Abstract
Studies in humans have established that polymorphisms in genes encoding the innate immune Toll-like receptors (TLRs) are associated with inflammatory atherosclerosis. In hyperlipidemic mice, TLR2 and TLR4 have been reported to contribute to atherosclerosis progression. Human and mouse studies support a role for the oral pathogen Porphyromonas gingivalis in atherosclerosis, although the mechanisms by which this pathogen stimulates inflammatory atherosclerosis via innate immune system activation is not known. Using a genetically defined apolipoprotien E-deficient (ApoE−/−) mouse model we demonstrate that pathogen-mediated inflammatory atherosclerosis occurs via both TLR2-dependent and TLR2-independent mechanisms. P. gingivalis infection in mice possessing functional TLR2 induced the accumulation of macrophages as well as inflammatory mediators including CD40, IFN-γ and the pro-inflammatory cytokines IL-1β, IL-6 and tumor necrosis factor-α in atherosclerotic lesions. The expression of these inflammatory mediators was reduced in atherosclerotic lesions from P. gingivalis-infected TLR2-deficient (TLR2−/−) mice. These studies provide a mechanistic link between an innate immune receptor and pathogen-accelerated atherosclerosis by a clinically and biologically relevant bacterial pathogen.
Key Words: Toll-like receptor 2, Inflammation, Porphyromonas gingivalis, Pro-inflammatory cytokines, Atherosclerosis
Introduction
A number of studies have established a fundamental role for chronic inflammation in atherosclerosis [1]. Chronic inflammation results from the continuous accumulation and activation of leukocytes and the activation of pro-inflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor-α (TNF-α) and IL-6 [2]. These cytokines stimulate the production of chemokines that ultimately leads to the recruitment and accumulation of monocytes [3]. Activation of pro-inflammatory cytokines occurs in part via the Toll-like receptors (TLRs), a family of innate immune recognition receptors, which detect conserved microbial patterns and endogenous ligands, and play a key role in innate immune signaling and initiating inflammatory responses [4]. Ligation of these receptors initiates the activation of nuclear factor-κB (NF-κB), resulting in the expression of a wide array of inflammatory genes. Recent studies have established that in humans, polymorphisms in genes encoding TLRs and genes involved in subsequent signaling cascades are associated with atherosclerosis [5]. Animal studies employing hyperlipidemic mice have shown that TLR2, TLR4 as well as the downstream signaling molecules myeloid differentiation factor (MyD88) and IL-1 receptor-associated kinase 4 (IRAK4) play an important role in the progression of atherosclerosis [6,7,8,9].
Human epidemiological studies support a role for several pathogenic bacteria, including Chlamydophila pneumoniae and Porphyromonas gingivalis in atherosclerosis progression [10,11]. C. pneumoniae and P. gingivalis have also been reported to accelerate atherosclerosis in various animal models [10,12]. P. gingivalis induces a local host inflammatory response in the oral cavity, resulting in oral bone destruction, which is manifested as chronic inflammatory periodontal disease. This disease affects approximately 100 million people in the United States [13]. While a number of studies have focused on defining which bacterial factors and inflammatory events contribute to P. gingivalis-mediated periodontal disease, how P. gingivalis induces and maintains atherosclerosis is not known. Recently, the requirement for TLR2 in mediating inflammatory disease at local sites of TLR2 stimulation [14] or oral infection with P. gingivalis [12,15] has been documented. In this report we demonstrate that TLR2 contributes to inflammatory responses in atherosclerotic lesions following P. gingivalis infection in a genetically defined ApoE−/− mouse model of atherosclerosis, but that the pro-atherogenic effects of P. gingivalis infection can also occur via TLR2-independent mechanisms.
Materials and Methods
Mouse Strains
Male ApoE−/− and C57BL/6 mice were obtained from the Jackson Laboratoryand TLR2−/− mice (C57BL/6 background) were provided by Dr. S. Akira (Osaka University). ApoE−/− TLR2−/− mice were generated as described [16]. Mice were confirmed by genotyping and age-matched mouse groups used for all experiments. Mice were cared for in accordance with Boston University Institutional Animal Care and Use Committee procedures.
Oral Challenge
Two independent experiments were performed with ApoE−/− (n = 13) and ApoE−/− TLR2−/− (n = 14) mice fed a normal chow diet (Harlan Teklad, Madison, Wisc, USA; Global 2018). Three independent experiments were performed with C57BL/6 (n = 42) and TLR2−/− (n = 22) mice fed a high fat diet (Harlan Teklad; TD.88137). Experimentally induced P. gingivalis oral bone loss was performed as described [12]. Six-week-old male mice were treated with a 2-week regimen of oral antibiotics to reduce the normal oral bacterial population. Mice were then challenged by oral application of vehicle or P. gingivalis strain 381 (1 × 109 CFU) at the buccal surface of the maxilla 5 times a week for 3 weeks. ApoE−/− and ApoE−/− TLR2−/− mice were euthanized 13 weeks after the final oral challenge (24 weeks of age). C57BL/6 and TLR2−/− mice were euthanized 25 weeks after the final oral challenge (36 weeks of age). Oral bone loss was measured as described [17].
