Abstract
Objective
Absence of dysplasia in the excised specimen following loop electrosurgical excision procedure (LEEP) for treatment of cervical intraepithelial neoplasia (CIN) 2/3 is an occasional finding of uncertain clinical significance. We evaluated several factors including age, liquid-based Pap (LBP) test, human papillomavirus (HPV) load before treatment, and HPV typing as predictors for absence of dysplasia. Absence of dysplasia in LEEP specimens was analyzed in terms of factors for recurrent disease after LEEP conization
Methods
In total, 192 women (mean age, 39.3±8.4 years; range, 24 to 70 years) with biopsy-proven CIN 2/3 were treated by LEEP conization. Age, LBP test, histological grade, HPV load, and HPV DNA typing were evaluated as possible predictors of the absence of residual dysplasia or recurrent disease.
Results
Of the LEEP specimens, 34 (17.7%) showed no dysplasia in preoperative biopsies from patients with proven CIN 2/3. Low HPV load (<100 relative light units [RLU]) was significantly related to the absence of dysplasia in LEEP specimens, using logistic regression. Margin involvement and high HPV load (≥400 RLU) were significant factors for recurrence.
Conclusion
Absence of dysplasia in LEEP specimens occurred in 17.7% of our specimens. Prediction of the absence of dysplasia in LEEP specimens was associated with low HPV load. Residual/recurrent disease after LEEP was associated with a positive resection margin and high viral load, and was not associated with absence of dysplasia in LEEP specimens. Even if there is no dysplasia in conization specimens, close follow-up for residual/recurrent disease is needed.
Keywords: Dysplasia, LEEP, Margin involvement, Human papillomavirus, Viral load
INTRODUCTION
Cervical cancer remains one of the major problems in female health worldwide and is preventable by early detection and treatment of precancerous, high-grade cervical intraepithelial neoplasm (CIN 2/3). Progression to high-grade CIN or invasive cancer in women with human papillomavirus (HPV) infection requires persistent high-risk infection.1-3
The loop electrosurgical excision procedure (LEEP) is recommended for treatment of CIN 2/3 lesions. LEEP was introduced in 1989 to treat cervical dysplastic lesions that could be completely visualized at the time of colposcopy. During recent years, indications have extended beyond cases that involve the exocervix to include lesions with margins not defined at colposcopy.4 Advantages of LEEP include safety, cost-effectiveness, histological evaluation, and performance under local anesthesia in an office setting.5
Although dysplasia is identified by colposcopic biopsy, specimens from LEEP some times show no residual dysplasia in the excised tissue. There have only been a few studies on absence of dysplasia in LEEP specimens, and the clinical significance of this phenomenon has not been determined.
Known risk factors for recurrence of cervical dysplasia after conization are well known. Specifically, age, cytological grade, menopausal status, resection margin involvement, and HPV load are all risk factors for recurrent disease after CIN treatment.6,7
The aim of this study was to establish the rate of absence of dysplasia in LEEP specimens after treatment of CIN 2/3, and the predictive factors involved. In addition to the prediction of no dysplastic lesion, the impact on recurrence was analyzed by comparing other factors, including age, preoperative cytology, punch biopsy grade, HPV status, and margin involvement.
MATERIALS AND METHODS
1. Study population
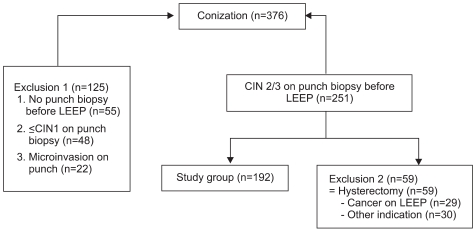
During the study period, 376 LEEPs were performed in the Department of Obstetrics and Gynecology, Soonchunhyang University Bucheon Hospital, Korea, between January 2003 and December 2005. Inclusion criteria were CIN 2/3 on colposcopic punch biopsy, and LEEP treatment and follow-up, without additional hysterectomy. The indications of colposcopy guided biopsy were mainly an abnormal cervical cytology, positive high-risk HPV infection in the absence of an abnormal cytology, abnormal cervicographic finding, irregular cervical contour, and postcoital bleeding. According to the inclusion criteria, 192 patients were selected for this study (Fig. 1). At the pretreatment visit, all women were subjected to an interview regarding clinical, social, and demographic data. After being interviewed, a gynecological examination was performed followed by liquid-based Pap (LBP) test, hybrid capture II (HC2) assay, HPV DNA typing, and colposcopic examination of the cervix.
