Abstract
Several newer magnetic resonance imaging (MRI) techniques are increasingly being applied to the study of white matter development and pathology across the lifespan. These techniques go beyond traditional macrostructural volumetric methods and provide valuable information about underlying tissue integrity and organization at the microstructural and biochemical levels. We first provide an overview of white matter development and discuss the role of white matter and myelin in cognitive function. We also review available studies of development that have employed traditional volumetric measures. Then, we discuss the contributions of four newer imaging paradigms to our understanding of brain development and aging. These paradigms are Diffusion Tensor Imaging (DTI), Magnetization Transfer Imaging (MTI), T2-Relaxography, and Magnetic Resonance Spectroscopy (MRS). Studies examining brain development during childhood and adulthood as well as studies of the effects of aging are discussed.
Keywords: White matter, MRI, Diffusion tensor imaging, DTI, Magnetization transfer imaging, MTI, Magnetic resonance spectroscopy, T2 relaxography, Development
1. Introduction
Cerebral white matter develops rapidly during the perinatal period and infancy, continues to mature into the second and even third decades of life and undergoes further change as part of the aging process (Pfefferbaum et al., 1994; Courchesne et al., 2000; Iwasaki et al., 1997). Characterizing these changes in brain structure is essential to our understanding of brain function across the lifespan. Until recently, knowledge of white matter development and maturation was limited to that derived from pathological studies and neuroimaging studies utilizing volumetric measures from conventional Magnetic Resonance Imaging (MRI). Several newer in vivo magnetic resonance (MR) imaging methods that provide more information about microstructure of cerebral white matter have become available. These newer methods have the potential to advance our understanding of white matter development over the lifespan beyond previous studies which were limited to measuring macrostructure.
This article will provide a framework within which to understand the potential applications of these brainimaging methodologies to the study of development and aging. First, we review the function and development of myelin. Next, we discuss the role of myelin specifically and white matter more generally in brain function. We then provide an overview of previous developmental and aging research using conventional MR volumetric methodology. Finally, we describe the white matter neuroimaging methods and summarize findings relevant to the study of development and aging. Four methods are described: Diffusion Tensor Imaging (DTI), Magnetization Transfer Imaging (MTI), T2 Relaxography, and Magnetic Resonance Spectroscopy (MRS). We discuss the merits of the individual techniques and the potentially unique contributions that each of these methods offers to the study of white matter in a developmental context.
2. Myelin
The myelin sheath is a multi-layered structure consisting of alternating protein and lipid layers. Myelin sheaths are formed by oligodendrocytes and exist in a symbiotic relationship with neurons (Brady et al., 1999). Fully developed myelin contains cholesterol (28%), galactocerebroside (22%), phosphatidylethanolamine (12%), phosphatidylcholine (11%), sphingomyelin (8%), phosphatidylserine (5%), sulfatide (4%), phosphatidlyinositol (1%), fatty acids, and proteins (Inder and Huppi, 2000). The MR methods available for evaluating white matter microstructure are all sensitive to myelin development and integrity and are all related to the properties of water within the myelin sheath, in the interstitial space outside of the sheath, and in cerebrospinal fluid (CSF) (Barkovich, 2000).
The signal in MR imaging varies depending on the parameters of the pulse sequence and the tissue being examined. Two important components of the signal (T1 and T2) are related to the relaxation times of the nuclei of interest, typically hydrogen. Protons in different brain tissue have different T1 and T2 relaxation times. The contrast in a conventional MR image results from the “mapping” of these differences in relaxation times (McRobbie et al., 2003). T1 and T2 relaxation times are relevant to the study of development and aging because they change along with changes in brain water content. Furthermore, they change as myelin develops. “Bound” water within the myelin sheath has shorter T1 and T2 relaxation times while “free” water in the extracellular space has longer T1 and T2 relaxation times (Whittall et al., 1997). Early in brain development, bound water is likely responsible for the shortening of T1 relaxation time seen during white matter development. T2 relaxation time, which also shortens with development, is more related to extracellular water and water within the axon itself. During development, changes in extracellular water may reflect displacement of water by myelin as it matures and takes up space. Changes in axonal water during development may reflect displacement by microtubules and microfilaments inside the axon (Barkovich, 2000).
Myelination of axons, which is essential for efficient neural transmission, proceeds in an orderly spatial and temporal sequence. In humans, posterior regions are myelinated prior to anterior regions (Kinney et al., 1988; Barkovich et al., 1988; Hayakawa et al., 1991). Cerebral myelination is rapid and extensive during the first 2 years of human life, but the process continues slowly into adolescence and young adulthood (Holland et al., 1986). The last fibers to be myelinated are inter-cortical associational neurons (Yakolev and Lecours, 1967). Myelination in the frontal lobes occurs into adolescence and early adulthood. Although clear links between this frontal myelination and cognition have not been well-established, it is noteworthy that significant cognitive changes in frontal-executive functioning also occur during adolescence and early adulthood. This association does not demonstrate the link, but clearly suggests that further investigation is warranted in this area.
Myelin also undergoes a number of significant changes over the long term including deterioration with age, as evident in studies of non-human primates. One common form of deterioration is localized splitting of the myelin lamellae, which results in cytoplasm enclosures (Peters, 2002a). “Balloons” can also form in the myelin sheath as a result of cavitation of the cytoplasm (Feldman and Peters, 1998). Both of these types of abnormalities, which can occur as a result of damage or genetic factors, also occur commonly in the aging brain. “Redundant myelin” is another problem, evidenced by studies showing that axons in older primate brains have more layers of myelin in the sheath than younger brains (Peters et al., 2001). In humans, myelin volume appears to increase with age until the sixth or seventh decade, likely as a result of the addition of redundant myelin. By the eighth decade in humans, myelin begins to break down significantly. Analogous studies of aging in non-human primates have demonstrated that these myelin abnormalities are correlated with cognitive deficits and that the relationship is probably mediated by a decrease in conduction velocity of the affected axons (Peters, 2002b). This loss of conduction velocity with aging has been seen in other non-primate species (Xi et al., 1999). Lowered conduction velocity is also likely to be a significant factor in the cognitive deterioration seen in human aging (Peters, 2002a, b).
Although myelin breaks down with age, studies of nonhuman primates show that new myelin is generated even in old age (Peters, 2002b). Oligodendrocytes, the cells responsible for myelin creation and maintenance, increase in numbers with age in rhesus monkeys (Peters et al., 1991), perhaps because of the increasing burden of maintaining myelin that is degenerating. Oligodendrocytes are heterogeneous and there is some evidence to suggest that those involved in later myelination produce myelin that may be more vulnerable to breakdown than myelin produced earlier in development (reviewed in Bartzokis et al., 2004). The increased vulnerability of late-myelinating regions such as frontal and temporal regions may underlie the changes in processing speed, executive functioning, and memory seen in normal human aging and, to a greater degree, in age-associated diseases such as Alzheimer’s disease.
3. The role of cerebral white matter in brain function
Cerebral white matter comprises three major types of fibers: commissural fibers (corpus callosum, anterior and posterior commissures, and hippocampal commissure) that mainly connect homologous regions in the right and left hemispheres; association fibers that mainly connect regions within the same hemisphere both locally and at a distance; and projection fibers that consist of afferent and efferent connections between cortex and deep gray matter structures. Historically, white matter has been thought to be primarily involved in motor and sensory functioning. However, more recent evidence clearly indicates that white matter’s role as the “backbone” of neural networks in the brain is also critical to many important aspects of higher cognitive functioning including attention, executive functioning, non-verbal/visual-spatial processing, and general processing speed (Geschwind, 1965; Filley, 1998). Various neurodevelopmental and neurological conditions affecting white matter have been shown to be associated with a specific pattern of deficits referred to as a Non-verbal learning disability (NLD) (Rourke et al., 2002; Myklebust, 1975; Rourke, 1995). Rourke and others have suggested that this specific pattern of deficits may be related to right hemisphere specialization for these functions and the higher white matter to gray matter ratio in the right hemisphere, especially in frontal regions (Rourke, 1995; Gur et al., 1980; Weinberger et al., 1982). In contrast, many verbal language functions, which are more localized in regions of the left hemisphere, may be less dependent on overall white matter integrity. Recent voxel-based morphometry studies have provided new insights into the relationships between white-gray matter ratios and regional hemispheric specialization. For example, (Watkins et al., 2001) found higher gray-white ratios in specific left hemisphere language regions compared to homologous regions in the right hemisphere. Lastly, it is worth considering data from studies of adult patients with various types of white matter pathology including multiple sclerosis (Rao, 1995), toxic leukoencephalopathy (Filley and Kleinschmidt-DeMasters, 2001), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoence-phalopathy (CADASIL), and others (Pantoni and Garcia, 1995), which also provides evidence that white matter is particularly important to executive function, attention, processing speed, and motor skill.
