Abstract
Fluid overload is a frequent finding in critically ill patients suffering from acute kidney injury (AKI). To assess the impact of fluid overload on the mortality of AKI patients treated with continuous renal replacement therapy (CRRT), we used a registry of eighty-one critically ill patients with AKI initiated on CRRT assembled over an 18 month period to conduct a cross-sectional analysis using volume-related weight gain (VRWG) of ≥10 and ≥20% of body weight, and oliguria (20 ≤mL/hour) as the principal variables, with the primary outcome measure being mortality at 30 days. Mean Apache II scores were 27.5±6.9 with overall cohort mortality of 50.6%. Mean (±SD) VRWG was 8.3±9.6 kg, representing a 10.2±13.5% increase since admission. Oliguria was present in 65.4% of patients. OR for mortality on univariate analysis was increased to 2.62 (95% CI: 1.07-6.44) by a VRWG ≥10% and to 3.22 (95% CI: 1.23-8.45) by oliguria. VRWG ≥20% had OR of 3.98 (95% CI: 1.01-15.75; p=0.049) for mortality. Both VRWG ≥10% (OR 2.71, p=0.040) and oliguria (OR 3.04, p=0.032) maintained their statistically significant association with mortality in multivariate models that included sepsis and Apache II score. In conclusion, fluid overload is an important prognostic factor for survival in critically ill AKI patients treated with CRRT. Further studies are needed to elicit mechanisms and develop appropriate interventions.
Keywords: Acute Kidney Failure, Fluid balance, Hemofiltration, Mortality, Multiple Organ Failure, Volume overload
Introduction
Acute kidney injury (AKI) is common in critically ill patients and associated with increased morbidity, mortality and health care costs1-3, especially in the context of multi-organ failure4, 5. These patients have numerous physiologic derangements including hemodynamic instability and are therefore being increasingly treated with continuous renal replacement therapy (CRRT). Consequently, considerable effort is being put into identifying prognostic factors and treatment variables that may predict or modify survival in these patients6-13.
One important prognostic factor may be fluid accumulation. Absolute or relative hypovolemia results in decreased cardiac output, blood pressure and organ perfusion14; hence volume resuscitation is needed to maintain organ function15. However, overaggressive volume-resuscitation can cause tissue congestion and hypoxia, and is associated with worse survival in general Intensive Care Unit (ICU) populations16 and in patients with AKI17. Because many of these patients will need CRRT for volume management, we evaluated the association between mortality and volume-related weight gain (VRWG) at the time of initiating CRRT, in AKI patients treated with CRRT. We addressed this by analyzing demographic, clinical and survival data from an observational, single-center registry of patients treated with CRRT at our institution.
Methods
Study Design, Setting, and Population
The study was approved by the Institutional Review Board of the University of Mississippi Medical Center, Jackson, MS. Adult patients (≥18 years old) that were treated with CRRT in our medical, cardiothoracic, and surgical intensive care units (ICU) between January 2003 and June 2004 were prospectively enrolled during or shortly after initial nephrology consultation. The decision to initiate CRRT, as well as the timing, prescribed dose and modality, was at the discretion of the nephrologist and performed using Gambro Prisma machines and AN-69 polyacrylonitirile dialysis membranes. Patients were excluded if they had preexistent ESRD or if the time from the onset of AKI to initiation of CRRT was two weeks or greater.
Definitions and Variables of Interest
Patients were considered to have AKI if their serum creatinine increased by 0.5 mg/dl or greater from baseline or if they had an abnormal serum creatinine at presentation with no known baseline value. The creatinine level at initiation of CRRT, the change in creatinine from hospital admission to initiation of CRRT, and the days elapsed from the diagnosis of AKI to initiation of CRRT were recorded. The patients’ weights were obtained in a variety of settings: the emergency room, regular nursing floors, and the ICU. Standard hospital scales were used for ambulatory patients and bed scales for non-ambulatory or ICU patients. The first available documented weight on the hospital record was taken as the initial weight, the majority of which were in the ICUs. Subsequent daily weights were monitored with bed scales by the nursing staff and recorded on the care flow sheets. VRWG was defined as the difference between the initial weight and the weight at initiation of CRRT. From this, we calculated the percent weight gain. Oliguria was defined as an average urine output of less than 20 ml/hour for at least 12 hours before enrollment. The diagnosis of sepsis was obtained from the chart, and Apache II scores were calculated at the time of the renal consult.