Fig. 1.
Patients profile. LEEP: loop electrosurgical excision procedure, CIN: cervical intraepithelial neoplasia.
We determined age, HC2 viral load, and LBP test results as predictive factors of the absence of residual dysplasia in LEEP specimens. Age was divided into ≥40 and <40 years. HPV load was divided into high (≥100 relative light units [RLU]) and low (<100 RLU). Preoperative LBP test results were divided into low-grade and high-grade groups. HPV genotyping was divided into type 16 infection, and negative or non-type 16?high-risk infection. For the prediction of recurrence after conization, HPV load was divided into high (≥400 RLU) and low (<400 RLU) load.
The definition of recurrent disease during follow-up was biopsy-proven CIN≥2, as demonstrated by colposcopy-guided biopsy.
2. LBP
The LBP test (ThinPrep; Cytic Corp., Boxborough, MA, USA) was performed using a plastic soft spatula and endocervical cytobrush (Medland, Seoul, Korea). All specimens were stained using the Papanicolaou Method. Final cytological diagnosis was achieved using the Bethesda System. Diagnosis was classified as normal or inflammatory, atypical squamous cells (ASC), low-grade squamous intraepithelial lesion (LSIL), or high-grade squamous intraepithelial lesion (HSIL).
3. Detection of HPV
Samples for the HC2 assay were obtained using a cytobrush (Digene Cervical Sampler) during a second swab of the cervix, and transferred to a vial that contained Digene Specimen Transport Medium. The samples were tested with 13 oncogenic genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), and the tests were classified as positive at a relative light unit/cutoff (RLU/CO) ratio of ≥1 pg/mL. Light measurements were quantified using a luminometer, and were expressed by comparing the RLU of clinical samples with a positive control (PC). RLU/PC ratios were calculated as the ratio of the luminescence of the specimen to that of the 1.0 pg/mL HPV 16 cutoff standard. With this cutoff value, HC2 has shown a high sensitivity and negative predictive value, >90-95% in most reported studies.8
For HPV genotyping, a commercial HPV DNA chip (MyHPV Chip) was used. The HPV chip can detect 24 type-specific HPVs (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 54, 56, 58, 59, 66, and 68) and eight of the low-risk group (6, 11, 34, 40, 42, 43, 44, and 70). Target HPV DNA was amplified by PCR with primers (HPV and human β-globulin) and conditions provided by Mygene (Seoul, Korea), and labeled using Cy5-deoxyuridine triphosphate (NEM Life Science Products, Boston, MA, USA). The PCR product was hybridized on the chip at 40℃ for 2 h and washed with 3× SSPE (3.0 M sodium chloride, 0.2 M sodium hydrogen phosphate, 0.02 M EDTA, pH 7.4). Hybridized signals were visualized with a DNA chip scanner (GSI Lumonics; ScanArray Lite, Ottawa, Canada).
4. LEEP
The cervix was exposed using an adapted speculum that allowed smoke evacuation. Local anesthesia was induced with 2% lidocaine plus epinephrine injection at the 3, 6, 9, and 12 o'clock positions of the cervix, with a 31 G dental needle. The electrosurgical procedure was performed with a high-frequency electrical generator. The loop was selected according to the size of the area to be excised. If the exocervical lesion was too large to be accommodated by a single sweep, it was excised with two or more systematic sweeps, and the specimens were gathered into their original anatomical shape by the operator, before sending them to the pathology laboratory to establish the true excisional margins of the specimens. The excised wound base was cauterized by ball diathermy. When an endocervical extension was suspected, an additional apical specimen was taken using a small wire loop electrode. The 12 o'clock position in the excised specimen was marked by cutting.
