–conjugated secondary antibodies (Santa Cruz and Jackson), developed in ECL (Santa Cruz) and densitometry performed on digitized images. At least four samples were used at each time point. Pooled cardiac mitochondria were prepared and respiration measured at 35°C 17 using calibrated Clark mini-electrodes (Diamond General, Ann Arbor, MI) 18.
RNA and DNA
Cytoplasmic RNA was isolated and cDNA synthesized using the Invitrogen SUPERSCRIPT System. Cardiac mtDNA was isolated using NaI kits. Mouse-specific primers and probes were designed as reported 19 RNA samples (1μg) were reverse-transcribed and gene transcripts were amplified in triplicate with gene-specific primers 19, 20 and quantified by densitometry normalized to 18S rRNA or GAPDH . MtDNA copy number was determined as reported 21.
Metabolic measurements
Steady-state O2 consumption and CO2 production ( and ) were measured in resting mice at the same time of day 13 in individual metabolic chambers by collecting expired gas at a timed flow rate. Expired gas concentrations were measured by gas chromatography, and computed with standard formulae, and resting energy expenditure (REE) calculated with a modified Weir formula and expressed in kcal/d 22.
Voluntary exercise
Mice were housed individually in cages containing rodent exercise wheels 23 equipped with a digital magnetic counter activated by wheel rotation. To limit the variability in time spent running, individual mice were observed initially for 72 hours, and then assigned randomly to groups that were well-matched for running times. One group received injections of EPO, and the other NaCl, and running duration, running speed, and distance in kilometers were recorded daily.
Transthoracic echocardiography
Mice were lightly anesthetized with 1.5% isofluorane and the measurements made with a 707B 30 MHz ultrasonic probe and recorded on a VEVO 770 System (VisualSonics, Inc., Toronto).
Statistics
Grouped data (n=4-6) were expressed as means ± SEM. Statistical analyses was by two-way or repeated measures ANOVA using StatView (SAS Institute, 5.0.1; Chicago, IL). P≤0.05 was considered significant.
Please see the on-line supplement for detailed Materials and Methods.
Results
Mitochondrial biogenesis in reporter mice pre- and post-EPO
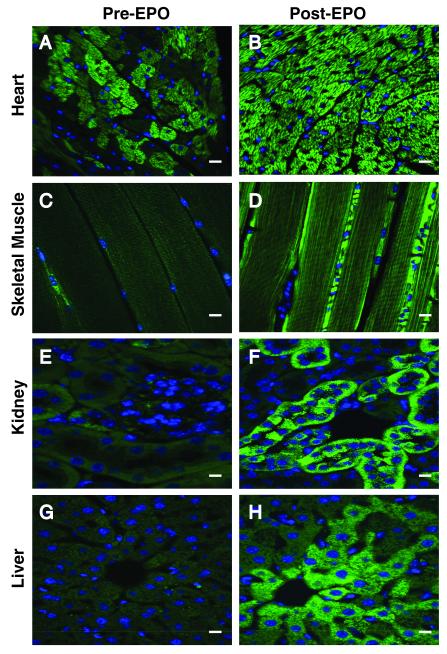
The distribution and mass of mitochondria in the tissues of mitochondrial-targeted GFP reporter mice were examined pre- and post- three days of daily EPO administration (Figure 1). In reporter mice pre-EPO, most of the tissues demonstrated fairly uniform, low-intensity, green fluorescence with rare bright cells containing abundant mitochondria. By post-EPO day 3 however, the heart showed intense mitochondrial fluorescence throughout the myocardium (A, B), while the other tissues showed modest, non-uniform enhancement of mitochondrial fluorescence, e.g. skeletal muscle blood vessels (C, D), renal tubules (E, F), and hepatocytes (G, H). Quantitatively, post-EPO at 3 days, mean cardiomyocyte fluorescence increased 2.8 fold from 36.6 ±5.8 units to 102.5 ±8.0 units per micron2. Since mitochondrial turnover in the heart requires about a week 24, we examined cardiac interfibrillar and subsarcolemmal mitochondria at higher resolution at day 7. These micrographs demonstrated an EPO effect on both populations, but in some subsarcolemmal areas mitochondrial density and giant mitochondria formation were especially prominent (Supplemental Material: Online Figure I). To confirm the increase in mitochondrial mass in the reporter mice, immunofluorescence microscopy was performed for citrate synthase pre- and post-EPO. Cardiac co-localization of citrate synthase with GFP expression (yellow-orange) pre and post-EPO demonstrated strong overlap in the staining pre- and post-EPO. (Online Figure II).
Figure 1. Mitochondrial density and distribution in mitochondrial reporter mouse tissues.
