Abstract
Lung diseases caused by bacteria are a leading cause of death in both immunocompromised and immunocompetent individuals as well as in children. Although neutrophil recruitment is critical to augment the host defence, excessive neutrophil accumulation results in life-threatening diseases, such as acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Therefore, it is important to modulate excessive neutrophil influx in ALI/ARDS to mitigate lung damage and mortality. A better understanding of the basic mechanisms underlying neutrophil influx is crucial to designing novel and innovative treatment strategies for ALI/ARDS. Recognition of bacteria in the lung is the critical first step leading to neutrophil influx. Pattern recognition receptors, such as Toll-like receptors and NOD-like receptors, play an important role in the recognition of bacterial pathogens. Understanding the molecular and cellular mechanisms associated with the recognition of bacterial pathogens by the host is critical for the development of effective therapeutic strategies to control parenchymal damage via modulating neutrophil accumulation in the lung.
Keywords: TLRs, NLRs, lung innate immunity, bacterial lung infection, acute lung injury, acute respiratory distress syndrome
INTRODUCTION
Bacterial infections in the lung represent an important cause of morbidity, mortality and healthcare expenditure. In fact, bacterial pneumonia is the third leading cause of mortality world-wide. Pneumonia is the result of overwhelming infection of micro-organisms such as viruses, bacteria, fungi and parasites. Neutrophil recruitment is the pathological hallmark of pneumonia caused by bacteria. Although neutrophils play an important role in the pulmonary host defence during infection, excessive neutrophil accumulation leads to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS).1,2 Therefore, the development of drugs that target the excessive neutrophil accumulation could slow the progression of lung damage, thereby decreasing mortality during bacterial pneumonia.
Infections at mucosal sites caused by a broad range of pathogens are primarily defended by the innate immune mechanisms. The key players of innate immunity are phagocytes, such as neutrophils, and macrophages. Recognition of the pathogen is the first step in a multistep paradigm leading to innate immunity in the lungs and this function is primarily performed by pattern recognition receptors, such as membrane bound Toll-like receptors (TLRs) and cytosolic receptors, such as nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), Rig I like receptors (RLR), C-lectin receptors (CLR) and Scavenger receptors. The TLRs were identified more than a decade ago, whereas cytosolic receptors have been only recently discovered.3 As a result, TLRs are the most extensively studied. In this article, we will review our current understanding of the TLRs and NLRs in the context of bacterial recognition in the lungs.
Neutrophils contribute to initial host defence in the lung during bacterial infections.4–6 Neutrophils are stored in bone marrow and are mobilised from the bone marrow into the circulation during infections. Neutrophil recruitment to the site of infection and inflammation is a multistep paradigm involving chemokine and cytokine production, up-regulation of cell adhesion molecules, margination of neutrophils, rolling and diapedesis.7 In the inflammatory foci, activated neutrophils phagocytose microbes, degranulate, generate free radicals and degrade the microbes. Although neutrophils serve as the primary defence, their short life-span (<6 h) renders it difficult to investigate molecular mechanisms upon interaction with bacterial pathogens. A recently described long-term bone-marrow culture system will advance our knowledge of neutrophil biology in the near future.8 Although neutrophils are considered as primary defenders of the innate immune system, a recent report has shown that neutrophil-derived interleukin (IL)-18, along with dendritic cell-derived IL-12, stimulate natural killer cells to release interferon (IFN)-γ,9 illustrating their role in the induction of adaptive immunity. In addition to the intracellular killing mechanisms of neutrophils, recent studies have shown that neutrophils form extracellular traps that can bind, confine and destroy extracellular bacteria (neutrophil extracellular traps; NETs). The NETs can be formed by activated neutrophils. In this context, it has been shown that LPS, phorbol myristate acetate (PMA) and chemokines, such as IL-8, can activate neutrophils to form NETs.10 These NETs are composed of chromatin meshwork containing several types of antimicrobial proteins from primary granules, including neutrophil elastase, cathepsin G and myeloperoxidase, secondary and tertiary granules, such as lacoferrins and gelatinase.10 Although the formation of NETs is an exciting mechanism to destroy extracellular pathogens, forming NETs may be deleterious to the host because of extracellular noxious components released from neutrophils which can induce severe lung damage.