5. Pathological examination
LEEP specimens were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. If more than one sweep was required, each specimen was processed independently. Histological examination of all specimens was performed by an experienced gynecologic pathologist (KE). All specimens were reviewed with special attention to the marginal status (exocervical or endocervical, clear or involved) and glandular involvement (present or absent). Biopsies were analyzed according to the World Health Organization criteria and classified as negative, CIN 1, CIN 2, CIN 3 or micro-invasive carcinoma. Microinvasive cervical carcinoma was defined as a lesion that invaded below the basement membrane to a depth of ≤5 mm, and that did not spread >7 mm horizontally.
6. Follow-up
Post-LEEP follow-up was performed every 3-6 months during the 2 years after LEEP, and annually thereafter. Cervical cytology and HPV tests were obtained at each visit, and referrals for colposcopy-guided biopsies were indicated for patients with abnormal cytological findings, regardless of HPV status.
7. Statistical analysis
Statistical analyses of the data were performed using SPSS ver. 12 (SPSS Inc., Chicago, IL, USA). Statistical tests used were Student's t-test, Fisher's exact test, and logistic regression analysis. All p-values were 2-sided and were deemed to indicate statistical significance at p<0.05.
RESULTS
1. Patient characteristics
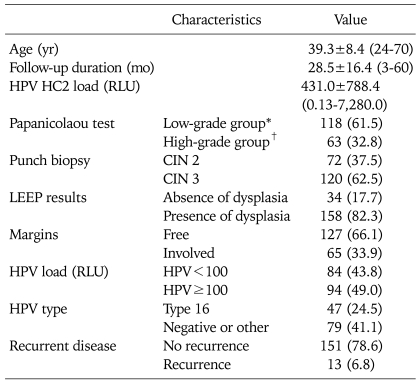
The average age of the 192 patients was 39.3±8.4 years (range, 24 to 70 years), and their characteristics are summarized in Table 1.
Table 1.
Patient characteristics (N=192)
Values are presented as average±SD (range) or number (%).
HPV: human papillomavirus, HC2: hybrid capture 2, RLU: relatively level of unit, CIN: cervical intraepithelial neoplasia, LEEP: loop electrosurgical excision procedure.
*Low grade group: normal, atypical squamous cells (ASC)-US, low-grade squamous intraepithelial lesion. †High grade group: ASC-H, high-grade squamous intraepithelial lesion, and cancer.
2. HPV and biopsy results
The Pap test before LEEP showed that 118 (61.5%) patients had low-grade (10 normal, 83 ASC-US, 25 LSIL) CIN, and 63 (32.8%) had high-grade CIN (63 ASC-H and HSIL or greater). Of the patients, 72 (37.5%) had CIN 2 and 120 (62.5%) had CIN 3, as determined from the punch biopsy specimens. The average preconization HPV viral load was 431.0±788.4 RLU. Patients were divided into two groups, based on preconization loads of <100 (84, 43.8%) or ≥100 (94, 49.0%) RLU, after deciding on a value of 100 RLU as a cutoff for the analysis. The HPV genotype analysis was divided into two groups according to the presence or absence of HPV 16 infection. HPV 16 infection was present in 47 (24.5%) of the patients and 79 (41.1%) of the patients were negative or had other type infections.
LEEP specimens showed no lesions in 34 (17.7%) and residual lesions in 158 (82.3%) cases. All fifty-nine cases of exclusion 2 who underwent the hysterectomy revealed dysplasia in the LEEP specimen. Provided that cases were included in terms of an absence of dysplasia in the LEEP specimen, the rate of an absence of dysplasia in the LEEP specimen with punch-biopsy proven CIN 2/3 was 13.5% (34/251). Residual lesions included CIN 1 (8, 4.2%), CIN 2 (26, 13.5%), CIN 3 (113, 58.9%), and microinvasive cancer (11, 5.7%). The status of the margins at the excision site was available in 192 participants: free margins were observed in 127 (66.1 %) and 65 (33.9%) patients had involved margins.
