Abstract
This article describes the development of a Pd-catalyzed reaction for the arylhalogenation (halogen = Cl or Br) of diverse α-olefins by oxidatively intercepting Mizoroki-Heck intermediates. These transformations afford synthetically useful 1,2- and 1,1-arylhalogenated products in good yields with good to excellent selectivities that can be modulated by changing the nature of the halogenating reagent and/or the reaction conditions. The selectivity of these reactions can be rationally tuned by: (i) controlling the relative rates of oxidative functionalization versus β-hydride elimination from equilibrating PdII-alkyl species and (ii) stabilization of organometallic PdII intermediates through the formation of π-benzyl adducts. These arylhalogenations exhibit modest to excellent levels of stereospecificity, and the key carbon-halogen bond-forming step proceeds with predominant retention of stereochemistry at carbon.
Keywords: Alkene, Arylhalogenation, Palladium, Catalysis, Heck, Mechanism, π-benzyl
Introduction
The Mizoroki-Heck reaction involves the Pd-catalyzed coupling of an alkene with an aryl halide or aryl metal species.1 This transformation is widely used in organic synthesis for the construction of carbon–carbon bonds,2 and significant effort has been devoted to both catalyst development1–3 and mechanistic investigations.1–4 As shown in eq. 1, the general mechanism of the Heck reaction involves formation of a σ-aryl Pd species followed by olefin insertion to generate σ-alkyl Pd intermediate A and then β-hydride elimination/olefin dissociation to release product B.
 |
(1) |
Methods for intercepting Mizoroki-Heck intermediate A via other fundamental organometallic reactions would provide attractive routes for the 1,2-arylfunctionalization of α-olefins (eq. 2). Such transformations would complement the traditional Mizoroki-Heck reaction by providing access to products containing one additional bond and one additional stereocenter as compared to B. Some success has been realized in this area by trapping intermediate A via alkene, alkyne, or CO insertion.5,6 However, the scope, generality, and range of products accessible from these reactions remains limited, in large part because the relative rate of β-hydride elimination to form B is often competitive in these processes.
 |
(2) |
On the basis of our interest in high oxidation state palladium chemistry, we thought that oxidation of A would provide an attractive alternative route for intercepting this intermediate. Indeed, very early studies by Heck demonstrated the feasibility of this transformation. For example, in 1968, he reported the Pd-catalyzed reaction of methylvinylketone with PhHgCl and CuCl2 to afford 1,2-arylchlorination product 1 in 80% yield (eq. 3).7 However, the overall synthetic utility of this transformation was limited by a modest substrate scope, competing β-hydride elimination/olefin dissociation (to form 2), and the requirement for aryl mercury reagents.
 |
(3) |
Sporadic subsequent reports have also suggested that Heck intermediate A can be intercepted under oxidative conditions to generate 1,1-difunctionalized products.8 For example, Tamaru and coworkers demonstrated the palladium-catalyzed 1,1-phenylchlorination of 1-octene with PhSnBu3 in the presence of CuCl2 (eq. 4).9,10 However, the yield of this transformation was not reported, and 1-octene was the only substrate examined.11 More recently, a similar Pd-catalyzed 1,1-phenylchlorination product 4 was unexpectedly observed in the reaction between 3, PhSnBu3, and CuCl2 (eq. 5).12 Again, this was an isolated example, and the reaction was not explored further.
 |
(4) |
 |
(5) |
These prior studies suggested that it might be possible to develop oxidative transformations for the predictable and selective conversion of α-olefins into 1,2 or 1,1-arylfunctionalized products of general structures C and D (eq. 6). Recent work from our group and others has shown that σ-alkyl Pd intermediates related to A can be intercepted with a wide variety of oxidants to install diverse functional groups, including C–Cl, C–Br, C–I, C–F, C–N, C–O, and C–C bonds. This has been demonstrated in the context of Pd-catalyzed ligand-directed C–H functionalization,13 where sp3 C–H activation has been followed by oxidative halogenation,14 amination,15 oxygenation,16 and arylation.17 In addition, a variety of sequences involving amino-, oxy- or halopalladation of olefins to generate σ-alkyl Pd intermediates followed by oxidative halogenation,18,19 amination,20 oxygenation,21 and arylation22 have been developed to afford diverse organic products.
 |
(6) |
We report herein the implementation of this strategy in the development of general oxidative Mizoroki-Heck reactions for the 1,2- and 1,1-arylhalogenation of alkenes.23 The full scope of both 1,2-and 1,1-arylchlorination and bromination reactions is described. Additionally, investigations of the mechanism and the stereospecificity of these transformations are discussed in detail.
Results
Reaction Design
In order to develop general and robust conditions for alkene arylfunctionalization, we needed a working mechanistic hypothesis for the formation of each of the products. As shown in Scheme 1, the Heck product B is known to be generated from σ-alkyl Pd intermediate A via β-hydride elimination/olefin dissociation. The 1,2-arylfunctionalization product C could be formed by direct oxidative functionalization of A. Finally, the isomeric 1,1-product D could derive from A via a sequence involving β-hydride elimination, olefin insertion with opposite regiochemistry, and finally oxidative functionalization of the resulting Pd-benzyl complex (E) (Scheme 1). This mechanistic proposal suggests that the ratio of products B, C, and D should be tunable by modifying the relative rates of four fundamental organometallic transformations: oxidative functionalization, β-hydride elimination, olefin dissociation, and olefin insertion.
Scheme 1.
Working Mechanistic Hypothesis for Formation of B-D
1,2-Arylchlorination Reactions
We reasoned that use of the highly reactive electrophilic chlorinating reagent PhICl2 should increase the rate of oxidative functionalization of A relative to that of β-hydride elimination, thereby promoting formation of 1,2-product C. Thus, we first studied the Pd-catalyzed reaction between alkene 5, PhICl2, and PhSnBu324 in a variety of common solvents. Unexpectedly, the 1,1-product (6b) was the major isomer observed at room temperature in all solvents examined (Table 1, entries 1–5). The most promising initial lead was in CH2Cl2, which showed formation of significant quantities of 6a (1 : 1.3 ratio of 6a : 6b, 46% overall yield, entry 5). Lowering the temperature to −78 °C and increasing the reaction concentration to 0.064 M in CH2Cl2 led to a significant enhancement in both selectivity for and yield of 6a (entry 8). The yield was improved further by increasing the amount of PhSnBu3 from 1.3 to 2.6 equiv (entry 9). Ultimately, optimal conditions were found to be: 10 mol % of PdCl2(PhCN)2, 2 equiv of PhICl2, 2.6 equiv of PhSnBu3 in CH2Cl2 (0.064 M) starting at −78 °C with gradual warming over 5 h to 25 °C. This afforded a quantitative yield of 6 as a 10 : 1 mixture of isomer 6a : 6b (entry 9).
Table 1.
