Abstract
Background
Breast cancer incidence is higher in US-born Hispanic women than foreign-born Hispanics, but no studies have examined how these rates have changed over time. To better inform cancer control efforts, we examined incidence trends by nativity and incidence patterns by neighborhood socioeconomic status (SES) and Hispanic enclave (neighborhoods with high proportions of Hispanics or Hispanic immigrants).
Methods
Information regarding all Hispanic women diagnosed with invasive breast cancer between 1988 and 2004 were obtained from the California Cancer Registry. Nativity was imputed from Social Security number for the 27% of cases with missing birthplace information. Neighborhood variables were developed from Census data.
Results
From 1988 to 2004, incidence rates for US-born Hispanics were parallel, but lower than, those of non-Hispanic whites, showing an annual 6% decline from 2002 to 2004. Foreign-born Hispanics had an annual 4% increase in incidence rates from 1995 to 1998 and a 1.4% decline thereafter. Rates were 38% higher for US- than foreign-born Hispanics, with elevations more pronounced for localized than regional/distant disease, and for women > 50 years of age. Residence in higher SES and lower Hispanic enclave neighborhoods were independently associated with higher incidence, with Hispanic enclave having a stronger association than SES.
Conclusions
Compared to foreign-born, US-born Hispanic women in California had higher prevalence of breast cancer risk factors, suggesting that incidence patterns largely reflects these differences in risk factors.
Impact
Further research is needed to separate the effects of individual- and neighborhood-level factors that impact incidence in this large and growing population.
INTRODUCTION
Hispanics or Latinos are the fastest growing and largest minority population in the United States (US) (1). They are composed of a diverse population from many countries (Mexico, Dominican Republic, Puerto Rico, Cuba, Costa Rica, Guatemala, Honduras, Nicaragua, Panama, El Salvador, Argentina, Bolivia, Chile, Colombia, Ecuador, Paraguay, Peru, Uruguay, and Venezuela) with 40% being foreign-born, according to the 2000 Census (2). Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death in Hispanic women, as in non-Hispanic white women in the US (3). Although incidence rates of invasive breast cancer are lower in Hispanic than non-Hispanic white women (88.3 per 100,000 vs. 134.0 per 100,000 person-years) (4), rates in Hispanics were previously found to increase more rapidly over time (1969–1987 (5), 1992–2000 (6)) than rates in non-Hispanic white women.
Breast cancer incidence in US-born Hispanics is higher than that in foreign-born Hispanics living in the US (7–9) or Hispanics living in Latin America (10, 11). These differences in breast cancer risk after migration to the US may relate to changes in lifestyle factors such as diet and physical activity, and reproduction (12). In a case-control study conducted in the San Francisco Bay Area, foreign-born Hispanics had a 50% lower risk of breast cancer than US-born Hispanics and lower prevalence of breast cancer risk factors, but their risk increased with length of residence in the US, younger age at migration and increasing level of acculturation (as measured by migration patterns and language usage) (13). Differences in the risk of breast cancer among US Hispanics by nativity were attenuated after adjustment for breast cancer risk factors among pre-menopausal women, but remained significantly higher among US-born post-menopausal women (13).
Neighborhood factors have been increasingly recognized to influence its residents’ diet, physical activity, and obesity levels (14–18), all documented risk factors for breast cancer. Although most studies have focused on neighborhood socioeconomic status (SES), with higher neighborhood SES associated with higher breast cancer incidence among Hispanics (19, 20), recent studies showed that, in Hispanics, living in a census tract with more immigrants was associated with lower consumption of high-fat foods and lower physical activity (14), and living in a census tract with more Hispanics was associated with a higher body mass index (16). No studies, to our knowledge, have investigated the independent effects of ethnic enclaves (neighborhoods with high proportions of Hispanics or Hispanic immigrants) and neighborhood SES on breast cancer incidence patterns.
To better inform cancer control efforts for the large and growing California Hispanic population, we examined: 1) breast cancer incidence trends over time by nativity (1988–2004), 2) breast cancer incidence patterns by neighborhood SES and Hispanic enclave (1998–2002), and 3) prevalence of breast cancer risk factors and measures of health care access from the Hispanic population in California to ecologically inform the observed patterns. Such an analysis has not been conducted, in part due to missing data regarding birthplace for a sizable proportion (~25%) of Hispanic cancer cases in cancer registry data (21, 22). Therefore, through statistical imputation of nativity for cancer cases with missing birthplace, and robust demographic methods to compute corresponding population estimates, we estimated invasive breast cancer incidence rates for California Hispanic women by nativity.
MATERIALS AND METHODS
Cancer cases
From the California Cancer Registry (CCR), we obtained information about all female California residents diagnosed with a first primary invasive breast cancer (International Classification of Disease for Oncology, 3rd Edition, (ICD-O-3) site codes C50.0–50.9) during the period January 1, 1988 through December 31, 2004. For each breast cancer case, we obtained registry information routinely abstracted from the medical record on age at diagnosis, sex, race/ethnicity, birthplace, stage at diagnosis (summarized as localized, regional, advanced, or unknown), and address of residence at the time of diagnosis, which in turn was geocoded by the CCR. Registry patient race/ethnicity and birthplace are collected primarily through self-report, by assumption of hospital personnel, or from inference through use of other information including race/ethnicity of parents, maiden name, surname, and birthplace, and from death records (22, 23). The North American Association of Central Cancer Registries Hispanic Identification Algorithm (NHIA) was used to improve classification of Hispanic ethnicity, which is based on surname, maiden names, and/or birthplace (24). For this study, 35,134, cases of NHIA-augmented Hispanic ethnicity, regardless of race, were identified.
To classify nativity (US- or foreign-born) of Hispanics, we used methods, published previously for Asians (25), involving two sources of birthplace information: 1) cancer registry-based data from medical records and/or death certificates (available for 70.4% (n=24,744) of cases: 92.4% from hospital medical records and 7.6% from death certificates); and 2) statistical imputation of nativity using the first five digits of the patient’s Social Security number (SSN) for those who had unknown birthplace (n=9,539, 27.2% of patients). Based on the first five digits of SSNs, which are linked to the state and year of issuance (26, 27), we imputed nativity as follows: we considered cases who received their SSN before age 21 years as US-born, and those who received their SSN at age ≥21 years as foreign-born. This age cut-point was determined from comparison to self-reported nativity in a series of previously interviewed Hispanic cancer cases (N=1,277) (21), and maximization of predictive value and minimization of misclassification based on receiver operating characteristic curves. There were 851 cases (2.4%) with missing or invalid SSNs and they were assigned a nativity based on the age nativity distribution of the overall sample.