Measurement of Serum Levels of Total Cholesterol and P. gingivalis-Specific Immunoglobulin G
Serum samples were obtained from individual mice at the time of sacrifice for analysis of cholesterol, immunoglobulin G (IgG) levels and pro-inflammatory mediators. Total cholesterol in individual serum samples was determined by a colormetric assay (Wako Chemicals USA Inc., Richmond, Va., USA). P. gingivalis-specific IgG antibody levels were determined in individual serum samples by ELISA with a detection limit of 0.19 ng/ml and performed as described [17]. Levels of pro-inflammatory mediators in serum were determined as described below.
Measurement of Cytokines in Serum
A semi-quantitative membrane-based Raybio Mouse Inflammation Antibody Array (Raybiotech Inc., Norcross, Ga., USA) was used to detect and evaluate a panel of 40 inflammatory mediators in serum collected from ApoE−/− and ApoE−/− TLR2−/− mice as described [18]. Samples for antibody arrays were prepared by pooling equal volumes of serum from 5–8 mice per group from two independent experiments. Arrays were blocked with blocking buffer, equal amounts of serum were added, and the arrays were incubated at room temperature for 2 h. Arrays were processed according to the manufacturer's instructions. The signal intensity from the antibody arrays was detected with an LAS-4000 luminescent image analyzer (Fujifilm). Densitometry was performed to determine the relative protein expression levels between groups. For each array the background signal was subtracted from individual intensity values and signal intensities between dual data points were averaged. To normalize the signal intensity across arrays, the averaged intensity value for a particular protein was multiplied to the ratio of the averaged positive control value of a reference array to the averaged positive control value of the array for the particular protein. The normalized intensity values were then averaged between the two studies for each group of mice. Relative expression, or fold change, in protein levels were determined by taking a ratio of the averaged normalized intensity values between the groups of interest. A fold change of greater than 1.5 was considered a measurable change as defined by the manufacturer.
Atherosclerotic Plaque Assessment
The aorta of each mouse was harvested from the aortic valve to the iliac bifurcation, opened longitudinally, and stained with Sudan IV [19,20,21,22]. Digital micrographs were taken of the aortic arch, and the total area of atherosclerotic plaque was determined from on-screen imaging using IPLabs (Scanalytics Inc., Fairfax, Va., USA) by an observer blinded to the identity of the samples. For the C57BL/6 high-fat diet-induced model of atherosclerosis, atherosclerotic plaque was only observed in the aortic arch and results were presented as total atherosclerotic plaque in the arch region.
Immunohistochemistry
C57BL/6 and TLR2−/− mice (2 from each group) were perfused with saline and the aortic arch with heart tissue was harvested and embedded. Five-micrometer serial cryosections were collected every 50 μm in the aortic sinus and immunohistochemistry was performed using anti-mouse F4/80 (Serotec, Oxford, UK) or isotype-matched antibodies (Serotec). Biotinylated anti-rat (mouse absorbed) IgG was used as secondary antibody (Vector Laboratories Inc., Burlingame, Calif., USA). Images were recorded using a digital camera attached to a light microscope.
Analysis of Markers of Inflammation in Aorta
Individual aortas from ApoE−/− and ApoE−/− TLR2−/− mice were homogenized in 1× Cell Lysis Buffer (Raybiotech Inc.) and samples centrifuged to pellet cellular debris and recover tissue lysate. Samples were prepared by pooling equal amounts of protein from 3–4 mice in each of the groups from two independent experiments. Semi-quantitative membrane-based Raybio mouse atherosclerotic antibody arrays (Raybiotech Inc.) were used to detect and evaluate a panel of 22 atherosclerosis mediators as described [18]. Equal amounts of protein (100 μg) were processed as described above for serum analysis.
Statistics
Normality of data sets was determined by plotting data into histograms and visually inspecting for or against standard bell-shaped curves. A Mann-Whitney U test was performed to compare two independent samples with GraphPad Prism 4 software (GraphPad Software Inc., San Diego, Calif., USA) with an α equal to 0.05 considered significant. Two-way ANOVA was performed for analysis of percent plaque between genotypes and infections. A value of p < 0.05 was considered significant.
Results
ApoE−/− TLR2−/− Mice Develop Less Atherosclerotic Plaque in Response to Pathogen Challenge
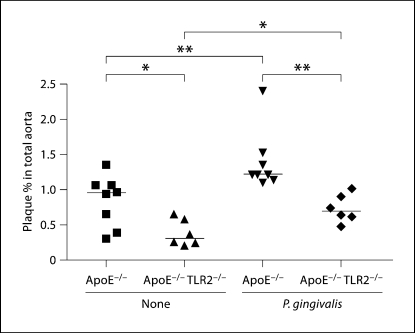
To evaluate the role of TLR2 in P. gingivalis-induced atherosclerosis, we utilized our recently generated ApoE−/− TLR2−/− mice [16] fed a normal chow diet. In agreement with our previous studies [22], oral infection of ApoE−/− mice with P. gingivalis resulted in an significant increase in the percent of total atherosclerotic plaque as compared to uninfected ApoE−/− mice (fig. 1; p < 0.01). P. gingivalis-infected ApoE−/− TLR2−/− mice exhibited an increase in the percent of total aortic atherosclerotic plaque as compared to uninfected ApoE−/− TLR2−/− mice (fig. 1; p < 0.05). However, we also observed that P. gingivalis-infected ApoE−/− TLR2−/− mice had a significant decrease in the percent of total aortic atherosclerotic plaque as compared to that observed in the P. gingivalis-infected ApoE−/− mice (fig. 1; p < 0.01). These results demonstrate that absence of TLR2 diminishes atherosclerosis in response to P. gingivalis infection, but that a TLR2-independent mechanism may also play a role in P. gingivalis-mediated atherosclerosis. Uninfected ApoE−/− TLR2−/− mice expressed a significant decrease in the percent of total atherosclerotic plaque as compared to that observed in uninfected ApoE−/− mice (fig. 1;p < 0.05). Two-way ANOVA determined that there were statistical differences when using infection or genotype as the variables, but the interaction was not significant (pgenotype = 0.0002 significant between ApoE−/− mice and ApoE−/− TLR2−/− mice; pinfection = 0.0014 significant between unchallenged mice and P. gingivalis-challenged mice; pinteraction = 0.4231). We did not observe any differences in body weight in the 4 groups of mice (data not shown).