Optimization of the 1,2-Arylchlorination Reaction
 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Temperature | Concentration | Yielda | 6a : 6ba |
| 1 | Dioxane | 25 °C | 0.032 M | 77% | 1 : 6.7 |
| 2 | THF | 25 °C | 0.032 M | 53% | 1 : 9.6 |
| 3 | Et2O | 25 °C | 0.032 M | 64% | 1 : 9.7 |
| 4 | C6H6 | 25 °C | 0.032 M | 55% | 1 : 8.9 |
| 5 | CH2Cl2 | 25 °C | 0.032 M | 46% | 1 : 1.3 |
| 6 | CH2Cl2 | 0 °C to 25 °C | 0.032 M | 55% | 1.9 : 1 |
| 7 | CH2Cl2 | −78 °C to 25 °C | 0.032 M | 56% | 8 : 1 |
| 8 | CH2Cl2 | −78 °C to 25 °C | 0.064 M | 82% | 10 : 1 |
| 9b | CH2Cl2 | −78 °C to 25 °C | 0.064 M | 100% | 10 : 1 |
Yield and ratio of isomers determined by 1H NMR spectroscopic analysis of the crude reaction mixture.
2.6 equiv of PhSnBu3.
These conditions proved general for the 1,2-arylchlorination of numerous α-olefins (Table 2). The reactions were tolerant of a wide variety of common functional groups, including esters, aromatic and alkyl halides, benzylic hydrogens, amides, and silyl ethers. Additionally, both electron rich and electron deficient arylstannanes were effective coupling partners. The products were obtained in good to excellent yields and with good selectivity for the 1,2-isomer. In general, the mass balance in these reactions consisted of small amounts of the 2,1-phenylchlorination, dichlorination, and/or β-hydride elimination products.
Table 2.
Substrate Scope for 1,2-Arylchlorination
 | |||||
|---|---|---|---|---|---|
| Entry | Alkene | Stannane | Major Product | Yielda | 1,2 : 1,1 |
| 1 |  |
PhSnBu3 |  |
72% | 8 : 1 |
| 2 | PhSnBu3 |  |
84% | 13 : 1 | |
| 3 |  |
PhSnBu3 |  |
96% | 9 : 1 |
| 4 | PhSnBu3 |  |
92% | 11 : 1 | |
| 5 |  |
p-FC6H4SnBu3 |  |
84% | 11 : 1 |
| 6 |  |
PhSnBu3 |  |
85% | 6: 1 |
| 7 |  |
p-BrC6H4SnBu3 |  |
96% | 7 : 1 |
| 8 |  |
PhSnBu3 |  |
86% | 8 : 1 |
| 9 |  |
PhSnBu3 |  |
68% | 6 : 1 |
| 10 |  |
PhSnBu3 |  |
86% | 10 : 1 |
| 11 |  |
p-CH3C6H4SnBu3 |  |
74% | 5 : 1 |
| 12 |  |
PhSnBu3 |  |
80% | 14: 1 |
Isolated yields and selectivities. Notably, in some cases, the isolated material contained minor impurities (typically 2,1-phenylchlorination or dichlorination products). See Supporting Information for details.
The 1,1 and 1,2-isomers were generally not separable by column chromatography, but could be isolated in pure form using HPLC.
The variation in 1,2 : 1,1 selectivity as a function of substrate may be due to differing interactions of polar functional groups within the substrate with the palladium catalyst.
1,1 Arylchlorination Reactions
We next sought conditions that would selectively provide 1,1-arylchlorinated products. The solvent screen in Table 1 showed that the reaction of 5 with PhICl2 significantly favored formation of the 1,1-product 6b in ethereal solvents. We reasoned that the use of a less reactive chlorinating reagent would further slow competing 1,2 functionalization and thus provide higher selectivity for 6b. Gratifyingly, the use of NCS or CuCl2 under otherwise identical conditions resulted in >20 : 1 selectivity for 6b in a variety of solvents (Table 3). While the yields were modest at room temperature (ranging from 10–58%), they could be significantly improved by conducting the reactions at −78 °C. Under optimal conditions (10 mol % of PdCl2(PhCN)2, 4 equiv of CuCl2, 1.3 equiv of PhSnBu3 in Et2O (0.032 M) starting at −78 °C with gradual warming over 5 h to 25 °C), 6b was obtained in 82% yield and >20 : 1 selectivity as determined by 1H NMR spectroscopy (entry 8).
Table 3.
Optimization of 1,1-Arylchlorination Reaction
 | ||||
|---|---|---|---|---|
| Entry | Solvent | Temperature | Yield CuCl2a | Yield NCSa |
| 1 | Dioxane | 25 °C | 13% | 34% |
| 2 | C6H6 | 25 °C | 28% | 48% |
| 3 | AcOH | 25 °C | 10% | 46% |
| 4 | CH2C12 | 25 °C | 32% | 36% |
| 5 | THF | 25 °C | 58% | 39% |
| 6 | THF | −78 °C to 25 °C | 75% | ndb |
| 7 | Et2O | 25 °C | 29% | 36% |
| 8 | Et2O | −78 °C to 25 °C | 82% | 44% |
Yield and ratio of isomers determined by 1H NMR spectroscopic analysis of crude reaction mixture.
nd = not determined
The substrate scope of 1,1-arylchlorination with CuCl2 was similar to that of the PhICl2 reactions. Aryl stannanes and α-olefins containing diverse functional groups were effective coupling partners, and the 1,1-isomer was consistently obtained in >20 : 1 selectivity (Table 4). In all cases, <10% of alkene products derived from β-hydride elimination/alkene dissociation were observed by 1H NMR spectroscopic analysis of the crude mixtures.25
Table 4.
Substrate Scope for 1,1-Arylchlorination25
 | |||||
|---|---|---|---|---|---|
| Entry | Alkene | Stannane | Major Product | Yielda | 1,2 : 1,1 |
| 1 | PhSnBu3 |  |
75% (53%) | 1 : >20 | |
| 2 |  |
PhSnBu3 | 83% (54%) | 1 : >20 | |
| 3 |  |
PhSnBu3 |  |
84% (71%) | 1 : >20 |
| 4 |  |
o-CH3C6H4SnBu3 | 87% (67%) | 1 : >20 | |
| 5 | PhSnBu3 | 86% (66%) | 1 : >20 | ||
| 6 | p-FC6H4SnBu3 |  |
76% (59%) | 1 : >20 | |
| 7 |  |
PhSnBu3 |  |
73% (71%) | 1 : >20 |
| 8 |  |
PhSnBu3 |  |
51% (41%) | 1 : >20 |
| 9 |  |
PhSnBu3 |  |
74% (55%) | 1 : >20 |
| 10 |  |
PhSnBu3 |  |
73% (71%) | 1 : >20 |
| 11 |  |
p-CH3C6H4SnBu3 |  |
78% (72%) | 1 : >20 |
| 12 |  |
PhSnBu3 |  |
94% (82%) | 1 : >20 |
| 13 |  |
p-BrC6H4SnBu3 |  |
82% (54%) | 1 : >20 |
Yield determined by 1H NMR spectroscopic analysis of crude reaction mixture. Isolated yields are in parentheses.