Neighborhood socioeconomic status and Hispanic enclave
We assigned a neighborhood measure of SES for the 96% of cases whose residential address at diagnosis could be accurately geocoded to a census tract. Cases that could not be precisely geocoded to a census tract were randomly assigned to a census tract within their county of residence. The measure assigned is a previously-described index that incorporates 2000 Census data on education, income, occupation, and housing costs, and has previously been associated with breast cancer incidence (19). Each breast cancer patient was assigned a neighborhood SES quintile based on the distribution of SES across census tracts in California. Because cancer registries do not collect individual-level information on patient SES, we could not assess multilevel effects of SES. To each breast cancer case, we also assigned a neighborhood Hispanic enclave index that was based on 2000 Census variables (% linguistically isolated, % linguistically isolated who speak Spanish, % speaking limited English, % speaking limited English who spoke Spanish, % of recent immigrants, % Hispanic, and % foreign-born) and derived using principal components analysis across block groups. This composite index explained 68% of the variability in the data. Block group values were averaged across census tracts.
General population data
From the 1990 and 2000 Census Summary File 3 (SF-3), we obtained population counts by sex, race/ethnicity, nativity, and five-year age group for the state of California. Data from the 20% Integrated Public-Use Microdata Sample of the Census also were used to estimate age- and nativity-specific population counts for Hispanics (28) by smoothing with a spline-based function. For inter- and post-censal years, we estimated the percent foreign-born using cohort component interpolation and extrapolation methods, adjusting estimates to the populations by age and year provided by the California Department of Finance for years 1988–1989 and by the US Census for years 1990–2004 due to data availability. Analyses of neighborhood SES and Hispanic enclave were limited to the 1998 to 2002 diagnosis years due to the availability of population information from the 2000 US Census.
Statistical analysis
SEER*Stat software (29) was used to compute age-adjusted incidence rates (standardized to the 2000 US standard million population) and 95% confidence intervals (CIs) for invasive breast cancer. We calculated incidence rate ratios (IRRs) comparing rates between US and foreign-born Hispanics. Joinpoint Regression software (30) was used to calculate the annual percent changes (APC) during the period 1988 and 2004. We calculated APCs by fitting a series of least squares regression lines to the natural logarithm of the age-adjusted incidence rates, using calendar year as a regression variable (30).
Age-adjusted prevalence estimates of selected breast cancer risk factors and health care access characteristics among Hispanic women in California by nativity were obtained from the 2001 and 2003 California Health Interview Surveys (CHIS) using the internet-based AskCHIS application. CHIS is a telephone survey of a geographically stratified sample, identified through random-digit-dialing that over-sampled under-represented geographic areas and racial/ethnic groups. The 2001 CHIS sample, which included more than 55,000 households and was conducted in multiple languages, including Spanish, represents the geographic and racial/ethnic diversity of California. For households that completed a screening interview (59.2% of the sample), the response rate for the adult 2001 interview across the state was 63.7%, for an overall response rate of 37.7% (31). CHIS prevalence estimates are weighted to the California Department of Finance estimates of the number of residents in each California county by age, race and sex, and the 2000 Census of Population counts from the US Census Bureau.
This project was approved by the institutional review board of the Northern California Cancer Center.
RESULTS
Foreign-born Hispanic women accounted for nearly half of the 35,134 Hispanic women diagnosed with invasive breast cancer in California from 1988 to 2004 (Table 1). They were more likely to be diagnosed with regional/distant disease and larger tumors (≥ 2 cm), and a higher proportion had unknown estrogen receptor status than US-born Hispanics.
Table 1.
Characteristics of Hispanic women diagnosed with invasive breast cancer by nativity, California, 1988–2004.
| Characteristics | Foreign-Born N=18,033 n (%) |
US-Born N=17,101 n (%) |
p-value |
|---|---|---|---|
| Age at diagnosis (years) | |||
| ≤44 | 4,590 (25.5) | 4,109 (24.0) | |
| 45–54 | 4,721 (26.2) | 4,363 (25.5) | |
| ≥ 55 | 8,722 (48.4) | 8,629 (50.5) | p <0.01 |
| Stage at diagnosis | |||
| Localized | 9,010 (50.0) | 9,340 (54.6) | |
| Regional | 7,133 (39.6) | 6,079 (35.5) | |
| Distant | 944 (5.2) | 865 (5.1) | |
| Unknown | 946 (5.2) | 817 (4.8) | p <0.01 |
| Histologic subtype | |||
| Ductal | 12,314 (68.3) | 11,987 (70.1) | |
| With lobular component | 2,159 (12.0) | 2,245 (13.1) | |
| Other | 3,560 (19.7) | 2,869 (16.8) | p <0.01 |
| Estrogen Receptor (ER) status* | |||
| ER positive | 6,864 (50.8) | 6,887 (56.1) | |
| ER negative | 3,050 (22.6) | 2,728 (22.2) | |
| Missing | 3,596 (26.6) | 2,652 (21.6) | p <0.01 |
| Tumor size | |||
| < 2 cm | 6,414 (35.6) | 7,162 (41.9) | |
| ≥ 2 cm | 9,921 (55.0) | 8,498 (49.7) | |
| Unknown | 1,698 (9.4) | 1,441 (8.4) | p <0.01 |
Limited to 25,777cases diagnosed from 1994–2004.