3. Predictors of absence of residual disease
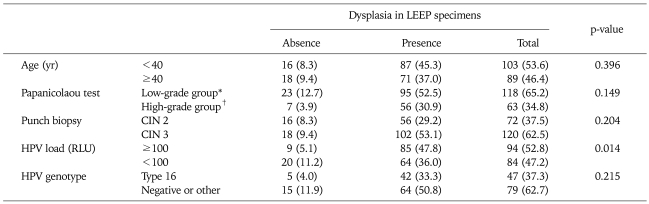
Table 2 shows risk factors for the absence of dysplasia in LEEP specimens. Of 192 specimens, 34 (17.7%) showed an absence of dysplasia in the excised specimen; 16 of the 34 patients with no dysplasia were aged <40 years, and 18 were aged ≥40 years (p=0.396).
Table 2.
Risk factors predicting absence of dysplasia in LEEP specimens for treatment of CIN 2/3
Values are presented as number (%).
LEEP: loop electrosurgical excision procedure, CIN: cervical intraepithelial neoplasia, HPV: human papillomavirus, RLU: relatively level of unit.
*Low grade group: normal, atypical squamous cells (ASC)-US, low-grade squamous intraepithelial lesion. †High grade group: ASC-H, high-grade squamous intraepithelial lesion, and cancer.
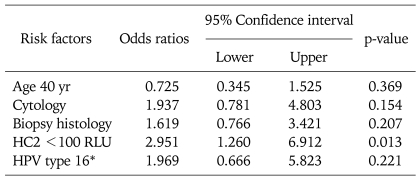
Preoperative punch biopsy grade and HPV genotype (type 16 vs. other types or negative cases) were not related to the absence of dysplasia in LEEP specimens (p=0.204 and 0.215, respectively). Only low HPV load (cutoff=100 RLU) was significantly related to prediction of the absence of dysplasia in LEEP specimens (p=0.014). The odds ratio (OR) of HPV load <100 RLU of HC2 was 2.951 (95% confidence interval, 1.260 to 6.912; p=0.013) Table 3.
Table 3.
Logistic regression analysis of risk factors predicting absence of dysplasia in LEEP specimens
LEEP: loop electrosurgical excision procedure, HC2: hybrid capture 2, RLU: relatively level of unit, HPV: human papillomavirus.
*HPV type 16 versus other type including negative cases.
4. Predictors of recurrent disease during follow-up
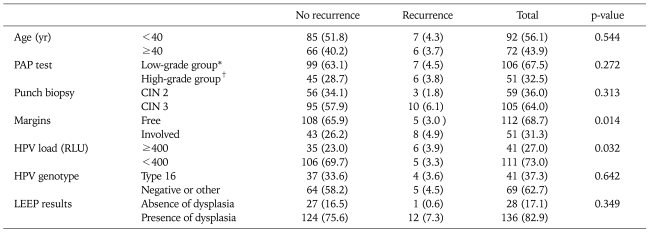
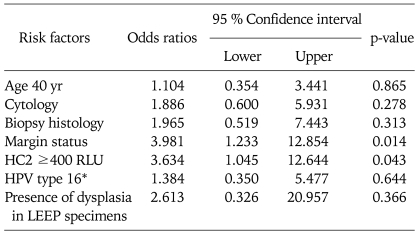
Table 4 shows the risk factors in relation to recurrent disease. Thirteen patients (6.8%) developed recurrent disease during follow-up. Age, Pap test prior to LEEP, punch biopsy, and HPV genotype were not associated with recurrence after LEEP. Margin status and high HPV load were significant independent factors related to recurrence after LEEP. Among 151 patients with no recurrence, 108 of 151 (65.9%) had free margin status and 43 of 151 (26.2%) had histological abnormalities (p=0.014). High HPV load (≥400 RLU) also was significantly related to recurrence (p=0.032). Logistic regression analysis showed that margin involvement and high preoperative HPV load were also significantly related to residual CIN and/or recurrence (OR, 3.981 and 3.634, respectively) (Table 5).