Reporter mice expressing a GPF-labeled mitochondrial localization sequence received EPO (4,000 U/kg/d) for three consecutive days and the sections were compared by fluorescence microscopy pre- and at day 3 post-EPO. Pre-EPO, green punctuate fluorescence, representing mitochondria, was present diffusely and staining was enhanced in sporadic clusters of cells. Post-EPO, the heart showed intense green fluorescence throughout the myocardium (A, B). More focal and less pronounced responses were found in skeletal muscle (C, D), kidney (E, F), and liver (G, H). Scale bars are 10 microns.
EPO responses in Wt, eNOS-/- and Akt1-/- mice
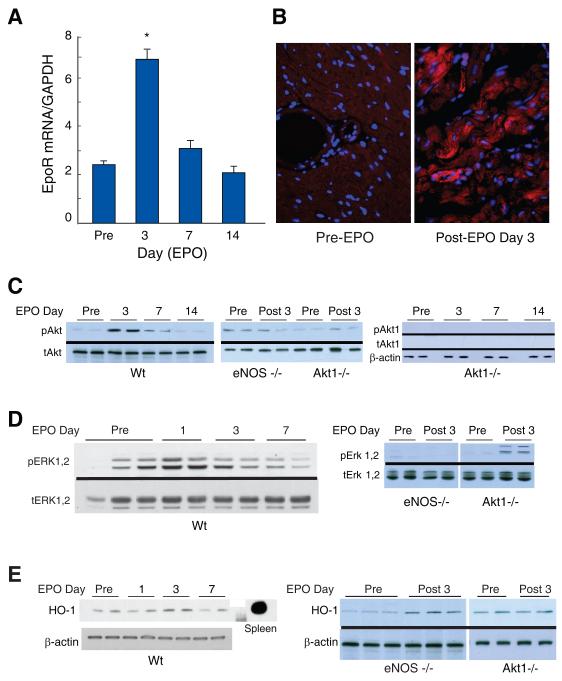
Three mouse strains were compared for intactness of erythropoiesis: Wt, eNOS-/- and Akt1-/- (Table 1). Hemoglobin content responded comparably to EPO and to hypoxia over 7 days in Wt and eNOS-/- strains, but lagged behind in the Akt1-/- mice. In Wt mice, EpoR transcript levels in the heart tripled by EPO day 3 and returned to control by day 14 (Figure 2A). EpoR protein at baseline was detected by immunofluorescence in vascular endothelium and cardiomyocyte plasma membranes, but this labeling was sharply enhanced by EPO administration (Figure 2B).
Table 1.
Blood hemoglobin concentration after hypoxic hypoxia or EPO administration
| Time (days) | Wt | eNOS -/- | Akt1-/- |
|---|---|---|---|
| 0 | 14.3 ± 0.2 | 14.1 ± 0.3 | 13.7 ± 0.3 |
|
| |||
| 7 | 17.7 ± 0.3* | 16.1 ± 0.2* | -- |
| (16.3 ± 0.4*) | (15.9 ± 0.4*) | -- | |
|
| |||
| 14 | 19.7 ± 0.3* | 18.8 ± 1.0* | -- |
| (17.0 ± 1.4*) | (16.6 ± 0.7*) | (15.1 ± 0.2*) | |
Mean ± SEM hemoglobin values in g/dL(n=4-9 Wt mice) measured after exposure to 18,000 feet (0.5 ATA) of altitude for 0, 7, or 14 days or after the three day EPO regimen (values in parentheses
P<0.05 vs. day 0).
Figure 2. Cardiac EpoR expression and Akt1, Erk 1,2, and HO-1 activation by EPO.
Cardiac EpoR transcript levels by real time RT-PCR at 3 days post-EPO administration to Wt mice. A. EpoR mRNA was detected pre-EPO and increased by post-EPO day 3 (*P < 0.05 vs. control; n=4 at each point). B. EpoR localization in mouse hearts pre- and post-EPO treatment (red fluorescence) with DAPI nuclear counterstain (blue). C. EPO activation of Akt1 in mouse heart. Phospho/total Akt was increased in Wt heart post-EPO day 3 and fell to control by day 14. Phospho/total Akt was unresponsive to EPO in eNOS -/- mice. Negative control are heart tissue from Akt1-/- mice probed with anti-pAkt and anti-Akt1 specific antibodies. D. Phospho/total Erk 1,2 activation in Wt, eNOS-/- and Akt1-/- hearts, pre- and post-EPO day 3. E. HO-1 protein expression in Wt, eNOS-/- and Akt1-/- mouse hearts.