Toll-like receptors
The TLRs play a pivotal role against a variety of exogenous and endogenous pathogens. The TLRs are type 1 integral membrane glycoproteins which have an extracellular LRR domain and signalling cytoplasmic domain homologous to IL-1 receptor (IL-1R) and termed Toll–IL-1 receptor (TIR) domain.11 There are 12 TLRs in mice and 10 TLRs in humans that have been reported so far.3 The TLRs function as homodimers with the exception of TLR2 which dimerises with TLR1 and TLR6 with different ligand specificity. The TLRs activate similar, but not identical, molecules as in the IL-1R signalling cascade.12 Stimulation of TLR ligands to their receptors recruit adaptor molecules to the cytoplasmic TIR domain and trigger the downstream signalling cascade, activation of NF-κB and production of pro-inflammatory cytokines and chemokines. TLR1, TLR2, TLR4, TLR5 and TLR6 are located on the cell surface and TLR3, TLR7 and TLR9 are located on the endosomal membrane. TLR1–TLR2 and TLR2–TLR6 heterodimers recognize lipoteichoic acid (LTA) and lipoproteins from Gram-positive bacteria and TLR4 recognizes lipopolysaccharide (LPS) of Gram-negative bacteria. TLR2 gene deficient mice showed high susceptibility to Staphylococcus aureus or Streptococcus pneumoniae upon challenge.13 However, it protects only partially against Legionella pneumophila.14–16 The TLR4 ligand LPS binds to TLR4 in association with LPS binding protein (LBP), CD14 and MD2.3,17 TLR4 plays an important role in the pulmonary immunity against Klebsiella pneumoniae,18 Haemophillus influenzae,19 and Strep. pneumoniae.20 During Acinetobacter baumanii21 and Pseudomonas aeruginosa22 infections, both TLR2 and TLR4 are shown to play an important role in the host defence. TLR5 is important for the recognition of flagellin, a constituent of flagella in motile bacteria. Mutations of TLR5 are associated with susceptibility to L. pneumophila23 infections. TLR3, TLR7 and TLR8 recognize viral RNAs and their synthetic analogues. TLR9 is stimulated by bacterial unmethylated CpG DNA and protects the host against Gram-negative as well as Gram-positive bacterial infections.24–26 Furthermore, TLR11 is important for the recognition of profilin and uropathogenic bacteria.3
Engagement of microbial components to the TLRs triggers the downstream signalling pathways and induction of genes involved in the host defence. There are five distinct adaptor molecules (MyD88, TRIF, TIRAP, TRAM and SARM) that are associated with TLR signalling which are recruited to the cytoplasmic TIR domain of the TLRs. Among the adaptor molecules, MyD88 is essential for almost all TLRs and it is recruited to the TIR domain by another adaptor molecule, TIRAP, in the TLR2 and TLR4 signalling cascade. TLR3 and TLR4 signalling involves the MyD88-independent pathway where the other adaptor, TRIF plays an essential role. TRAM is a key player in TRIF-mediated, MyD88-independent signalling. Recruitment of MyD88 facilitates the association of IL-1R associated kinases (IRAKs), IRAK4 and IRAK1, to the receptor complex; during this process, IRAK4 and IRAK1 become activated and promote the interaction of TRAF6 with the complex. This complex interacts again with another preformed complex comprising of TAK1, TAB1 and TAB2.3 This clustering activates IKK and, subsequently, activates NF-κB. Activation of TAK1 also activates mitogen-associated protein kinase (MAPK) and Janus kinase (JNK). This activation results in the expression of growth factors, chemokines and cytokines, and cell adhesion molecules. There are four different IRAKs (IRAK-1, IRAK-2, IRAK-M, and IRAK-4) that have been identified in mice and humans. Interestingly, recent studies have shown that IRAK-M serves as a negative regulator of TLR signalling and IRAK-M gene deficient mice show increased inflammatory response.27
Several studies have elucidated the roles of adaptor molecules involved in the TLR pathways, such as MyD88-dependent cascade (MyD88 and TIRAP) and MyD88-independent cascade (TRIF and TRAM) in bacterial infections. For example, MyD88 is essential for the host defence against Strep. pneumoniae,28aureus,29 Escherichia coli,30 K. pneumoniae,6 H. influenzae,31 P. aeruginosa,29,32 and L. pneumophila,15,33 whereas TIRAP, an upstream molecule in the MyD88 cascade, is important for pulmonary host defence against E. coli and K. pneumoniae.6,30 In general, MyD88 has been shown to be more critical for protection against bacterial infections than single or multiple TLRs (Table 1). Upon E. coli30 and P. aeruginosa34 challenge, TRIF plays an important role in the host defence. Regarding the relative importance of MyD88 and TRIF, MyD88 plays a much more important role than TRIF against K. pneumoniae.35 These findings suggest that pathogens utilize both MyD88-dependent and MyD88-independent cascades in the host via different bacterial components.
Table 1.