A number of studies using different methodologies have demonstrated a relationship between white matter integrity and neurocognitive skill within the context of aging in humans. As in the studies of development, a significant relationship exists between white matter integrity and specific neurocognitive functions including executive functions, attention span, and processing speed (Filley, 1998; Pantoni and Garcia, 1997; de Groot et al., 1998). For example, in a sample of healthy elderly patients, Ylikoski et al. showed a significant relationship between the severity of leukoaraiosis (seen on conventional T2-weighted MR images as hyperintense white matter lesions) and performance on neuropsychological measures of attention and processing speed (Ylikoski et al., 1993). Fukui showed a relationship between white matter hyperintensities and poor performance on a speeded naming task (Fukui et al., 1994). In a large sample of elderly subjects, white matter hyperintensities were found to correlate with performance on a speeded task (Longstreth et al., 1996). Unfortunately, many of the studies showing these relationships are cross-sectional rather than longitudinal and have other methodological issues (Ferro and Madureira, 2002). Nevertheless, there is compelling evidence to suggest that much more detailed study of the white matter changes that accompany aging is warranted.
4. Volumetric studies
Studies utilizing MR measurements of tissue volume have evolved from analyses relying on hand-outlined volumes (Filipek et al., 1994) to more sophisticated analyses incorporating automated segmentation (Inder and Huppi, 2000; Good et al., 2001; Fischl et al., 2002; Ashburner and Friston, 2005; Ashburner and Friston, 2000). Segmentation routines typically rely on the MR signal intensity as well as “knowledge” about typical tissue distribution within the brain in order to classify individual voxels as gray matter, white matter, and cerebrospinal fluid (CSF) (Pfefferbaum et al., 2000). Studies have shown that MR volumetric measures are reliable (Iwasaki et al., 1997) and sensitive to the rapid growth in white matter volume during the first year of life (Holland et al., 1986; Huppi et al., 1998a), which corresponds to the known time frame for rapid myelination as evidenced by pathologic studies. MR volumetric studies looking at development across the lifespan have shown fairly reliable changes in tissue volumes (Rivkin, 2000). Giedd et al. found significant increases in corpus callosum volume with age in a sample of subjects between 4 and 18 years old (Giedd et al., 1996). Within the context of a longitudinal study, Giedd et al. demonstrated that white matter volume increases in a roughly linear fashion in most regions of the brain into at least the early 20 s (Giedd, 2004). Others have found similar results. A combined longitudinal/cross-sectional analysis of subjects ages 4–20 demonstrated that white matter volume increases with age (Giedd et al., 1999). White matter density in several regions including the internal capsule and arcuate fasciculus was shown to be significantly correlated with age in a sample of children 4–17 years old (Paus et al., 1999). In a study of patients ages 4 months to 28 years, Iwasaki et al. found that total white matter volume increased quickly until age 10 and then continued to increase more slowly into adolescence (Iwasaki et al., 1997). Pfefferbaum et al. found that while gray matter volume peaked at around age 4, white matter volume increased until around age 20 (Pfefferbaum et al., 1994). In their older subjects (21–70 years), they noted a steady decline in gray matter but no significant decrease in white matter volume. Courchesne et al. quantified the rapid growth in white matter during early childhood as a 74% increase in volume by age 15 (Courchesne et al., 2000). Furthermore, they demonstrated continued, slower growth of white matter into the fourth decade. In addition, they noted a 13% decrease in white matter volume among their oldest subjects (70–80 years). Good et al. did not observe a significant decrease in overall white matter volume with age but did report some localized loss of white matter tissue volume primarily in the posterior limbs of the internal capsule (Good et al., 2001). They suggest that, although myelin deteriorates with age, white matter bulk increases with capillary expansion and swelling of perivascular spaces, perhaps “canceling out” each other and resulting in very little net change in white matter volume. Bartzokis et al. noted a slow increase in frontal and temporal white matter volume into the 40 s (peaking at around 45 years of age) and, in contrast to Good et al., noted a subsequent decline in volume in older subjects (Bartzokis et al., 2001). This discrepancy could potentially be related to differences in the samples: Good et al. examined 465 men and women who were selected from a large pool of 1761 community volunteers and whose MRIs were screened for any significant abnormalities. In contrast, Bartzokis et al. seem to have had a more inclusive set of entrance criteria and did not mention MRI abnormalities as an exclusionary criteria. Bartzokis et al. also studied only men, whereas the Good et al. study included both sexes. Lastly, the men in the Bartzokis et al. study were somewhat older (19–76 years) than the men in the Good et al. study (17–68 years). Nevertheless, using a different methodology—examining transverse relaxation time (R2 = 1/T2 × 1000 ms/s), which changes as a result of myelin breakdown—Bartzokis et al. showed a similar developmental pattern of decline in frontal white matter integrity after the age of 38 years (Bartzokis et al., 2003).
Looking across studies, it seems clear that white matter volume does change well beyond adolescence, increasing in most studies at least into the fifth decade. In the later years, the volumetric findings are less clear, but most investigators seem to agree that there are important white matter changes occurring, even if they are not measurable with standard volumetric techniques. Lastly, it is noteworthy that in the majority of studies examining the relationship between age and white and gray matter volumes during development, white matter volume increases are accompanied by corresponding decreases in gray matter volume (Paus et al., 2001).
5. Diffusion tensor imaging
DTI measures the magnitude and directionality of water diffusion in tissue. Without barriers, the random Brownian movement of water molecules is uniform in all directions or “isotropic.” In the presence of barriers such as cell membranes, fibers, and myelin, the diffusion can be greater in one direction than others and is termed “anisotropic.” MR is well suited to the quantitative and non-invasive measurement of diffusion (Stejskal and Tanner, 1965) and was developed into an imaging technique by Le Bihan et al. (1986). Using MR, an apparent diffusion coefficient (ADC) can be computed. In a set of animal experiments, it was found that the ADC was higher in the direction parallel to white matter fibers because water diffuses relatively freely parallel to the fibers (Moseley et al., 1990). In contrast, the ADC was low in the direction perpendicular to the fibers because water diffusion is restricted by axonal membranes, microtubules, neurofilaments, and myelin (Beaulieu, 2002). The development of DTI provided a method to measure the magnitude and direction of water diffusion without concern for the orientation of the sample in the magnet (Pierpaoli and Basser, 1996; Pierpaoli et al., 1996). The “tensor” in DTI refers to a mathematical construct for representing the magnitude of directional water diffusion in a three-dimensional volume (Moseley et al., 2002).