To assess EpoR signaling, cardiac Akt phosphorylation was examined and Akt1 Ser-473 and was found to increase post-EPO on day 3 and return to control on day 7 (Figure 2C). In eNOS-/- mice, Akt1 was not activated after EPO (Figure 2C shows two Akt Westerns; middle blot shows day 3 with antibodies to total and phosphor Akt; right blot shows pre 3, 7 and 14 days post-EPO using Akt1 specific antibodies). Erk1,2 was strongly activated by EPO in Wt mice, but was activated minimally in Akt1-/- mice (Figure 2D). EPO also induced heme oxygenase-1 (HO-1) protein, which is involved in the regulation of cardiac mitochondrial biogenesis 25. HO-1 expression, like Akt, was highest in Wt mice at day 3 (Figure 2E). HO-1 was also increased in eNOS-/- mice, but not in Akt1-/- mice (Figure 2E). EPO thus exploits eNOS in the heart to activate Akt and Erk1/2, and Akt1 acts upstream of Erk1/2 and HO-1.
Respiration, REE, and voluntary exercise
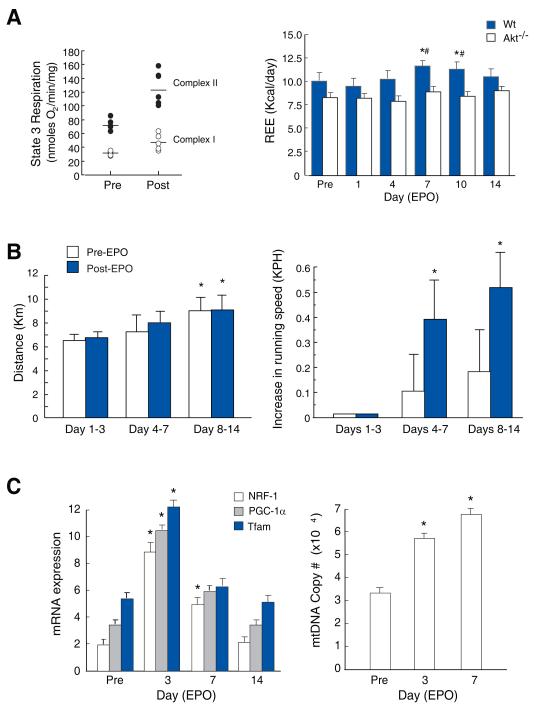
EPO did not affect mitochondrial RCR in the heart, but at day 3 post-EPO, State 3 respiration increased significantly (Figure 3A; left panel), accompanied by ~30% increases in resting energy expenditure (REE) (Figure 3A; right) at days 7 and 10 (P<0.05). Compared with Wt mice, basal REE was marginally depressed in Akt1-/- mice (P= 0.06), and did not respond to EPO (Figure 3A; right). REE was checked in two eNOS-/- mice, and predictably did not respond to EPO, so this experiment was not repeated.
Figure 3. REE, voluntary exercise, and mitochondrial biogenesis post-EPO administration in mice.
A. Left panel: State 3 respiration in control and EPO-treated Wt mouse heart mitochondria with malate/glutamate (open circles) or succinate (closed circles) substrate (P < 0.05 pre vs. post EPO for State 3). Right panel: REE measured from oxygen uptake (VO2) and carbon dioxide production (VCO2) before and after EPO in Wt and Akt1-/- mice (*P < 0.05 vs. day 0, # P < 0.05 Wt vs. Akt1). REE failed to increase in Akt1-/- mice after EPO. B. Voluntary exercise on calibrated wheels. Data are averages for days 1-3, 4-7, and 8-14. Left panel: All mice were self-trained by increasing their running distance. Right panel: Running speed increases after EPO (* P< 0.05). C. Activation of cardiac mitochondrial biogenesis. NRF-1, PGC-1α and Tfam mRNA levels, and mtDNA copy number measured pre- and post-EPO in Wt control mouse hearts (*P<0.05 vs. control; n=4).
Voluntary exercise in untrained Wt mice was quantified over two weeks using ergometer-calibrated running wheels. Typically, most sedentary mice allowed to run ad lib over this interval will increase distance and running time at constant speed 22. Control and EPO-treated mice demonstrated comparable running distances (Figure 3B), but during the second week, EPO-treated mice spontaneously covered the same distances in less time (P<0.05; Fig 3B right), but did not significantly increase their total running distance.
Post-EPO transcriptional activation of cardiac mitochondrial biogenesis
EPO stimulated the expression of cardiac regulatory genes for mitochondrial biogenesis, including NRF-1, the PGC-1α co-activator, and mitochondrial transcription factor-A (Tfam; Figure 3C). NRF-1, PGC-1α and Tfam mRNA increased 4-fold on post-EPO day 3 and returned to control by day 14, while mtDNA copy number doubled (Figure 3C right panel; P<0.05).