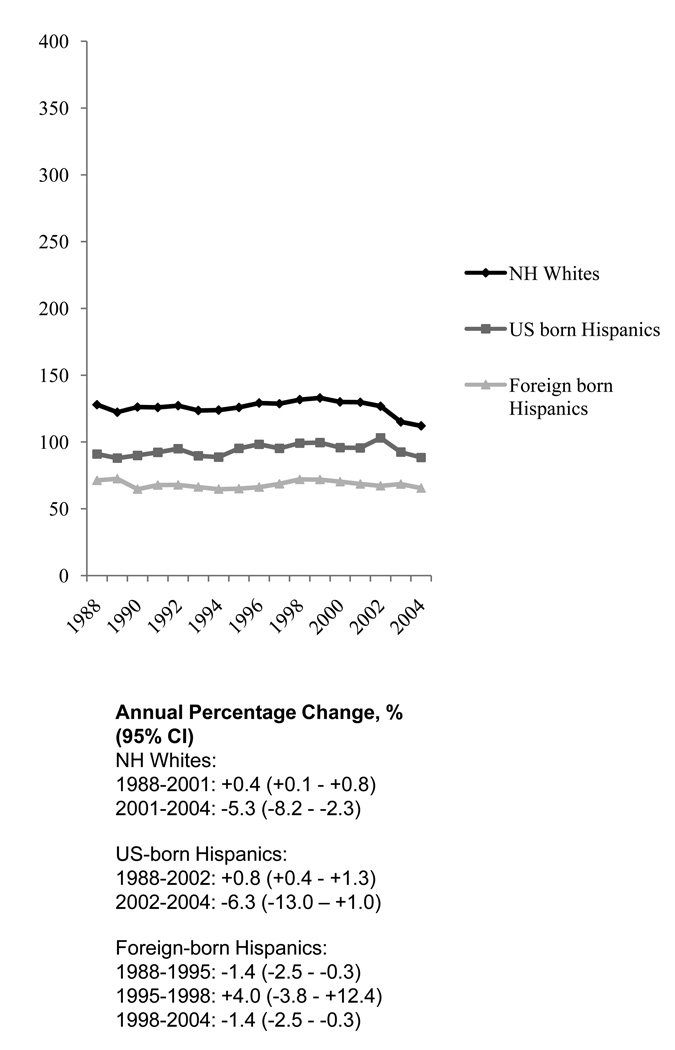
Over the period 1988–2004, non-Hispanic white women had incidence rates of invasive breast cancer that were 34% higher than US-born Hispanics and 84% higher than foreign-born Hispanics (Table 2; Figure 1). Overall, incidence rates were 38% higher in US-born than foreign-born Hispanic women, with a larger difference found for localized disease (45% higher) than for regional/distant disease (29% higher). The incidence rate of localized disease was 41% higher in US-born Hispanics and 24% higher in foreign-born Hispanics than rates of regional distant disease.
Table 2.
Age-adjusted incidence rates of invasive breast cancer by nativity in non-Hispanic (N-H) white and Hispanic women, California, 1988–2004: Incidence of breast cancer per 100,000 person-years, age-standardized to the 2000 United States Population, with 95% confidence intervals (95% CI).
| Race/ethnicity | Cases (N) | Rate | 95% CI | Rate Ratio* | 95% CI |
|---|---|---|---|---|---|
| N-H White† | 212,087 | 125.7 | 125.1–126.2 | ||
| Hispanic‡ | 35,134 | 78.3 | 77.4–79.1 | ||
| Foreign-born | 18,033 | 68.2 | 67.2–69.3 | reference | -- |
| US-born | 17,101 | 93.8 | 92.4–95.3 | 1.38 | 1.35–1.41 |
| Stage at diagnosis | |||||
| Localized | |||||
| Foreign-born | 9,010 | 35.7 | 35.0–36.5 | reference | |
| US-born | 9,340 | 52.0 | 50.9–53.1 | 1.45 | 1.41–1.50 |
| Regional/Distant | |||||
| Foreign-born | 8,077 | 28.8 | 28.1–29.5 | reference | |
| US-born | 6,944 | 37.0 | 36.1–37.9 | 1.29 | 1.24–1.33 |
| Unknown | |||||
| Foreign-born | 946 | 3.7 | 3.4–4.0 | reference | |
| US-born | 817 | 4.8 | 4.5–5.2 | 1.30 | 1.18–1.44 |
Ratio of the incidence rate in US-born to the incidence rate in foreign-born.
N-H White population was 142,863,244.
Foreign-born Hispanic population was 34,259,869; US-born Hispanic population was 45,755,983.
Figure 1.
Age-adjusted incidence rates of invasive breast cancer in non-Hispanic (N-H) white and Hispanic women by nativity, California, 1988–2004: incidence of Breast Cancer per 100,000 Person-Years, Age-standandized to the 2000 United States population.
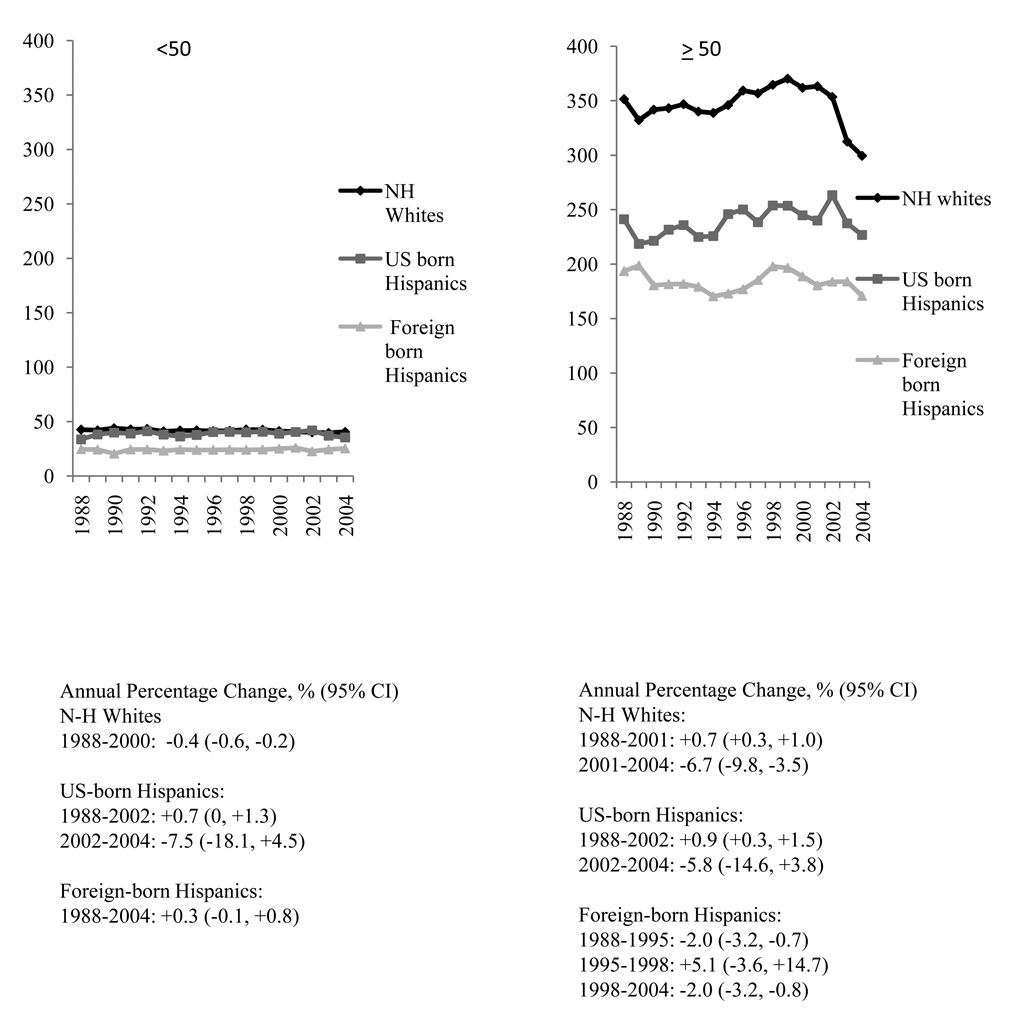
Yearly incidence trends were stable in Hispanic women < 50 years of age, with the lowest rates found in foreign-born Hispanics, and slightly higher, but similar rates in US-born Hispanics and non-Hispanic whites (Figure 2). In US-born Hispanics ≥ 50 years of age, rates increased by 0.9% from 1988 to 2002, and were stable thereafter (2002–2004). In foreign-born Hispanics, rates either decreased (1988–1995) or were stable (1995–1998) before decreasing by 2.0% from 1998 to 2004. To determine whether incidence trends were correlated with changes in the population-level prevalence of menopausal hormone therapy (HT) use, we limited analyses to women 50 to 74 years of age with estrogen receptor-positive invasive breast cancer; in this group, incidence rates significantly increased from 1994 to 2004 for both US-born (APC: +3.4, 95% CI: +1.9, +4.9) and foreign-born (APC: +3.2, 95% CI: +2.1, +4.3) Hispanics. Between 2001 and 2004, rates of estrogen receptor-positive breast cancer in non-Hispanic white women decreased dramatically, although not statistically significantly (APC: −6.3, 95% CI: −12.3, +0.2).
Figure 2.
Age-adjusted incidence rates of invasive breast cancer in non-Hispanic (N-H) white and Hispanic females by nativity and age group, California, 1988–2004: incidence of Breast Cancer per 100,000 Person-Years, Age-standardized to the 2000 United States population.
