Abstract
Objective
Notch is known as a transmembranous receptor family with four homologous forms - Notch 1, Notch 2, Notch 3, and Notch 4 and related to cell fate regulation and angiogenesis. The purpose is to investigate the effect of follicular stimulating hormone (FSH) on the Notch 1 expression and proliferation in ovarian cancer cells.
Methods
Human ovarian cancer cell line, SK-OV-3 and FSH were used. XTT cell proliferation and cell migration assay were carried out with FSH 100 mIU/mL and Notch 1 siRNA. Western blots and reverse transcriptase-polymerase chain reactions (RT-PCR) were carried out to determine the expression level of the Notch 1 protein and mRNA with FSH treatment in 0, 1, 5, 10, 100, 200, 300 mIU/mL concentrations. Immunofluorescent (IF) stains were performed in SK-OV-3 cell cultures with FSH 100 mIU/mL. Student-t tests were used in statistical analyses.
Results
The SK-OV-3 have Notch 1 receptors in their natural status. FSH stimulated SK-OV-3 cells in XTT cell proliferation and cell migration assays and notch 1 siRNA inhibited. The expression level of Notch 1 protein and mRNA were increased in a dose dependent pattern according to FSH concentrations compared to untreated cells. IF stains also showed brighter Notch1 expressions in the FSH treated cells compared to the control cells.
Conclusion
FSH enhances proliferation & migration and Notch 1 signaling in SK-OV-3 cells. The Notch signaling probably supports one of the cell proliferating mechanisms of FSH in ovarian cancer cells.
Keywords: Follicular stimulating hormone, Notch 1, Ovarian cancer
INTRODUCTION
Ovarian cancer is such an aggressive disease that the overall mortality rate reaches to about 50%.1 Notch proteins are a family of single-pass transmembranous receptors that regulate cell fate decisions during development, including the differentiation of arterial versus venous fates in the vascular system.2 There are four human Notch transmembranous receptors and five known ligands, which must be presented by adjoining cells to activate signaling. If activated Notch proteins undergo sequential internal cleavage and translocation into the nucleus, they will form transcriptional active complexes, and induce transcription of a group of effecter proteins including Hes 1, hairy enhancer-of-split related with YRPW motif 1 (Hey1), Hey 2, Hey L, and others.3 Notch receptors and vascular endothelial growth factors have important roles in the angiogenesis, especially in tumors.4
Follicular stimulating hormone (FSH) is a glycoprotein composed with α and β subunits.5 It is synthesized and secreted by the pituitary gland and stimulates pre-ovulatory follicles of the ovaries and is related to steroidogenesis.6 It is used in the ovulation induction of infertile women.7 Some ovarian cancer cells have the receptors for FSH.8 The promoting activity of proliferation and multiple gene expression of FSH in ovarian cancer cells has been known.9-11
The Notch 1 expression in ovarian tumors was reported recently12 and FSH can change the ovarian cancer cells into various conditions. We designed this study to see the FSH effect on cell proliferation and the Notch 1 expression in ovarian cancer cells, SK-OV-3.
MATERIALS AND METHODS
1. Cell lines and culture conditions
The SK-OV-3 cell line was purchased from the Korean Cell Bank. Cells were maintained in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS; Sigma). For monolayer cultures, the cell lines were maintained on Falcon tissue culture dishes (Becton Dickinson, Franklin Lakes, NJ, USA). The cells were cultured in a humidified atmosphere of 5% CO2: 95% air at 37℃. FSH (Follimon, LG Bioscience, Iksan Korea), which was added in various concentrations (0, 1, 5, 10, 50, 100, 200, 300 mIU/mL). After 24 hours of FSH treatment, cells were harvested to perform the procedures.
2. Cell counting
The cell numbers were counted before and after the FSH treatments with the hematocrit counting method. The cells were seeded at a density of 2×105 cells in 100 mm culture dishes. In order to correct the effect of cell number differences we divided the density of western blotting by the counted cell numbers and multifold one thousand.