Fig. 1.
Plaque percent in total aortic area in ApoE−/− and ApoE−/− TLR2−/− mice following P. gingivalis challenge. Percentages of Sudan IV-stained plaque in total aortic area of unchallenged ApoE–/– mice (8), unchallenged ApoE–/– TLR2–/– mice (6), P. gingivalis-challenged ApoE−/− mice (8) and P. gingivalis-challenged ApoE−/− TLR2−/− mice (6) are shown. Each point represents a single mouse and each horizontal line represents median plaque percent in total aortic area in each group. Unchallenged (none) or P. gingivalis challenged. ∗ p < 0.05; ∗∗ p < 0.01. Data were analyzed by the Mann-Whitney U test.
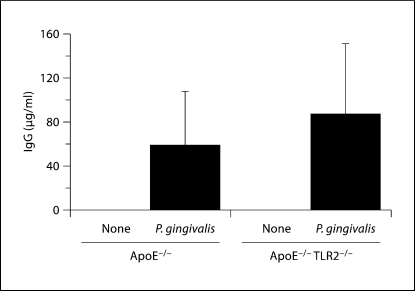
High titers of P. gingivalis-specific IgG were detected in ApoE−/− and ApoE−/− TLR2−/− mice challenged with P. gingivalis andabsent in sham-challenged mice (fig. 2). There were no statistically significant differences in pathogen-specific titers between challenged ApoE−/− and challenged ApoE−/− TLR2−/− mice (p >0.05). Thus, TLR2 deficiency impacted the inflammatory atherosclerotic response to P. gingivalis oral challenge, but did not alter the IgG response to whole P. gingivalis.
Fig. 2.
ApoE−/− and ApoE−/− TLR2−/− mice generate P. gingivalis-specific IgG antibodies in response to oral challenge. Data represent median and interquartile deviation of P. gingivalis-specific IgG titers in individual serum samples obtained from orally challenged mice. There was no significant difference between P. gingivalis-infected ApoE−/− mice and P. gingivalis-infected ApoE−/− TLR2−/− mice by the Mann-Whitney U test. Unchallenged (none) or P. gingivalis challenged.
TLR2 Deficiency Attenuates P. gingivalis-Induced Inflammatory Mediators in Aortic Lesions
To examine the inflammatory composition of aortic lesions obtained from P. gingivalis-infected mice, we next utilized semi-quantitative membrane-based antibody arrays to detect atherosclerosis mediators. Atherosclerotic inflammatory lesions from P. gingivalis-infected ApoE−/− mice expressed increased levels of pro-inflammatory cytokines, including IL-1α, IL-1β, IL-6 and TNF-α as well as inflammatory mediators not previously observed, including MIP-3α, RANTES, MCSF, CD40 and IFN-γ, as compared to uninfected ApoE−/− mice (table 1). Moreover, we observed a decrease in the expression of several of these inflammatory mediators in aortic tissue obtained from P. gingivalis-infected ApoE−/− TLR2−/− mice as compared to infected ApoE−/− mice (table 1). These included CD40, IFN-γ, GM-CSF, IL-1β, IL-2, IL-3, IL-13 and basic fibroblast growth factor (bFGF).
Table 1.
Atherosclerotic mediator expression in aortic lesions
| Inflammatory markers | Fold induction following P. gin-givalis infection1 | Fold reduction following P. gingivalis infection in TLR2-deficient mice2 |
|---|---|---|
| Monocyte recruitment/activation | ||
| MIP-3α | 10.2 | NS |
| RANTES | 6.0 | NS |
| M-CSF | 3.5 | NS |
| CD40 | 3.2 | 1.8 |
| MCP-1 | 2.3 | NS |
| IFN-γ | 2.0 | 1.7 |
| GM-CSF | 1.5 | 1.5 |
| G-CSF | NS | NS |
| Eotaxin | NS | NS |
| Endothelial cell activation | ||
| L-selectin | 2.8 | NS |
| P-selectin | 2.4 | NS |
| Cytokines | ||
| IL-lα | 5.7 | NS |
| IL-13 | 3.6 | 1.8 |
| TNF-1α | 2.4 | NS |
| IL-β | 2.2 | 1.6 |
| IL-5 | 1.6 | NS |
| IL-6 | 1.5 | NS |
| IL-2 | 1.5 | 2.1 |
| IL-3 | NS | 1.5 |
| IL-4 | NS | NS |
| Angiogenesis | ||
| bFGF | 6.5 | 2.6 |
| VEGF | 1.8 | NS |
Aortic tissue samples were examined as described in ‘Materials and Methods' using semi-quantitative membrane-based Ray-bio mouse atherosclerotic antibody array (Raybiotech Inc.) to evaluate a panel of 22 atherosclerosis mediators in aortic tissue. The intensity of signals was quantified by densitometry and fold differences were determined. NS = Fold change was less than 1.5.