Stage-specific incidence rates (1998–2002) by neighborhood SES and Hispanic enclave are shown in Table 3. Incidence rates increased with each quintile increase in neighborhood SES and decrease in Hispanic enclave, with more pronounced differences found for localized compared to regional/distant stage disease. Rates in the highest versus lowest SES quintile and in the lowest versus highest Hispanic enclave quintile were 222% and 202% higher, respectively, for localized disease and 44% and 41% higher, respectively, for regional/distant disease. When both neighborhood SES and Hispanic enclave were considered together, Hispanic women in high-SES, low-enclave neighborhoods had 56% higher rates than women in low-SES, high-enclave neighborhoods; for localized disease and regional/distant disease, rates were 75% and 34% higher, respectively. Living in a Hispanic enclave appeared to be a more important predictor of breast cancer incidence rates than neighborhood SES, as incidence rate differences were larger moving from low to high Hispanic enclave neighborhoods than when moving from low to high SES neighborhoods, when holding the other variable constant.
Table 3.
Age-adjusted incidence rates of invasive breast cancer by neighborhood socioeconomic status (SES), Hispanic enclave and stage at diagnosis in Hispanic females (n=12,563), California, 1998–2002: Incidence of breast cancer per 100,000 person-years, age-standardized to the 2000 United States population, with 95% confidence intervals (95% CI).
| Race/ethnicity | Cases (N) | Rate | 95% CI | Rate Ratio* | 95% CI |
|---|---|---|---|---|---|
| SES (quintiles) | |||||
| All stages of diagnosis | |||||
| 1 (lowest) | 3,710 | 66.2 | 64.0–68.5 | Reference | |
| 2 | 3,129 | 80.8 | 77.8–83.8 | 1.22 | 1.16–1.28 |
| 3 | 2,474 | 88.3 | 84.7–92.0 | 1.33 | 1.26–1.41 |
| 4 | 1,899 | 103.1 | 98.4–108.0 | 1.56 | 1.47–1.65 |
| 5 (highest) | 1,351 | 118.8 | 112.3–125.6 | 1.79 | 1.68–1.92 |
| Localized stage at diagnosis | |||||
| 1 (lowest) | 1,743 | 32.3 | 30.7–33.9 | Reference | |
| 2 | 1,635 | 43.4 | 41.2–45.6 | 1.34 | 1.25–1.44 |
| 3 | 1,304 | 48.1 | 45.4–50.9 | 1.49 | 1.38–1.61 |
| 4 | 1,056 | 58.7 | 55.1–62.4 | 1.82 | 1.68–1.97 |
| 5 (highest) | 797 | 71.7 | 66.7–77.1 | 2.22 | 2.03–2.43 |
| Regional/distant stage at diagnosis | |||||
| 1 (lowest) | 1,727 | 29.4 | 28.0–30.9 | Reference | |
| 2 | 1,310 | 32.5 | 30.7–34.4 | 1.10 | 1.02–1.19 |
| 3 | 1,024 | 34.6 | 32.5–36.9 | 1.18 | 1.08–1.28 |
| 4 | 760 | 39.6 | 36.7–42.6 | 1.35 | 1.23–1.47 |
| 5 (highest) | 507 | 42.3 | 38.6–46.3 | 1.44 | 1.29–1.60 |
| Hispanic enclave index (quintile) | |||||
| All stages of diagnosis | |||||
| 1 (highest) | 4,333 | 67.5 | 65.4–69.6 | Reference | |
| 2 | 3,230 | 78.5 | 75.7–81.4 | 1.16 | 1.11–1.22 |
| 3 | 2,264 | 100.2 | 95.9–104.6 | 1.48 | 1.41–1.56 |
| 4 | 1,598 | 105.9 | 100.6–111.5 | 1.57 | 1.48–1.67 |
| 5 (lowest) | 1,138 | 121.0 | 113.8–128.5 | 1.79 | 1.67–1.92 |
| Localized stage at diagnosis | |||||
| 1 (highest) | 2,125 | 34.5 | 32.9–36.0 | Reference | |
| 2 | 1,668 | 41.6 | 39.4–43.7 | 1.21 | 1.13–1.29 |
| 3 | 1,221 | 55.7 | 52.5–59.0 | 1.62 | 1.50–1.74 |
| 4 | 887 | 59.8 | 55.8–64.0 | 1.74 | 1.60–1.88 |
| 5 (lowest) | 634 | 69.6 | 64.1–75.5 | 2.02 | 1.84–2.22 |
| Regional/distant stage at diagnosis | |||||
| 1 (highest) | 1,985 | 29.5 | 28.1–30.9 | Reference | |
| 2 | 1,387 | 32.4 | 30.7–34.3 | 1.10 | 1.02–1.18 |
| 3 | 915 | 38.5 | 35.9–41.2 | 1.31 | 1.20–1.42 |
| 4 | 621 | 39.6 | 36.4–43.0 | 1.34 | 1.22–1.48 |
| 5 (lowest) | 420 | 41.6 | 37.5–45.9 | 1.41 | 1.26–1.58 |
| Combined SES and Hispanic enclave † | |||||
| All stages of diagnosis | |||||
| Low SES, high enclave | 5,948 | 69.5 | 67.7–71.4 | Reference | |
| High SES, low enclave | 4,109 | 108.5 | 105.1–112.0 | 1.56 | 1.50–1.63 |
| Low SES, low enclave | 891 | 96.7 | 90.1–103.5 | 1.39 | 1.29–1.50 |
| High SES, high enclave | 1,615 | 81.2 | 77.2–85.5 | 1.17 | 1.10–1.24 |
| Localized stage at diagnosis | |||||
| Low SES, high enclave | 2,920 | 35.3 | 34.0–36.7 | Reference | |
| High SES, low enclave | 2,284 | 62.0 | 59.4–64.7 | 1.75 | 1.66–1.86 |
| Low SES, low enclave | 458 | 50.8 | 46.0–55.8 | 1.44 | 1.29–1.59 |
| High SES, high enclave | 873 | 45.1 | 42.1–48.3 | 1.28 | 1.18–1.38 |
| Regional/distant stage at diagnosis | |||||
| Low SES, high enclave | 2,691 | 30.1 | 28.9–31.3 | Reference | |
| High SES, low enclave | 1,610 | 40.3 | 38.3–42.5 | 1.34 | 1.25–1.43 |