1,2 and 1,1 Arylbromination Reactions
We next pursued analogous palladium-catalyzed arylbromination reactions using CuBr2 and NBS as electrophilic brominating reagents (PhIBr2 is not readily available).26,27 As shown in Table 5, NBS was poorly effective for this transformation. In contrast, CuBr2 efficiently promoted the formation of both 1,1-arylbromination product 28b and 1,2-arylbromination product 28a. The isomer ratio in these CuBr2-mediated reactions could be tuned by simply changing the solvent. For example, 1,1-product 28b was formed in high (82%) yield and with >20 : 1 selectivity in Et2O (entry 6). However, moving from Et2O to THF under similar conditions resulted in a reversal of selectivity to afford 28a as the major product (28a : 28b = 3 : 1), albeit in modest (49%) yield (Table 5, entry 8). This is in notable contrast to the trend observed for arylchlorination, where the use of CuCl2 in THF favored 1,1-products. Subsequent optimization revealed that increasing the concentration to 0.128 M resulted in 80% yield of a 10 : 1 mixture of 28a : 28b (entry 10). Under these conditions, the mass balance consisted of 29 (20% yield), which is the product of 1,2-addition of Ph and ring opened THF to the alkene.28
Table 5.
Optimization of Arylbromination Reaction
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Solvent | Temperature | Concentration | Yield CuBr2a |
28a : 28ba |
Yield NBSa |
28a : 28ba |
| 1 | Dioxane | 25 °C | 0.032 M | 29% | 1 : >20 | 19% | 1 : >20 |
| 2 | C6H6 | 25 °C | 0.032 M | 24% | 1 : 13 | 8% | 1 : >20 |
| 3 | AcOH | 25 °C | 0.032 M | 16% | 1 : >20 | 0% | ndb |
| 4 | CH2Cl2 | 25 °C | 0.032 M | 27% | 1 : >20 | 6% | 1 : >20 |
| 5 | Et2O | 25 °C | 0.032 M | 46% | 1 : >20 | 8% | 1 : >20 |
| 6 | Et2O | −78 °C to 25 °C | 0.032 M | 82% | 1 : >20 | ndb | ndb |
| 7 | THF | 25 °C | 0.032 M | 25% | 1 : >20 | 11% | 1 : >20 |
| 8c | THF | −78 °C to 25 °C | 0.032 M | 49% (17%) | 3 : 1 | ndb | ndb |
| 9c | THF | −78 °C to 25 °C | 0.064 M | 50% (5%) | 10 : 1 | ndb | ndb |
| 10c | THF | −78 °C to 25 °C | 0.128 M | 80% (20%) | 10 : 1 | ndb | ndb |
Yield and ratio of isomers determined by 1H NMR spectroscopic analysis of crude reaction mixture. The yield of 29 is shown in parentheses where applicable.
nd = not determined.
1.9 equiv of PhSnBu3.
As shown in Table 6 and Table 7, the optimal conditions for formation of the 1,1- and 1,2-arylbrominated products were general for numerous α-olefins. These transformations exhibited a scope and functional group tolerance similar to the arylchlorination reactions. Notably, THF-addition products analogous to 29 were formed as significant by-products (5–20% yield as determined by 1H NMR spectroscopic analysis of the crude reaction mixtures) in the 1,2-arylbrominations.29
Table 6.
Substrate Scope for 1,2-Arylbromination29
 | ||||
|---|---|---|---|---|
| Entry | Alkene | Major Productb | Yielda | 1,2 : 1,1a |
| 1 |  |
73% (54%) | 23 : 1(15 : 1) | |
| 2 |  |
 |
86% (64%) | 17: 1 (15: 1) |
| 3 |  |
 |
72% (56%) | 14 : 1 (11 : 1) |
| 4 |  |
 |
84% (60%) | 13 : 1 (15: 1) |
| 5 |  |
87% (63%) | 14 : 1 (20 : l)c | |
| 6 |  |
87% (63%) | 14 : 1 (22 : 1) | |
| 7 |  |
65% (52%) | 12 : 1 (10: 1) | |
Yield and ratio of isomers determined by 1H NMR spectroscopic analysis of crude reaction mixture. Isolated yields and selectivities are in parentheses.
Between 5 and 20% of THF-addition products analogous to 29 was observed by crude 1H NMR spectroscopic analysis with all substrates.
Isolated ratio reflects 1,2 product to a mixture of 1,1 and 1,4 products.
The 1,1 and 1,2-isomers were generally not separable by column chromatography, but could be isolated in pure form using HPLC.
The variation in 1,2 : 1,1 selectivity as a function of substrate may be due to differing interactions of polar functional groups within the substrate with the palladium catalyst.
Table 7.
Substrate Scope for 1,1-Arylbromination29
 | ||||
|---|---|---|---|---|
| Entry | Alkene | Major Product | Yielda | 1,2 : 1,1a |
| 1 |  |
 |
66% (56%) | 1 : >20 |
| 2 | 85% (70%) | 1 : 15 | ||
| 3 |  |
70% (67%) | 1 : >20 | |
| 4 | 88% (60%) | 1 : >20 | ||
| 5 |  |
 |
79% (69%) | 1 : >20 |
| 6 |  |
 |
50% (41%)b | 1 : >20 |
| 7 |  |
 |
80% (68%) | 1 : >20 |
| 8 | 64% (45%) | 1:>20 | ||
Yield and selectivity determined by 1H NMR spectroscopic analysis of crude reaction mixture. Isolated yields are in parentheses.
Approximately 10% of the Heck byproduct was observed by crude NMR.
Mechanistic Investigations: General Goals
We next turned our efforts to investigating the mechanism of these transformations. In particular, we sought to: (i) interrogate the proposed reaction pathways for formation of the 1,2- and the 1,1-products, (ii) understand the role of oxidant and solvent in imparting 1,2- versus 1,1 selectivity, (iii) gain mechanistic insights into the high selectivity for benzylic functionalization with CuCl2 and CuBr2 in Et2O, and (iv) probe the stereochemical course of the key carbon-halogen bond-forming step of these transformations. The results of these mechanistic studies are detailed below, and their implications are discussed later in the manuscript.