Role of innate immune molecules in acute respiratory bacterial infection
| Phenotypea |
|||||
|---|---|---|---|---|---|
| Infection | Survival | Neutrophil influxb | Bacterial burdenc | Bacterial disseminationd | |
| TLR | |||||
| TLR2 | Acinetobacter baumannii 21 | ↓ | ↓ | ND | ND |
| Legionella pneumophila 14 – 16 | ↓ | ↓ | ↑ | ↑ | |
| Pseudomonas aeruginosa 22 | ND | NS | ↑ early | ND | |
| Streptococcus pneumoniae 13 | ↓ | ↓ | ↑ | ↑ | |
| TLR4 | Acinetobacter baumannii 21 | ↓ | ↓ | ↑ | ↑ |
| Haemophillus influenzae 19 | ↓ | ↓ | ↑ | ND | |
| Klebsiella pneumoniae 18 | ↓ | ND | ↑ | ND | |
| Pseudomonas aeruginosa 22 | N | ↓ late | NS | ND | |
| Streptococcus pneumoniae 20 | ↓ | ↓ | ↑ | ND | |
| TLR5 | Legionella pneumophila 23 | ND | ↓ early | NS | ND |
| TLR9 | Klebsiella pneumoniae 25 | ↓ | NS | ↑ | ↑ |
| Legionella pneumophila 24 | ↓ | NS | ↑ | ND | |
| Streptococcus pneumoniae 26 | ↓ | NS | ↑ | ↑ | |
| TLR adaptor | |||||
| MyD88 | Escherichia coli 30 | ↓ | ↓ | ND | ND |
| Haemophilus influenzae 31 | ND | ND | ↓ | ND | |
| Klebsiella pneumoniae 35 | ↓ | ↓ | ↓ | ↓ | |
| Legionella pneumophila15,33 | ↓ | ↓ | ↑ | ↑ | |
| Pseudomonas aeruginosa6,29,32 | ↓ | ↓ | ↑ | ↑ | |
| Staphylococcus aureus 29 | ND | ↓ | ND | ND | |
| Streptococcus pneumoniae 28 | ↓ | ↓ | ↑ | ↑ | |
| TIRAP | Klebsiella pneumoniae6,30 | ↓ | ↓ | ↑ | ↑ |
| Escherichia coli6,30 | ND | ↓ | ↑ | ND | |
| TRIF | Escherichia coli 30 | ↓ | ↓ | ↑ | ↑ |
| Pseudomonas aeruginosa 34 | ND | ↓ | ↑ | ND | |
| Klebsiella pneumoniae 35 | ↓ | ↓ | ↑ | ↑ | |
| NLR | |||||
| NALP3 | Klebsiella pneumoniae 46 | ↓ | ↓ | ND | ND |
| IPAF | Pseudomonas aeruginosa 52 | NS | ND | ↑ | ↑ |
| Transcription factor | |||||
| NF-κB p50 | Escherichia coli58,59 | ↓ | ↓ | NS | ND |
| NF-κB ReLa | Streptococcus pneumoniae 60 | ND | ↓ | ↑ | ND |
| STAT3 | Escherichia coli 63 | ND | ↓ | ↑ | ND |
| STAT4 | Klebsiella pneumoniae 64 | ↓ | ND | ↑ | ↑ |
| Pseudomonas aeruginosa 65 | ND | NS | ↓ | ND | |
| Cytokine | |||||
| IL-23 | Pseudomonas aeruginosa 74 | ND | ↓ | NS | NS |
| Klebsiella pneumoniae 72 | ↓ | ND | ND | ND | |
| IL-17 | Klebsiella pneumoniae 73 | ↓ | ↓ | ↑ | ND |
| TNF-α | Streptococcus pneumoniae 61 | ↓ | ↓ | ↑ | ND |
| IL-1 | Streptococcus pneumoniae 61 | ↓ | ↓ | ↑ | ND |
| CXC chemokine receptor | |||||
| CXCR2 | Pseudomonas aeruginosa 79 | ↓ | ↓ | ND | ND |
| Legionella pneumophila 81 | ↓ | ↓ | NS | ND | |
| Norcadia asteroides 80 | ↓ | ↓ | ↑ | ND | |
| CXC chemokine | |||||
| KC$ | Klebsiella pneumoniae 83 | ↓ | ↓ | ND | ND |
| MIP2# | Klebsiella pneumoniae 84 | ↓ | ↓ | ↑ | ↑ |
| Norcadia asteroides 80 | NS | NS | NS | NS | |
| Lungkine | Klebsiella pneumoniae 85 | ↓ | ↓(24 h)* ↑ (48 h)* | ↑ | ND |
Phenotype was determined mainly by using gene-deficient or mutant mice after infection.
Neutrophil influx was determined in BALF and/or lung parenchyma.
Bacterial burden was measured as CFUs in the lungs.
Bacterial dissemination was measured as CFUs in blood or spleen.
Significant in airspaces but not in lung parenchyma.
Studied with transgenic mice.
Abs only.
ND, not determined; NS, no significant difference.