Table 4.
Risk factors for predicting recurrent disease
Values are presented as number (%).
PAP: papanicolaou, CIN: cervical intraepithelial neoplasia, HPV: human papillomavirus, RLU: relatively level of unit, LEEP: loop electrosurgical excision procedure.
*Low grade group: normal, atypical squamous cells (ASC)-US, low-grade squamous intraepithelial lesion. †High grade group: ASC-H, high-grade squamous intraepithelial lesion, and cancer.
Table 5.
Logistic regression analysis of risk factors for predicting recurrent disease after LEEP treatment
LEEP: loop electrosurgical excision procedure, HC2: hybrid capture 2, RLU: relatively level of unit, HPV: human papillomavirus.
*HPV type 16 versus other type including negative cases.
One patient among the 28 with absence of dysplasia in LEEP specimens showed recurrence of CIN 3 during follow-up 44 months later after LEEP. In detail, she was 26-year old, the preoperative pap HSIL, preoperative HPV HC2 viral load 0.24 RLU, HPV genotype 39 and 56 positive, and punch biopsy before LEEP CIN 2. Whether dysplasia was present in LEEP specimens was not significantly related to recurrence (p=0.349).
DISCUSSION
High-grade CIN 2/3 has the potential to develop into cervical cancer if untreated.9 Conservative treatment for high-grade CIN 2/3 has been shown to reduce the risk of developing invasive cervical carcinoma. LEEP has been used widely as an effective treatment, and has achieved cure rates similar to those for other conservative treatments.10
Although the absence of residual lesions in LEEP specimens is not uncommon, there have been few reported studies on this phenomenon to date. In our study, the rate of the absence of dysplasia in LEEP specimens was 17.7% (34/192) in women with CIN 2/3 proven by colposcopy-guided biopsy before conization, similar to that in a previous report (16.4%).11 Sherman et al.12 found that the rate of the absence of residual disease in LEEP specimens was higher (33%) in women with low-grade Pap results, including ASC-US or LSIL.
There are several reasons for the absence of residual dysplasia in LEEP specimens. First, the CIN lesion is focal and small, and it is removed completely by punch biopsy.11,13 Sherman et al.12 observed that the number of cases of CIN 3 missed by enrollment colposcopy in the ALTS study was very small. The median length of CIN3 in the ALTS study was only 6.5 mm, and one third of lesions were so small that colposcopy did not leave any residual CIN 3 to be detected by LEEP.12 Second, the remaining small lesion after punch biopsy may undergo spontaneous regression. Third, CINs are missed and not removed by LEEP. This group can be filtered to have residual disease during follow-up. Fourth, the wrong pathological report can be obtained; the pathologist may fail to observe the area that contained the CIN.
We found no statistically significant differences in age, LBP test, or punch biopsy results in the prediction of the absence of dysplasia in LEEP specimens. In contrast, Li et al.11 reported that lesions in women with CIN 2, satisfactory colposcopy, and three or more biopsies, were more likely to be excised completely at the time of colposcopy-directed biopsy, resulting in negative LEEP specimens. In our study, low HPV load before biopsy (<100 RLU cutoff) was a significant predictive factor for the absence of dysplasia in LEEP specimens. The result differed from that of Kinney and Cohn14 who did not find that HPV DNA, Pap cytology, or colposcopy before LEEP was a predictive factor for absence of residual dysplasia in LEEP specimens.