Statistical Analysis
The principal outcome was mortality at 30 days. The main comparisons were: a) patients with VRWG ≥10% vs. those that gained less <10%; and b) those who gained ≥20% vs. those gaining <20%. In a separate analysis, we evaluated the mortality in patients gaining <10%, between ≥10 and <20%, and greater ≥20%. Additional variables included age, gender, chart diagnosis of sepsis, Apache II scores, ICU location, creatinine at CRRT initiation, absolute change of creatinine and days elapsed (as described in previous section). Cross-sectional analysis of selected variables was conducted for association with mortality. Chi-square tests were used for bivariate analysis of association between selected categorical variables and mortality. Independent two-sample T-test was performed to assess the association of continuous variables with mortality. Multivariate binary logistic regression analysis was conducted for more complex associations. Data were analyzed using both SPSS (version 15) and Minitab (version 13).
Results
We have previously reported the general characteristics of the survivors vs. non-survivors of our study cohort in detail10. Our overall cohort consisted of 81 patients, 24 of which were women. Their mean age was 51.4 years (range: 23-85) and overall mortality rate was 50.6%. Oliguria was present in 53 patients (65.4%). Of forty-one deceased patients, 32 had oliguria; unadjusted odds ratio for death was 3.22 (95% CI 1.23-8.45; p=0.018) for these patients. The overall mean VRWG was 8.3 kg (range: −10.5 to +45.9 kg) representing a mean percent weight gain of 10.2% (range: −11 to +81%). Thirty-eight patients (46.9%) had VRWG ≥10% and 13 patients (16%) had ≥20% VRWG. There were no significant associations between oliguria and percent weight gains (p=0.215), Apache II scores and weight changes (Pearson r 0.101, p=0.368), between chart diagnosis of sepsis and death (p=0.557), and between gender and death (p=0.943) on univariate analysis.
We first assessed the association of VRWG with mortality using a VRWG of 10% and 20% as our cutoff points. The cohort statistics stratified with VRWG of <10 and ≥10% are presented in Table 1. The average VRWG was 1.4±4.6% and 20.3±13.3 in the <10% and ≥10% groups, respectively. The group with the VRWG ≥10% had a significantly higher risk of dying then the reference group of <10% (OR for death 2.62, 95% CI: 1.07-6.44; p=0.046) that was not associated with differences in age, gender, Apache II scores, or in the presence of sepsis or oliguria. Serum creatinine at the time of initiation of CRRT was lower in the patients with VRWG of ≥10% (perhaps reflecting dilution) and there was a tendency for these patients to have more days elapsed from AKI diagnosis to initiation of CRRT (p=0.06). Table 2 shows the cohort statistics according to VRWG of <20 and ≥20%. The average VRWG was 6.0±7.4% in the <20% group and 32.2±17.1 in the ≥20% group. Odds ratio for death in the patients with a VRWG of ≥20% (vs. reference group of <20%) was even higher: 3.98 (95% CI: 1.01-15.75; p=0.049). Otherwise, there were no differences between groups except that a smaller percentage of patients who had ≥20% WRWG were women. As shown in Figure 1, separating the cohort into three categories of VRWG (<10%; ≥10 but <20%; ≥20% VRWG) was associated with a progressive increase of mortality: 39.5% (17 of 43), 56% (14 of 25) and 76.9% (10 of 13). Accordingly, against a reference group of VRWG<10%, OR for death was increased to 1.95 (95% CI 0.72-5.28; p=0.191) in the group with intermediate weight gains and to 5.10 (95% CI 1.22-21.25; p=0.025) in the group with severe (≥20%) weight gains.
Table 1.