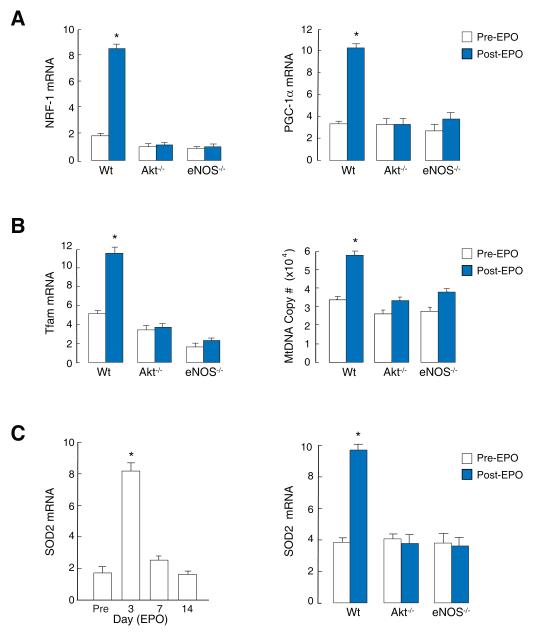
NRF-1, PGC-1α and Tfam mRNA levels did not respond to EPO in eNOS -/- or Akt1-/- mice and mtDNA copy number was unaffected (Figure 4A and 4B), establishing the requirement for eNOS. SOD2 transcript levels also increased after EPO in Wt but not in eNOS -/- or Akt1-/- mice (Figure 4C).
Figure 4. Activation of mitochondrial biogenesis by EPO is dependent on Akt and eNOS.
A. Cardiac mRNA expression for NRF-1 and PGC-1α in Wt mice at day 3 post-EPO is absent in eNOS -/- and Akt1-/- mice. B. Increases in Tfam mRNA and mtDNA copy number in Wt mice at day 3 post-EPO is absent in eNOS -/- and Akt1-/- mice. C. SOD2 mRNA expression in Wt mouse heart pre- and post- EPO (left), and pre- and at post-EPO day 3 in Wt, eNOS -/- and Akt1-/- mice relative to GAPDH (right) (* P<0.05 vs. control; n=4).
Cardiac ultrasonography
Cardiac chamber size, wall thickness, and function were evaluated in Wt mice by echocardiography at day 0 and at day 7 after EPO. A small increase in intraventricular septal diameter in diastole was noted after EPO, but no changes in left ventricular mass index or in fractional shortening at comparable heart rates (Table 2).
Table 2.
Transthoracic echocardiography measurements in Wt mice pre- and post-EPO
| Measurement | Pre-EPO | Post-EPO | P value |
|---|---|---|---|
| BW | 25.8 ±0.7 | 26.4 ±0.5 | 0.46 |
| LVEDD | 3.0 ±0.2 | 3.2 ±0.3 | 0.24 |
| LVESD | 1. 5 ±0.2 | 1.8 ±0.3 | 0.32 |
| IVSTS | 1.4 ±0.1 | 1.5 ±0.1 | 0.59 |
| IVSTD | 0.8 ±0.1 | 1.0 ±0.1 | 0.03* |
| PWTD | 1.4 ±0.2 | 1.3 ±0.2 | 0.76 |
| PWTS | 1.9 ±0.9 | 1.5 ±0.2 | 0.26 |
| FS | 0.51 ±0.1 | 0.45 ±0.1 | 0.61 |
| HR | 458 ±27 | 484 ±6 | 0.44 |
| LVMI | 135 ±11 | 121 ±5 | 0.42 |
BW, body weight; LVEDD, left ventricular end-diastolic dimension (mm); LVESD, left ventricular end systolic dimension (mm); IVSTS, interventricular septal thickness in systole (mm); IVSTD, interventricular septal thickness in diastole (mm); PWTD, posterior wall thickness in diastole (mm); PWTS, posterior wall thickness in systole (mm), FS, fractional shortening; HR, heart rate (beats per min); LVMI, left ventricular mass index (external LV diameter diastole3- LV end-diastolic dimension3) × 1.055 (mg). Values are mean ± SEM for n=4.