Many studies have reported risk factors for residual and/or recurrent disease after treatment of CIN using LEEP. Risk factors analyzed include age, parity, menopausal status, lesion grade, glandular extension, and margin status.15,16 Many studies have revealed that marginal involvement in LEEP specimens, particularly endocervical margins, is a major risk factor for recurrence.17,18 In our study, margin status was a significant independent factor in relation to recurrence after LEEP. Reasons for recurrence after complete excision may include the possibility of multifocal lesions, inadequate specimens, and HPV DNA persistence.19,20 Older age has been shown to be a predictor for recurrence in other studies.21 In contrast, our results showed that age was not related to recurrence. High HPV load before LEEP may also be associated with recurrent disease.7 HPV persistence during follow-up after conization is significantly related to recurrence,22 as is high preoperative viral load in the negative margin in the excised cervix.23 A significant relationship was also found between high HPV load and recurrent disease in our study. HPV type 16 infection has been found to increase the risk of recurrence after treatment for CIN.24 In our study, HPV type 16 infection was not related to recurrence. Absence of dysplasia in LEEP specimens was not a predictor of recurrence, nor did it guarantee no recurrence at follow-up. Thus, close follow-up in cases without dysplasia after LEEP is needed, as in other patients who have undergone LEEP.
Limitations of this study include its' retrospective nature, and that the size of the cervical lesion during colposcopic examination and LEEP was not analyzed. However, the study had a reasonable sample size, and all women were managed by a single expert colposcopist with over 15 years' experience.
In conclusion, the results of the present study suggest that low HPV load before conization of the cervix is indicative of the absence of dysplasia in LEEP specimens. Also, high viral load before conization and margin involvement in LEEP conization were significant independent predictors of recurrence after conization. The absence of dysplasia in the LEEP specimens was not a predictor of recurrence or a guarantee for no recurrence at follow-up. Close follow-up of cases with no dysplasia in LEEP specimens is still needed, as it is in other patients who have undergone LEEP.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee HB, Lee YS, Lee KH, Kim CJ, Park JS. A case of advanced gynecologic pelvic tumors showing the diagnostic utility of HPV analysis. J Gynecol Oncol. 2009;20:251–253. doi: 10.3802/jgo.2009.20.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snijders PJ, Hogewoning CJ, Hesselink AT, Berkhof J, Voorhorst FJ, Bleeker MC, et al. Determination of viral load thresholds in cervical scrapings to rule out CIN 3 in HPV 16, 18, 31 and 33-positive women with normal cytology. Int J Cancer. 2006;119:1102–1107. doi: 10.1002/ijc.21956. [DOI] [PubMed] [Google Scholar]
- 3.Park JS, Hwang ES, Park SN, Ahn HK, Um SJ, Kim CJ, et al. Physical status and expression of HPV genes in cervical cancers. Gynecol Oncol. 1997;65:121–129. doi: 10.1006/gyno.1996.4596. [DOI] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 5.Eduardo AM, Dinh TV, Hannigan EV, Yandell RB, Schnadig VJ. Outpatient loop electrosurgical excision procedure for cervical intraepithelial neoplasia: can it replace cold knife conization? J Reprod Med. 1996;41:729–732. [PubMed] [Google Scholar]
- 6.Brockmeyer AD, Wright JD, Gao F, Powell MA. Persistent and recurrent cervical dysplasia after loop electrosurgical excision procedure. Am J Obstet Gynecol. 2005;192:1379–1381. doi: 10.1016/j.ajog.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Alonso I, Torne A, Puig-Tintore LM, Esteve R, Quinto L, Campo E, et al. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2-3. Gynecol Oncol. 2006;103:631–636. doi: 10.1016/j.ygyno.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Clavel C, Cucherousset J, Lorenzato M, Caudroy S, Nou JM, Nazeyrollas P, et al. Negative human papillomavirus testing in normal smears selects a population at low risk for developing high-grade cervical lesions. Br J Cancer. 