Clinical Variables and Outcomes in Patients with VRWGs of <10% vs. ≥10%
| Clinical Variables | All | VRWG <10% | VRWG ≥10% | P value |
|---|---|---|---|---|
| Number | 81 | 43 | 38 | N/A |
| Average weight gain (%) | 1.4 ±4.6 | 20.3 ±13.3 | ||
| Age (years) | 50.4 ±16.5 | 52.6 ±17 | 0.547 | |
| Female Gender | 27.9% | 31.5% | 0.718 | |
| Apache II scores | 27.2 ±6.9 | 27.9 ±6.8 | 0.657 | |
| Creatinine at CRRT initiation | 5.5 ±2.4 | 4.5 ±1.8 | 0.039 | |
| Change in creatinine (mg/dL) | 1.8 ±2.2 | 2.4 ±1.7 | 0.167 | |
| Days elapsed until CRRT | 3.1 ±2.9 | 4.4 ±3 | 0.061 | |
| Sepsis | 55.8% | 63.1% | 0.500 | |
| Oliguria | 62.8% | 68.4% | 0.593 | |
| ICU Service | 0.192 | |||
| Cardiac ICU-Cardiology (n=9) | 16.3% | 5.3% | ||
| Cardiac ICU-Surgery (n=14) | 11.7% | 23.7% | ||
| Medical ICU (n=42) | 55.8% | 47.4% | ||
| Surgical ICU (n=16) | 16.3% | 23.7% | ||
|
| ||||
| Mortality | 50.6% | 39.5% | 63.2% | 0.029 |
Apache II scores were calculated at time of renal consult.
Table 2.
Clinical Variables and Outcomes in Patients with VRWG of <20% vs. ≥20%
| Clinical Variables | All | VRWG <20% | VRWG ≥20% | P value |
|---|---|---|---|---|
| Number | 81 | 68 | 13 | n/a |
| Average weight gain (%) | 6.0 ±7.4 | 32.2 ±17.1 | ||
| Age (years) | 51.9 ±16.7 | 49.2 ±17 | 0.616 | |
| Female Gender | 23.5% | 61.5% | 0.008 | |
| Apache II scores | 27.3 ±6.8 | 28.8 ±7.2 | 0.480 | |
| Creatinine at CRRT initiation | 5.2 ±2.2 | 4.2 ±1.6 | 0.058 | |
| Change in creatinine (mg/dL) | 2.0 ±2.0 | 2.5 ±1.5 | 0.334 | |
| Days elapsed until CRRT | 3.5 ±2.9 | 4.7 ±3.1 | 0.241 | |
| Sepsis | 57.4% | 69.2% | 0.401 | |
| Oliguria | 63.2% | 76.9% | 0.295 | |
| ICU Service | 0.546 | |||
| Cardiac ICU-Cardiology (n=9) | 10.3% | 15.4% | ||
| Cardiac ICU-Surgery (n=14) | 19.1% | 7.7% | ||
| Medical ICU (n=42) | 52.9% | 46.2% | ||
| Surgical ICU (n=16) | 17.6% | 30.8% | ||
|
| ||||
| Mortality | 50.6% | 45.6% | 76.9% | 0.017 |
Apache II scores were calculated at time of renal consult.
Figure 1.
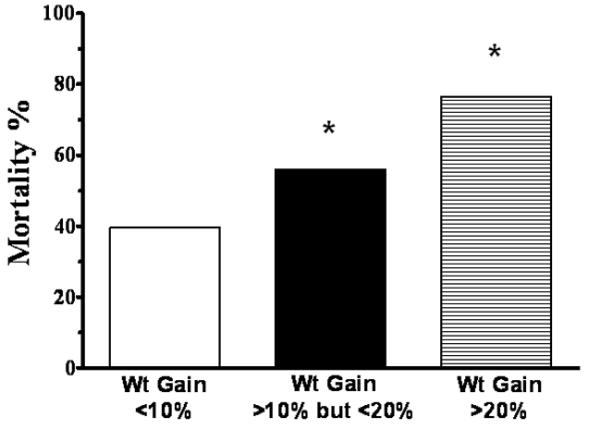
Association between progressive increases in VRWG and Mortality.
We next performed multivariate modeling to assess the association of other potential risk factors for death in these patients (Table 3). When analyzed together, oliguria (p=0.021) and ≥10 weight gain (p=0.042) maintained independent significance. When sepsis and Apache II scores were included in the modeling, both oliguria (OR 3.04, p=0.032) and ≥10% weight gain (OR 2.71, p=0.040) maintained significance. The effect of sepsis and Apache II scores remained non-significant on multivariate analysis. The combined presence of oliguria and ≥10% weight gain explained approximately 12% of the observed mortality. Including creatinine at CRRT initiation and days elapsed until CRRT into the logistic regression model abolished the association with ≥10% VRWG (p=0.133; OR 2.14 with 95% CI 0.79-5.76) but not with oliguria (p=0.018; OR 3.64; 95% CI 1.25-10.6) (r2 0.16).