3. Notch 1 small interfering RNA transfection
siRNA-mediated silencing of the Notch 1 gene was performed as described elsewhere, with slight modification. Briefly, cells were transfected with 10 microliter of siRNA duplexes against Notch 1 using Lipofectamine Plus reagent (Invitrogen, Paisley, UK) according to the manufacturer's instructions.
4. XTT assay
Cell proliferation assays were done by XTT assay. Because the cells were grown well in FSH 100 mIU/mL the concentration was used for comparing tests. After seeding cells (106 cells/cm2), incubation of cancer cells (RPMI1640 media in 10% FBS for SK-OV-3) for 24 hours in 96-well culture plate (BD, Frankin Lakes, NJ, USA) were done. Prepared XTT (2,3-Bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt) and phenazine methosulfate (PMS) reagent (WEL GENE, Seoul, Korea) were thawed completely in temperature 37℃ and Welcount Cell Viability Assay Kits (WEL GENE) were used. One mL of XTT reagent was mixed in 20 microliters PMS reagent. After adding 20% mixed media (20 microliter) into wells cautiously, plates were enrolled to mix the media. After the mixing, cells were cultured in 37℃, 5% CO2 incubator (MCO-20AIC, Sanyo, Japan) for 2 hours. XTT formazan solutions were made to spread evenly through light tapping. Using the microplate reader (Molecular devices, Minneapolis, MN, USA), we measured the absorbance (450 to 690 nm).
5. Cell migration assay
After culturing cells for 24 hours and 2 washings with PBS, serum free media was added. Slits using 200 microliter pipette tips were made on the cells after the confluent growing state. FSH 100 mIU/mL was treated on the cells except for the control plates. Cell migration distances were calculated after measuring the distance at every 12, 24, and 48 hours using a microscope. Every test was performed more than 3 times.
6. RNA extraction and RT-PCR
The total RNA was extracted using the RNeasy/QIAamp Kit (QIAGEN, Basel, Switzerland). Contaminating genomic DNA was removed by DNase I treatment according to the manufacturer's instructions (RNase-Free DNase Set, QIAGEN). Reversed transcription was performed using 1 microgram total RNA, 1 microgram primer oligo dT primers (Roche, Rotkreuz, Switzerland), 1.3 microliter PCR nucleotide Mix, 10 mM deoxynucleotide triphosphate (Roche), 28 U rRNasin (Promega, Wallisellen, Switzerland), 5 microliter M-MLV 5×buffer (Promega), 200 UM-MLV reverse transcriptase (Promega) in a final volume of 25 microliter. PCR was done at least as duplicates as follows: 5 microliter cDNA as template were added to a mastermix made of 4 microliter 5×PCR buffer+Mg (Bioneer, Daejeon, Korea), 3 microliter glycerol, 4 microliter PCR nucleotides mix, 1 mM deoxynucleotide triphosphate (Bioneer), 0.8 U Tag DNA polymerase (Bioneer), sense and antisense primers with a final concentration of 260 nM. Thermocycler conditions are described elsewhere. The annealing temperature was optimized for each primer pair and thirty five to thirty seven cycles were applied. The Notch 1 primer sequence for the RT-PCR is sense: 5'-AGATCAACCTGGATGACTGTGCCAGCAG-3', antisense: 5'-ASGACATGACCAGTGGCTACGTGTGCAC-3'. The glyceraldehydes-3-phosphate dehydrogenase (GAPDH) primers were used for quality control of RT-PCR for each cDNA generated, sense: 5-GAGCTGAACGGGAAGCTCACTGG-3; antisense: 5-CAACTGTGAGGAGGGGAGATTCA G-3. PCR products were separated on a 1 percent agarose gel containing ethidium bromide. The densities of the bands were measured by the program. The density values were analyzed statistically.