Deuterium Labeling
Deuterium labeled 1-octene-(1,1-d2) was utilized as a substrate for both the 1,2- and 1,1-phenylhalogenations. Reaction with PhICl2 or with CuBr2/THF afforded 40a–Cl/Br as the sole 1,2-phenylhalogenated products (eq. 7). Less than 5% deuterium incorporation was observed at any other site along the alkyl chain.
 |
(7) |
The reaction of 1-octene-(1,1-d2) was also examined with CuCl2 and CuBr2/Et2O. In both cases, 40b–Cl/Br was the only 1,1-phenylhalogenated product formed (eq. 8). Again, less than 5% of other isomers were observed, indicating clean migration of the deuterium from the 1- to the 2-position.8
 |
(8) |
4-(4-Chlorophenyl)-1-butene
The reactivity of 4-(4-chlorophenyl)-1-butene (33) was examined under both 1,2- and 1,1-arylhalogenation conditions. With CuCl2 and CuBr2/Et2O, mixtures of two isomeric products were formed (eq. 9). In both cases, the major isomer was the expected 1,1-product 41-Cl/Br. However, a minor amount of a second benzylic halogenation product 42-Cl/Br was also observed. The ratio of 41-Cl : 42-Cl was 4 : 1 in the chlorination reaction and the ratio of 41-Br : 42-Br was 7 : 1 under the bromination conditions as determined by 1H NMR spectroscopic analysis of the crude reaction mixtures.
 |
(9) |
The use of PhICl2 or CuBr2/THF as the oxidant produced 1,2-arylhalogenated products 43-Cl/Br in 62/81% crude yield (eq. 10). The analogous 1,1-products were also formed (17/4% crude yield) along with traces (2/2%) of the 1,4-arylfunctionalized compounds 42-Cl/Br (eq. 10). Notably, isomers derived from chlorination/bromination at other sites in the middle of the alkyl chain were not detected in these transformations.
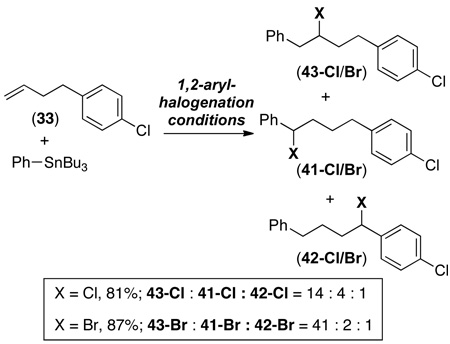 |
(10) |
Styrene Substrates
The Pd-catalyzed reaction of 4-fluorostyrene with PhSnBu3 under 1,2-arylhalogenation conditions afforded the expected 1,2-isomer (44-Cl/Br) with >20 : 1 selectivity (Table 8, entries 1 and 3). Intriguingly, the 1,2-product 44-Cl/Br was also favored in this transformation with CuCl2 or CuBr2/Et2O as the oxidant (selectivity = 1.4 : 1 in the chlorination and 9 : 1 in the bromination reaction) (Table 8, entries 2 and 4). This experiment was repeated with styrene as the alkene substrate and (p-FC6H4)SnBu3, and, again, 1,2-arylhalogenated products predominated in all four cases (Table 8, entries 5–8).
Table 8.
Reactions of Styrene Substrates
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Oxidant/Solvent | X | Y | Z | Yield | Ratio 1,2 : 1,1 |
| 1 | PhICl2/CH2Cl2 | Cl | F | H | 50%a | >20 : 1a |
| 2 | CuCl2/Et2O | Cl | F | H | 48%b | 1.4 : 1b |
| 3c | CuBr2/THF | Br | F | H | 45%b | >20 : 1b |
| 4d | CuBr2/Et2O | Br | F | H | 69%b | 9 : 1b |
| 5 | PhICl2/CH2Cl2 | Cl | H | F | 79%a | > 20 : 1a |
| 6 | CuCl2/Et2O | Cl | H | F | 50%b | 2.2 : 1b |
| 7c | CuBr2/THF | Br | H | F | 30%b | > 20 : 1b |
| 8d | CuBr2/Et2O | Br | H | F | 50%b | 24 : 1b |
Yield and selectivity determined based on isolated material.
Yield and selectivity determined by 1H NMR spectroscopic analysis of crude reaction mixture.
Reaction conditions were optimized to −78 °C to 25 °C with 2 equiv of organostannane to increase the yield – there was no difference in selectivity compared to the reaction at 0 °C.
Pd(acac)2 used as [Pd].
Vinylnaphthalene Substrate
The Pd-catalyzed reaction between vinylnaphthalene and (p-FC6H4)SnBu3 was also explored, and the 1,2-arylhalogenation product 46-Cl/Br was the major isomer observed under all four sets of conditions (Table 9).30 The regioselectivity with CuCl2 and CuBr2/Et2O increased significantly in comparison to that observed in the corresponding reactions of styrene. For example, upon changing from styrene to vinylnaphthalene, the 1,2 : 1,1 selectivity changed from 2.2 : 1 to >50 : 1 for chlorination with CuCl2 and from 24 : 1 to >50 : 1 for bromination with CuBr2/Et2O.
Table 9.
Reactions of Vinylnaphthalene
 | ||||
|---|---|---|---|---|
| Entry | Oxidant/Solvent | X | Yielda | Ratio 1,2 : 1,1a |
| 1 | PhICl2/CH2Cl2 | Cl | 60%b | > 50 :1 |
| 2 | CuCl2/Et2O | Cl | 76% | > 50 : 1 |
| 3c | CuBr2/THF | Br | 12%d | > 50 : 1 |
| 4c | CuBr2/Et2O | Br | 48%d | > 50 : 1 |
Yield and selectivity determined by 1H NMR spectroscopic analysis of crude reaction mixture.
Mass balance in 1,2-chlorination is accounted for by formation of 25% of the 1,2-dichloro product.
Pd(acac)2 used as [Pd].
Mass balance in arylbromination reactions is predominantly accounted for by incomplete conversion (15% conversion of vinylnaphthalene in entry 3 and 70% conversion of vinylnaphthylene in entry 4).
Solvent Effects in Arylbromination
To explore the role of solvent in the 1,1- versus 1,2-arylbrominations with CuBr2/THF, the reactions were examined in 2-methyltetrahydrofuran (Me-THF) and 2,5-dimethyltetrahydrofuran (Me2-THF). As shown in Table 10, changing the solvent resulted in a dramatic decrease in selectivity for the 1,2-product. With Me-THF, a 1.5 : 1 ratio of 28a : 28b was obtained, with similar results (28a : 28b = 1.8 : 1) in Me2-THF.
Table 10.
Effect of Substituted THF-Derivatives on 1,2- versus 1,1-Phenylbromination with CuBr2
Yield and selectivity determined by 1H NMR spectroscopic analysis of crude reaction mixture.
Me-THF = 2-methyltetrahydrofuran.
Me2-THF = 2,5-dimethyltetrahydrofuran.
These reactions were also examined in a series of aprotic solvents with dielectric constants (ε) ranging from 7.58 to 2.25. As shown in Table 11, there is a rough correlation between product ratio and ε, with more of 1,2-product 28a generally formed in higher dielectric solvents.
Table 11.