Table 3.
The Effect of Oliguria, VRWG, Sepsis, and Apache II Scores on Mortality During Multivariate Modeling
| Clinical Variable | exp (B) ±95% Confidence Intervals |
p-value | R2 |
|---|---|---|---|
| Oliguria and ≥10% Volume-Related Weight Gain | |||
| Oliguria | 3.23 (1.19-8.73) | 0.021 | 0.12 |
| ≥10% VRWG | 2.64 (1.04-6.70) | 0.042 | |
|
| |||
| Oliguria, ≥10% Volume-Related Weight Gain, Sepsis and Apache II scores | |||
| Oliguria | 3.04 (1.10-8.36) | 0.032 | 0.13 |
| ≥10% VRWG | 2.71 (1.05-6.99) | 0.040 | |
| Sepsis | 0.63 (0.22-1.78) | 0.379 | |
| Apache II scores | 0.96 (0.89-1.03) | 0.219 | |
We then analyzed our data according to a three-way separation of the cohort (VRWG < 10%; ≥10 but <20%; ≥20% VRWG) in logistic regression. The OR of mortality increased 2.17 (95% CI: 1.11-4.26; p=0.024) for each step-increase across VRWG categories as well as for oliguria (OR 2.93; 1.06-8.11; p=0.038), but not for Apache II (p=0.24) and sepsis (p=0.35). Including creatinine at CRRT initiation and days elapsed until CRRT into logistic regression did not show an independent association with death (p=0.456 and 0.135, respectively) but abolished the association with VRWG with death (p=0.079; 95% CI 0.93-3.76), while the association with oliguria persisted (p=0.022; OR 3.53; 95% CI 1.20-10.34). Finally, we analyzed the OR between the two extreme cohorts (<10% VRWG vs. ≥20% VRWG; total of 56 patients) in multiple logistic regression models. With inclusion of oliguria, sepsis and Apache II scores, OR for death were markedly increase with VRWG ≥20% against the reference group of VRWG <10% (5.13; 95% CI 0.89 – 21.16; p=0.069), albeit formal significance was lost. Results with inclusion of multiple co-variates results are shown in Table 4.
Table 4.
The Effect of VRWGs (<10% vs. ≥20%) on Mortality During Multivariate Modeling
| Clinical Variable | exp (B) ±95% Confidence Intervals |
p-value |
|---|---|---|
| Oliguria | 1.90 (0.53-6.80) | 0.325 |
| VRWGs (<10% vs. ≥20%) | 4.03 (0.88-18.38) | 0.072 |
| Sepsis | 0.64 (0.18-2.27) | 0.490 |
| Apache II scores | 1.02 (0.94-1.12) | 0.605 |
| Creat inine at CRRT initiation (mg/dL) | 0.92 (0.71-1.20) | 0.553 |
| Days elapsed until CRRT | 1.10 (0.90-1.36) | 0.353 |
R2 0.147.
Discussion
Volume management is an essential component in maintaining hemodynamic stability, tissue perfusion and organ function. Several studies have demonstrated that early volume administration is critical to reverse tissue hypoperfusion and influence prognosis15. However, when volume resuscitation is excessive, it may be detrimental. Indeed, fluid overload increases morbidity and mortality in patients with acute respiratory distress syndrome18, 19, sepsis20, and surgical ICU patients21, 22 and lesser fluid gains are associated with better outcomes in abdominal compartment syndrome23, 24 and after colon resection21. Because patients with AKI have impaired ability to regulate volume, they may be particularly susceptible to volume-related increases in morbidity and mortality. Some data suggests that this may be true in pediatric patients; fluid overload at the initiation of CRRT is associated with increased mortality2, 7, 8, but even less information is available in adult patients with AKI. Recently, Payen et al. used the “Sepsis Occurrence in Acutely ill Patients” (SOAP) database17 to evaluate whether a positive fluid balance is associated with worse outcomes in patients admitted to the ICU. They reported that patients with AKI, non-survivors had a more positive fluid balance than the survivors. The PICARD cohort group recently extended these findings25. They analyzed the data from their cohort of critically ill adult patients with AKI and found that patients with fluid overload had a significantly higher mortality, irrespective of renal replacement therapy.