Fold induction in atherosclerotic markers in aortic lesions obtained from P. gingivalis-infected ApoE−/- mice with respect to uninfected ApoE−/- mice.
Fold reduction in P. gingivalis-inf ected ApoE−/- TLR2−/- with respect to P. gingivalis-infected ApoE−/- mice.
ApoE−/− TLR2−/− Mice Develop Decreased Levels of Serum Inflammatory Mediators in Response to P. gingivalis Oral Challenge
We next utilized semi-quantitative membrane-based antibody arrays to detect inflammatory mediators in serum collected from P. gingivalis-infected ApoE−/− and ApoE−/− TLR2−/− mice. Serum samples obtained from P. gingivalis-infected ApoE−/− mice expressed increased levels of several inflammatory mediators as compared to uninfected ApoE−/− mice (table 2). These included IL-6, CD30 ligand (CD30L) and tissue inhibitor of metalloproteinase 1 (TIMP-1). Serum samples obtained from P. gingivalis-infected ApoE−/− TLR2−/− mice expressed decreased levels of the pro-inflammatory cytokines, IL-1β, IL-6 and TNF-α, as well as decreased levels of CD30L, TIMP-1, Fas ligand (FasL), soluble TNF receptor 1 (sTNFR1), IL-2, eotaxin and lymphotactin, as compared to infected ApoE−/− mice (table 2). Comparison of the levels of these inflammatory mediators in serum samples of uninfected ApoE−/− versus uninfected ApoE−/− TLR2−/− mice revealed no differences in these two groups of mice (data not shown). Collectively, these results indicate TLR2 deficiency impacted the inflammatory response to P. gingivalis oral challenge as detected by a decrease in inflammatory mediators in serum and in atherosclerosis lesions.
Table 2.
Inflammatory mediator expression in serum
| Inflammatory markers | Fold induction following P. gin-givalis infection1 | Fold reduction following P. gingivalis infection in TLR2-deficient mice2 |
|---|---|---|
| Interleukins | ||
| IL-1β | NS | 1.6 |
| IL-2 | NS | 1.5 |
| IL-6 | 1.6 | 1.9 |
| Chemokines | ||
| Eotaxin | NS | 1.6 |
| Lymphotactin | NS | 1.5 |
| TNF superfamily | ||
| TNF-α | NS | 1.5 |
| Fas L | NS | 2.0 |
| CD30L | 1.7 | 1.8 |
| sTNFRI | NS | 1.7 |
| TIMP superfamily | ||
| TIMP-1 | 2.3 | 1.7 |
| TIMP-1 | 2.3 | 1.7 |
Pooled serum was examined as described in ‘Materials and Methods' using semi-quantitative membrane-based Raybio mouse inflammation antibody arrays (Raybiotech Inc.) to detect and evaluate a panel of 40 inflammatory mediators. The intensity of signals was quantified by densitometry and fold differences were determined. NS = Fold change was less than 1.5. The fold changes of eotaxin-2, fractalkine, GCSF, GM-CSF, IFN-7, IL-la, IL-3, IL-4, IL-9, IL-10, IL-12p40p70, IL-12p70, IL-13, IL-17,1-TAC, KC, Leptin, LIX, MCP-1, MCSF, MIG, MlP-la, MIP-I7, RANTES, SDF-1, TCA-3, TECK, TIMP-2 and sTNFRII were less than 1.5.
Fold induction in inflammatory mediators in serum ob tained from P. gingivalis-infected ApoE−/- mice with respect to uninfected ApoE−/- mice.
Fold reduction in P. gingivalis-infected ApoE−/- TLR2−/- with respect to P. gingivalis-infected ApoE−/- mice.
Local Oral Inflammatory Bone Loss in ApoE−/− TLR2−/− Mice in Response to P. gingivalis Oral Challenge
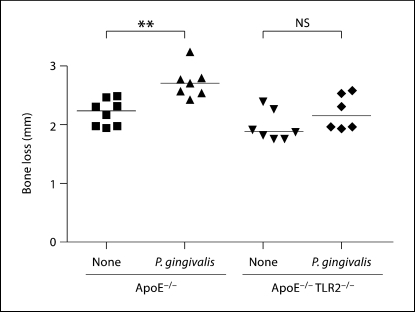
We have previously demonstrated that mice deficient in TLR2 on a C57BL/6 background did not develop oral bone loss in response to P. gingivalis oral challenge [12]. To confirm that P. gingivalis oral challenge resulted in oral bone loss in the ApoE mouse model, we measured oral bone loss in P. gingivalis-infected ApoE−/− and ApoE−/− TLR2−/− mice. As expected, we observed an increase in the distance between the alveolar bone crest (ABC) and the cement enamel junction (CEJ) in ApoE−/− mice orally challenged with P. gingivalis (fig. 3; p < 0.01). In contrast, P. gingivalis-infected ApoE−/− TLR2−/− mice did not exhibit a similar increase in the distance between the ABC and the CEJ (fig. 3). These results confirmed that TLR2 was required for oral inflammatory bone loss in response to P. gingivalis infection in ApoE−/− mice.
Fig. 3.