Surfactant proteins (SPs) are a complex of lipoproteins that enhance pathogen clearance and primarily are expressed in the epithelial lining of the lung. The host-defence functions of surfactant are primarily mediated by SP-A and SP-D, which are members of the collectin family of proteins. These collectins opsonise bacterial pathogens in order to facilitate their phagocytic clearance. The surfactant proteins A and D potentially bind to several receptors and activate a number of signalling cascades, among which TLR2 and TLR4 are important. Activation of TLRs by surfactant proteins initiates a conserved series of responses that culminate in the production of inflammatory cytokines.36
NOD-like receptors
The NLRs comprise a large family of intracellular receptors that are characterized by the NOD family of proteins. They regulate both inflammation and apoptosis. There has been about 23 NLRs reported to date. The general structure of these proteins includes an amino-terminal caspase recruitment domain (CARD), pyrin, or baculovirus inhibitor repeat domains, a central NOD, and carboxyl-terminal leucine-rich repeats (LRRs) that detect specific PAMPs.37,38 The family contains NALP (NACHT-, LRR-, and pyrin-domain containing proteins), NOD, CIITA (class II transactivator), IPAF (ICE-protease activating factor) and NAIP (neuronal apoptosis inhibitor protein). These receptors sense intracellular pathogens. The best characterized of the NODs are NOD1 and NOD2, where NOD1 has one CARD domain and NOD2 has two CARD domains in their amino terminus and they recognise different muropeptides (γ-D-glutamyl-mesodiaminopimelic acid and muramyl dipeptide) from the bacterial cell wall although how these peptides enter the cytosol is not clear.37,38 The importance of NODs has been widely studied through their mutations associated with chronic inflammatory diseases, such as Crohn's disease. NOD1 is ubiquitously expressed whereas NOD2 is primarily found in antigen-presenting cells. Upon ligand binding to the NODs, the CARD-containing adaptor molecule serine/threonine kinase RIP2 (also known as RICK, CARDIAK, CCK, and Ripk2) transduces signals for antimicrobial inflammatory responses independent of TLR signalling.39 RIP2 gene-deficient mice have shown increased susceptibility to intracellular Listeria monocytogenes.40 Similar observations were reported in NOD1 and NOD2 gene-deficient mice against List. monocytogenes infection.39 NOD1 is critical for the host immunity during P. aeruginosa infection41 and NOD2 in Mycobacterium tuberculosis.42 NOD1 and NOD2 activation results in nuclear factor (NF)-kB and MAPK activation and NOD-mediated NF-kB activation is essential for the protection of host during Strep. pneumoniae infection.43
Besides NODs, there are other NLRs that play an important role in the antimicrobial host defence. The NALP subfamily has PYRIN instead of CARD in their N-terminus and has 14 members. NALP1, NALP2 and NALP3 form a multiprotein complex called `inflammasome' comprising ASC (apoptosis-associated speck-like protein containing a CARD) and caspase. This complex activates caspase1 and leads to the cleavage of pro-IL-1β, pro-IL-18 and pro-IL-33 to their biologically active forms. NALP3 inflammasome has been shown to be essential for host defence against L. pneumophila,44 Francisella tulerensis,45 and K. pneumoniae,46 whereas NALP1 is important for the host defence against Bacillus anthracis.47 IPAF is another NLR that has a CARD domain and forms an inflammasome that recognises the bacterial flagellin of motile bacteria such as Salmonella, Shigella, Legionella, Francisella and Pseudomonas.45,48–53
Transcription factors
Several transcription factors such as NF-kB, AP-1, STAT and IRFs are involved in the regulation of host defence mechanism. NF-kB and STAT proteins have been studied in detail. However, NF-kB is considered as the central mediator of immune mechanisms. The TLR and NOD pathways converge to activate NF-kB-dependent gene transcription to mediate immune responses. TLR2 and TLR4 were both found to activate NF-kB, TLR2 in response to lipopeptides, and TLR4 in response to LPS stimulation, a response that is enhanced by the presence of CD14 and LBP.54,55 Furthermore, TLR5 regulates NF-kB activation during flagellin-induced sepsis.56 Similarly, TLR3 activated by poly I:C mediates NF-kB activation.57 The NF-kB family of structurally related transcription factors occur as homodimers or heterodimers in the cytoplasm of almost all mammalian cells.58 The NF-kB commonly refers to p50–RelA heterodimer, which is one of the most avidly forming dimers present in cells. They control a large number of normal cellular processes, such as immune and inflammatory responses, developmental processes, cellular growth, and apoptosis. These NF-kB proteins are related through a highly conserved DNA-binding/dimerization domain called the Rel homology (RH) domain. The NF-kB protein activity is primarily regulated by interaction with inhibitory IkB proteins. In cells, NF-kB is present in a latent, inactive, IkB-bound complex form in the cytoplasm. The NF-kB–IkB interaction prevents NF-kB nuclear localisation as well as DNA binding ability. When a cell receives a signal, it can trigger multiple signalling cascades that can activate the serine-specific IkB kinase (IKK) and eventually ubiquitinate the IkB and promote its degradation. This facilitates activation of NF-kB, nuclear localisation and DNA binding. NF-kB activation turns on several pro-inflammatory genes, more specifically the neutrophil chemo-attractants and result in neutrophil accumulation at the site of infection.58 Studies of NF-kB have shown that endogenous NF-kB is essential for the host defence against E. coli and Strep. pneumoniae pneumonia.59,60 It has been shown that NF-kB activity is affected by cytokine receptor signalling such as TNF-α and IL-1 receptors during pneumococcal infection.61 During P. aeruginosa34 and K. pneumoniae35 infection TRIF, an adaptor molecule for TLR3 and TLR4, has been shown to reduce NF-kB activation and chemokine expression.