7. Western blotting
Cells were lysed in RIPA buffer (150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium desoxycholate, 0.1% sodium deoxy sulfate, 0.5 mM Tris, pH 8.0) on ice for 30 minutes, followed by centrifugation for 20 minutes at 4℃. Quantification was done by Bradford assay (Bio-Rad). In all, 50 microgram total cellular protein per lane was size fractionated on a 7% tris-acetate gel (Invitrogen, Paisley, UK) for Notch 1 detection and bottled onto nitrocellulose (Pontan; Schleicher and Schuell, Kassel, Germany). Equal loading and transfer efficiency was visually checked by Ponceau staining. Membranes were blocked for 3 hours at room temperature with 5% w/v nonfat dry milk/TBS and Tween-20 (0.05% w/v). Membranes were incubated with rabbit polyclonal anti-Notch 1 antibodies (H-131, Santa Cruz, Nunningen, Switzerland) for the detection of the extracellular domain of Notch 1. HeLa cells were used as positive controls for Notch 1 expression.13 Experiments were repeated more than 3 times. The densities of the bands were measured by the program. The density values were analyzed statistically.
8. Immunofluorescent (IF) staining
Prepared cells of 24-hour FSH 100 mIU/mL treatment were used for IF staining. Primary polyclonal Notch1 antibody from rabbit (Abcam, Swiss) concentration was 1 : 100 for 2 hours. Washings were done four times for 5 minutes each. Secondary antibody concentration was 1 : 200 for 1 hour. 5% BSA/PBS was used as a blocking solution for 2 hours. Washings were done four times for 10 minutes each. Photographs were obtained with a fluorescent microscope.
9. Stastistic analysis
Data are expressed as means±standard deviation. Student-t tests were used in analyzing data. A p-values below 0.05 was considered statistically significant.
RESULTS
1. SKOV-3 Cell proliferations were increased under FSH influence
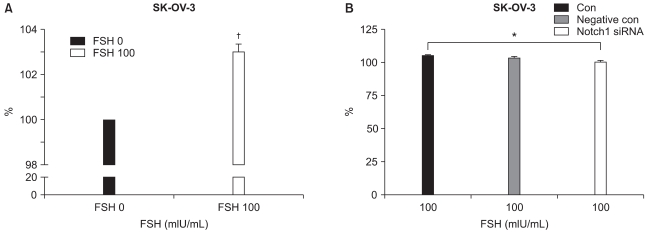
To see the FSH effect on ovarian cancer cell, cell proliferation assay with XTT assays were carried out. Mean values of XTT assays were 100±0 and 103±1.25 (N=12; p=0.001) in control and FSH 100 mIU/mL (Fig. 1A). FSH stimulates SKOV-3 cells to proliferate more than cells of control.
Fig. 1.
The XTT assays of ovarian cancer cell line, SK-OV-3. (A) The cell proliferation effect of follicular stimulating hormone (FSH, 100 mIU/mL) in SK-OV-3 is significantly higher than the control (FSH 0 mIU/mL), 103 vs. 100 (p=0.0001, N=12). (B) Cell proliferations were inhibited significantly by Notch 1 siRNA in FSH 100 mIU/mL treated conditions 104.83 to 100.37 (p=0.009, N=8). *,†statistically significant, p<0.01.
2. FSH's cell proliferating effect was inhibited by Notch 1 siRNA
To see the Notch 1 specific activity in response to FSH, Notch 1 gene was inhibited with siRNA in FSH (100 mIU/mL) treated condition and XTT assays were performed. The mean values of XTT assays were 104.83±3.23 (control), 103.35±2.59 (negative control), and 100.37±3.65 (siRNA treated). The p-value was 0.0086 between control vs. siRNA treated ones (N=8) (Fig. 1B). The FSH effects on SKOV-3 cells were suppressed by siRNA. Notch signaling may have relationship with FSH signaling.