Oral bone loss in response to P. gingivalis challenge in ApoE−/− and ApoE−/− TLR2−/− mice. ApoE−/− and ApoE−/− TLR2−/− mice were orally challenged with P. gingivalis. Linear measurements of bone loss (14 sites) were obtained from the maxillary molars of each mouse. Uninfected ApoE−/− mice (8), P. gingivalis-challenged ApoE−/− mice (6), unchallenged ApoE−/− TLR2−/− mice (7), and P. gingivalis-challenged ApoE−/− TLR2−/− mice (6), respectively. Each point represents total bone loss from 14 sites of a single mouse and horizontal line represents median oral bone loss in each group. Unchallenged (none) or P. gingivalis challenged. ∗∗ p < 0.01 by the Mann-Whitney U test. NS = No significant difference. Two outliers were removed from P. gingivalis-challenged ApoE−/− group and P. gingivalis-challenged ApoE−/− TLR2−/− group.
TLR2 Deficiency on a C57BL/6 Background Attenuates P. gingivalis-Induced Inflammatory Cells in Aortic Lesions and Atherosclerosis
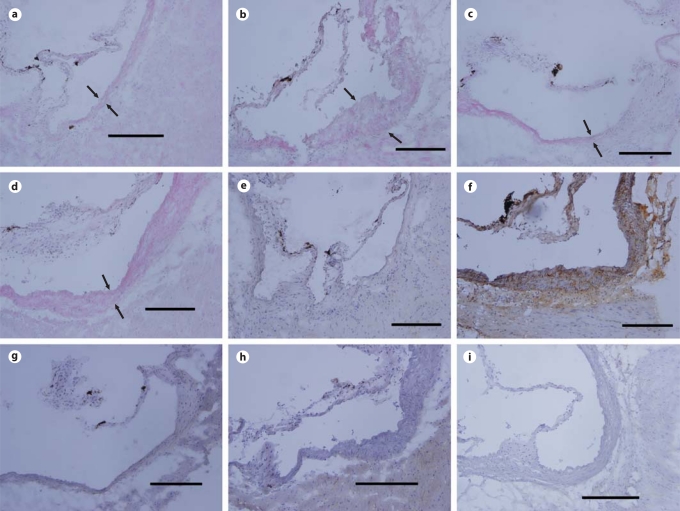
To confirm the results obtained above and to determine if TLR2 deficiency on a C57BL/6 background was associated with decreased atherosclerosis in response to P. gingivalis oral challenge, we utilized a diet-induced model of atherosclerosis in C57BL/6 and TLR2−/− mice. P. gingivalis infection of C57BL/6 mice resulted in an increase in inflammatory cells in aortic sinus samples as compared to that observed in samples obtained from uninfected C57BL/6 mice (fig. 4). We observed an increase in macrophage-specific staining between the luminal and adventitial surfaces of the aortic sinus samples obtained from P. gingivalis-infected C57BL/6 mice as compared to samples obtained from uninfected C57BL/6 mice (fig. 4). In contrast, aortic sinus samples obtained from P. gingivalis-infected TLR2−/− mice exhibited less staining for inflammatory cells and macrophage-specific staining as compared to infected C57BL/6 mice (fig. 4). Aortic sinus samples obtained from uninfected TLR2−/− mice exhibited less inflammatory cells and macrophage-specific staining as compared to uninfected C57BL/6 mice. No background staining was observed for the isotype control IgG (fig. 4). Taken together, these data indicate that TLR2 deficiency resulted in reduced macrophage accumulation in the aortic sinus in response to P. gingivalis oral challenge.
Fig. 4.
Hematoxylin and eosin as well as macrophage staining of cryosections of aortic lesions. Representative staining of aortic sinus plaques from uninfected C57BL/6 mice (a and e), P. gingivalis-infected C57BL/6 (b and f), uninfected TLR2−/− mice (c and g), and P. gingivalis-infected TLR2−/− mice (d and h). Sections were stained with hematoxylin and eosin (a–d), macrophage marker F4/80 (e–h) and isotype control (i). Arrows point to the luminal and adventitial surfaces of the aorta (a–d). Scale bars = 200 μm.
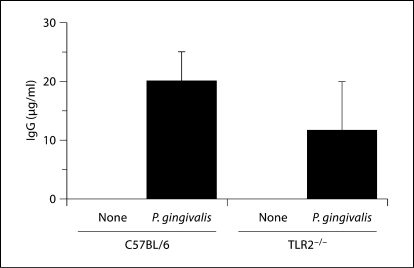
In the C57BL/6 mice fed a high-fat diet we observed atherosclerotic lesions on the aortic arch as measured by an statistically significant increase (p < 0.01) in Sudan IV-stained lipid accumulation as compared to C57BL/6 mice on a normal-chow diet (table 3). In P. gingivalis orally challenged C57BL/6 mice fed a high-fat diet we observed an increase in the percent atherosclerotic plaque in the aortic arch as compared to uninfected C57BL/6 mice on a normal-chow diet or fed a high-fat diet (table 3). In agreement with the results obtained in the ApoE−/− mouse model, P. gingivalis orally challenged TLR2−/− mice exhibited less atherosclerotic plaque in the aortic arch as compared to C57BL/6 mice (table 3). P. gingivalis-specific IgG titers were detected in mice challenged with P. gingivalis and absent in sham-infected mice (fig. 5), and titers were similar in P. gingivalis-infected C57BL/6 and TLR2-deficient mouse strains (p >0.05). Thus, as in the ApoE model, TLR2 deficiency on a C57BL/6 background did not affect the humoral immune response to P. gingivalis oral infection.