| Low SES, low enclave | 346 | 35.9 | 32.0–40.0 | 1.19 | 1.05–1.34 |
| High SES, high enclave | 681 | 32.8 | 30.3–35.5 | 1.09 | 1.00–1.19 |
Ratio of the incidence rate in US-born to the incidence rate in foreign-born.
Low SES includes quintiles 1 and 2; high SES includes quintiles 3, 4 and 5; low enclave includes quintiles 3, 4 and 5; high enclave includes quintiles 1 and 2.
Population data from the 2001 CHIS (Table 4) show that US-born Hispanic female respondents were more likely than foreign-born Hispanics to have graduated from high school and college, to have a body mass index below 25 kg/m2 (women <50 years of age) or below 30 kg/m2 (women ≥ 50 years of age), to have been physically active in the past 30 days, to have reported consuming alcohol in the past month, particularly two or more drinks per day, have had menarche before 12 years of age, and to have had their first child after the age of 30 years or to be nulliparous. Significantly more US-born Hispanic women had reported undergoing mammographic screening within the last two years, and had a usual source of medical care. To estimate the prevalence of combined estrogen and progestin HT use for menopausal symptoms, we examined CHIS estimates of any HT use in non-pregnant women aged 40 years and older who did not report having a hysterectomy (32). Compared to foreign-born Hispanics, higher percentages of US-born Hispanic women used estrogen/ progestin-containing HT in 2001; however, use was much lower than that observed for non-Hispanic white women (24.5%, 95% CI: 23.4% – 25.6%). In 2003 CHIS data, the percentages of women using estrogen/ progestin-containing HT were comparable in US-born (8.1%, 95% CI: 4.5% – 11.6%) and foreign-born (6.1%, 95% CI: 4.1% – 8.2%) Hispanics, again lower than those in non-Hispanic white women (10.7%, 95% CI: 9.6% – 11.8%).
Table 4.
Prevalence (percentage, 95% confidence interval) of selected characteristics by nativity in California Hispanic women, California Health Interview Survey, 2001.
| Characteristics | Nativity |
|
|---|---|---|
| US-Born % (95% CI) |
Foreign-Born % (95% CI) |
|
| Education | ||
| Some high school or less | 24.0 (21.5–26.5) | 67.7 (65.9–69.6) |
| High school graduate | 32.9 (30.6–35.3) | 16.2 (14.8–17.7) |
| Some college | 30.5 (28.2–32.8) | 11.2 (10.0–12.5) |
| College graduate or higher | 12.6 (11.1–14.1) | 4.8 (4.1–5.5) |
| Body mass index <25 in women <50 years | 48.9 (45.9–52.0) | 39.7 (37.3–42.2) |
| Body mass index ≥30 in women ≥50 years | 31.5 (27.1–35.9) | 37.7 (32.8–42.7) |
| No vigorous/moderate physical activity in past 30 days | 31.9 (29.5–34.3) | 59.1 (57.1–61.2) |
| Drank alcohol in past month | 51.5 (48.9–54.1) | 28.3 (26.4–30.1) |
| ≥2 drinks/day* | 60.3 (56.8–63.7) | 39.2 (35.3–43.0) |
| Age at menarche <12 years | 30.3 (27.9–32.7) | 17.2 (15.6–18.8) |
| Age at first live birth ≥30 years | 5.9 (4.9–7.0) | 4.1 (3.5–4.8) |
| Number of live births | ||
| 0 | 30.5 (28.0–33.0) | 10.8 (9.5–12.2) |
| 1–2 | 36.7 (34.3–39.1) | 35.0 (33.0–37.0) |
| ≥3 | 32.8 (30.4–35.2) | 54.2 (52.1–56.3) |
| Mammogram screening history | ||
| 2 years or less | 55.3 (52.4–58.1) | 45.8 (43.5–48.2) |
| More than 2 years ago | 13.0 (11.0–15.1) | 11.6 (10.0–13.2) |
| Never had a mammogram | 31.7 ( 29.0–34.3) | 42.5 (40.2–44.9) |
| Type of usual source of medical care (adults) | ||
| Doctor’s office/ health maintenance organization/ Kaiser | 71.6 (69.1–74.0) | 41.3 (39.3–43.3) |
| Community clinic/ government clinic/ community hospital | 15.0 (13.1–17.0) | 35.3 (33.3–37.3) |
| Emergency Room/ urgent care | 1.2 (0.7–1.7) | 1.7 (1.1–2.2) |
| Some other place | 0.6 (0.2–1.0) | 0.5 (0.2–0.8) |
| No usual source of care | 11.6 (9.7–13.5) | 21.2 (19.4–23.0) |
| Currently takes hormone supplements for menopause symptoms | 28.3 (25.1–31.4) | 15.1 (13.1–17.2) |
| Among women without a hysterectomy** | 15.5 (12.4–18.5) | 10.1 (8.2–12.0) |
Among women who drank alcohol in the past month.