A DTI data set includes diffusion-weighted images with the diffusion sensitized in non-collinear directions. A minimum of six non-collinear images is needed but, often, 12 or more images are collected in order to increase the accuracy of the measure. A diffusion tensor matrix is constructed from the collected data and three eigenvectors are calculated using matrix diagonalization (Basser and Pierpaoli, 1998). Scalar measures can be derived from these eigenvectors. The sum of all three eigenvalues (λ1 + λ2 + λ3) is the “trace” of the diffusion tensor. The first and largest of these eigenvalues, λ1, is a representation of the water diffusivity parallel to the axonal fibers and is referred to as the axial diffusivity (Basser, 1995). The second and third eigenvalues, λ2 and λ3, represent water diffusion in the planes orthogonal to the long axis of the axon and are typically averaged (λ2 + λ3)/2 to yield a measure of radial diffusivity. Relative anisotropy (RA) is a ratio of the anisotropic part of the tensor to the isotropic part. Fractional anisotropy (FA) represents the fraction of the magnitude of tensor that is due to anisotropic water diffusion (Basser et al., 1994; Masutani et al., 2003). The equation listed below (1) reflects the computation of FA. In the formula, λM represents the mean diffusivity or (λ1 + λ2 + λ3)/3:
| (1) |
Mean diffusivity decreases while FA increases with brain maturation as a result of a number of changes to axons including development of the axon structure, changes to the axonal membrane, and myelination (Neil et al., 1998; Nomura et al., 1994; Sakuma et al., 1991). Within the myelin sheath itself, water movement becomes increasingly anisotropic with development. Initially, it was thought that myelin was the primary contributor to anisotropy in axons (Rutherford et al., 1991). It has since been demonstrated that other changes to the axons also contribute significantly to increasing anisotropy with development. The contribution of myelin to diffusion anisotropy has been investigated in a number of in vitro studies that found similar degrees of diffusion anisotropy in myelinated and unmyelinated axons (Beaulieu and Allen, 1994a, b). These studies of excised, non-mammalian axons may be limited in terms of generalizability to intact mammalian systems. However, studies of “pre-myelinated” neurons in mammalian species, including rats, have corroborated the general finding that myelin is not necessary in order for fibers to have significant diffusion anisotropy (Prayer et al., 2001; Wimberger et al., 1995).
DTI studies of human brain maturation have revealed that this methodology is highly sensitive to very early changes during brain development in newborns (Neil et al., 1998). Diffusivity changes rapidly during these early periods. ADC is significantly higher in newborns than in older infants. During the first 3 or 4 months of life, ADC drops by more than 25% (Morriss et al., 1999). By 3 years of age, ADC values are similar to adult values. Other DTI measures, such as FA, are also sensitive to very early developmental changes in newborns (Gilmore et al., 2004) but are, perhaps, uniquely sensitive to further changes in white matter over longer periods of development. For example, Klingberg et al. demonstrated lower anisotropy in frontal white matter in children (mean age 10) compared to adults (mean age 27) (Klingberg et al., 1999). Schmithorst et al. found significant correlations between age and white matter trace values (inverse correlations) as well as FA measures (positive correlations) in multiple white matter regions in subjects ranging in age from 5 to 18 years (Schmithorst et al., 2002). Nagy et al. reported significant correlations between cognitive measures (reading and working memory) and FA in specific left-frontal and temporal white matter regions (Nagy et al., 2004). Age, which ranged from 7 to 18 years, was a significant correlate in this study, and the authors suggest the findings may reflect a relationship between brain development and cognitive skill development.
Looking across studies, it becomes clear that imaging methodologies such as DTI do not measure a single characteristic of white matter development. Rather, DTI reflects underlying changes in tissue at a number of levels (Neil et al., 2002). Although DTI was initially seen as primarily a measure of myelin status, it is clear that DTI reflects other aspects of axonal integrity and the organization and alignment of groups of axons and fibers in white matter tissue (Beaulieu, 2002; Neil et al., 2002). Findings which demonstrate that diffusion anisotropy is present well before myelination corroborate this (Inder and Huppi, 2000; Huppi et al., 1998b). As neurons mature and myelinate, the space between neurons decreases, resulting in restricted diffusion and increased anisotropy. It is also important to note that measures such as DTI likely reflect different aspects of development during different developmental phases. For example, changes in DTI observed during the neonatal period may be more a function of overall changes in brain water content and physical growth of axons while, in contrast, the changes in DTI seen in early childhood and adolescence are likely more reflective of myelination.
Specific DTI measures may be differentially sensitive to aspects of white matter change with development and with disease processes. In a study of dysmyelination, Song et al. examined shiverer mice, which have a mutation in myelin basic protein resulting in incomplete myelination, but normal axons (Song et al., 2002). These authors showed that radial diffusivity, but not axial diffusivity, was sensitive to the dysmyelination in this model. Axial diffusivity was nearly identical in animals with and without myelin. Shiverer mice showed significantly lower RA and higher radial diffusivity than control mice. Interestingly, these authors noted that the nearly complete lack of myelin in these mice only resulted in a 20% increase in radial diffusivity, again pointing toward the importance of nonmyelin factors in white matter diffusion anisotropy. Further evidence for the specificity of these DTI measures was provided by a study of retinal ischemia in mice (Song et al., 2003). The authors demonstrated that optic nerve axonal degeneration, which occurred quickly after ischemia, was associated with an increase in axial diffusivity. Demyelination, which occurred later, was associated with an increase in radial diffusivity. Most recently, Song et al. (2005) demonstrated that radial diffusivity was sensitive to both demyelination and re-myelination in mice who were fed cuprizone. Cuprizone, which affects oligodendrocytes, resulted in demyelination. This was reflected in increased radial diffusivity in the corpus callosum. After removal of dietary cuprizone, re-myelination was reflected in decreasing radial diffusivity values (returning to normative levels).
DTI studies have demonstrated white matter abnormalities in a number of neuropsychiatric conditions including schizophrenia, major depression, and HIV infection, as well as chronic alcohol and cocaine abuse (Lim and Helpern, 2002; Moeller et al., 2005; Lim et al., 2002). A number of studies have also examined the utility of DTI measures in the study of normal and abnormal aging (Moseley et al., 2002). In a study of men ranging in age from 23 to 76 years, Pfefferbaum et al. noted a significant decline in FA with age in all white matter regions studied (Pfefferbaum et al., 2000). Decline was most notable in the genu of the corpus callosum and in the centrum semiovale but less prominent in the splenium. Sullivan et al. demonstrated similar findings of decreasing FA with age and showed a relationship between slowing of motor speed and decreasing FA in their sample (Sullivan et al., 2001). In a relatively large study of subjects ages 23–85 years, Pfefferbaum and Sullivan (2003) found that DTI trace increased with age and FA decreased with age, both suggesting deterioration of white matter over time. A recent study examining FA and age found the characteristic decline with age and also showed that FA correlated with slowed response times in older subjects on a visual task (Madden et al., 2004). In a review of DTI studies of aging, Moseley concludes that these observed declines in FA with age across various studies are likely related to the changes in axons, loss of myelin, and increase in extracellular space that accompanies normal aging (Moseley, 2002).
In a transgenic mouse model of Alzheimer’s Disease (using PDAPP mice that over-express amyloid precursor protein), Song et al. found that DTI measures were sensitive to amyloid plaque depositions in older mice but not younger mice (Song et al., 2004) with differences seen in radial diffusivity but not in axial diffusivity. In human studies of patients with probable Alzheimer’s disease, significant changes in diffusion in white matter have been observed (Rose et al., 2000; Sandson et al., 1999). In a study of early Alzheimer’s disease, Choi et al. found reduced FA, increased mean diffusivity, and increased radial diffusivity in superior frontal white matter (Choi et al., 2005). These authors noted that their findings supported the theory of “retrogenesis”, which suggests that the pathologic processes in Alzheimer’s disease proceed in an opposite manner to normal developmental patterns (Reisberg et al., 1999). This and other evidence (Braak and Braak, 1996) suggests that white matter regions which mature later in life (i.e. superior frontal and temporal regions) may be affected first in pathologic conditions such as Alzheimer’s disease.