Studies of isolated cardiomyocytes
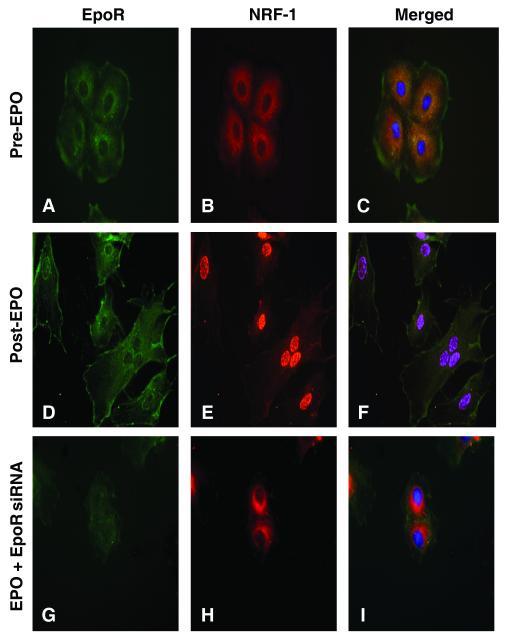
To investigate the requirement for EpoR, we selected NRF-1 for examination, which activates more than 100 genes involved in mitochondrial biogenesis, including the genes necessary for mtDNA replication. By immunofluorescence, NRF-1 was localized mainly to the cytoplasm of control H9C2 cardiomyocytes (Figure 5A-C), while after EPO (Figure 5D-F) NRF-1 staining was found primarily in nuclei. After EpoR knockdown (siRNA data not shown), NRF-1 protein did not accumulate in the nucleus after EPO, and NRF-1 remained sequestered in the cytoplasm (Figure 5G-I).
Figure 5. EpoR requirement for EPO-mediated nuclear NRF-1 accumulation in cardiomyocytes.
H9C2 rat cardiomyocytes were transfected with scrambled (Scr) or EpoR siRNA followed by EPO treatment (1000 U/ml). After 24h, fluorescence microscopy was performed using anti-NRF-1 (red) and EpoR (green). Panels A-C: Scr RNA Pre-EPO. Panels D-F: ScrRNA, Post-EPO; Panels G-I: Post-EPO + siRNA. Nuclear NRF-1 translocation by EPO requires EpoR expression.
Discussion
The novel aspects of this work disclose that EPO stimulates cardiac mitochondrial proliferation through a highly-regulated, receptor-mediated, eNOS/Akt1-dependent cascade that activates the transcriptional program of mitochondrial biogenesis. This activity occurs over approximately 3 to 7 days in mice and leads to a higher State 3 respiration, REE, and running speed in advance of a rising hematocrit, which increases at 1-2% per day. Although the EPO response requires eNOS 26, the sites of microvascular NO production and NO signaling on a paracrine or a cell-by-cell basis were not evaluated, although we did detect an unexplained increase post-EPO in EpoR receptor density in the heart and in cardiomyocytes 27, but found no short-term structural evidence of left ventricular hypertrophy.
The interpretation of these studies has some limitations. We did not formally assess vascular EpoR expression or changes in capillarity in the heart, and although changes in capillary density post-EPO have been reported10, such responses, if present here, could not be definitively linked to EpoR-mediated regulation of mitochondrial biogenesis at our current level of discernment. In terms of metabolism, the heart in resting mice consumes ~30% of the O2 with a well-defined preference for myocardial β-oxidation over glucose oxidation. Higher Complex II activity would help support the re-oxidation of FADH2, which requires more O2, but this could quantitatively account for the higher REE only if resting myocardial O2 utilization had doubled. Thus, it is likely that respiration in other organs contributed a modest but unknown amount to the increase in REE 4, 27. Similar constraints apply to the interpretation of the spontaneous exercise data, which demonstrate effectiveness for our EPOS protocol, but where the roles of eNOS, Akt1 and changes in skeletal muscle oxidative capacity were not assessed. Skeletal muscle fiber-type-switching would also be of interest, but could reflect the modified sub-maximal exercise behavior and not a direct effect of EPO, which among other factors would depend on EpoR activity in skeletal muscle. Finally, the abundant response by subsarcolemmal mitochondria was not investigated in detail, but these mitochondrial populations do serve different cellular functions and are differently regulated. Overall however, there is good temporal and quantitative correlation among the molecular, functional, and structural manifestations of cardiac mitochondrial biogenesis after EPO administration.
EPO’s therapeutic potential in acute coronary and cerebral ischemia 28 involves anti-apoptotic, anti-inflammatory, and angiogenic effects 28, 29 for which the pro-survival serine/threonine kinase Akt1/2 is a key effector 11. Here, Akt1 was required for EPO to stimulate cardiac mitochondrial biogenesis in keeping with the activating role of Akt/NRF-1 mitochondrial biogenesis 14, 30. The transcriptional program is also activated by eNOS 15, 26, 31 and as an aside, we note that eNOS must be active for EPO to prevent apoptosis and limit experimental cardiac infarct size 9. EPO-induced mitochondrial biogenesis conforms to eNOS-inducible metabolic gene regulation, e.g. by CREB, and the requirement for Akt1 fits its role in growth, metabolism, and cell survival. Also, Akt, eNOS, and HO-1 exhibit an elaborate integration: Akt activates eNOS by calcium-independent phosphorylation 32, and NO activates PI-3K, an important Akt regulator, and under some conditions, Akt itself 33. The Wt and eNOS-/- mice also up-regulate HO-1, the latter without new mitochondrial biogenesis, implying that HO-1 is upstream of eNOS in this case.