The JAK/STAT pathway is an important mechanism for a wide array of cytokines and growth factors. Intracellular activation of the JAK/STAT pathway occurs when ligand binding induces the multimerization of receptor subunits. Activated JAKs subsequently phosphorylate and activate STATs. Similar to NF-kB, STATs are also latent transcription factors that reside in the cytoplasm until activated. Phosphorylated and dimerised STATs enter the nucleus and bind specific regulatory sequences to activate or repress transcription of target genes.62 During E. coli pneumonia, IL-6 has been shown to activate STAT3 to promote neutrophil recruitment into the lungs.63 STAT4 is an essential component of the innate immune defence against K. pneumoniae64 and P. aeruginosa65 infections and bacterial clearance of K. pneumoniae.64
Cytokines
Cytokines are a diverse group of soluble proteins or peptides that bring biological responses at very low concentrations. These cytokines have autocrine, paracrine or endocrine activities to bring about immune functions. Specific binding of these cytokines to their cognate receptors results in signal transduction via secondary messengers, up-regulation or down-regulation of their receptors, cell proliferation and secretion. The major pro-inflammatory cytokines or the acute phase cytokines that are studied extensively are tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-8 and IFN-γ. TNF-α, IL-1β and IL-6 play an important role in vasodilatation, increasing the vascular permeability and up-regulation of cellular adhesion molecules. IL-8 and IL-1β act as a potent chemokines and IFN-γ promotes the intracellular phagocytic killing of microbes. During Gram-negative bacterial infection, TLR4 has been shown to regulate the expression of TNF-α and IL-1β.66,67 whereas, during L. pneumophila infection, TLR5 and TLR9 mediate the expression of TNF-α.23,24 On the other hand, anti-inflammatory cytokines IL-10, TGF-β and IL-1Ra play a critical role to dampen the inflammatory response and resolve inflammation and aid in healing.12 IL-12 is a pro-inflammatory cytokine that has been shown to have beneficial effects in host-acquired immunity. Effective delivery of IL-12 to the murine lung during K. pneumoniae infection protects the mice.68 During L. pneumophila infection, TLR9 seems to regulate IL-12 production in lungs.24 The IL-12/IFN-γ axis is important for intracellular bacterial host defence and phagocytic destruction of bacteria.
Recent studies have shown an emerging role of IL-23 in innate host defence during bacterial pneumonia. IL-23 is a heterodimer that has a p40 subunit identical to the IL-12 and a unique p19 subunit.69 IL-23 is predominantly a cytokine that is produced by antigen-presenting cells and has been shown to stimulate the production IL-17 (IL-17A and IL-17F) by Th17 and γδ T-cells in a TLR-dependent manner. These cytokines promote the production of many other cytokines and chemokines.70,71 IL-17 signalling is critical for the pulmonary host defence against, and survival during, K. pneumoniae infection.72,73 Studies have also shown that IL-23 plays a critical role in the pulmonary immunity against P. aeruginosa infection.74 The IL-23/IL-17 axis has been shown to regulate the expression of various other pro-inflammatory cytokines. In addition to IL-17A there are IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. IL-17 cytokine binds to type I cell surface receptor called IL-17R which has three different variants (IL-17RA, IL-17B and IL-17C). IL-17 signalling is critical for host defence against extracellular bacteria by regulating chemokine gradients for neutrophil emigration into infected tissue.75 Recent reports demonstrate that the IL-17 family of cytokines can be regulated by IL-23, IL-15 and IL-12.71
Chemokines
Chemokines are cytokines that induce leukocyte infiltration into the site of infection/inflammation. Chemokines promote the expression of cellular adhesion molecules and extravasation of leukocytes. There are four types of chemokines depending on the presence of cysteine towards the N-terminus (C, CC, CXC and CX3C chemokines).76 The members of ELR+CXC chemokines are neutrophil chemo-attractants. In humans there are seven chemokines (IL-8; NAP-2; GRO α, β and γ; ENA-78 and GCP-2) reported.77 Among these, IL-8 is considered the most potent neutrophil chemo-attractant. In mice, keratinocyte-derived chemokine (KC), macrophage inflammatory protein (MIP)-2, LPS-induced CXC chemokine (LIX/CXCL5), and lungkine are the four CXC chemokines associated with neutrophil recruitment to the lungs.78 However, no rodent homologue of human IL-8 has been identified yet. KC and MIP-2 are myeloid cell derived chemokines, whereas lungkine and LIX are secreted by bronchial epithelial cells and type II alveolar epithelial cells, respectively. There are two receptors CXCR1 and CXCR2 which bind to CXC chemokines and present in both humans and mice. Between these receptors, CXCR2 binds to all ELR+CXC chemokines and is essential for neutrophil infiltration during bacterial infection, it is involved in the host defence and