Among non-pregnant women aged 40 years and older who did not report having a hysterectomy
DISCUSSION
Over the 17-year study period from 1988 to 2004, incidence rates of invasive breast cancer were 38% higher for US-born Hispanic than foreign-born Hispanic women, with elevations more pronounced for localized than regional/distant stage disease. The greater incidence differential between US-born and foreign-born Hispanics for localized disease may reflect higher mammography screening rates (33) or higher levels of having a usual source of medical care among US-born Hispanics compared to foreign-born Hispanics (34), consistent with CHIS population data.
This is the first study, to our knowledge, to describe trends in breast cancer incidence over time in US- and foreign-born Hispanic women, as prior reports (7–9) examined incidence rates by nativity over a single time period. The trend analyses showed that, while differences in incidence between non-Hispanic whites and US-born and foreign-born Hispanics were consistent over the 17-year period, the differences were considerably more pronounced in women over 50 years of age, primarily post-menopausal women. In fact, among younger women, rates in US-born Hispanics were similar to those in non-Hispanic whites. As we have observed in US-born Asian women (25), age-specific incidence rates did not increase as rapidly in Hispanic women, regardless of nativity, as they did in non-Hispanic whites. In women over 50 years of age, the rates of invasive breast cancer decreased significantly after 1998 in foreign-born Hispanics and non-significantly after 2002 in US-born Hispanics. However, we did not observe decreases in the incidence of estrogen receptor positive breast cancer in US- or foreign-born Hispanics after 2002, as seen in non-Hispanic whites (32), even though the use of combined estrogen and progestin HT decreased by 48% in US-born Hispanics and 40% in foreign-born Hispanics from 2001 to 2003, possibly due to the lower use of HT in Hispanics.
Incidence differences by nativity may be due to differences in population distributions of breast cancer risk factors, as we found that US-born Hispanic women have a higher prevalence of certain risk factors, including advanced education, lower body mass index among women <50 years, nulliparity, late age at first birth, early menarche, alcohol consumption and use of estrogen/ progestin-containing HT for menopausal symptoms, as compared with foreign-born Hispanics. Other breast cancer risk factors, including physical inactivity and obesity in women ≥50 years, were less common in US-born Hispanics and are therefore unlikely to have contributed to the higher incidence rates of breast cancer in this group.
We also found that both living in a higher SES neighborhood and Hispanic enclave were important predictors of invasive breast cancer incidence rates in Hispanic women in California. Even though the two measures were highly correlated, neighborhood SES and living in a Hispanic enclave were independently associated with breast cancer incidence, with Hispanic enclave having a somewhat stronger association with breast cancer incidence than neighborhood SES. Our findings are consistent with a study using SEER cancer registry data that found higher breast cancer incidence (1988–1992) in Hispanic women living in US Census tracts with fewer Hispanics and higher incomes (35). Our neighborhood measures likely capture both individual and neighborhood components of SES and acculturation; however, as cancer registries do not collect individual-level data on education or other measures of SES, we could not distinguish between the individual and neighborhood effects. Case-control studies have shown that women living in high SES neighborhoods had an increased risk of developing breast cancer above and beyond their individual SES (36). Neighborhood SES may influence health through characteristics of the social (e.g., crime, social support, attitudes towards health), physical (e.g., pollution), and built (e.g., availability of health services, healthy food and recreation) environments of the neighborhood (37).
Living in a Hispanic enclave may be an indicator of low acculturation to the U.S., as Hispanic immigrants have been found to initially reside in segregated enclaves, and over time, intermingle with people of other race/ethnicities in the host country (38, 39). Residence in enclaves may also be an indicator of resource availability and/or social support. Immigrants living in enclaves may be more likely to maintain the advantageous health behaviors, such as a healthier diet (14, 17), if ethnic food sources or other resources, such as services in their native language, are more readily available. One study found that Mexican-Americans living in Census tracts with higher percentages of Mexican-Americans consumed more traditional foods (e.g., corn, tomatoes, and legumes) and less of certain foods (e.g., some fruits, broccoli) than their counterparts living in less concentrated tracts (40). There also may be fewer barriers in accessing medical care in areas with a high percentage of Hispanics (41). Indeed, one study found that Mexican American immigrants living in areas with more Spanish speakers or Hispanic immigrants had better access to health care; however, this association was not seen for US-born Mexican Americans living in these areas (42). On the other hand, Hispanic enclaves may be more likely to lack access to quality food and grocery stores (18), quality medical care (43) or a safe and walkable environment that promotes physical activity (14, 44). Our study lacked the population estimates for computing rates that account for both nativity and ethnic enclave, limiting our ability to differentiate these effects. Future research should differentiate the individual- and neighborhood-level effects, particularly the aspects of ethnic enclaves that positively or negatively affect breast cancer risk.
High acculturation to the U.S., as typically measured by duration of residence in the US and English language usage, has been found to be associated with a higher breast cancer risk in Hispanic women (13). Similarly, higher neighborhood SES has been associated with a higher incidence of breast cancer among Hispanics (19, 20). Both high acculturation and high SES are correlated with certain lifestyle and reproductive risk factors that are related to higher breast cancer risk, including earlier age at menarche, later age at first birth, nulliparity or fewer children, and later age at menopause (12, 13, 20). Additionally, higher physical activity has been associated with higher SES (45), while shorter duration of breast feeding, increased HT use, higher caloric intake, increased alcohol consumption, sedentary lifestyle, and larger body mass have been associated with higher acculturation (12, 13, 20).