DTI certainly has great potential to provide unique data about white matter maturation over the lifespan. At this point, the relationship between newer measures, like DTI, and volumetric measures is not especially well understood. Within the context of development and aging, both measures potentially provide an assessment of structural maturation and integrity. While volumetric measures provide information about macrostructural changes such as fiber growth and large-scale changes in myelination, DTI provides a finer-grained assessment of structural integrity. During early development, DTI may be more sensitive than volumetrics to axonal transitions from unmyelinated to myelinated. DTI may also be more sensitive to abnormalities in early myelination and fiber organization than volumetrics. In later development and aging, DTI and volumetrics may potentially combine to yield information that neither method alone can provide. For example, DTI’s sensitivity to myelin breakdown with aging, combined with volumetric data providing evidence of redundant lamination, may ultimately help clarify some of the inconsistent findings concerning white matter changes during aging that have been reported in the literature thus far. In any case, it is clear that volumetrics and DTI both have tremendous potential for increasing our understanding of brain development especially when utilized in the context of longitudinal studies and/or cross-sectional studies examining wide age ranges.
Future advancements will come from continued integration of multiple imaging modalities, refinements to DTI data collection methods, and continued implementation of non-human studies that validate the methodology and help clarify the relationships between underlying physiology and DTI measures.
6. DTI tractography
Interest in DTI tractography has developed rapidly in recent years (Moseley et al., 2002). Each voxel in a DTI data set contains vector information that reflects the directionality and magnitude of diffusion in the underlying tissue. Tractography uses algorithms to determine if one voxel’s effective diffusion vector is likely “connected” with the neighboring voxel (Parker et al., 2002). Visual representations of fibers spanning these voxels are then constructed using a maximum-likelihood method. Using oversampling techniques and interpolation, representations of fibers are constructed at greater spatial resolution than the actual images collected. These methods yield images which correspond to the known underlying anatomy. Although this methodology is promising and the images are often strikingly detailed, there are significant limitations to the technique (reviewed in Ramnani et al., 2004). For example, while tractography works well in deep white matter structures with high FA, such as corpus callosum, it is not as effective in less central regions of white matter where fiber directionality is more diverse, such as at the junction with gray matter. In addition, caution is needed in the application and interpretation of current methods because of the artifacts that arise when relying on “coarsely sampled, noisy, voxel-averaged direction field data” and the fact that these artifacts may show “phantom connections… that do not exist anatomically” (Basser and Jones, 2002). Smaller voxel sizes do improve tractography results but do not get around the problems caused by voxels containing curved, crossing, or branching fibers (Basser et al., 2000). Newer DTI methods, such as high angular resolution diffusion imaging (HARDI) (Tuch et al., 2002, 2003) and Q-Ball imaging (Tuch, 2004)do have potential yielding more robust measures of fiber integrity, even when there is intravoxel crossing, branching, or curving. In a recent review, Ramnani et al. (2004) discuss several new approaches to the complexities of imaging increasingly finer white matter tracts and less central tracts including the use of global probability density functions. Behrens et al. (2003) have provided an elegant example of probabilistic tractography methods in mapping tracts that connect specific thalamic regions with specific gray matter locations in cortex. Johansen-Berg et al. (2004) utilized both probabilistic tractography techniques and functional MRI to map distinct tracts and cortical regions involved in a cognitive task. In summary, much work remains in solving the challenges inherent to tractography, but it is certainly a very promising technique that will likely contribute greatly to our understanding of connectivity within the brain.
7. Magnetization transfer imaging (MTI)
MTI is a brain imaging technique that relies on transfer of magnetization between protons in different water “pools” within the tissue. Protons that are bound to macromolecular structures (e.g., myelin and cell membranes) are characterized by restricted motion while protons in free water have relatively unrestricted motion (Wolff and Balaban, 1989). The interaction between protons in these two pools produces the contrast that allows for tissue differentiation. MTI provides a quantitative measure of macromolecular structural integrity of the tissue. This most commonly used MT measure is the magnetization transfer ratio (MTR), which is described below (Wolff and Balaban, 1994). The technique is particularly relevant to the study of disease because it is capable of detecting subtle neuropathological changes in vivo before it manifests on conventional MRI. White matter MTR decreases are seen in conditions with myelin or axonal loss including multiple sclerosis, central pontine myelinolysis, cerebral ischemia, systemic lupus, HIV, and traumatic brain injury (Ernst, 1999; Gass et al., 1994; Kado, 2001; Bosma, 2000; Sinson, 2001). MTI has been used to demonstrate white matter abnormalities in multiple sclerosis and seems to be highly sensitive to white matter changes that are associated with neurocognitive deficits (Filippi et al., 2000). In multiple sclerosis, MTI typically shows effects of the disease both in areas of white matter lesion as well as in normal appearing white matter (NAWM) (Hiehle et al., 1994). Findings have also been observed in schizophrenia (Bagary et al., 2003; Kiefer et al., 2004). In general, MTR may be more sensitive to certain types of neuropathology than conventional MR volumetric imaging (Bagary et al., 2003; Foong et al., 2001).
MT contrast is achieved by saturating macromolecule protons using an off-resonance radio frequency (RF) pulse. These macromolecule protons then interact with protons in neighboring free water, reducing the MR signal from the tissue. The formula for computing MTR is listed below (2). In this formula, M0 represents the MR signal intensity without the saturation pulse and MS represents the signal after the saturation pulse:
| (2) |
MTR differs by tissue type, allowing for differentiation of tissue in the resulting image. Tissues with a high number of restricted or bound protons will show a large MT effect. Myelinated white matter, which contains a large number of bound proteins in lipids, has a higher MTR than gray matter (Mehta et al., 1995; Mehta et al., 1996). Within white matter, myelin water contributes disproportionately to the MTR effect. For example, using bovine optic nerves and MT modeling, (Stanisz et al., 1999) demonstrated that the MT effect was largely a result of water bound to myelin macromolecules as opposed to water in the axon or in the extracellular space. This suggests that MTR is a relatively specific measure of myelin status.
MTR increases with brain myelination, probably as a result of increasing interactions between “bound” water and glycolipids, cholesterol, and galactocerebrosides in myelinating neurons (Barkovich, 2000; Engelbrecht et al., 1998). In young children, myelination of white matter is reflected in increasing MTR values during the first year and a half to 2 years of life (Engelbrecht et al., 1998; van Buchem et al., 2001). MTR measures within specific white matter fiber bundles show that changes in MTR correspond to the well-established pattern of posterior to anterior myelination in the brain (Rademacher et al., 1999). Also, MTR was found to be higher in commissural and projection fibers compared with association fibers. In addition, MTR reflects the very early myelination of projection fibers (within the first month of life) (Rademacher et al., 1999). MTR appears to be relatively stable throughout adulthood (Mehta et al., 1995). Armstrong et al. (2004) found that MTR was stable throughout adulthood but noted a trend suggesting a slight increase in regional white matter MTR with age, especially in the occipital and temporal regions. Other studies have demonstrated decreasing MTR with age (Ge et al., 2002; Tanabe et al., 1997), but these studies did not remove the potentially biasing effects of white matter hyperintensities (Armstrong et al., 2004). Armstrong et al. suggest that, if the effects of hyperintensities are controlled, MTR increases slowly until age 60 or 70 as a result of myelin accumulation (redundant lamination). Further MTI studies in healthy older subjects (70s and 80s) are warranted to determine if MTI is sensitive to the significant deterioration in myelin status (e.g., myelin breakdown, splitting, ballooning, etc.) that has been shown to occur in those decades (Svennerholm et al., 1997).
Although MTR is the most commonly used measure of magnetization transfer, its interpretation is not entirely straightforward because it relies on a simple two-pool model (a free-water pool and a bound-water pool). The resulting MTR actually reflects a combination of various relaxation and exchange properties. (Sled and Pike, 2001) also suggest that MTR values are heavily dependent on specific pulse sequence characteristics and indicate that MTR results are difficult to compare across studies. As an alternative, they have proposed a quantitative MT method which yields measures of the fractional size of the restricted water pool, the magnetization exchange rate, the T2 of the restricted pool, and the relaxation of the free water pool. Sled et al. (2004) have demonstrated that the size of the restricted pool reflects different aspects of myelin than MTR. They also showed that this alternative measure was more sensitive to regional variations in myelin than was MTR. Thus, quantitative MT measures may have the potential to provide more detailed information about properties of the underlying white matter tissue.