2004;90:1803–1808. doi: 10.1038/sj.bjc.6601726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol. 2000;43:352–362. doi: 10.1097/00003081-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Prendiville W, Cullimore J, Norman S. Large loop excision of the transformation zone (LLETZ): a new method of management for women with cervical intraepithelial neoplasia. Br J Obstet Gynaecol. 1989;96:1054–1060. doi: 10.1111/j.1471-0528.1989.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 11.Li ZG, Qian de Y, Cen JM, Chen GD, Shu YH. Three-step versus "see-and-treat" approach in women with high-grade squamous intraepithelial lesions in a low-resource country. Int J Gynaecol Obstet. 2009;106:202–205. doi: 10.1016/j.ijgo.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of cervical intraepithelial neoplasia 3 lesions in the atypical squamous cells of undetermined significance low-grade squamous intraepithelial lesion triage study: implications for subject safety and lead-time bias. Cancer Epidemiol Biomarkers Prev. 2003;12:372–379. [PubMed] [Google Scholar]
- 13.Berdichevsky L, Karmin R, Chuang L. Treatment of high-grade squamous intraepithelial lesions: a 2- versus 3-step approach. Am J Obstet Gynecol. 2004;190:1424–1426. doi: 10.1016/j.ajog.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 14.Kinney SR, Cohn AR. Predicting "no residual dysplasia" in loop electrosurgical exision specimens. 2006 Biennial Meeting of ASCCP. J Low Genit Tract Dis. 2006;10:173. [Google Scholar]
- 15.Husseinzadeh N, Shbaro I, Wesseler T. Predictive value of cone margins and post-cone endocervical curettage with residual disease in subsequent hysterectomy. Gynecol Oncol. 1989;33:198–200. doi: 10.1016/0090-8258(89)90551-9. [DOI] [PubMed] [Google Scholar]
- 16.Phelps JY, 3rd, Ward JA, Szigeti J, 2nd, Bowland CH, Mayer AR. Cervical cone margins as a predictor for residual dysplasia in post-cone hysterectomy specimens. Obstet Gynecol. 1994;84:128–130. [PubMed] [Google Scholar]
- 17.Soutter WP, de Barros Lopes A, Fletcher A, Monaghan JM, Duncan ID, Paraskevaidis E, et al. Invasive cervical cancer after conservative therapy for cervical intraepithelial neoplasia. Lancet. 1997;349:978–980. doi: 10.1016/s0140-6736(96)08295-5. [DOI] [PubMed] [Google Scholar]
- 18.Paraskevaidis E, Kalantaridou SN, Paschopoulos M, Zikopoulos K, Diakomanolis E, Dalkalitsis N, et al. Factors affecting outcome after incomplete excision of cervical intraepithelial neoplasia. Eur J Gynaecol Oncol. 2003;24:541–543. [PubMed] [Google Scholar]
- 19.Nagai Y, Maehama T, Asato T, Kanazawa K. Persistence of human papillomavirus infection after therapeutic conization for CIN 3: is it an alarm for disease recurrence? Gynecol Oncol. 2000;79:294–299. doi: 10.1006/gyno.2000.5952. [DOI] [PubMed] [Google Scholar]
- 20.Costa S, De Simone P, Venturoli S, Cricca M, Zerbini ML, Musiani M, et al. Factors predicting human papillomavirus clearance in cervical intraepithelial neoplasia lesions treated by conization. Gynecol Oncol. 2003;90:358–365. doi: 10.1016/s0090-8258(03)00268-3. [DOI] [PubMed] [Google Scholar]
- 21.Moore BC, Higgins RV, Laurent SL, Marroum MC, Bellitt P. Predictive factors from cold knife conization for residual cervical intraepithelial neoplasia in subsequent hysterectomy. Am J Obstet Gynecol. 1995;173:361–366. doi: 10.1016/0002-9378(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 22.Park JY, Bae J, Lim MC, Lim SY, Lee DO, Kang S, et al. Role of high risk-human papilloma virus test in the follow-up of patients who underwent conization of the cervix for cervical intraepithelial neoplasia. J Gynecol Oncol. 2009;20:86–90. doi: 10.3802/jgo.2009.20.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam K, Chung S, Kim J, Jeon S, Bae D. Factors associated with HPV persistence after conization in patients with negative margins. J Gynecol Oncol. 2009;20:91–95. doi: 10.3802/jgo.2009.20.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gok M, Coupe VM, Berkhof J, Verheijen RH, Helmerhorst TJ, Hogewoning CJ, et al. HPV16 and increased risk of recurrence after treatment for CIN. Gynecol Oncol. 2007;104:273–275. doi: 10.1016/j.ygyno.2006.10.011. [DOI] [PubMed] [Google Scholar]