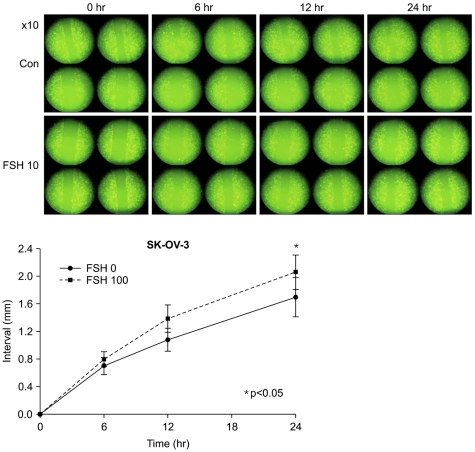
3. FSH stimulated ovarian cancer cells to migrate more
In FSH 0 vs. 100 mIU/mL, moved distances of cells 6, 12, 24 hour after FSH treatment were 0.699±0.25 cm vs. 0.796±0.21 cm (p=0.158); 1.076±0.33 cm vs. 1.384±0.40 cm (p=0.138); 1.696±0.57 cm vs. 2.054±0.50 cm (p=0.043) respectively (Fig. 2). Moved distances of FSH treated cells were longer than the ones of control at 24 hour of incubations.
Fig. 2.
Cell migration assay by slit tests shows that follicular stimulating hormone (FSH) 100 mIU/ mL made more distant movement of cells dependent on time than the control (FSH 0 mIU/mL).
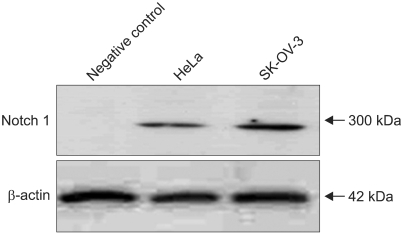
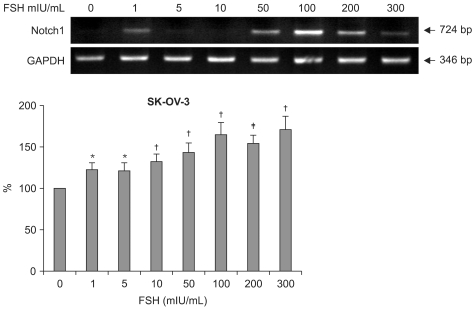
4. Notch 1 mRNA up-regulated by FSH dose dependently in SKOV-3 cells
After seeing the Notch 1 protein expressions in gynecologic cancer cells, HeLa and SKOV-3 (Fig. 3), semi-quantitative RT-PCRs were performed with SKOV-3 cells. The mRNA of Notch 1 gene expressions levels by RT-PCR were as follows. In control (FSH 0 mIU/mL), the mean density was 116.44±23.22 (p=0.078). In FSH 1 mIU/mL, the mean density was 114.58±22.97 (p=0.098). In FSH 5 mIU/mL, the mean density was 123.77±25.60 (p=0.092). In FSH 10 mIU/mL, the mean density was 133.49±33.05 (p=0.065). In FSH 100 mIU/ mL, the mean density was 149.26±46.93 (p=0.043). In FSH 200 mIU/mL, the mean density was 142.99±32.49 (p=0.048). In FSH 300 mIU/mL, the mean density was 156.44±46.07 (p=0.022) (Fig. 4). Dose dependent increase means the FSH's effects on SKOV-3 cells are real in RNA level.
Fig. 3.
The Notch 1 protein was examined by western blotting. HeLa and SK-OV-3 cells produce Notch 1 protein (300 KD) spontaneously without any drug treatment.
Fig. 4.
RT-PCRs of Notch 1 mRNA in SK-OV-3 cells showing that follicular stimulating hormone (FSH) increases the gene expressions of Notch 1 in a dose dependent pattern. The lanes mean the concentration of the FSH 0, 1, 5, 10, 50, 100, 200, and 300 mIU/mL respectively. *statistically significant, p<0.05, †,‡statistically significant, p<0.01.