8. T2 relaxography (T2R)
T2R is an imaging modality that can provide a measure of the myelin water fraction within tissue (MacKay et al., 1994). In brain, the T2 signal is made up of several components with distinct T2 relaxation times (Laule et al., 2004). These components are each thought to be related to specific “pools” of water. A fast relaxing T2 component (~ 10–50 ms) is attributed to water trapped between the hydrophobic bilayers that form the myelin sheath (Jones et al., 2004; Menon and Allen, 1991). A long component (>1 s) is related to water in CSF. A third, intermediate component (~70–100 ms) is due to intracellular and extracellular water. The sum of the three components described above reflects total brain water. By collecting a multiple echo sequence during which the signal is refocused at different times, these different T2 components can be individually quantified (Whittall et al., 1997; MacKay et al., 1994). A myelin water fraction is defined as the ratio of the fast-relaxing T2 component (10–50 ms) to total water signal. The first studies using this methodology collected 32 or more echoes (from 10 to 320 ms) in order to quantify the T2 relaxation curve (Whittall et al., 1997; MacKay et al., 1994). Typically the determination of the T2 spectrum is performed using a non-negative least squares (NNLS) algorithm but faster linear combinations of multiple echo data have also been used (Jones et al., 2004).
Studies examining subjects with multiple sclerosis have found reduced myelin water fraction not only in demyelinated lesions (MacKay et al., 1994) but also in NAWM (Laule et al., 2004; Moore et al., 2000; Vavasour et al., 1998). In these studies, myelin water fraction in NAWM was reduced by 16% on average. Flynn et al. (Basset et al., 2003) found that patients with schizophrenia had a white matter myelin fraction that was 26% lower than controls. In that study, frontal lobe myelin fraction was inversely correlated with age in control subjects but not in patients, suggesting that T2R is sensitive to normal myelination during development and to myelin abnormalities in neuropsychiatric disorders. An investigation of multiple sclerosis which compared MTR data and T2R data found that both were useful measures of white matter status but MTR and T2R were not correlated with each other, suggesting that they measure different aspects of white matter in this population (Vavasour et al., 1998. Gareau et al., 2000) found reduced MTR and reduced T2R-derived myelin fraction in an animal model of multiple sclerosis (experimental allergic encephalomyelitis in guinea pigs), but demonstrated that the two methods measured somewhat different aspects of white matter change in this model. Specifically, changes in MTR occurred in parallel with the clinical signs of disease and MTR returned to nearly normal levels with treatment. In contrast, the myelin water percentage as determined by T2R, was found to be uniformly reduced throughout the illness course and was not reactive to treatment. The authors concluded that changes in MTR were reflective of the inflammatory-induced changes occurring in NAWM as a result of the disease and T2R was specifically sensitive to loss of myelin.
The Gareau et al. study suggests that T2R has applicability as an indicator of myelin pathology. It is less clear, however, how T2R will apply to the study of development across the lifespan. We are not yet aware of any studies of normal development that have utilized T2R methodology. A related but different methodology, T2 Relaxometry, has been utilized in a few studies. In contrast to T2R, which is a multi-exponential method, T2 Relaxometry measures a single mono-exponential value from the tissue. One study of young children who were born prematurely (mean age = 7) revealed longer T2 relaxation times in cerebral white matter compared to age-matched children who were born full-term (Abernethy et al., 2003). Another T2 Relaxometry study, which examined a small group of healthy children ages 3 months to 13 years, found that three-pool modeling revealed changes in myelin that corresponded very well to known patterns of myelination during development (Lancaster et al., 2003). Thus, both T2 Relaxometry and T2R may be useful methods for the study of myelin growth during important developmental periods such as adolescence and may also be applicable to the study of myelin changes during aging.
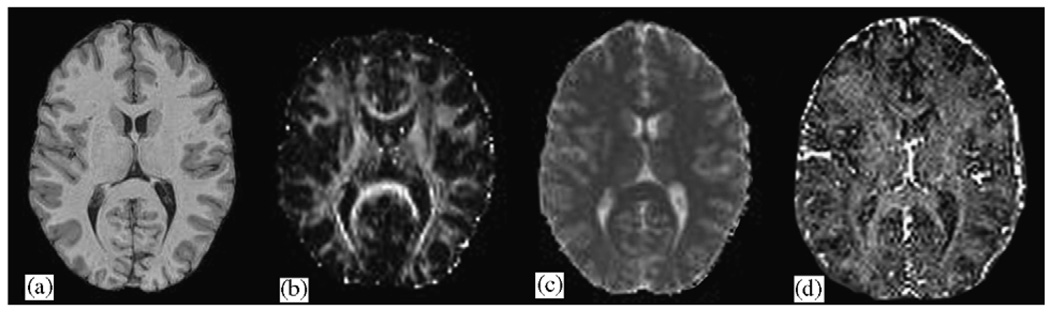
Until recently, T2R has typically been performed with a 32-echo sequence resulting in a 26 min acquisition time and limited to a single slice. Newer methods utilizing as few as three echoes have been developed (Vidarsson et al., 2005). These methods result in a 5 min acquisition time and also allow for collection of multiple slices. Fig. 1 contains an example of a myelin fraction image collected using a three-echo sequence. It is likely that multi-slice T2R imaging will steadily improve in the near future, allowing researchers much more flexibility in designing studies.
Fig. 1.
Comparison of imaging methodologies: (a) standard T1-weighted image; (b) Diffusion Tensor Imaging (DTI) Fractional Anisotropy (FA) map; (c) Magnetization Transfer Ratio (MTR) image computed from a Magnetization Transfer Imaging (MTI) series; (d) T2R myelin fraction image.
9. Magnetic resonance spectroscopy
MRS is unique among MR imaging methodologies because it provides a measure of the chemical composition of tissue. A number of metabolites, including N-acetyl aspartate (NAA), phosphocreatine, choline, myo-inositol, glutamine, glutamate, GABA, glucose, taurine, scylloinositol, and lactate can be measured in the brain with proton MRS. MRS utilizes a set of RF pulses to perturb nuclei within various molecules and subsequently measures the resulting resonant signal. Because the frequency of the signal depends on the specific chemical structure and environment of the molecule, a spectrum can be constructed to indicate the amplitude of the signal for various frequencies. From this spectrum, the level of various metabolites can be calculated. As typically implemented for brain measurements, MRS is performed for a single voxel placed within a region of interest in the brain. However, metabolites can be measured in larger areas of tissue, whole slices, and even whole brain volumes with spectroscopic chemical shift imaging (CSI) (Brown et al., 1982; (Kuker et al., 2004; Guimaraes et al., 1999).
Because NAA is found only in neurons, it is often used as a marker of neuronal integrity (Birken and Oldendorf, 1989). NAA levels are frequently found to be lower than normal in conditions resulting in injury to brain tissue (Friedman et al., 1998; Davie et al., 1994; Lu et al., 1996). NAA levels can also serve as a marker of development as levels are known to change systematically with age. Because choline level reflects cellular density and is related to cell membrane integrity (choline is a precursor of phosphatidylcholine—a major constituent of cell membrane), NAA level is frequently contrasted with choline level in the form of a ratio: NAA/Cho as a single measure of neuronal integrity. Kadota et al. found that, while NAA/Cho levels decreased gradually from childhood to old age in gray matter, the pattern was different in white matter (Kadota et al., 2001). Specifically, NAA/Cho levels increased throughout childhood and peaked in the second or third decade. There were regional differences for the peak in NAA/Cho levels: parietal white matter peaked at 15.9 years, pre-central motor cortex at 17.6 years, and frontal association white matter at 21.9 years. Interestingly, they also noted a 1–4 year maturational advantage in right hemisphere white matter compared to left hemisphere white matter. Kadota et al. also found that, starting in the third decade, NAA/Cho levels begin to decline steadily.