Effect of Aprotic Solvents on 1,2 versus 1,1 Arylbromination with CuBr2a
 | ||||
|---|---|---|---|---|
| Entry | Solvent | ε | Yieldb | Ratio 1,2 : 1,1b |
| 1 | THF | 7.58 | 49%c | 3.4 : 1 |
| 2 | 1,2 Dimethoxyethane | 7.2 | 14% | > 20:1 |
| 3 | EtOAc | 6.02 | 63% | 1 : 8 |
| 4 | CHCl3 | 4.81 | 45% | 1: > 20 |
| 5 | Et2O | 4.33 | 82% | 1 : 24 |
| 6 | Anisole | 4.33 | 23%d | 1: > 20 |
| 7 | iPr2O | 3.9 | 76% | 1 : 5.9 |
| 8 | MTBE | 2.6 | 56% | 1: > 20 |
| 9 | Dioxane | 2.25 | 30% | 1 : 20 |
These reactions were performed at 0.032 M to maximize solubility and improve reproducibility across this broad range of solvents.
Determined by 1H NMR spectroscopy.
1.9 equiv PhSnBu3 used.
0 °C → 25 °C.
Stereochemistry
Finally, we examined the stereochemical outcome of these transformations with alkenes cis-48 and trans-48. With cis-48, all of the reactions produced arylfunctionalization product 49-Cl/Br as the major diastereomer with modest to excellent levels of selectivity (Table 12, entries 1–3).31 Compound 49-Cl/Br is the result of net syn addition of Ph and X across the alkene.31,32 With trans 48, the stereoselectivity was significantly diminished, particularly with PhICl2 (Table 12, entries 4–6).32 However, in all cases, the major product was diastereomer 50-Cl/Br, which is again the product of syn Ph/X addition.
Table 12.
Arylhalogenation of cis-48 and trans-48a
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Oxidant | Solvent | Yieldb,c | Ratio 49 : 50b |
| 1 | cis-48 | PhICl2 | CH2Cl2 | 40% | 12 : 1 |
| 2 | cis-48 | CuCl2 | Et2O | 51% | >30 : 1 |
| 3 | cis-48 | CuBr2 | Et2O | 41% | 5 : 1 |
| 4 | trans-48 | PhICl2 | CH2Cl2 | 21% | 1 : 1.6 |
| 5 | trans-48 | CuCl2 | Et2O | 45% | 1 : 8 |
| 6 | trans-48 | CuBr2 | Et2O | 9% | 1 : 8 |
Conditions: 2 equiv PhSnBu3, 10 mol % PdCl2(PhCN)2, 0.032 M, −78 → 23 °C, 36 h.
Yield and selectivity determined by 1H NMR spectroscopic analysis of crude reaction mixture.
The mass balance in these reactions is accounted for by recovered starting material as well as the corresponding Mizoroki-Heck product. See Supporting Information for full details.
Discussion
1,2-Arylhalogenation Reactions
All of the mechanistic experiments presented above are consistent with the mechanism proposed in Scheme 1. In this scenario, 1,2-arylhalogenation products are generated via oxidative halogenation of Mizoroki-Heck intermediate A. The deuterium labeling studies in eq. 7 are in line with this proposal, as they show clean formation of 40-Cl/Br without accompanying migration of the deuterium label.
The 1,2-arylbrominations are particularly intriguing because simply moving from THF to Et2O reverses product selectivity. We were thus very interested to understand the role of solvent in these transformations. As shown in Table 10, changing the solvent from THF to Me-THF or Me2-THF resulted in a steep drop in selectivity for the 1,2-product 28a. Me-THF and Me2-THF are expected to have very similar polarity but significantly poorer coordinating capabilities than THF.33 Thus, this result suggests that THF coordination (either to Pd34 or Cu35) may play a key role in switching the selectivity in this system. The results in Table 11 suggest that solvent polarity may also be an important factor for selectivity, with more polar solvents favoring the 1,2 product. Polar coordinating solvents are likely to enhance the solubility of CuBr2, which should increase the rate of oxidative bromination relative to that of β-hydride elimination, thereby providing improved selectivity for 1,2-product 28a.
1,1-Arylhalogenation Reactions
These mechanistic studies provide evidence that the 1,1-products are derived from the β-hydride elimination/reinsertion/oxidation mechanism shown in Scheme 1 and in Scheme 2, path 1. The migration of deuterium from the 1 to the 2 position to generate 40b-Cl/Br (eq. 8) is fully consistent with this mechanism. In addition, the formation of 1,4-addition product 42-Cl/Br from the reactions of 4-(4-chlorophenyl)-1-butene with CuX2/ether (eq. 9) provides strong evidence for equilibrating β-hydride elimination/reinsertion steps prior to oxidative cleavage.
Scheme 2.
Two Possible Mechanisms for Formation of 1,1-Products
A possible alternative pathway involving a standard Mizoroki-Heck mechanism followed by hydrohalogenation of the resulting alkene (Scheme 2, path 2) can be ruled out based on the data in Table 8. In path 2, the reaction of styrene/(p-FC6H4)SnBu3 should produce the same alkene product as that of 4-fluorostyrene/PhSnBu3. Accordingly, the mechanism in path 2 should afford identical mixtures of 44 to 45 for both reactions. However, as shown in Table 8, the ratio of 44 : 45 changed depending on the alkene/stannane used (entries 2 versus 6 and 4 versus 8).
Selective 1,1-arylfunctionalization with CuX2/ether appears to derive from a preference for oxidative functionalization of PdII–benzyl intermediates (like E) versus PdII–alkyl intermediates (like A) (Scheme 2). This preference is reflected in the sole formation of 42 and 41 (as opposed to isomers resulting from halogenation at other sites along the alkyl chain) in reactions of 4-(4-chlorophenyl)-1-butene with CuX2/ether (eq. 9). Additionally, the observation that 1,2-products predominate in the functionalization of styrene derivatives with CuX2/ether (Table 8) is consistent with fast oxidative functionalization of an initially formed PdII-benzyl species in these systems.
We hypothesize that the selectivity for Pd-benzyl functionalization under equilibrating conditions derives from a stabilizing π-benzyl interaction (F-π, eq. 11). This proposal is supported by the experiments in Table 8 and Table 9, which compare CuX2/ether reactions of styrene to those of vinylnaphthalene. With both CuCl2 and CuBr2, the selectivity for 1,2-functionalization increases significantly upon moving from styrene to vinylnaphthalene (from 2.2 : 1 to >50 : 1 with CuCl2, and from 24 : 1 to >50 : 1 with CuBr2). In both cases, initial alkene insertion should generate a PdII-benzyl intermediate F-σ/π (eq. 11) or PdII-naphthyl intermediate G-σ/π (eq. 12). Thus, the increase in 1,2-product with vinylnaphthalene suggests that kOx/kβ-H (where kOx = rate constant for direct oxidative functionalization and kβ-H = rate constant for β-hydride elimination) is significantly larger for intermediate G than for F. Importantly, this is consistent with literature precedent, which has shown that π-naphthyl complexes are more kinetically reactive towards nucleophilic functionalization than their π-benzyl counterparts.36
 |
(11) |
 |
(12) |
Stereochemistry
The stereochemical course of oxidatively-induced sp3-carbon–halogen bond-formation is of interest from a fundamental mechanistic perspective. Additionally, the future development of asymmetric arylfunctionalization reactions hinges upon the ability to achieve clean retention or inversion of stereochemistry at carbon during C–X coupling. Without high stereochemical fidelity in this step, the new stereocenter will be eroded over the course of the reaction.