Table 3.
Atherosclerotic plaque formation in a diet-induced model of atherosclerosis
| Oral P. gingivalis | Mouse strain | Diet | Plaque in aortic arch, % |
|---|---|---|---|
| Uninfected | C57BL/6 | chow | 0.011 ± 0.027∗∗ |
| C57BL/6 | Western | 0.17 ± 0.22∗ | |
| TLR2−/- | Western | 0.47 ± 0.52 | |
| Infected | C57BL/6 | Western | 0.55 ± 0.71∗ |
| TLR2−/- | Western | 0.39 ± 0.79 | |
The data are presented as the mean ± SD. Statistical analysis was performed by the two-tailed Mann-Whitney U test with a = 0.05. pgenotype > 0.05; pinfection > 0.05 by two-way ANOVA.
p < 0.05 significant between unchallenged C57BL/6 mice fed normal chow and P. gingivalis-challenged C57BL/6 mice.
p < 0.01. C57BL/6 mice fed normal chow (n = 6) presented significantly lower formation of atherosclerotic plaque in aortic arch compared to C57BL/6 fed Western high-fat diet (n = 22), unchallenged TLR2−/- mice (n = 10), P. gingivalis orally challenged C57BL/6 mice (n = 23), and P. gingivalis orally challenged TLR2−/- mice (n = 10).
Fig. 5.
C57BL/6 and TLR2−/− mice generate P. gingivalis-specific IgG antibodies in response to oral challenge. Data represent median and interquartile deviation of P. gingivalis-specific IgG titers in individual serum samples obtained from orally challenged mice. There was no significant difference between P. gingivalis-infected C57BL/6 mice and P. gingivalis-infected TLR2−/− mice by the Mann-Whitney U test. Unchallenged (none) or P. gingivalis challenged.
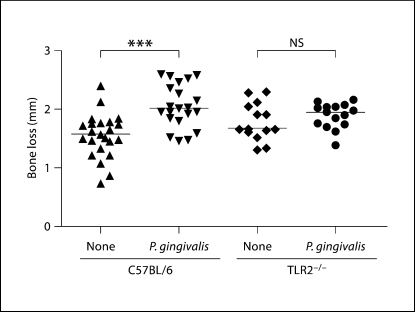
As expected, C57BL/6 mice orally challenged with P. gingivalis exhibited an increase in oral bone loss as measured by ABC-CEJ distance as compared to uninfected mice (fig. 6; p < 0.001). Confirming our previous results [12], TLR2−/− mice orally challenged with P. gingivalis did not exhibit appreciable oral bone loss as compared to uninfected mice (fig. 6; p > 0.05). As expected, C57BL/6 mice fed a high-fat diet displayed statistically significant increases in body weight (p < 0.0001) and serum cholesterol levels (p < 0.001) as compared to C57BL/6 mice fed a normal-chow diet (data not shown). We did not observe statistically significant differences in either body weight or serum cholesterol levels in the 4 groups of C57BL/6 mice fed a high-fat diet (data not shown). Collectively, these results demonstrate that TLR2 deficiency on a C57BL/6 background was associated with both decreased atherosclerosis and oral bone loss in response to P. gingivalis oral challenge.
Fig. 6.
Oral bone loss in response to P. gingivalis challenge in C57BL/6 and TLR2−/− mice. Linear measurements of bone loss (n = 14 sites) were obtained from the maxillary molars of each mouse. Uninfected C57BL/6 mice (23), P. gingivalis-infected C57BL/6 mice (21), unchallenged TLR2−/− mice (14) and P. gingivalis-challenged TLR2−/− mice (15), respectively. Each point represents total bone loss from 14 sites of a single mouse and horizontal line represents median oral bone loss in each group. Unchallenged (none) or P. gingivalis challenged. ∗∗∗ p < 0.001 by the Mann-Whitney U test. NS = No significant difference.
Discussion
In this study, we demonstrate that TLR2 contributes to the induction of pro-inflammatory responses in atherosclerotic lesions in response to infection with the oral pathogen P. gingivalis. We also observed that while the absence of TLR2 diminished atherosclerosis in response to P. gingivalis infection, a TLR2-independent mechanism may also play a role in P. gingivalis-mediated atherosclerosis. Since our studies utilized an oral challenge regimen with live bacteria and not simply a purified TLR2 ligand, these results are not unexpected. Indeed, numerous in vitro studies have documented a role for TLR4 in P. gingivalis-mediated inflammatory responses [12,23]. Thus, it is highly probable that several innate immune receptors respond to various bacterial products in the context of in vivo challenge with an intact viable bacterial pathogen [24]. TLR2 deficiency resulted in a reduction in aortic sinus lipid accumulation, and in inflammatory cell and macrophage infiltration into the inflammatory lesion in response to P. gingivalis when compared with that observed in mice possessing TLR2 but did not impact the IgG response to P. gingivalis challenge. We also determined that oral infection with P. gingivalis stimulated expression of pro-inflammatory cytokines and pro-inflammatory molecules involved in endothelial cell activation (L-selectin, P-selectin, VEGF) as well as monocyte recruitment and activation (MIP-3α, RANTES, M-CSF, CD40, MCP-1, IFN-γ, GM-CSF) in the aortic arch of mice. Importantly, we observed a decrease in the expression of inflammatory mediators in aortic tissue samples obtained from P. gingivalis-infected mice deficient in TLR2 as compared to P. gingivalis-infected mice possessing TLR2.