5. FSH stimulated Notch 1 expressions of SKOV-3 dose dependently
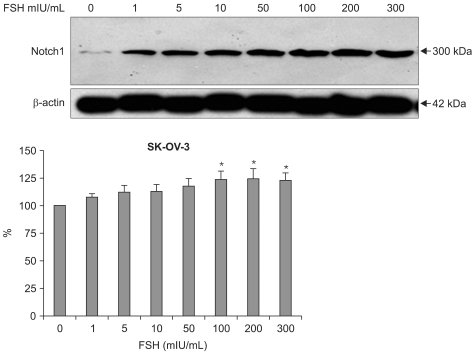
Notch 1 protein expressions levels by western blotting were as follows. In control (FSH 0 mIU/mL), mean band density was 100±0. In FSH 1 mIU/mL, mean band density was 107.69±7.30 (p=0.0781). In FSH 5 mIU/mL mean band density was 112.33±12.82 (p=0.0980). In FSH 10 mIU/mL mean band density was 11.04±13.24 (p=0.0924). In FSH 50 mIU/mL mean band density was 117.70±15.66 (p=0.0648). In FSH 100 mIU/mL mean band density was 123.65±18.10 (p=0.0432). In FSH 200 mIU/mL mean band density was 124.86±19.72 (p=0.0479). In FSH 300 mIU/mL mean band density was 123.34±14.37 (p=0.0221) (Fig. 5). Dose dependent increasing pattern of Notch 1 reveals that the FSH's upregulatory effect of Notch 1 on SKOV-3 cells in RNA levels are true.
Fig. 5.
The western blotting of Notch 1 protein (300 KD) in SK-OV-3 shows follicular stimulating hormonal (FSH) stimulatory effect. According to the FSH concentrations, the protein expressions are increasing. *statistically significant, p<0.05.
DISCUSSION
We examined the stimulatory effect of FSH on cancer cell proliferation by XTT assay. On XTT cell proliferation assay, the ovarian cancer cell line SK-OV-3 cell had grown significantly under the FSH 100 mIU/mL influence, 100 vs. 103 (Fig. 1A, p=0.02). siRNA of Notch 1 made significant cell proliferation inhibition in XTT assay as 104.83 (control), 103.35 (negative control, p=0.061) and 100.37 (siRNA treated, p=0.0086) in FSH 100 mIU/mL treated condition (Fig. 1B). Concerning cell migration by slit test, the cells moved more in FSH 100 mIU/mL treated cells (Fig. 2). Cell migration distances were significantly different over 24 hour incubation periods. These results suggest that FSH stimulated cell proliferation and migration in ovarian cancer cells. The FSH effect can be suppressed by adding siRNA of Notch 1.
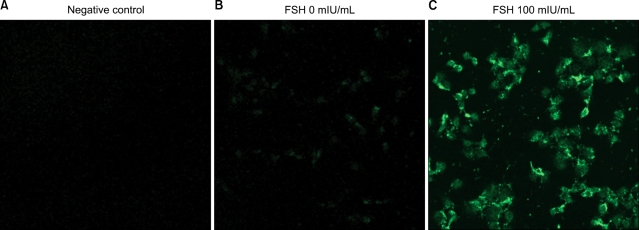
Next, we tested the cancer cells to determine whether they have Notch1 molecules by western blotting. The notch 1 expression was observed in HeLa (known already) and SK-OV-3 without FSH stimulation (Fig. 3). And the enhanced Notch 1 gene expression phenomenon in mRNA level by FSH was observed using semi-quantitative RT-PCR in ovarian cancer cells, SK-OV-3. The significant differences were made in every FSH concentration 1, 5, 10, 50, 100, 200 and 300 mIU/mL (Fig. 4). And the enhanced Notch 1 protein expression in SK-OV-3 by the FSH in a dose dependent pattern was also observed with western blotting. FSH concentrations 100, 200 and 300 mIU/mL produced significant outcomes (Fig. 5). 100 mIU/mL of FSH made an obvious difference in brightness with immunofluorescent staining (Fig. 6). FSH seems to stimulate ovarian cancer cells to produce more Notch 1. In ovarian cancer, reports of the Notch protein signaling are already known.12 It is also well known that cervical cancer, breast cancer, lung cancer and lymphoma cells are closely related with Notch signaling.13-16 But its relation to FSH was not known until now in ovarian cancer.
Fig. 6.
The Notch1 immunofluorescent staining of SK-OV-3 cells with follicular stimulating hormone (100 mIU/ml). There are marked differences in fluorescence brightness. Negative control shows no signal (A), cells of right box (C) are much brighter than the cells of left one (B) (×200).