Spectroscopy is relevant to the current discussion because of its sensitivity to myelin status (Kuker et al., 2004). The ratio of choline to creatine (Cho/Cre) is particularly relevant to the study of white matter development. During periods of extensive myelination early in life, the Cho/Cre ratio declines quickly, presumably because choline is being incorporated into macromolecule components of myelin (Kreis et al., 1993). Elevations in white matter Cho/Cre have been observed in children with mild developmental delays at the age of 2 years, presumably as a result of under-myelination (Filippi et al., 2002). Charles et al. found that the choline, creatine, and NAA levels were all lower in the gray matter of older subjects than younger subjects, but found no differences in white matter between older and younger subjects (Charles et al., 1994).
MRS provides in vivo measurements of important brain chemicals. Since these chemicals are in the millimolar concentration level, the signal to noise ratio is low when compared to measuring water signal (50 M), as is done with MRI. Also, the low signal-to-noise ratio limits resolution to the level of cubic centimeters. Despite these current limitations, MRS provides unique information about tissue status and integrity that is unavailable from any other methodology. MRS is emerging as an important tool in the study of both development and aging and it will very likely yield important data in the years to come.
10. Summary
In recent years, there has been a steady increase in interest in white matter imaging in the study of disease as well as in normal development and aging. This interest is being fueled by the recognition of white matter’s role in cognition, by the evidence for very significant changes in white matter structure over the course of the lifespan, and by the development of new tools that permit noninvasive examination and quantification of white matter properties beyond those of conventional MRI.
To date, the methods reviewed here have provided exciting new insights into the development and aging of white matter. The most dramatic changes in white matter occur during infancy. Rapid myelination during this period is reflected in changes in nearly all measures of white matter including volumetrics, diffusivity, myelin water, and macromolecular metrics. During the early childhood years, the various imaging methods diverge somewhat in terms of their sensitivity to further developmental change. For example, FA seems to be sensitive to changes in white matter organization well into the early adult years, but measures of mean diffusivity are less sensitive beyond early childhood. Perhaps this reflects the fact that axons are developed and organized into fiber bundles early on in childhood, but more subtle changes to myelin, especially regional changes, continue to occur into adulthood. Volumetric measures continue to reflect changes into the fourth and fifth decades and, perhaps, even beyond. These may also reflect changes in myelination level, especially regionally. MTR may stabilize earlier in adulthood than other measures and, thus, may be a more useful measure of developmental change in white matter during the early years. The type of macromolecular changes to which MTR is sensitive may not occur much beyond early stages of development. MRS may be uniquely sensitive to changes that occur very early on during infancy, but there are suggestions that it may also be useful in the study of myelin breakdown that occurs in aging. The study of aging during the later decades, using various neuroimaging methods, is beginning to yield results, but there are challenges to overcome. One of these challenges is finding ways to parse the changes in MR signals that are due to deterioration or breakdown of white matter constituents from the changes in MR signals that are due to compensatory processes such as redundant myelination and changes in signal due to age-associated phenomena such as small bleeds, etc. It is for these reasons that the combination of multiple imaging modalities may be especially useful.
The application of multiple imaging methods in the same subject has not been common. Clearly, this has tremendous potential to allow for a more detailed understanding of brain development, organization, pathology, recovery, etc. Combining measures of cognition with the various imaging methodologies is also critical to maximizing their potential, given our limited understanding of structure–function relationships in the context of development. Thus, continued application of these new imaging techniques, in conjunction with carefully designed studies of brain function during early childhood, adolescence, and adulthood, will contribute greatly to our understanding of brain development across the lifespan.
Acknowledgments
The authors wish to thank Bryon Mueller for his suggestions and his assistance with the preparation of this paper.
Contributor Information
Jeffrey R. Wozniak, Email: jwozniak@umn.edu.
Kelvin O. Lim, Email: kolim@umn.edu.
References
- Abernethy LJ, et al. Magnetic resonance imaging and T2 relaxometry of cerebral white matter and hippocampus in children born preterm. Pediatric Research. 2003;54(6):868–874. doi: 10.1203/01.PDR.0000091285.84577.4E. [DOI] [PubMed] [Google Scholar]
- Armstrong CL, et al. Age-related, regional, hemispheric, and medial-lateral differences in myelin integrity in vivo in the normal adult brain. American Journal of Neuroradiology. 2004;25(6):977–984. [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6, Part 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bagary MS, et al. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Archives of General Psychiatry. 2003;60(8):779–788. doi: 10.1001/archpsyc.60.8.779. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Concepts of myelin and myelination in neuroradiology. American Journal of Neuroradiology. 2000;21(6):1099–1109. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, et al. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166(1, Part 1):173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, et al. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Archives of General Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, et al. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of Neurology. 2003;60(3):393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, et al. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging. 2004;25(7):843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR in Biomedicine. 2002;15(7–8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magnetic Resonance in Medicine. 1998;39(6):928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, et al. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Basset ASCE, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. American Journal of Psychiatry. 2003;160(9):1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Allen PS. Water diffusion in the giant axon of the squid: implications for diffusion-weighted MRI of the nervous system. Magnetic Resonance in Medicine. 1994a;32(5):579–583. doi: 10.1002/mrm.1910320506. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magnetic Resonance in Medicine. 1994b;31(4):394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- Behrens TE, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience and Biobehavioral Review. 1989;13(1):23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Bosma G. Evidence of central nervous system damage in patients with neuropsychiatric systemic lupus erythematosus, demonstrated by magnetization transfer imaging. Arthritis and Rheumatism. 2000;43:48–54. doi: 10.1002/1529-0131(200001)43:1<48::AID-ANR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathologica (Berlin) 1996;92(2):197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- Brady ST, et al. Formation of compact myelin is required for maturation of the axonal cytoskeleton. Journal of Neuroscience. 1999;19(17):7278–7288. doi: 10.1523/JNEUROSCI.19-17-07278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TR, Kincaid BM, Ugurbil K. NMR chemical shift imaging in three dimensions. Proceedings of the National Academy of Science USA. 1982;79(11):3523–3526. doi: 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles HC, et al. Proton spectroscopy of human brain: effects of age and sex. Programme of Neuropsychopharmacol Biology and Psychiatry. 1994;18(6):995–1004. doi: 10.1016/0278-5846(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Choi SJ, et al. Diffusion tensor imaging of frontal white matter microstructure in early Alzheimer’s disease: a preliminary study. Journal of Geriatric Psychiatry and Neurology. 2005;18(1):12–19. doi: 10.1177/0891988704271763. [DOI] [PubMed] [Google Scholar]
- Courchesne E, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Davie CA, et al. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117(Pt 1):49–58. doi: 10.1093/brain/117.1.49. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Breteler MM. Cognitive correlates of cerebral white matter changes. Journal of Neural. Transmission Supplement. 1998;53:41–67. doi: 10.1007/978-3-7091-6467-9_5. [DOI] [PubMed] [Google Scholar]
- Engelbrecht V, et al. Age-dependent changes in magnetization transfer contrast of white matter in the pediatric brain. American Journal of Neuroradiology. 1998;19(10):1923–1929. [PMC free article] [PubMed] [Google Scholar]