The present study may be subject to some limitations. The imputation of immigrant status based on SSN, although an improvement over random assignment, is subject to some error. Given the similar sensitivity and specificity of the method, however, it is likely that misclassification as foreign-born was balanced with misclassification as US-born to produce accurate case counts overall for incidence rate calculations. The impact on overall case counts within groups defined by nativity is likely small, given that nativity was only imputed for 30% of cancer cases, and prior research showed high accuracy for cancer registry birthplace data (21, 22). Due to the sensitive nature of immigration status, it is also possible that undocumented immigrants may report being US-born rather than foreign-born; however, this would likely affect both our numerator and denominator, thus minimizing the effect of misclassification of nativity. Although only 2.4% of Hispanics in our study had missing or invalid SSNs, it is possible that some undocumented immigrants used false SSNs, as 2.8 million undocumented/illegal immigrants were estimated in California in 2006 (90% of these undocumented immigrants were from Latin America, with 65% from Mexico alone) (46). Due to the increasing population of undocumented immigrants, the Social Security Administration has been identifying mismatched SSNs; in 2008, they found 4% of reports to be mismatched (47). If false SSNs systematically classified foreign-born Hispanics as US-born, the incidence differences between foreign-born and US-born women observed here would be underestimated.
Our population estimates for Hispanics by nativity may also be subject to error, particularly for specific age-groups, which may have biased overall or age-adjusted incidence rates. While we relied on US Census data, the most definitive source of data for estimating populations, all population counts stratified by nativity are based on a sample of the population. To determine whether the assumptions underlying the methods we used for estimating annual populations were accurate, we compared our 2004 population estimates to those from the 2005 American Community Survey, a 2.3% stratified sample of the California population (48), and found a 1.0% and 2.3% difference between the population estimates for US-born and foreign-born Hispanics, respectively. Lastly, the incidence in foreign-born Hispanics could be underestimated if immigrants returned home for medical care and their cancer were diagnosed in another country.
Our cancer registry data on Hispanic ethnicity data may also be subject to misclassification. However, several studies have shown registry classification of Hispanic ethnicity to be good (~80% sensitivity and positive predictive values) (22, 49). Our use of the North American Association of Central Cancer Registries Hispanic Identification Algorithm allows us improve the identification of Hispanics (24), a group typically undercounted. In a recent study of breast cancer patients in the Los Angeles region, Hispanic classification using the NHIA algorithm, compared to self-report, had 97.7% sensitivity and 90.7% specificity (50). Although Hispanics are heterogeneous with regard to country of origin and cancer incidence rates have been found to vary for national subpopulations (11), we were not able to consider incidence trends in subpopulations because 56.8% Hispanics in our study did not have information on ancestry of origin. Our findings largely reflect the experience of Hispanics of Mexican descent, who comprise 77% of California’s Hispanic population (1). Indeed, our incidence rates (1999–2001) in foreign-born Hispanics (70.2/100,000 (95% CI: 67.9–72.6) were similar to rates from Pinheiro et al. in primarily first generation/foreign-born Mexicans (71.9/100,000 (95% CI: 53.1–95.2)) (11).
Our findings of consistently lower incidence rates of invasive breast cancer among foreign-born than US-born Hispanics suggest that nativity is a measure of acculturation that captures population distributions of breast cancer risk factors, and is important for assessing population-based patterns and trends in breast cancer incidence. In addition, our finding that foreign-born Hispanics were more likely to be diagnosed with later stage disease than US-born Hispanics suggests that measures of acculturation and SES also likely impact health care access and utilization of medical care. Future studies should not only examine individual-level measures of breast cancer risk factors and how they change with acculturation, but also examine individual- and neighborhood-level socioeconomic and cultural influences on health behaviors, health care access and utilization of medical care. A better understanding of breast cancer incidence rate variations in US-born and foreign-born Hispanic women will help identify modifiable risk factors relevant to breast cancer prevention in all women.
ACKNOWLEDGEMENTS
The authors thank Dr. Myles Cockburn, Dr. Tim Miller, Ms. Rita Leung, Ms. Sarah Shema, Ms. Laura Perez, Ms. Laura McClure, and Ms. Jane Pham for their help with this manuscript. This study was supported by a grant from the Safeway Foundation and a Surveillance, Epidemiology and End Results Rapid Response Surveillance Study under contract N01-PC-35136. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
REFERENCES
- 1.Guzman B. Census 2000 Brief. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2001. The Hispanic Population: 2000. [Google Scholar]
- 2.Malone N, Baluja KF, Costanzo JM, Davis CJ. Census 2000 Brief. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2003. The Foreign-Born Population: 2000. [Google Scholar]
- 3.O'Brien K, Cokkinides V, Jemal A, et al. Cancer statistics for Hispanics, 2003. CA Cancer J Clin. 2003;53:208–226. doi: 10.3322/canjclin.53.4.208. [DOI] [PubMed] [Google Scholar]
- 4.Horner M, Ries L, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 5.Eidson M, Becker TM, Wiggins CL, Key CR, Samet JM. Breast Cancer among Hispanics, American Indians and Non-Hispanic Whites in New Mexico. Int J Epidemiol. 1994;23:231–237. doi: 10.1093/ije/23.2.231. [DOI] [PubMed] [Google Scholar]
- 6.Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M. Trends in Breast Cancer by Race and Ethnicity. CA Cancer J Clin. 2003;53:342–355. doi: 10.3322/canjclin.53.6.342. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DB, Karagas MR. Cancer in First and Second Generation Americans. Cancer Res. 1987;47:5771–5776. [PubMed] [Google Scholar]
- 8.Pike MC, Kolonel LN, Henderson BE, et al. Breast cancer in a multiethnic cohort in Hawaii and Los Angeles: risk factor-adjusted incidence in Japanese equals and in Hawaiians exceeds that in whites. Cancer Epidemiol Biomarkers Prev. 2002;11:795–800. [PubMed] [Google Scholar]