The physiological responses to EPO are conventionally attributed to hemoglobin biosynthesis and erythropoiesis, which increase red cell mass, and thus hematocrit and arterial O2 content (CaO2) 27, 34. In erythroid progenitor cells, EPO is also involved in the regulation of iron uptake through the posttranscriptional induction of transferrin receptor 35. EPO also directly stimulates the transcriptional and post-transcriptional expression of 5-aminolevulinic acid synthase (ALAS-E), which resides in mitochondria and is the rate-limiting step in heme biosynthesis 36 and necessary for cytochrome assembly.
Clinically, EPO treatment mitigates the anemia of chronic kidney disease, although EPO increases mortality in cancer patients 37 and when the target hemoglobin is set too high 38. In human athletes, EPO enhances endurance by improving maximum O2 uptake in direct relation to hematocrit 34; however, a role for EPO in maintaining cardiac function in hypoxia and anemia, the main settings for its production, although long suspected, has been unconfirmed.
In the context of hypoxia, the regulation of cardiac mitochondrial density by EPO implies that a distinct spatial and functional arrangement of the organelles is necessary for optimal aerobic work performance at steady-state convective oxygen transport (cardiac output times CaO2) as hematocrit, the principal determinant of blood viscosity, increases. Because blood viscosity is governed by hematocrit (at constant shear rate), the inherent matching of cardiac mitochondrial mass to erythrocyte O2 carrying capacity, despite the heart’s normally high oxygen extraction ratio, and barring limitations in coronary blood flow, would serve to protect cardiac oxidative phosphorylation and therefore maintain peripheral tissue O2 delivery during hypoxia. By comparison, in anemia of non-renal origin, the cardiac structure-function implications are less clear because endogenous EPO secretion tends to be elevated and mitochondrial volume density is not known to be affected.
In conclusion, cardiac EpoR activation after EPO administration activates mitochondrial biogenesis in normal mice prior to significant increases in circulating hemoglobin concentration. Ultimately, the physiological increase in mitochondrial density serves aerobic cardiac performance at a higher blood viscosity, but the effectiveness of this would be contingent on a higher myocardial O2 uptake stemming from an adjustment in aerobic capacity for contractile function designed for use in hypoxia 6, 11, 39. In addition to the new physiological role for EPO, our findings have latent implications for the treatment of patients with ischemic cardiomyopathy and anemia, often associated with chronic kidney disease, who may be candidates for stabilization of ventricular and renal function by the administration of erythropoietic agents 40, but who may also have myocardium at risk under conditions of an elevated cardiac O2 demand.
What is known?
- The renal hormone erythropoietin (EPO) regulates the production of red blood cells and acts to protect the heart, brain, and other tissues from certain types of injuries, such as ischemia-reperfusion, but by unknown mechanisms.
- EPO supplementation also improves exercise performance by increasing the supply of red blood cells (RBCs) and possibly by improving capillary density.
- The EPO receptor is expressed in several types of non-erythroid cells, but the reasons for this are poorly understood.
What new information does this article contribute?
- The administration of EPO stimulates the heart to produce new mitochondria through EPO-receptor-dependent activation of the transcriptional program of mitochondrial biogenesis.
- EPO induces mitochondrial biogenesis in the heart before circulating RBC mass increases, and leads to increases in both resting energy expenditure and running speed in mice.
- The mechanism of EPO-dependent mitochondrial biogenesis requires endothelial nitric oxide synthase and the pro-survival kinase Akt-1.
Novelty and Significance
The renal hormone EPO is employed clinically to treat anemia in patients with chronic cardiac and kidney diseases; however EPO may offer benefits beyond an improvement in hematocrit. Here, we investigated whether EPO influences the heart’s capacity to produce mitochondria in mice. After three days of EPO administration, mice developed sharp increases in mitochondrial DNA content and mitochondrial density throughout the myocardium, which peaked by seven days— preceding and surpassing effects on hepatic renal and skeletal muscle, and occurring before significant increases in blood hemoglobin content. This response was associated with increases in peak mitochondrial respiration rates, resting energy expenditure, and spontaneous running speed. EPO activated cardiac mitochondrial biogenesis through the endothelial nitric oxide synthase and the Akt1 pro-survival kinase by enhancing gene expression for the activators nuclear respiratory factor-1 (NRF-1), PGC-1α and mitochondrial transcription factor-A. An EPO receptor requirement was demonstrated by receptor-silencing in cardiomyocytes, which blocked the nuclear translocation of NRF-1 mediated by EPO. These new findings indicate a physiological and protective role for EPO, acting through its cell surface receptor and NO-Akt1 signal transduction, in matching the mitochondrial mass in the heart to the body’s oxygen transport capacity as the circulating erythrocyte mass expands.