As summarized in Table 12, the arylfunctionalization of cis-48 and trans-48 favors products of syn addition. The insertion of alkenes into Pd–Ph bonds is well-known to occur with high syn-selectivity.37 Thus, this result indicates that C–X bond-formation occurs primarily with retention of configuration at carbon (eq. 13).
 |
(13) |
Carbon–halogen formation most likely occurs via initial 1 or 2 e−oxidation of PdII–alkyl species A with PhICl2 or CuX2 to form a PdIV or PdIII intermediate.38,39,40 The observed retention of configuration is consistent with direct C–X bond-forming reductive elimination from this high oxidation state Pd center (eq. 14, path 1)41 or with a phenyl group assistance mechanism involving double inversion (eq, 14, path 2).41b Both of these pathways have been proposed in the literature for related transformations.41
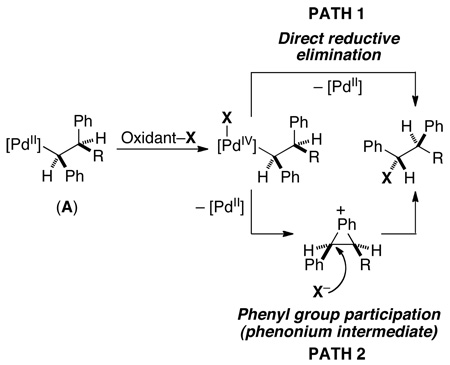 |
(14) |
While syn addition predominated in every case, modest levels of stereospecificity were observed in some reactions, particularly with trans-48 and PhICl2 (Table 12, entry 4). There are several potential explanations for these observations. First, selectivity would be eroded if oxidative functionalization occurred with competing retention and inversion of configuration at carbon. Literature studies of related oxidatively-induced carbon-heteroatom coupling reactions at Pd suggest that both retention41 and inversion42 are possible. Further, small modifications of the reaction conditions can sometimes lead to large changes in the stereochemical outcome.43 However, such competing pathways would most likely affect reactions of cis-48 and trans-48 to similar degrees and thereby provide similar levels of stereochemical erosion with both the cis and trans substrates. Thus, this explanation does not readily account for the dramatically different results for cis-48/PhICl2 (12 : 1 isomer ratio) versus trans-48/PhICl2 (1 : 1.6 isomer ratio).
A second possible explanation for formation of mixtures of diastereomers would be competing isomerization of the alkene starting material under the reaction conditions. This scenario would lead to an “apparent” erosion of stereospecificity even if C–X coupling proceeded with clean retention. Olefin isomerization is likely to be particularly problematic with trans-48, since it undergoes arylfunctionalization at a slower rate than cis-48.44 Thus, the observation of lower diastereoselectivities in reactions of trans-48 is consistent with a contribution from this pathway. In addition, 1H NMR spectroscopic analysis of the reaction between trans-48/PhSnBu3, and PhICl2 at 50% conversion revealed the presence of detectable quantities (2%) of cis-48. Furthermore, reaction of cis-48 at 23 °C with 2 equiv of PhICl2 and 2 equiv of PhSnBu3 resulted in 18% yield and an 8 : 1 ratio of 49-Cl : 50-Cl) along with 17% recovery of trans-48. These results clearly indicate that alkene isomerization can occur to a significant extent under the PhICl2/CH2Cl2 reaction conditions. While this side reaction undoubtedly needs to be addressed, the observation of good levels of stereospecificity with the cis substrates provides impetus for moving forward in the development of asymmetric versions of these transformations.
Summary
In summary, we have developed Pd-catalyzed reactions for the arylchlorination and arylbromination of α-olefins by oxidatively intercepting Mizoroki-Heck intermediate A (Scheme 1). The selectivity of these reactions can be rationally tuned by controlling the relative rates of oxidative functionalization versus β-hydride elimination from equilibrating PdII-alkyl species and by π-benzyl stabilization of PdII intermediates. This work provides a mechanistic basis for the future development of synthetically useful aryloxygenation, arylamination, arylhalogenation, and diarylation reactions using a variety of oxidants and transmetalating reagents. Additionally, the insights gained from these studies will be valuable for the design of enantioselective versions of these transformations. Work in all of these areas is ongoing in our laboratory and will be reported in due course.
Supplementary Material
Acknowledgements
This work was supported by NIH NIGMS (R01GM073836). We also gratefully acknowledge Boehringer Ingelheim and Bristol Myers Squibb for unrestricted funding. DK thanks Bristol Myers Squibb for a graduate fellowship. We thank Dr. Jeff Kampf for performing all of the X-ray crystallographic studies.
Footnotes
SUPPORTING INFORMATION AVAILABLE. Experimental details and spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Mizoroki T, Mori K, Ozaki A. Bull. Chem. Soc. Jpn. 1971;44:581. [Google Scholar]; (b) Heck RF, Nolley JP. J. Org. Chem. 1972;37:2320. [Google Scholar]; (c) Oestreich M, editor. The Mizoroki-Heck Reaction. Chichester, UK: John Wiley & Sons Ltd; 2009. [Google Scholar]; (d) Cabri W, Candiani I. Acc. Chem. Res. 1995;28:2. [Google Scholar]; (e) Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 2.(a) Link JT. The Intramolecular Heck Reaction. In: Overman LE, editor. Organic Reactions. Vol. 60. Hoboken, NJ: John Wiley & Sons, Inc; 2002. [Google Scholar]; (b) de Meijere A, Meyer FE. Angew. Chem. Int. Ed. Engl. 1994;33:2379. [Google Scholar]; (c) Brase S, de Meijere A. Cross-Coupling of Organyl Halides with Alkenes: the Heck Reaction. In: de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Second Ed. Weinheim, Germany: Wiley-VCH Verlag GmbH; 2004. [Google Scholar]; (d) Dounay AB, Overman LE. Chem. Rev. 2003;103:2945. doi: 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]; (e) Majumdar KC, Ansary I, Sinha B, Chattopadhyay B. Synthesis. 2009:3593. [Google Scholar]
- 3.For examples, see: Beletskaya IP, Cheprakov AV. Focus on Catalyst Development and Ligand Design. In: Oestreich M, editor. The Mizoroki-Heck Reaction. Chichester, UK: Wiley; 2009. Yoo KS, O'Neill J, Sakaguchi S, Giles R, Lee JH, Jung KW. J. Org. Chem. 2010;75:95. doi: 10.1021/jo901977n. Lamblin M, Nassar-Hardy L, Hierso J-C, Fouquet E, Felpin F-X. Adv. Synth. Catal. 2010;352:33.