Our present study focused on the sickest of AKI patients: those requiring CRRT. These patients are usually hypotensive, and have impaired tissue perfusion and oliguria. Consequently, they usually receive aggressive volume resuscitation putting them at risk of volume overload. In fact, oliguria and fluid gains of ≥10% of body weight were common in our cohort (65% and 47%, respectively). The severity of illness of our patient cohort is underscored by the fact that the overall mortality in these patients was higher (50.6%) than that in the general ICU population with AKI that was analyzed by the SOAP investigators (35.7%). Our central finding was that despite being similar in all other aspects (severity of illness, sepsis, oliguria, etc.) the patients with VRWG ≥10% had a higher mortality than those with <10% (63% vs. 39%). Moreover, the mortality appears to further increase with VRWG ≥20%, suggesting a dose response effect of progressive fluid accumulation.
Our present study could not discern whether VRWG contributed to the increased mortality or selected out those with a higher risk of death. Fluid overload can directly worsen outcomes by increasing interstitial pressure and impairing tissue perfusion/oxygenation. In fact, it is directly associated with adverse clinical endpoints such as increased ventilator dependency, skin breakdown with sacral decubiti and poor wound healing21. It also dilutes the serum creatinine, which may delay the detection of AKI and the initiation of therapy; indeed, we observed lower creatinine and a delay in initiating CRRT in the patients with higher VRWG. It is, therefore, tempting to speculate that the excessive mortality observed in the high VRWG was due to volume overload. However, because severely ill patients develop vasodilatation, capillary leak, third spacing26, and hemodynamic instability, which prompts aggressive fluid resuscitation, we cannot exclude the possibility that excessive VRWG is simply a surrogate of hypotension, capillary leak and severity of illness. One argument against this possibility is that the impact of VRWG on mortality did not diminish in our multivariate analysis in which we adjusted for severity of illness. Regardless, the severity of illness scoring systems are known to perform poorly in cohorts with AKI4, 27, thus our study may not be robust enough to detect associations between severity of illness and mortality. Therefore, our results must be interpreted cautiously. Further studies are clearly warranted to determine whether excessive VRWG is a culprit or marker of mortality and whether different fluid resuscitation strategies alter mortality in these patients.
Another strong prognostic factor of worse outcomes in our study was oliguria; mortality was 60.4% compared to 32.1% in non-oliguric subjects. This is consistent with previous reports; oliguria is consistently associated with worse outcomes in patients with AKI3, 4, 17. However, the mechanism by which oliguria leads to worse outcomes is not known. It is tempting to speculate that oliguric patients have worse outcomes because they may are susceptible to developing fluid overload. By the same token, increased mortality in the fluid overload groups may be the result of a higher incidence of oliguria. However, this was not the case in our study as the incidence of oliguria was not different between the groups, yet there was a large difference in mortality. Our results found that oliguria and weight gain were independent predictors of mortality by mechanisms that remain to be elucidated.
The limitations of our study include the relatively small size, single center design and lack of randomization. Oliguria was not defined based on weight (mL/kg/hour) and not identical to the current definition of the Acute Kidney Injury Network. Sepsis was not based on standardized definitions, recovered as “chart diagnosis” only and subject to physicians’ bias. Weights were subject to the imprecision frequently encountered in clinical practice. The dichotomous separation of weight gain at 10%, while a meaningful value and quoted in the literature 2, 8, 22 was, in fact, a post hoc separation of the cohort. The cohort studied is a specific subset of ICU patients, those with AKI receiving CRRT. Thus our conclusions are strictly limited this specific cohort and may not be directly extrapolated to other populations or to weight gains taking place after CRRT initiation. The practice of ICU care, CRRT and fluid management is steadily evolving, posing an important external bias on our observations. In particular, delays in obtaining renal consults may have influenced timing of CRRT initiation and led to some of the extreme weight gains observed. Further studies are needed to determine a) whether fluid overload actively contributes to the increased mortality, and b) whether targeted strategies that prevent, ameliorate or reverse fluid overload, will improve survival.