Supplementary Material
Online Figure I: Cardiac mitochondria pre- and post-EPO (day 7) in mitochondrial reporter mice. Pre-EPO sections are shown in panels A and B (100 and 1,000 magnification scale bars are 50 and 5 microns, respectively). Comparable images are shown in panels C and D at post-EPO day 7.
Online Figure II: Mitochondrial citrate synthase expression in mitochondrial reporter mice. Reporter mice expressing the GPF mitochondrial localization tag received EPO (4,000 U/kg/d) for three days and hearts were compared by fluorescence microscopy pre- and post-EPO. These tissues were then labeled with anti-citrate synthase (red fluorescence), and the images merged to demonstrate co-localization (yellow-orange). Citrate synthase staining matched the distribution of GFP, and was present with light diffuse staining pre-EPO, that increased nearly uniformly post-EPO at days 3 and 7. Scale bars are 5 microns.
Acknowledgements
The authors thank Martha Salinas and Craig Marshall for technical assistance and G. Allen Johnson, Ph.D. for support of the echocardiography.
Source of Funding. NIH R01 AI0664789 and HL 090679 (CAP).
Abbreviations
- Akt
protein kinase B
- CREB
cAMP-responsive element-binding protein
- EPO
erythropoietin
- EpoR
erythropoietin receptor
- ERK1,2
extracellular kinase 1,2
- FADH2
flavin adenine dinucleotide
- GFP
green fluorescent protein
- HO-1
heme oxygenase-1
- JAK2
Janus kinase-2
- NRF-1
nuclear respiratory factor-1
- eNOS
endothelial nitric oxide synthase
- PGC-1α
PPAR-gamma co-activator 1-alpha
- RBC
red blood cell
- REE
resting energy expenditure
- STAT
signal transducer and activator of transcription
- Tfam
mitochondrial transcription factor-A
Footnotes
Disclosures. The research described in this article has been reviewed by the Health Effects and Environmental Research Laboratory, United States Environmental Protection Agency, and approved for publication. Approval does not signify that the contents reflect the views and the policies of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sawada K, Krantz SB, Kans JS, Dessyrpis EN, Sawyer S, Glick AD, Civin CL. Purification of human erythroid colony-forming units and demonstration of specific binding of erythropoietin. J Clin Invest. 1987;80:357–366. doi: 10.1172/JCI113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrades RM, Roca J, Campistol JM, Diaz O, Barbera JA, Torregrosa JV, Masclans JR, Cobos A, Rodriguez-Roisin R, Wagner PD. Effects of erythropoietin on muscle O2 transport during exercise in patients with chronic renal failure. J Clin Invest. 1996;97:2092–2100. doi: 10.1172/JCI118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 4.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CH, Hsu CY, Chen HW, Tsai MS, Cheng HJ, Chang CH, Lee YT, Chen WJ. Erythropoietin improves the postresuscitation myocardial dysfunction and survival in the asphyxia-induced cardiac arrest model. Shock. 2007;28:53–58. doi: 10.1097/shk.0b013e31802f0218. [DOI] [PubMed] [Google Scholar]
- 7.Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 9.Burger D, Lei M, Geoghegan-Morphet N, Lu X, Xenocostas A, Feng Q. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res. 2006;72:51–59. doi: 10.1016/j.cardiores.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Madeddu P, Emanueli C. Switching on reparative angiogenesis: essential role of the vascular erythropoietin receptor. Circ Res. 2007;100:599–601. doi: 10.1161/01.RES.0000261610.11754.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi H, Miura T, Ishida H, Miki T, Tanno M, Yano T, Sato T, Hotta H, Shimamoto K. Limitation of infarct size by erythropoietin is associated with translocation of Akt to the mitochondria after reperfusion. Clin Exp Pharmacol Physiol. 2008;35:812–819. doi: 10.1111/j.1440-1681.2008.04925.x. [DOI] [PubMed] [Google Scholar]
- 12.Carraway MS, Suliman HB, Kliment C, Welty-Wolf KE, Oury TD, Piantadosi CA. Mitochondrial biogenesis in the pulmonary vasculature during inhalational lung injury and fibrosis. Antioxid Redox Signal. 2008;10:269–275. doi: 10.1089/ars.2007.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haden DW, Suliman HB, Carraway MS, Welty-Wolf KE, Ali AS, Shitara H, Yonekawa H, Piantadosi CA. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2007;176:768–777. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 15.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 16.Shitara H, Kaneda H, Sato A, Iwasaki K, Hayashi J, Taya C, Yonekawa H. Non-invasive visualization of sperm mitochondria behavior in transgenic mice with introduced green fluorescent protein (GFP) FEBS Lett. 2001;500:7–11. doi: 10.1016/s0014-5793(01)02574-1. [DOI] [PubMed] [Google Scholar]