- 4.For examples, see: Amatore C, Jutand A. J. Organomet. Chem. 1999;576:254. Knowles JP, Whiting A. Org. Biomol. Chem. 2007;5:31. doi: 10.1039/b611547k. Carrow BP, Hartwig JF. J. Am. Chem. Soc. 2010;132:79. doi: 10.1021/ja909306f.
- 5.For examples of CO insertion see: Sugihara T, Coperet C, Owczarczyk Z, Harring LS, Negishi E-i. J. Am. Chem. Soc. 1994;116:7923. Artman GD, III, Weinreb SM. Org. Lett. 2003;5:1523. doi: 10.1021/ol034314d. For examples of alkyne and alkene instertion see: Schweizer S, Song ZZ, Meyer FE, Parsons PJ, de Meijere A. Angew. Chem. Int. Ed. 1999;38:1452. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1452::AID-ANIE1452>3.0.CO;2-P. Tietze LF, Kahle K, Raschke T. Chem. Eur. J. 2002;8:401. doi: 10.1002/1521-3765(20020118)8:2<401::AID-CHEM401>3.0.CO;2-C.
- 6.For complementary routes to the 1,2-arylfunctionalization of alkenes, see: Wolfe JP. Synlett. 2008:2913. Chemler SR, Fuller PH. Chem. Soc. Rev. 2007;36:1153. doi: 10.1039/b607819m.
- 7.Heck RF. J. Am. Chem. Soc. 1968;90:5538. [Google Scholar]
- 8.For related Pd-catalyzed 1 1-diarylation reaction of terminal alkenes, see: Urkalan KB, Sigman MS. Angew. Chem. Int. Ed. 2009;48:3146. doi: 10.1002/anie.200900218.
- 9.(a) Tamaru Y, Hojo M, Higashimura H, Yoshida Z. Angew. Chem. Int. Ed. Engl. 1986;25:735. [Google Scholar]; (b) Tamaru Y, Hojo M, Kawamura S, Yoshida Z. J. Org. Chem. 1986;51:4089. [Google Scholar]
- 10.For a related Pd-catalyzed 1, 1-acetoxyarylation of α, β-unsaturated olefins, see: Rodriguez A, Moran WJ. Eur. J. Org. Chem. 2009:1313.
- 11.For synthesis of heterocycles via 1,1 arylfunctionalization reactions, see ref. 9b.
- 12.Parrish JP, Jung YC, Shin SI, Jung KW. J. Org. Chem. 2002;67:7127. doi: 10.1021/jo020159p. [DOI] [PubMed] [Google Scholar]
- 13.For a review on ligand directed C–H functionalization see: Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e.
- 14.(a) Giri R, Chen X, Yu J-Q. Angew. Chem. Int. Ed. 2005;44:2112. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]; (b) Kalyani D, Dick AR, Anani WQ, Sanford MS. Tetrahedron. 2006;62:11483. doi: 10.1021/ol060747f. [DOI] [PubMed] [Google Scholar]; (c) Giri R, Wasa M, Breazzano SP, Yu J-Q. Org. Lett. 2006;8:5685. doi: 10.1021/ol0618858. [DOI] [PubMed] [Google Scholar]; (d) Hull KL, Anani WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]
- 15.Thu H-Y, Yu W-Y, Che C-M. J. Am. Chem. Soc. 2006;128:9048. doi: 10.1021/ja062856v. [DOI] [PubMed] [Google Scholar]
- 16.(a) Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]; (b) Giri R, Liang J, Lei JQ, Li JJ, Wang DH, Chen X, Naggar IC, Guo C, Foxman BM, Yu JQ. Angew. Chem. Int. Ed. 2005;44:7420. doi: 10.1002/anie.200502767. [DOI] [PubMed] [Google Scholar]; (c) Desai LV, Malik HA, Sanford MS. Org. Lett. 2006;8:1141. doi: 10.1021/ol0530272. [DOI] [PubMed] [Google Scholar]; (d) Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]; (e) Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]; (f) Wang DH, Hao XS, Wu DF, Yu JQ. Org. Lett. 2006;8:3387. doi: 10.1021/ol061384m. [DOI] [PubMed] [Google Scholar]; (g) Neufeldt SR, Sanford MS. Org. Lett. 2010;12:532. doi: 10.1021/ol902720d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (b) ref. 16e. [Google Scholar]; (c) Giri R, Maugel N, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ. J. Am. Chem. Soc. 2007;129:3510. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; (d) Wasa M, Engle KM, Yu JQ. J. Am. Chem. Soc. 2009;131:9886. doi: 10.1021/ja903573p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Deprez NR, Sanford MS. J. Am. Chem. Soc. 2009;131:11234. doi: 10.1021/ja904116k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.For examples, see: Helaja J, Gottlich R. Chem. Commun. 2002:720. doi: 10.1039/b201209j. Lei A, Lu X, Liu G. Tetrahedron Lett. 2004;45:1785. Manzoni MR, Zabawa TP, Kasi D, Chemler SR. Organometallics. 2004;23:5618. Michael FE, Sibbald PA, Cochran BM. Org. Lett. 2008;10:793. doi: 10.1021/ol702922c. Wu T, Yin G, Liu G. J. Am. Chem. Soc. 2009;131:16354. doi: 10.1021/ja9076588. Christie SDR, Warrington AD, Lunniss CJ. Synthesis. 2009:148. Doroski TA, Cox MR, Morgan JB. Tetrahedron Lett. 2009;50:5162.
- 19.For examples, see: El-Qisairi A, Hamed O, Henry PM. J. Org. Chem. 1998;63:2790. doi: 10.1021/jo005627e. references therein. Hamed O, Henry PM. Organometallics. 1998;17:5184. El-Qisairi AK, Qaseer HA, Henry PM. J. Organomet. Chem. 2002;656:168. El-Qisairi AK, Qaseer HA, Katsigras G, Lorenzi P, Trivedi U, Tracz S, Hartman A, Miller JA, Henry PM. Org. Lett. 2003;5:439. doi: 10.1021/ol0273093.
- 20.For examples, see: Streuff J, Hovelmann CH, Nieger M, Muniz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y. Muniz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f. Muniz K, Hovelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a. Muniz K, Streuff J, Chavez P, Hovelmann CH. Chem. Asian J. 2008;3:1248. doi: 10.1002/asia.200800148. Sibbald PA, Michael FE. Org. Lett. 2009;11:1147. doi: 10.1021/ol9000087. Qiu S, Xu T, Zhou J, Guo Y, Liu G. J. Am. Chem. Soc. 2010;132:2856. doi: 10.1021/ja909716k.