An important observation from our studies was the contribution of TLR2 for the induction of CD40, IFN-γ and IL-1β directly in atherosclerotic lesions in response to P. gingivalis oral challenge. CD40 is a member of the TNF superfamily of cell surface proteins and engagement of CD40 induces the expression of potent pro-inflammatory cytokines [25]. It has been reported that polymorphisms in genes coding for CD40 are associated with atherosclerosis in humans [26]. IFN-γ is a potent immunostimulatory cytokine, which coordinates an array of cellular functions through transcriptional regulation of immunologically relevant genes. Furthermore, CD40 and IFN-γ have been demonstrated to promote and modify inflammatory atherosclerosis in mouse models [27]. The contribution of TLR2 for IL-1 expression in atherosclerotic lesions is noteworthy, since this pro-inflammatory cytokine has been associated with several human chronic inflammatory diseases. Gene polymorphisms in IL-1 receptor antagonist (IL-1ra) and IL-1β are associated with human atherosclerosis [28] and periodontal disease [29], respectively. In ApoE−/− mouse models, IL-1 has been shown to play a role in atherosclerosis in both the absence and presence of bacterial pathogens [30,31]. Current studies in our laboratory are focused on the role of IL-1 signaling in P. gingivalis-induced chronic inflammation at both local (oral bone loss) and distant (atherosclerosis) sites of infection.
Oral challenge with P. gingivalis also resulted in an increase in IL-6, CD30L and TIMP-1 in serum. Increased expression of IL-6 and TIMP-1 in response to P. gingivalis challenge is in agreement with previous studies [32] and may be due to the activation of CD30L [33]. We also found that mice deficient in TLR2 expressed decreased levels of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in response to P. gingivalis oral challenge as compared to mice possessing functional TLR2. These results indicate that TLR2 also contributes to the production of circulating levels of inflammatory mediators in response to oral infection with P. gingivalis. Interestingly, we also observed that P. gingivalis infection resulted in greater changes in the levels of inflammatory mediators present in atherosclerotic plaque samples as compared to the levels of inflammatory mediators observed in serum at the time of sacrifice and tissues processing. These results suggest that while P. gingivalis- induced systemic inflammation may contribute to atherosclerosis, the ability to modulate the levels of inflammatory mediators present in atherosclerotic plaque may ultimately be responsible for the induction of atherosclerosis.
Our results extend in vitro studies in which the ability of P. gingivalis to induce an inflammatory response in macrophages and endothelial cells has been documented [12,34]. In addition to the ability of P. gingivalis ligands to signal for pro-inflammatory responses in endothelial cells, this pathogen invades, survives and replicates within these cells [35]. Although we could not detect P. gingivalis in blood or aortic tissue of infected mice (data not shown), these results were not unexpected. We previously reported that P. gingivalis could be detected by 16S PCR in both blood and aortic tissue during the oral infection period, but not at later time points after bacterial challenge [22]. Our previous studies support the notion that activation of the endothelium via innate immune signaling pathways may be critical for inflammation at distant sites and may not be associated with bacterial colonization per se. Our in vitro studies have established that priming of endothelial cells by invasive P. gingivalis infection leads to induction of TLR-dependent inflammatory responses [36]. We have also recently demonstrated, using in vivo MRI analysis together with ex vivo immunohistochemistry, that P. gingivalis infection results in the progression of atherosclerotic plaque accumulation that is associated with the accumulation of lipids, macrophages and T cells [unpubl. data]. Thus, chronic and exogenous stimulation of the endothelium with P. gingivalis may sensitize the endothelium to other endogenous TLR ligands and may impact inflammatory outcomes in atherosclerosis.
In this study we also established that P. gingivalis oral infection in the diet-induced model of atherosclerosis in C57BL/6 mice resulted in a statistically significant increase in atherosclerotic plaque as compared to uninfected mice. P. gingivalis-challenged TLR2−/− mice developed less atherosclerotic plaque as compared to P. gingivalis-infected C57BL/6 mice, although this did not reach statistical significance. High-fat dietary supplementation could potentially mask the TLR2 dependency of bacterial challenge by the contribution of endogenous ligands present in hyperlipidemic conditions [8] which could impact immunological responses underlying atherosclerosis such as abnormally shifting T helper cell populations in these animals [37]. Thus, it is highly probable that the effects of the TLR2 mutation in C57BL/6 background were confounded by the high-fat diet used.
During the completion of our study, a study assessing the role of exogenous stimulation of TLR2 in atherosclerosis was reported [38], although this study utilized an artificial route of P. gingivalis administration which must be considered in the context of our results. It is well established that local or intravenous administration of a TLR agonist will induce an inflammatory-mediated sequelae. Since P. gingivalis colonization and infection occurs in the oral cavity of humans, we used a natural route of biological exposure to P. gingivalis. Our approach was to characterize local inflammatory oral bone loss in response to P. gingivalis challenge and inflammatory mediator expression directly in atherosclerotic lesions and in serum samples.
In summary, while a number of studies have focused on defining which bacterial factors and inflammatory events contribute to P. gingivalis-mediated inflammatory periodontal disease, few studies have focused on how this relates to chronic inflammatory atherosclerosis. In this study we demonstrate that pathogen-induced inflammatory responses in atherosclerotic lesions by a clinically and biologically relevant bacterial pathogen occur via TLR2 dependent and independent mechanisms. Our results point to a key role for TLR2 in macrophage recruitment, endothelial cell activation and pro-inflammatory cytokine responses to P. gingivalis in inflammatory atherosclerosis, although it remains to be determined how specific innate immune cells contribute to this response. Understanding the specific cell signaling networks involved in chronic inflammation will provide a promising avenue for novel therapies for chronic inflammatory disorders including atherosclerosis.