In ovarian cancer cell activity, this report is important in several aspects. First, if the cell proliferation is positively correlated with FSH concentration, the cancer cells grow well under the influence of the FSH elevating environments.11 This process could be related to the carcinogenic process in ovarian cancer. Second, FSH stimulation can be a recurrence precipitating mechanism in ovarian cancer patients after primary therapy. Remaining ovarian cancer cells can grow easily in an FSH elevated milieu after ovarian cancer cytoreductive surgery or adjuvant chemotherapy. If a low FSH level is maintained, it may be helpful in epithelial ovarian cancer patients. It could not be in all of the ovarian cancer cells because FSH receptors are not present in all of the ovarian cancer cells.8 But it is known that FSH receptors are more prevalent in ovarian cancer cells than in benign or normal ovarian tissues.11 Third, the FSH effect can be related with Notch signaling because notch 1 expresses more with a high concentration of FSH and Notch 1 siRNA inhibited the proliferation effect.
In conclusion, FSH acts as a stimulator of Notch 1 production in ovarian cancer cells and it is probably related to cancer cell growth. It is also likely that the elevated FSH is an aggravator of ovarian cancer, and Notch signaling could be a mediator of the process. Co-inhibiting FSH and Notch signaling may be helpful in ovarian cancer treatment. Further studies in genetics and in vivo research remain for the future of this theory.
ACKNOWLEDGEMENTS
This article is granted by the Hallym University Foundation of South Korea, grant number 01-2005-24.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Berg JW, Lampe JG. High-risk factors in gynecologic cancer. Cancer. 1981;48:429–441. doi: 10.1002/1097-0142(19810715)48:1+<429::aid-cncr2820481303>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 3.Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 4.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 5.Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15:378–389. doi: 10.1210/mend.15.3.0603. [DOI] [PubMed] [Google Scholar]
- 6.Monniaux D, Huet C, Besnard N, Clement F, Bosc M, Pisselet C, et al. Follicular growth and ovarian dynamics in mammals. J Reprod Fertil Suppl. 1997;51:3–23. [PubMed] [Google Scholar]
- 7.van der Meer M, Hompes PG, Scheele F, Schoute E, Veersema S, Schoemaker J. Follicle stimulating hormone (FSH) dynamics of low dose step-up ovulation induction with FSH in patients with polycystic ovary syndrome. Hum Reprod. 1994;9:1612–1617. doi: 10.1093/oxfordjournals.humrep.a138761. [DOI] [PubMed] [Google Scholar]
- 8.Yoo HR, Yoon MK, Park YH, Park HR, Jang PR. The effects of gonadotropins on the development of ovarian cancer. Korean J Obstet Gynecol. 2004;47:1698–1705. [Google Scholar]
- 9.Simon WE, Albrecht M, Hansel M, Dietel M, Holzel F. Cell lines derived from human ovarian carcinomas: growth stimulation by gonadotropic and steroid hormones. J Natl Cancer Inst. 1983;70:839–845. [PubMed] [Google Scholar]
- 10.Wimalasena J, Dostal R, Meehan D. Gonadotropins, estradiol, and growth factors regulate epithelial ovarian cancer cell growth. Gynecol Oncol. 1992;46:345–350. doi: 10.1016/0090-8258(92)90230-g. [DOI] [PubMed] [Google Scholar]
- 11.Ji Q, Liu PI, Chen PK, Aoyama C. Follicle stimulating hormone-induced growth promotion and gene expression profiles on ovarian surface epithelial cells. Int J Cancer. 2004;112:803–814. doi: 10.1002/ijc.20478. [DOI] [PubMed] [Google Scholar]
- 12.Hopfer O, Zwahlen D, Fey MF, Aebi S. The Notch pathway in ovarian carcinomas and adenomas. Br J Cancer. 2005;93:709–718. doi: 10.1038/sj.bjc.6602719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zagouras P, Stifani S, Blaumueller CM, Carcangiu ML, Artavanis-Tsakonas S. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc Natl Acad Sci U S A. 1995;92:6414–6418. doi: 10.1073/pnas.92.14.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 15.Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin Cancer Biol. 2004;14:357–364. doi: 10.1016/j.semcancer.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]