- 9.Menck HR, Henderson BE, Pike MC, Mack T, Martin SP, SooHoo J. Cancer incidence in the Mexican-American. J Natl Cancer Inst. 1975;55:531–536. doi: 10.1093/jnci/55.3.531. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Facts & Figures for Hispanics/Latinos 2006–2008. Atlanta, Georgia: American Cancer Society; 2006. [Google Scholar]
- 11.Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162–2169. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 12.Andreeva VA, Unger JB, Pentz MA. Breast cancer among immigrants: a systematic review and new research directions. J Immigr Minor Health. 2007;9:307–322. doi: 10.1007/s10903-007-9037-y. [DOI] [PubMed] [Google Scholar]
- 13.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14:2905–2913. doi: 10.1158/1055-9965.EPI-05-0483. [DOI] [PubMed] [Google Scholar]
- 14.Osypuk TL, Roux AV, Hadley C, Kandula NR. Are immigrant enclaves healthy places to live? The Multi-ethnic Study of Atherosclerosis. Soc Sci Med. 2009;69:110–120. doi: 10.1016/j.socscimed.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health. 2003;93:1552–1558. doi: 10.2105/ajph.93.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do DP, Dubowitz T, Bird CE, Lurie N, Escarce JJ, Finch BK. Neighborhood context and ethnicity differences in body mass index: a multilevel analysis using the NHANES III survey (1988–1994) Econ Hum Biol. 2007;5:179–203. doi: 10.1016/j.ehb.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubowitz T, Subramanian SV, Acevedo-Garcia D, Osypuk TL, Peterson KE. Individual and neighborhood differences in diet among low-income foreign and U.S.-born women. Womens Health Issues. 2008;18:181–190. doi: 10.1016/j.whi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36:74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 20.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Yin R, Coull BA. Race/ethnicity and changing US socioeconomic gradients in breast cancer incidence: California and Massachusetts, 1978–2002 (United States) Cancer Causes Control. 2006;17:217–226. doi: 10.1007/s10552-005-0408-1. [DOI] [PubMed] [Google Scholar]
- 21.Gomez SL, Glaser SL. Quality of cancer registry birthplace data for Hispanics living in the United States. Cancer Causes Control. 2005;16:713–723. doi: 10.1007/s10552-005-0694-7. [DOI] [PubMed] [Google Scholar]
- 22.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–187. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 23.Gomez SL, Le GM, West DW, Satariano WA, O'Connor L. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. Am J Public Health. 2003;93:1685–1688. doi: 10.2105/ajph.93.10.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NAACCR Latino Research Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2] Springfield, IL: North American Association of Central Cancer Registries; 2005. Sep, [Google Scholar]
- 25.Gomez SL, Quach T, Horn-Ross P, et al. Uncovering hidden disparities: disaggregating breast cancer incidence rates in Asian women by immigrant status. Am J Public Health. 2010 doi: 10.2105/AJPH.2009.163931. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block G, Matanoski GM, Seltser RS. A method for estimating year of birth using social security number. Am J Epidemiol. 1983;118:377–395. doi: 10.1093/oxfordjournals.aje.a113645. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruggles S, Sobek M, Alexander T, et al. Integrated Public Use Microdata Series: version 4.0 [Machine-readable database] Minneapolis, MN: Minnesota Population Center; 2009. [producer and distributor] [Google Scholar]
- 29.Surveillance Research Program, National Cancer Institute SEER*Stat software. ( www.seer.cancer.gov/seerstat) Version 6.5.2.
- 30.Joinpoint Regression Program Version 3.3.1. Bethesda: National Cancer Institute; [Google Scholar]
- 31.California Health Interview Survey. The CHIS 2001 Sample: Response Rate and Representativeness. Los Angeles: UCLA Center for Health Policy Research; 2003. [Google Scholar]
- 32.Keegan TH, Chang ET, John EM, et al. Breast Cancer Res. Vol. 9. 2007. Recent changes in breast cancer incidence and risk factor prevalence in San Francisco Bay area and California women: 1988 to 2004; p. R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 34.Just The Facts: Immigrants and Health. Public Policy Institute of California; 2008. [Google Scholar]
- 35.Eschbach K, Mahnken JD, Goodwin JS. Neighborhood Composition and Incidence of Cancer among Hispanics in the United States. Cancer. 2005;103:1036–1044. doi: 10.1002/cncr.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster TF, Hoffman K, Weinberg J, Vieira V, Aschengrau A. Community- and individual-level socioeconomic status and breast cancer risk: multilevel modeling on Cape Cod, Massachusetts. Environ Health Perspect. 2008;116:1125–1129. doi: 10.1289/ehp.10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55:111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charles CZ. The dynamics of racial residential segregation. Ann Rev Sociol. 2003;29:167–207. [Google Scholar]
- 39.Massey DS, Denton NA. Spatial assimilation as a socioeconomic outcome. Am Soc Rev. 1985;50:94–106. [Google Scholar]
- 40.Reyes-Ortiz CA, Ju H, Eschbach K, Kuo YF, Goodwin JS. Neighbourhood ethnic composition and diet among Mexican-Americans. Public Health Nutr. 2009;12:2293–2301. doi: 10.1017/S1368980009005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas JS, Phillips KA, Sonneborn D, et al. Variation in access to health care for different racial/ethnic groups by the racial/ethnic composition of an individual's county of residence. Med Care. 2004;42:707–714. doi: 10.1097/01.mlr.0000129906.95881.83. [DOI] [PubMed] [Google Scholar]
- 42.Gresenz CR, Rogowski J, Escarce JJ. Community demographics and access to health care among U.S. Hispanics. Health Serv Res. 2009;44:1542–1562. doi: 10.1111/j.1475-6773.2009.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayanga AJ, Kaiser HE, Sinha R, Berenholtz SM, Makary M, Chang D. Residential segregation and access to surgical care by minority populations in US counties. J Am Coll Surg. 2009;208:1017–1022. doi: 10.1016/j.jamcollsurg.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 44.Moore LV, Diez Roux AV, Evenson KR, McGinn AP, Brines SJ. Availability of recreational resources in minority and low socioeconomic status areas. Am J Prev Med. 2008;34:16–22. doi: 10.1016/j.amepre.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen IH, Kaplan GA. Poverty area residence and changes in physical activity level: evidence from the Alameda County Study. Am J Public Health. 1998;88:1709–1712. doi: 10.2105/ajph.88.11.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Illegal Immigrants. Public Policy Institute of California; 2008. [Google Scholar]
- 47.False Social Security Numbers Used by Undocumented Workers. U.S. Immigration Support; 2009. [Google Scholar]
- 48.American Community Survey (ACS) Maryland: U.S: Census Bureau; 2005. [Google Scholar]
- 49.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18:2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]