neutrophil recruitment upon challenge of P. aeruginosa,79 Nocardia asteroides,80 and L. pneumophila.81 It has been shown that TLR2 down-regulates CXCR2 and impairs neutrophil migration during polymicrobial sepsis.82 Studies have also shown that KC and MIP-2 play an important role in neutrophil accumulation during bacterial infection by using blocking peptides.83,84 However, lungkine is not important for neutrophil recruitment into the lung parenchyma during pneumonia.85 Unlike KC and MIP-2, CXCL5, also known as LIX, is a relatively newly reported CXC chemokine/neutrophil chemo-attractant. Up-regulation of CXCL5 increases neutrophil recruitment during infections with P. aeruginosa, K. pneumoniae, L. pneumophila and Bordetella bronchiseptica. Using TLR2 gene-deficient mice, it has been shown that TLR2 regulates the expression of KC and MIP-2 during LTA induced lung inflammation.86 However, TLR4 is important for the regulation of KC and MIP-2 production during LPS-induced inflammation.67,87 Furthermore, in flagellin challenged mice, TLR5 is critical for the production of KC and MIP-2.88 Although LPS-induced inflammation has also shown that LIX is an important molecule for neutrophil influx in the lungs,89 the role of LIX in neutrophil infiltration in the lungs during bacterial infections is unclear. Recently, a CC chemokine, monocyte chemo-attractant protein 1 (MCP1) which is primarily a monocyte chemokine, has been shown to play a role in neutrophil chemotaxis.90
Conclusions
Bacterial lung diseases are an essential public health concern. Neutrophils are amongst the first cells to reach the site of bacterial infection in order to clear bacteria. Our understanding of the molecular mechanisms that regulate neutrophil recruitment during infection/inflammation has improved substantially over recent years. Emerging studies indicate complex roles for TLRs and NLRs in neutrophil accumulation during pneumonia (Fig. 1). Although antibiotics are the rational treatment for pneumonias, antibiotic-resistant Strep. pneumoniae, H. influenzae, and S. aureus have been isolated from patients suffering from lower respiratory tract infections. The emergence of antibiotic-resistant pulmonary bacteria and the growing number of immunocompromised individuals have made the treatment of these infections difficult. As most of the TLR studies have been performed in murine models, the efficacy and safety of TLR therapies may not extrapolate to human responses. This is because: (i) of differences between the human and murine immune system; (ii) of differences in the activation profile of human and mouse, such as TLR8; and (iii) murine investigations are performed on in-bred strains that have minimal genetic variation. Though TLR9 agonists, such as CpG oligodeoxynucleotides have been shown to protect against numerous infectious agents in murine models, no human clinical studies have been reported, to our knowledge, using TLR9 agonists in bacterial infections. In addition, TLR3, TLR7, TLR8 and TLR9 can be activated upon intracellular bacterial infection, resulting in the production of IFN-α and, therefore, these receptors can be targeted to control bacterial infections. The studies using TLR adaptor-deficient mice in response to bacterial infection reveal the potential for using cell-permeable compounds to attenuate cytokine/chemokine production in order to reduce excessive neutrophil-mediated parenchymal damage. Unlike TLRs, NLRs have recently been identified and, therefore, their therapeutic potential to reduce bacteria-mediated neutrophil influx in the lungs remains to be evaluated. The future challenge will be to apply our current understanding of TLRs and NLRs to design therapeutic methods to attenuate uncontrolled neutrophil migration to the lungs in ALI/ARDS patients.
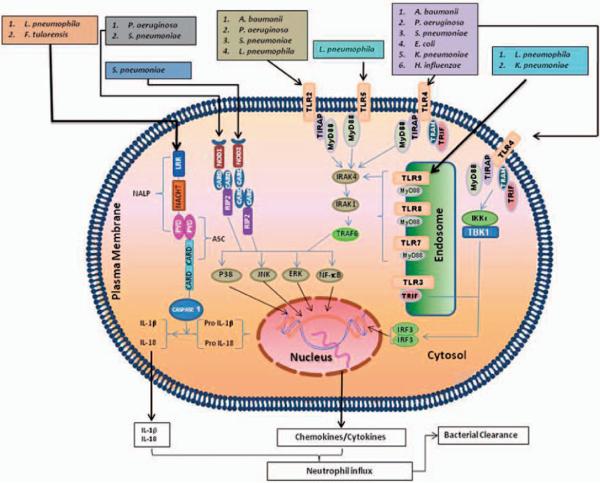
Fig. 1.
Respiratory pathogens are recognised by membrane bound and cytoplasmic pattern recognition receptors. Plasma membrane-bound TLRs (TLR2, TLR4 and TLR5) and endosome membrane-bound TLRs (TLR3, TLR7, TLR8 and TLR9) recognise bacterial pathogens in the lungs. TLR2, TLR4, TLR5, TLR6, TLR7 and TLR9 recruit MyD88 whereas TLR2 and TLR4 recruit both TIRAP and MyD88. TLR3 and TLR4 recruit TRIF to induce downstream signalling cascades. Binding of pathogens and/or PAMPs to TLRs leads to complex signalling cascades, which result in transcription of pro-inflammatory mediators and activation of MAP kinases. Cytosolic NODs recognise bacterial pathogens in the lung and mediate signalling via RIP2, whereas NALPs use ASC to induce signalling cascades, which result in transcription of pro-inflammatory mediators and activation of MAP kinases. These pro-inflammatory mediators, including chemokines, recruit neutrophils to the lung in order to clear the causative organism during bacterial infection.
Acknowledgements
This work was supported by a research grant from the American Lung Association (RG-22442-N), a scientist award from the Flight Attendant Medical Research Institute (YCSA-062466), and grants from the NIH (R01 HL-091958 and R01 HL-091958S1 via ARRA) to SJ.