- Ernst T. Progressive multifocal leukoencephalopathy and human immunodeficiency virus–associated white matter lesions in AIDS: magnetization transfer MR imaging. Radiology. 1999;210:539–543. doi: 10.1148/radiology.210.2.r99fe19539. [DOI] [PubMed] [Google Scholar]
- Feldman ML, Peters A. Ballooning of myelin sheaths in normally aged macaques. Journal of Neurocytology. 1998;27(8):605–614. doi: 10.1023/a:1006926428699. [DOI] [PubMed] [Google Scholar]
- Ferro JM, Madureira S. Age-related white matter changes and cognitive impairment. Journal of Neurological Sciences. 2002:203–204. 221–225. doi: 10.1016/s0022-510x(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Filipek PA, et al. The young adult human brain: an MRI-based morphometric analysis. Cerebral Cortex. 1994;4(4):344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Filippi M, et al. Changes in the normal appearing brain tissue and cognitive impairment in multiple sclerosis. Journal of Neurology Neurosurgery and Psychiatry. 2000;68(2):157–161. doi: 10.1136/jnnp.68.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi CG, et al. Developmental delay in children: assessment with proton MR spectroscopy. American Journal of Neuroradiology. 2002;23(5):882–888. [PMC free article] [PubMed] [Google Scholar]
- Filley CM. The behavioral neurology of cerebral white matter. Neurology. 1998;50(6):1535–1540. doi: 10.1212/wnl.50.6.1535. [DOI] [PubMed] [Google Scholar]
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. New England Journal of Medicine. 2001;345(6):425–432. doi: 10.1056/NEJM200108093450606. [DOI] [PubMed] [Google Scholar]
- Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Foong J, et al. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124(Part 5):882–892. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- Friedman SD, et al. Brain injury and neurometabolic abnormalities in systemic lupus erythematosus. Radiology. 1998;209(1):79–84. doi: 10.1148/radiology.209.1.9769816. [DOI] [PubMed] [Google Scholar]
- Fukui T, et al. Cognitive functions in subjects with incidental cerebral hyperintensities. European Neurology. 1994;34(5):272–276. doi: 10.1159/000117055. [DOI] [PubMed] [Google Scholar]
- Gareau PJ, et al. Magnetization transfer and multicomponent T2 relaxation measurements with histopathologic correlation in an experimental model of MS. Journal of Magnetic Resonance and Imaging. 2000;11(6):586–595. doi: 10.1002/1522-2586(200006)11:6<586::aid-jmri3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gass A, et al. Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Annals of Neurology. 1994;36(1):62–67. doi: 10.1002/ana.410360113. [DOI] [PubMed] [Google Scholar]
- Ge Y, et al. Age-related total gray matter and white matter changes in normal adult brain. Part II: quantitative magnetization transfer ratio histogram analysis. American Journal of Neuroradiology. 2002;23(8):1334–1341. [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, et al. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Research and Developmental Brain Research. 1996;91(2):274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, et al. 3 Tesla magnetic resonance imaging of the brain in newborns. Psychiatry Research. 2004;132(1):81–85. doi: 10.1016/j.pscychresns.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Good CD, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1, Part 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, et al. Echoplanar chemical shift imaging. Magnetic Resonance in Medicine. 1999;41(5):877–882. doi: 10.1002/(sici)1522-2594(199905)41:5<877::aid-mrm4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gur RC, et al. Differences in the distribution of gray and white matter in human cerebral hemispheres. Science. 1980;207(4436):1226–1228. doi: 10.1126/science.7355287. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, et al. Normal brain maturation in MRI. European Journal of Radiology. 1991;12(3):208–215. doi: 10.1016/0720-048x(91)90074-6. [DOI] [PubMed] [Google Scholar]
- Hiehle JF, Jr, et al. Correlation of spectroscopy and magnetization transfer imaging in the evaluation of demyelinating lesions and normal appearing white matter in multiple sclerosis. Magnetic Resonance in Medicine. 1994;32(3):285–293. doi: 10.1002/mrm.1910320303. [DOI] [PubMed] [Google Scholar]
- Holland BA, et al. MRI of normal brain maturation. American Journal of Neuroradiology. 1986;7(2):201–208. [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Annals of Neurology. 1998a;43(2):224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Huppi PS, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatric Research. 1998b;44(4):584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Inder TE, Huppi PS. In vivo studies of brain development by magnetic resonance techniques. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(1):59–67. doi: 10.1002/(SICI)1098-2779(2000)6:1<59::AID-MRDD8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Iwasaki N, et al. Volumetric quantification of brain development using MRI. Neuroradiology. 1997;39(12):841–846. doi: 10.1007/s002340050517. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, et al. Linear combination of multiecho data: short T2 component selection. Magnetic Resonance in Medicine. 2004;51(3):495–502. doi: 10.1002/mrm.10713. [DOI] [PubMed] [Google Scholar]
- Kado H. Abnormal magnetization transfer ratios in normalappearing white matter on conventional MR images of patients with occlusive cerebrovascular disease. American Journal of Neuroradiology. 2001;22:922–927. [PMC free article] [PubMed] [Google Scholar]
- Kadota T, Horinouchi T, Kuroda C. Development and aging of the cerebrum: assessment with proton MR spectroscopy. American Journal of Neuroradiology. 2001;22(1):128–135. [PMC free article] [PubMed] [Google Scholar]
- Kiefer C, et al. Differentiating hippocampal subregions by means of quantitative magnetization transfer and relaxometry: preliminary results. Neuroimage. 2004;23(3):1093–1099. doi: 10.1016/j.neuroimage.2004.07.066. [DOI] [PubMed] [Google Scholar]
- Kinney HC, et al. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology and Experimental Neurology. 1988;47(3):217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Klingberg T, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10(13):2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic Resonance in Medicine. 1993;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Kuker W, et al. Modern MRI tools for the characterization of acute demyelinating lesions: value of chemical shift and diffusion-weighted imaging. Neuroradiology. 2004;46(6):421–426. doi: 10.1007/s00234-004-1203-5. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, et al. Three-pool model of white matter. Journal of Magnetic Resonance Imaging. 2003;17(1):1–10. doi: 10.1002/jmri.10230. [DOI] [PubMed] [Google Scholar]
- Laule C, et al. Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. Journal of Neurology. 2004;251(3):284–293. doi: 10.1007/s00415-004-0306-6. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- Lim KO, Helpern JA. Neuropsychiatric applications of DTI—a review. NMR in Biomedicine. 2002;15(7–8):587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- Lim KO, et al. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biological Psychiatry. 2002;51(11):890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Lu D, et al. Proton MR spectroscopy of the basal ganglia in healthy children and children with AIDS. Radiology. 1996;199(2):423–428. doi: 10.1148/radiology.199.2.8668788. [DOI] [PubMed] [Google Scholar]
- MacKay A, et al. In vivo visualization of myelin water in brain by magnetic resonance. Magnetic Resonance in Medicine. 1994;31(6):673–677. doi: 10.1002/mrm.1910310614. [DOI] [PubMed] [Google Scholar]
- Madden DJ, et al. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21(3):1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Masutani Y, et al. MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. European Journal of Radiology. 2003;46(1):53–66. doi: 10.1016/s0720-048x(02)00328-5. [DOI] [PubMed] [Google Scholar]
- McRobbie DW, et al. MRI from Picture to Proton. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- Mehta RC, Pike GB, Enzmann DR. Magnetization transfer MR of the normal adult brain. American Journal of Neuroradiology. 1995;16(10):2085–2091. [PMC free article] [PubMed] [Google Scholar]
- Mehta RC, Pike GB, Enzmann DR. Magnetization transfer magnetic resonance imaging: a clinical review. Topics in Magnetic Resonance Imaging. 1996;8(4):214–230. [PubMed] [Google Scholar]
- Menon RS, Allen PS. Application of continuous relaxation time distributions to the fitting of data from model systems and excised tissue. Magnetic Resonance in Medicine. 1991;20(2):214–227. doi: 10.1002/mrm.1910200205. [DOI] [PubMed] [Google Scholar]
- Moeller FG, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moore GR, et al. A pathology-MRI study of the short-T2 component in formalin-fixed multiple sclerosis brain. Neurology. 2000;55(10):1506–1510. doi: 10.1212/wnl.55.10.1506. [DOI] [PubMed] [Google Scholar]
- Morriss MC, et al. Changes in brain water diffusion during childhood. Neuroradiology. 1999;41(12):929–934. doi: 10.1007/s002340050869. [DOI] [PubMed] [Google Scholar]