- 17.Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res. 2004;64:279–288. doi: 10.1016/j.cardiores.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Taylor DE, Ghio AJ, Piantadosi CA. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch Biochem Biophys. 1995;316:70–76. doi: 10.1006/abbi.1995.1011. [DOI] [PubMed] [Google Scholar]
- 19.Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA. Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem. 2003;278:41510–41518. doi: 10.1074/jbc.M304719200. [DOI] [PubMed] [Google Scholar]
- 20.Suliman HB, Welty-Wolf KE, Carraway MS, Schwartz DA, Hollingsworth JW, Piantadosi CA. Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. FASEB J. 2005;19:1531–1533. doi: 10.1096/fj.04-3500fje. [DOI] [PubMed] [Google Scholar]
- 21.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 22.Matarese LE. Indirect calorimetry: technical aspects. J Am Diet Assoc. 1997;97:S154–160. doi: 10.1016/s0002-8223(97)00754-2. [DOI] [PubMed] [Google Scholar]
- 23.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 24.Hoppel CL, Tandler B, Fujioka H, Riva A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol. 2009;41:1949–1956. doi: 10.1016/j.biocel.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini H, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundby C, Hellsten Y, Jensen MB, Munch AS, Pilegaard H. Erythropoietin receptor in human skeletal muscle and the effects of acute and long-term injections with recombinant human erythropoietin on the skeletal muscle. J Appl Physiol. 2008;104:1154–1160. doi: 10.1152/japplphysiol.01211.2007. [DOI] [PubMed] [Google Scholar]
- 28.Fliser D, Bahlmann FH, deGroot K, Haller H. Mechanisms of disease: erythropoietin--an old hormone with a new mission? Nat Clin Pract Cardiovasc Med. 2006;3:563–572. doi: 10.1038/ncpcardio0609. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T, Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006;71:684–694. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deora AA, Win T, Vanhaesebroeck B, Lander HM. A redox-triggered ras-effector interaction. Recruitment of phosphatidylinositol 3′-kinase to Ras by redox stress. J Biol Chem. 1998;273:29923–29928. doi: 10.1074/jbc.273.45.29923. [DOI] [PubMed] [Google Scholar]
- 34.Lundby C, Robach P, Boushel R, Thomsen JJ, Rasumssen P, Koskolou M, Calbet JA. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol. 2008;105:581–587. doi: 10.1152/japplphysiol.90484.2008. [DOI] [PubMed] [Google Scholar]
- 35.Zoller H, Decristoforo C, Weiss G. Erythroid 5-aminolevulinate synthase, ferrochelatase and DMT1 expression in erythroid progenitors: differential pathways for erythropoietin and iron-dependent regulation. Br J Haematol. 2002;118:619–626. doi: 10.1046/j.1365-2141.2002.03626.x. [DOI] [PubMed] [Google Scholar]
- 36.Weiss G, Houston T, Kastner S, Johrer K, Grunewald K, Brock JH. Regulation of cellular iron metabolism by erythropoietin: activation of iron-regulatory protein and upregulation of transferrin receptor expression in erythroid cells. Blood. 1997;89:680–687. [PubMed] [Google Scholar]
- 37.Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kuzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M. Venous. thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 38.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 39.Joyeux-Faure M, Ramond A, Beguin PC, Belaidi E, Godin-Ribuot D, Ribuot C. Early pharmacological preconditioning by erythropoietin mediated by inducible NOS and mitochondrial ATP-dependent potassium channels in the rat heart. Fundam Clin Pharmacol. 2006;20:51–56. doi: 10.1111/j.1472-8206.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 40.Silverberg DS, Wexler D, Iaina A, Schwartz D. The correction of anemia in patients with the combination of chronic kidney disease and congestive heart failure may prevent progression of both conditions. Clin Exp Nephrol. 2009;13:101–106. doi: 10.1007/s10157-008-0074-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure I: Cardiac mitochondria pre- and post-EPO (day 7) in mitochondrial reporter mice. Pre-EPO sections are shown in panels A and B (100 and 1,000 magnification scale bars are 50 and 5 microns, respectively). Comparable images are shown in panels C and D at post-EPO day 7.
Online Figure II: Mitochondrial citrate synthase expression in mitochondrial reporter mice. Reporter mice expressing the GPF mitochondrial localization tag received EPO (4,000 U/kg/d) for three days and hearts were compared by fluorescence microscopy pre- and post-EPO. These tissues were then labeled with anti-citrate synthase (red fluorescence), and the images merged to demonstrate co-localization (yellow-orange). Citrate synthase staining matched the distribution of GFP, and was present with light diffuse staining pre-EPO, that increased nearly uniformly post-EPO at days 3 and 7. Scale bars are 5 microns.