References
- 1.Mizgerd JP. Lung infection – a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Garvy BA, Harmsen AG. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation. 1996;20:499–512. doi: 10.1007/BF01487042. [DOI] [PubMed] [Google Scholar]
- 5.Tateda K, Moore TA, Deng JC, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 6.Jeyaseelan S, Young SK, Yamamoto M, et al. Toll/IL-1R Domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol. 2006;177:538–547. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 7.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14:123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 8.Zemans RL, Briones N, Young SK, et al. A novel method for long term bone marrow culture and genetic modification of murine neutrophils via retroviral transduction. J Immunol Methods. 2009;340:102–115. doi: 10.1016/j.jim.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spörri R, Joller N, Hilbi H, Oxenius A. A novel role for neutrophils as critical activators of NK cells. J Immunol. 2008;181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill LA, Bowie AG. The family of five: Tir-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 12.Moldoveanu B, Otmishi P, Jani P, et al. Inflammatory mechanisms in the lung. J Inflamm Res. 2009;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Dessing MC, Florquin S, Paton JC, van der Poll T. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell Microbiol. 2008;10:237–246. doi: 10.1111/j.1462-5822.2007.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuse ET, Tateda K, Kikuchi Y, et al. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol. 2007;56:305–312. doi: 10.1099/jmm.0.46913-0. [DOI] [PubMed] [Google Scholar]
- 15.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and Toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 16.Archer KA, Alexopoulou L, Flavell RA, Roy CR. Multiple MyD88-dependent responses contribute to pulmonary clearance of Legionella pneumophila. Cell Microbiol. 2009;11:21–36. doi: 10.1111/j.1462-5822.2008.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai S, Zemans RL, Young SK, Worthen GS, Jeyaseelan S. MD-2-dependent and -independent neutrophil accumulation during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2009;40:701–709. doi: 10.1165/rcmb.2008-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of Toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect Immun. 2005;73:532–545. doi: 10.1128/IAI.73.1.532-545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Moser C, Louboutin JP, et al. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol. 2002;168:810–815. doi: 10.4049/jimmunol.168.2.810. [DOI] [PubMed] [Google Scholar]
- 20.Dessing MC, Schouten M, Draing C, Levi M, von Aulock S, van der Poll T. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J Infect Dis. 2008;197:245–252. doi: 10.1086/524873. [DOI] [PubMed] [Google Scholar]
- 21.Knapp S, Wieland CW, Florquin S, et al. Differential roles of CD14 and Toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med. 2006;173:122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- 22.Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol. 2005;175:3927–3934. doi: 10.4049/jimmunol.175.6.3927. [DOI] [PubMed] [Google Scholar]
- 23.Hawn TR, Berrington WR, Smith IA, et al. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J Immunol. 2007;179:6981–6987. doi: 10.4049/jimmunol.179.10.6981. [DOI] [PubMed] [Google Scholar]
- 24.Bhan U, Trujillo G, Lyn-Kew K, et al. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect Immun. 2008;76:2895–2904. doi: 10.1128/IAI.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhan U, Lukacs NW, Osterholzer JJ, et al. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J Immunol. 2007;179:3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 26.Albiger B, Dahlberg S, Sandgren A, et al. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 27.Deng JC, Cheng G, Newstead MW, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albiger B, Sandgren A, Katsuragi H, et al. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol. 2005;7:1603–1615. doi: 10.1111/j.1462-5822.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 29.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol. 2004;172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- 30.Jeyaseelan S, Young SK, Fessler MB, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 31.Wieland CW, Florquin S, Maris NA, et al. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable Haemophilus influenzae from the mouse lung. J Immunol. 2005;175:6042–6049. doi: 10.4049/jimmunol.175.9.6042. [DOI] [PubMed] [Google Scholar]
- 32.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol. 2007;292:L312–L322. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 33.Archer KA, Roy CR. MyD88-dependent responses involving Toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect Immun. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Power MR, Li B, Yamamoto M, Akira S, Lin TJ. A role of Toll–IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J Immunol. 2007;178:3170–3176. doi: 10.4049/jimmunol.178.5.3170. [DOI] [PubMed] [Google Scholar]
- 35.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 37.Ting JPY, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 38.Franchi L, McDonald C, Kanneganti TD, Amer A, Núñez G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 39.Sirard J-C, Vignal C, Dessein R, Chamaillard M. Nod-like receptors: cytosolic watchdogs for immunity against pathogens. PLoS Pathog. 2007;3:e152. doi: 10.1371/journal.ppat.0030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 41.Travassos LH, Carneiro LA, Girardin SE, et al. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol Chem. 2005;280:36714–36718. doi: 10.1074/jbc.M501649200. [DOI] [PubMed] [Google Scholar]