- Moseley ME, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176(2):439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Moseley M, Bammer R, Illes J. Diffusion-tensor imaging of cognitive performance. Brain and Cognition. 2002;50(3):396–413. doi: 10.1016/s0278-2626(02)00524-9. [DOI] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging—a review. NMR in Biomedicine. 2002;15(7–8):553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Myklebust HR. Nonverbal learning disabilities: assessment and intervention. In: Myklebust HR, editor. Progess in Learning Disabilities. New York: Grune & Stratton; 1975. [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Neil JJ, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209(1):57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Neil J, et al. Diffusion tensor imaging of normal and injured developing human brain—a technical review. NMR in Biomedicine. 2002;15(7–8):543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Nomura Y, et al. Diffusional anisotropy of the human brain assessed with diffusion-weighted MR: relation with normal brain development and aging. American Journal of Neuroradiology. 1994;15(2):231–238. [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger’s report. A review. Stroke. 1995;26(7):1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Cognitive impairment and cellular/vascular changes in the cerebral white matter. Annals of the New York Academy of Sciences. 1997;826:92–102. doi: 10.1111/j.1749-6632.1997.tb48463.x. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Wheeler-Kingshott CA, Barker GJ. Estimating distributed anatomical connectivity using fast marching methods and diffusion tensor imaging. IEEE Transactions on Medical Imaging. 2002;21(5):505–512. doi: 10.1109/TMI.2002.1009386. [DOI] [PubMed] [Google Scholar]
- Paus T, et al. Structural maturation of nerual pathways in children and adolescents: in vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Paus T, et al. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. Journal of Neurocytology. 2002a;31(8–9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neuroscience and Biobehavioral Review. 2002b;26(7):733–741. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anatomical Record. 1991;229(3):384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. Journal of Comparative Neurology. 2001;435(2):241–248. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003;49(5):953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, et al. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magnrtic Resonance in Medicine. 2000;44(2):259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, et al. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Prayer D, et al. Visualization of nonstructural changes in early white matter development on diffusion-weighted MR images: evidence supporting premyelination anisotropy. American Journal of Neuroradiology. 2001;22(8):1572–1576. [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, et al. Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. Neuroimage. 1999;9(4):393–406. doi: 10.1006/nimg.1998.0416. [DOI] [PubMed] [Google Scholar]
- Ramnani N, et al. New approaches for exploring anatomical and functional connectivity in the human brain. Biological Psychiatry. 2004;56(9):613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Rao SM. Neuropsychology of multiple sclerosis. Current Opinion in Neurology. 1995;8(3):216–220. doi: 10.1097/00019052-199506000-00010. [DOI] [PubMed] [Google Scholar]
- Reisberg B, et al. Retrogenesis: clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer’s and other dementing processes. European Archives Psychiatry and Clinical Neuroscience. 1999;249 Suppl. 3:28–36. doi: 10.1007/pl00014170. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ. Developmental neuroimaging of children using magnetic resonance techniques. Mental Retardation and Development Disabilities Research Reviews. 2000;6(1):68–80. doi: 10.1002/(SICI)1098-2779(2000)6:1<68::AID-MRDD9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Rose SE, et al. Loss of connectivity in Alzheimer’s disease: an evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. Journal of Neurology Neurosurgery and Psychiatry. 2000;69(4):528–530. doi: 10.1136/jnnp.69.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke BP. Introduction: the NLD syndrome and the white matter model. In: Rourke BP, editor. Syndrome of Nonverbal Learning Disabilities: Neurodevelopmental Manifestations. New York: The Guilford Press; 1995. pp. 1–26. [Google Scholar]
- Rourke BP, et al. Child clinical/pediatric neuropsychology: some recent advances. Annual Review of Psychology. 2002;53:309–339. doi: 10.1146/annurev.psych.53.100901.135204. [DOI] [PubMed] [Google Scholar]
- Rutherford MA, et al. MR imaging of anisotropically restricted diffusion in the brain of neonates and infants. Journal of Computer Assisted Tomography. 1991;15(2):188–198. doi: 10.1097/00004728-199103000-00002. [DOI] [PubMed] [Google Scholar]
- Sakuma H, et al. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology. 1991;180(1):229–233. doi: 10.1148/radiology.180.1.2052700. [DOI] [PubMed] [Google Scholar]
- Sandson TA, et al. Diffusion-weighted magnetic resonance imaging in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorder. 1999;10(2):166–171. doi: 10.1159/000017099. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, et al. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinson G. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: correlation with clinical outcome after traumatic brain injury. American Journal of NeuroRadiology. 2001;22:143–151. [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Pike GB. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magnetic Resonance in Medicine. 2001;46(5):923–931. doi: 10.1002/mrm.1278. [DOI] [PubMed] [Google Scholar]
- Sled JG, et al. Regional variations in normal brain shown by quantitative magnetization transfer imaging. Magnetic Resonance in Medicine. 2004;51(2):299–303. doi: 10.1002/mrm.10701. [DOI] [PubMed] [Google Scholar]
- Song SK, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, et al. Diffusion tensor imaging detects age-dependent white matter changes in a transgenic mouse model with amyloid desposition. Neurobiology of Disease. 2004;15(3):640–647. doi: 10.1016/j.nbd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stanisz GJ, et al. Characterizing white matter with magnetization transfer and T(2) Magnetic Resonance in Medicine. 1999;42(6):1128–1136. doi: 10.1002/(sici)1522-2594(199912)42:6<1128::aid-mrm18>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Stejskal EO, Tanner JE. Spin diffusion measurements: spin echos in the presence of time-dependent field gradient. Journal of Chemical Physics. 1965;42:288–292. [Google Scholar]
- Sullivan EV, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Bostrom K, Jungbjer B. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathologica (Berlin) 1997;94(4):345–352. doi: 10.1007/s004010050717. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, et al. Volumetric method for evaluating magnetization transfer ratio of tissue categories: application to areas of white matter signal hyperintensity in the elderly. Radiology. 1997;204(2):570–575. doi: 10.1148/radiology.204.2.9240555. [DOI] [PubMed] [Google Scholar]
- Tuch DS, et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magnetic Resonance in Medicine. 2002;48(4):577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Tuch DS, et al. Diffusion MRI of complex neural architecture. Neuron. 2003;40(5):885–895. doi: 10.1016/s0896-6273(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Tuch DS. Q-ball imaging. Magnetic Resonance in Medicine. 2004;52(6):1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- van Buchem MA, et al. Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: a preliminary study. American Journal of Neuroradiology. 2001;22(4):762–766. [PMC free article] [PubMed] [Google Scholar]
- Vavasour IM, et al. A comparison between magnetization transfer ratios and myelin water percentages in normals and multiple sclerosis patients. Magnetic Resonance in Medicine. 1998;40(5):763–768. doi: 10.1002/mrm.1910400518. [DOI] [PubMed] [Google Scholar]
- Vidarsson L, et al. Echo time optimization for linear combination myelin imaging. Magnetic Resonance in Medicine. 2005;53(2):398–407. doi: 10.1002/mrm.20360. [DOI] [PubMed] [Google Scholar]
- Watkins KE, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cerebral Cortex. 2001;11(9):868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, et al. Asymmetrical volumes of the right and left frontal and occipital regions of the human brain. Annals of Neurology. 1982;11(1):97–100. doi: 10.1002/ana.410110118. [DOI] [PubMed] [Google Scholar]
- Whittall KP, et al. In vivo measurement of T2 distributions and water contents in normal human brain. Magnetic Resonance in Medicine. 1997;37(1):34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- Wimberger DM, et al. Identification of “premyelination” by diffusion-weighted MRI. Journal of Computer Assisted Tomography. 1995;19(1):28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magnetic Resonance in Medicine. 1989;10(1):135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer imaging: practical aspects and clinical applications. Radiology. 1994;192(3):593–599. doi: 10.1148/radiology.192.3.8058919. [DOI] [PubMed] [Google Scholar]
- Xi MC, et al. Changes in the axonal conduction velocity of pyramidal tract neurons in the aged cat. Neuroscience. 1999;92(1):219–225. doi: 10.1016/s0306-4522(98)00754-4. [DOI] [PubMed] [Google Scholar]
- Yakolev PI, Lecours AR. The myelogenic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Ylikoski R, et al. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Archives of Neurology. 1993;50(8):818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]