- 21.For examples, see: Alexanian EJ, Lee C, Sorensen EJ. J. Am. Chem. Soc. 2005;127:7690. doi: 10.1021/ja051406k. Liu G, Stahl SS. J. Am. Chem. Soc. 2006;128:7179. doi: 10.1021/ja061706h. Desai LV, Sanford MS. Angew. Chem. Int. Ed. 2007;46:5737. doi: 10.1002/anie.200701454. Li Y, Song D, Dong VM. J. Am. Chem. Soc. 2008;130:2962. doi: 10.1021/ja711029u. Wang A, Jiang H, Chen H. J. Am. Chem. Soc. 2009;131:3846. doi: 10.1021/ja900213d. Wang W, Wang F, Shi M. Organometallics. 2010;29:928.
- 22.Sibbald PA, Rosewall CF, Swartz RD, Michael FE. J. Am. Chem. Soc. 2009;131:15945. doi: 10.1021/ja906915w. [DOI] [PubMed] [Google Scholar]
- 23.For a preliminary account of this work, see: Kalyani D, Sanford MS. J. Am. Chem. Soc. 2008;130:2150. doi: 10.1021/ja0782798.
- 24.PhSnBu3 was chosen as the arylating reagent because it undergoes facile transmetallation with palladium(II) under mild conditions in the absence of strong bases or other additives. Stille JK. Angew. Chem. Int. Ed. Engl. 1986;25:508.
- 25.The 1,1-arylchlorination products typically underwent some decomposition during isolation/chromatographic purification (as reflected in the discrepancy between the isolated yield and the yield determined from analysis of the crude reaction mixtures).
- 26.ArIBr2 generally lacks stability and cannot be isolated. It is typically generated in situ from PhI(OAc)2 and a bromide source. Macdonald TL, Narasimhan N. J. Org. Chem. 1985;50:5000. Stang PJ, Zhdankin VV. Chem. Rev. 1996;96:1123. doi: 10.1021/cr940424+. Braddock DC, Cansell G, Hermitage SA. Synlett. 2004:461. Karade NN, Shirodkar SG, Dhoot BM, Waghmare PB. J. Chem. Res. 2005:274.
- 27.NIS, CuI, and I2 did not produce appreciable yields of 1,2- or 1,1-aryliodinated products under any conditions examined.
- 28.The catalyst Pd(Cl)2(MeCN)2 afforded slightly better yields than Pd(Cl)2(PhCN)2 for arylbromination.
- 29.The 1,2 and 1,1-arylbromination products typically underwent some decomposition during isolation/chromatographic purification (as reflected in the discrepancy between the isolated yield and the yield determined from analysis of the crude reaction mixtures).
- 30.For analogous reactions between substituted stannanes and vinylnaphthalene/styrene, see Table S5.
- 31.The stereochemistry of 49-Cl/Br and 50-Cl/Br was determined by 1H NMR spectroscopy (based on chemical shift and coupling constant analysis) by analogy to several related products that were characterized by X-ray crystallography. See Supporting Information for complete details.
- 32.Analogous results are not reported for CuBr2/THF because this reaction was extremely poor yielding under these conditions.
- 33.Wax MJ, Bergman RG. J. Am. Chem. Soc. 1981;103:7028. [Google Scholar]
- 34.For examples of PdII(THF) complexes, see: Uson R, Fornies J, Tomas M, Menjon B. Organometallics. 1985;4:1912. Sperrle M, Gramlich V, Consiglio G. Organometallics. 1996;15:5196. Yagyu T, Hamada M, Osakada K, Yamamoto T. Organometallics. 2001;20:1087. Clegg W, Eastham GR, Elsegood MRJ, Heaton BT, Iggo JA, Tooze RP, Whyman R, Zacchini S. Organometallics. 2002;21:1832. Kim Y, Verkade JG. J. Organomet. Chem. 2003;669:32.
- 35.For examples of CuII(THF) complexes, see: Breeze SR, Wang S. Inorg. Chem. 1993;32:5981. Amel’chenkova EV, Denisova TO, Nefedov SE. Russ. J. Inorg. Chem. 2006;51:1218.
- 36.(a) Becker Y, Stille JK. J. Am. Chem. Soc. 1978;100:845. [Google Scholar]; (b) Johns AM, Utsunomiya M, Incarvito CD, Hartwig JF. J. Am. Chem. Soc. 2006;128:1828. doi: 10.1021/ja056003z. [DOI] [PubMed] [Google Scholar]; (c) Johns AM, Tye JW, Hartwig JF. J. Am. Chem. Soc. 2006;128:16010. doi: 10.1021/ja067084h. [DOI] [PubMed] [Google Scholar]; (d) ref. 8. [Google Scholar]
- 37.Heck RF. J. Am. Chem. Soc. 1969;91:6707. [Google Scholar]
- 38.For the oxidation of PdII starting materials to PdIV with PhICl2, see: Lagunas M-C, Gossage RA, Spek AL, van Koten G. Organometallics. 1998;17:731. Whitfield SR, Sanford MS. J. Am. Chem. Soc. 2007;129:15142. doi: 10.1021/ja077866q.
- 39.For the oxidation of PdII starting materials to PdIII-dimers with PhICl2, see: Powers DC, Ritter T. Nat. Chem. 2009;1:302. doi: 10.1038/nchem.246.
- 40.For generation of high oxidation state Pd intermediates by oxidation of PdII starting materials with 1 e− oxidants, see: Lanci MP, Remy MS, Kaminsky W, Mayer JM, Sanford MS. J. Am. Chem. Soc. 2009;131:15618. doi: 10.1021/ja905816q.
- 41.For examples of oxidatively-induced carbon-heteroatom bond-forming reactions at Pd with retention of stereochemistry, see ref. 21c and: Coulson DR. J. Am. Chem. Soc. 1969;91:200. Backvall JE, Nordberg RE. J. Am. Chem. Soc. 1980;102:393. Zhu G, Ma S, Lu X, Huang Q. J. Chem. Soc. Chem. Commun. 1995:271. Zhu G, Lu X. J. Organomet. Chem. 1996;508:83. Yin G, Liu G. Angew. Chem. Int. Ed. 2008;47:5442. doi: 10.1002/anie.200801438.
- 42.For examples of oxidatively-induced carbon-heteroatom bond-forming reactions at Pd with inversion of stereochemistry, see refs 20c, 21b, and: Backvall JE. Tetrahedron Lett. 1977:467. Backvall JE. Tetrahedron Lett. 1978:163. Backvall JE, Bjorkman EE. J. Org. Chem. 1980;45:2893. Tong X, Beller M, Tse MK. J. Am. Chem. Soc. 2007;129:4906. doi: 10.1021/ja070919j. Lyons TW, Sanford MS. Tetrahedron. 2009;65:3211. doi: 10.1016/j.tet.2008.10.107.
- 43.For an example of different stereochemical outcomes as a function of reaction conditions, see: Wong PK, Stille JK. J. Organomet. Chem. 1974;70:121.
- 44.See Supporting Information (Table S3) for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