- 42.Ferwerda G, Girardin SE, Kullberg B-J, et al. NOD2 and Toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:e34. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opitz B, Püschel A, Schmeck B, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 44.Case CL, Shin S, Roy CR. Asc and Ipaf inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77:1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 46.Willingham SB, Allen IC, Bergstralh DT, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutterwala FS, Flavell RA. NLRC4/IPAF: a CARD carrying member of the NLR family. Clin Immunol. 2009;130:2–6. doi: 10.1016/j.clim.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mariathasan S, Newton K, Monack DM, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 49.Zamboni DS, Kobayashi KS, Kohlsdorf T, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki T, Franchi L, Toma C, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franchi L, Stoolman J, Kanneganti TD, Verma A,, Ramphal R, Núñez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 53.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janeway CA, Jr, Medzhitov R. Innate immunity: lipoproteins take their Toll on the host. Curr Biol. 1999;9:R879–R882. doi: 10.1016/s0960-9822(00)80073-1. [DOI] [PubMed] [Google Scholar]
- 55.Beutler B. Endotoxin, Toll-like receptor 4, and the afferent limb of innate immunity. Curr Opin Microbiol. 2000;3:23–28. doi: 10.1016/s1369-5274(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 56.Liaudet L, Szabó C, Evgenov OV, et al. Flagellin from Gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock. 2003;19:131–137. doi: 10.1097/00024382-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. Poly(dI·dC)-induced Toll-like receptor 3 (TLR3)-mediated activation of NF-κB and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 59.Alcamo E, Mizgerd JP, Horwitz BH, et al. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-{kappa}B in leukocyte recruitment. J Immunol. 2001;167:1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- 60.Mizgerd JP, Lupa MM, Kogan MS, Warren HB, Kobzik L, Topulos GP. Nuclear factor-{kappa}B p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am J Respir Crit Care Med. 2003;168:810–817. doi: 10.1164/rccm.200303-412OC. [DOI] [PubMed] [Google Scholar]
- 61.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-{kappa}B activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 63.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng JC, Zeng X, Newstead M, et al. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J Immunol. 2004;173:4075–4083. doi: 10.4049/jimmunol.173.6.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Sullivan R, Carrigan SO, Marshall JS, Lin TJ. Signal transducer and activator of transcription 4 (STAT4), but not IL-12 contributes to Pseudomonas aeruginosa-induced lung inflammation in mice. Immunobiology. 2008;213:469–479. doi: 10.1016/j.imbio.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Andonegui G, Zhou H, Bullard D, et al. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest. 2009;119:1921–1930. doi: 10.1172/JCI36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Togbe D, Schnyder-Candrian S, Schnyder B, et al. TLR4 gene dosage contributes to endotoxin-induced acute respiratory inflammation. J Leukoc Biol. 2006;80:451–457. doi: 10.1189/jlb.0206099. [DOI] [PubMed] [Google Scholar]
- 68.Greenberger M, Kunkel SL, Strieter RM, et al. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–3012. [PubMed] [Google Scholar]
- 69.Zhang Z, Hinrichs DJ, Lu H, Chen H, Zhong W, Kolls JK. After interleukin-12p40, are interleukin-23 and interleukin-17 the next therapeutic targets for inflammatory bowel disease? Int Immunopharmacol. 2007;7:409–416. doi: 10.1016/j.intimp.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 70.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 71.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 72.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–528. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol. 2007;292:L519–L528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aujla SJ, Dubin PJ, Kolls JK. Interleukin-17 in pulmonary host defense. Exp Lung Res. 2007;33:507–518. doi: 10.1080/01902140701756604. [DOI] [PubMed] [Google Scholar]
- 76.Lukacs NW, Hogaboam C, Campbell E, Kunkel SL. Chemokines: function, regulation and alteration of inflammatory responses. Chem Immunol. 1999;72:102–120. doi: 10.1159/000058729. [DOI] [PubMed] [Google Scholar]
- 77.Lukacs NW, Hogaboam C, Campbell E, Kunkel SL. Chemokines: function, regulation and alteration of inflammatory responses. Chem Immunol. 1999;72:102–120. doi: 10.1159/000058729. [DOI] [PubMed] [Google Scholar]
- 78.Baggiolinim M, Dewaldm B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 79.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun. 2000;68:4289–4296. doi: 10.1128/iai.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore TA, Newstead MW, Strieter RM, Mehrad B, Beaman BL, Standiford TJ. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. JImmunol. 2000;;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]
- 81.Tateda K, Moore TA, Newstead MW, et al. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alves-Filho J, Freitas A, Spiller F, et al. TLR2 signaling downregulates chemokine receptor CXCR2 and impairs neutrophil migration in severe polymicrobial sepsis. Crit Care. 2007;11(Suppl 4):P47. [Google Scholar]
- 83.Tsai WC, Strieter RM, Wilkowski JM, et al. Lung-specific-transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]
- 84.Greenberger MJ, Strieter RM, Kunkel SL, et al. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 85.Chen SC, Mehrad B, Deng JC, et al. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J Immunol. 2001;166:3362–3368. doi: 10.4049/jimmunol.166.5.3362. [DOI] [PubMed] [Google Scholar]
- 86.Knapp S, von Aulock S, Leendertse M, et al. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- 87.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 88.Feuillet V, Medjane S, Mondor I, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun. 2004;72:7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]