Acknowledgements
We would like to thank Dr. Lisa Sullivan and Dr. Michael Lavalley (Boston University School of Public Health) for their expert assistance with statistical analysis. This study was supported by NIH Public Health Service grant HL080387 (C.A.G.).
Footnotes
C.H. and A.G.M. contributed equally to this work.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 5.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 6.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 7.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rekhter M, Staschke K, Estridge T, Rutherford P, Jackson N, Gifford-Moore D, Foxworthy P, Reidy C, Huang XD, Kalbfleisch M, Hui K, Kuo MS, Gilmour RVlahos CJ. Genetic ablation of IRAK4 kinase activity inhibits vascular lesion formation. Biochem Biophys Res Commun. 2008;367:642–648. doi: 10.1016/j.bbrc.2007.12.186. [DOI] [PubMed] [Google Scholar]
- 10.Belland RJ, Ouellette SP, Gieffers J, Byrne GI. Chlamydia pneumoniae and atherosclerosis. Cell Microbiol. 2004;6:117–127. doi: 10.1046/j.1462-5822.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 11.Gibson FC, 3rd, Yumoto H, Takahashi Y, Chou HH, Genco CA. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J Dent Res. 2006;85:106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 12.Gibson FC, 3rd, Ukai T, Genco CA. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis. Front Biosci. 2008;13:2041–2059. doi: 10.2741/2822. [DOI] [PubMed] [Google Scholar]
- 13.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 14.Schoneveld AH, Oude Nijhuis MM, van Middelaar B, Laman JD, de Kleijn DP, Pasterkamp G. Toll-like receptor 2 stimulation induces intimal hyperplasia and atherosclerotic lesion development. Cardiovasc Res. 2005;66:162–169. doi: 10.1016/j.cardiores.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, 3rd, Genco CA. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson FC, 3rd, Genco CA. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infect Immun. 2001;69:7959–7963. doi: 10.1128/IAI.69.12.7959-7963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy AB, Srivastava SK, Ramana KV. Anti-inflammatory effect of aldose reductase inhibition in murine polymicrobial sepsis. Cytokine. 2009 doi: 10.1016/j.cyto.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Messas E, Batista EL, Jr., Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105:861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 21.Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 1998;97:780–785. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- 22.Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 23.Davey M, Liu X, Ukai T, Jain V, Gudino C, Gibson FC, 3rd, Golenbock D, Visintin A, Genco CA. Bacterial fimbriae stimulate proinflammatory activation in the endothelium through distinct TLRs. J Immunol. 2008;180:2187–2195. doi: 10.4049/jimmunol.180.4.2187. [DOI] [PubMed] [Google Scholar]
- 24.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor α signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phipps RP. Atherosclerosis: the emerging role of inflammation and the CD40-CD40 ligand system. Proc Natl Acad Sci USA. 2000;97:6930–6932. doi: 10.1073/pnas.97.13.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdon KP, Langefeld CD, Beck SR, Wagenknecht LE, Carr JJ, Rich SS, Freedman BI, Herrington DBowden DW. Variants of the CD40 gene but not of the CD40L gene are associated with coronary artery calcification in the Diabetes Heart Study (DHS) Am Heart J. 2006;151:706–711. doi: 10.1016/j.ahj.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis SE, Camp NJ, Dewberry RM, Gunn J, Syrris P, Carter ND, Jeffery S, Kaski JC, Cumberland DC, Duff GW, Crossman DC. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation. 1999;99:861–866. doi: 10.1161/01.cir.99.7.861. [DOI] [PubMed] [Google Scholar]
- 29.Kornman KS, Crane A, Wang HY, di Giovine FS, Newman MG, Pirk FW, Wilson TG, Jr., Higginbottom FL, Duff GW. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–77. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 30.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 32.Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, Lu Y, Papapanou PN, Schmidt AM. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1405–1411. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 33.Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157:3635–3639. [PubMed] [Google Scholar]
- 34.Takahashi Y, Davey M, Yumoto H, Gibson FC, 3rd, Genco CA. Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell Microbiol. 2006;8:738–757. doi: 10.1111/j.1462-5822.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto T, Yumoto H, Takahashi Y, Davey M, Gibson FC, 3rd, Genco CA. Pathogen-accelerated atherosclerosis occurs early after exposure and can be prevented via immunization. Infect Immun. 2006;74:1376–1380. doi: 10.1128/IAI.74.2.1376-1380.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yumoto H, Chou HH, Takahashi Y, Davey M, Gibson FC, 3rd, Genco CA. Sensitization of human aortic endothelial cells to lipopolysaccharide via regulation of Toll-like receptor 4 by bacterial fimbria-dependent invasion. Infect Immun. 2005;73:8050–8059. doi: 10.1128/IAI.73.12.8050-8059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madan M, Amar S. Toll-like receptor-2 mediates diet and/or pathogen associated atherosclerosis: proteomic findings. PLoS ONE. 2008;3:e3204. doi: 10.1371/journal.pone.0003204. [DOI] [PMC free article] [PubMed] [Google Scholar]