Conclusion
The present study extends previous ones that suggest that fluid overload (represented by VRWG in the present study) is a predictor of survival in critically ill patients with AKI requiring CRRT. The increased mortality in the patients with higher degrees of volume overload was not related to a higher incidence of oliguria or severity of illness scores, suggesting that the fluid overload may be contributing to the worse mortality.
Footnotes
Disclosures: Luis A. Juncos is supported in part by the National Institutes of Health grants RO1-DK073401.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uchino S, Kellum JA, Bellomo R, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19:1394–1399. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 3.Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI) Investigators. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–7. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 5.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 6.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 7.Symons JM, Chua AN, Somers MJ, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. 2007;2:732–738. doi: 10.2215/CJN.03200906. [DOI] [PubMed] [Google Scholar]
- 8.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 9.Kellum JA, Bellomo R, Ronco C, Mehta R, Clark W, Levin NW. The 3rd International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Int J Artif Organs. 2005;28:441–444. doi: 10.1177/039139880502800503. [DOI] [PubMed] [Google Scholar]
- 10.Brar H, Olivier J, Lebrun C, et al. Predictors of mortality in a cohort of intensive care unit patients with acute renal failure receiving continuous renal replacement therapy. Am J Med Sci. 2008;335:342–347. doi: 10.1097/MAJ.0b013e3181571f56. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SL, Somers MJ, Brophy PD, et al. The Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) Registry: design, development and data assessed. Int J Artif Organs. 2004;27:9–14. doi: 10.1177/039139880402700104. [DOI] [PubMed] [Google Scholar]
- 12.Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008;19:1233–1238. doi: 10.1681/ASN.2007111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera-Gutierrez ME, Seller-Perez G, Lebron-Gallardo M, Munoz-Bono J, Banderas-Bravo E, Cordon-Lopez A. Early hemodynamic improvement is a prognostic marker in patients treated with continuous CVVHDF for acute renal failure. ASAIO J. 2006;52:670–676. doi: 10.1097/01.mat.0000242162.35929.bc. [DOI] [PubMed] [Google Scholar]
- 14.Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. JAMA. 1994;271:226–233. [PubMed] [Google Scholar]
- 15.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Eng J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 16.Uchino S, Bellomo R, Morimatsu H, et al. Pulmonary artery catheter versus pulse contour analysis: a prospective epidemiological study. PAC/PiCCO Use and Likelihood of Success Evaluation [PULSE] Study Group. Critical Care (London, England) 2006;10:R174. doi: 10.1186/cc5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Critical Care (London, England) 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakr Y, Vincent JL, Reinhart K, et al. Sepsis Occurence in Acutely Ill Patients Investigators. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128:3098–3108. doi: 10.1378/chest.128.5.3098. [DOI] [PubMed] [Google Scholar]
- 19.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. N Eng J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 21.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Danish Study Group on Perioperative Fluid Therapy. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowell JA, Schifferdecker C, Driscoll DF, Benotti PN, Bistrian BR. Postoperative fluid overload: not a benign problem. Crit Care Med. 1990;18:728–733. doi: 10.1097/00003246-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;34:707–713. doi: 10.1007/s00134-007-0969-4. [DOI] [PubMed] [Google Scholar]
- 24.McNelis J, Marini CP, Jurkiewicz A, et al. Predictive factors associated with the development of abdominal compartment syndrome in the surgical intensive care unit. Arch Surg. 2002;137:133–136. doi: 10.1001/archsurg.137.2.133. [DOI] [PubMed] [Google Scholar]
- 25.Bouchard J, Soroko SB, Chertow GM, et al. Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 26.van der Heijden M, Verheij J, van Nieuw Amerongen GP, Groeneveld AB. Crystalloid or colloid fluid loading and pulmonary permeability, edema, and injury in septic and nonseptic critically ill patients with hypovolemia. Crit Care Med. 2009;37:1275–1281. doi: 10.1097/CCM.0b013e31819cedfd. [DOI] [PubMed] [Google Scholar]
- 27.Fiaccadori E, Maggiore U, Lombardi M, Leonardi S, Rotelli C, Borghetti A. Predicting patient outcome from acute renal failure comparing three general severity of illness scoring systems. Kidney Int. 2000;58:283–292. doi: 10.1046/j.1523-1755.2000.00164.x. [DOI] [PubMed] [Google Scholar]


