Abstract
Background
Prenatal alcohol exposure, which is associated with macrostructural brain abnormalities, neurocognitive deficits, and behavioral disturbances, is characterized as fetal alcohol syndrome (FAS) in severe cases. The only published study thus far using diffusion tensor imaging (DTI) showed microstructural abnormalities in patients with FAS. The current study investigated whether similar abnormalities are present in less severely affected, prenatally exposed patients who did not display all of the typical FAS physical stigmata.
Methods
Subjects included 14 children, ages 10 to 13, with fetal alcohol spectrum disorders (FASD) and 13 matched controls. Cases with full-criteria FAS, mental retardation, or microcephaly were excluded. Subjects underwent MRI scans including DTI.
Results
Although cases with microcephaly were excluded, there was a trend toward smaller total cerebral volume in the FASD group (p = 0.057, Cohen’s d effect size = 0.73). Subjects with FASD had greater mean diffusivity (MD) in the isthmus of the corpus callosum than controls (p = 0.013, effect size = 1.05), suggesting microstructural abnormalities in this region. There were no group differences in 5 other regions of the corpus callosum. Correlations between MD in the isthmus and facial dysmorphology were nonsignificant.
Conclusions
These results suggest that even relatively mild forms of fetal alcohol exposure may be associated with microstructural abnormalities in the posterior corpus callosum that are detectable with DTI.
Keywords: Diffusion Tensor Imaging (DTI); Magnetic Resonance Imaging (MRI); Brain; Fetal Alcohol (FAS, FASD)
Fetal alcohol syndrome (FAS) is associated with intellectual deficits, mental retardation (Streissguth, 1986; Streissguth et al., 1991a, 1991b), and other neurocognitive deficits including attention (Coles et al., 1997; Olson et al., 1998), learning and memory (Kerns et al., 1997; Laforce et al., 2001; Mattson and Riley, 1999; Mattson et al., 1998), language (Korkman et al., 2003), executive functioning (Connor et al., 2000; Mattson et al., 1999), and motor skills (Wass et al., 2002). These cognitive effects are also seen in non-FAS, alcohol-exposed individuals without the full FAS phenotype (Burden et al., 2005; Howell et al., 2006; Mattson et al., 1997; Mattson et al., 1998; Mattson et al., 1999; Sampson et al., 2000; Schonfeld et al., 2001). As a result, the term fetal alcohol spectrum disorders (FASD) is now commonly used to describe affected individuals (Barr and Streissguth, 2001; Centers for Disease Control and Prevention, 2005; Sokol et al., 2003). The goal of the current study was to use diffusion tensor imaging (DTI) to examine white matter microstructure in prenatally exposed FASD children without full-criteria FAS.
Neuropathological studies of FAS have revealed significant structural brain abnormalities including microcephaly, cerebellar malformations, and neuronal migration errors (Clarren and Smith, 1978; Jones et al., 1973). Swayze et al. (1997) reported frequent midline anomalies in FAS including corpus callosum malformations and cavum septi pellucidi in addition to ventricular enlargement and microcephaly. Magnetic resonance imaging has demonstrated macrostructural effects associated with all levels of alcohol exposure (Riley et al., 2004). Small brain size has been consistently reported (Archibald et al., 2001; Mattson et al., 1992; Mattson et al., 1994; Riikonen et al., 1999; Riikonen et al., 2005) as have regional volume differences (Archibald et al., 2001). Studies using voxelbased morphometry show abnormalities in perisylvian regions, temporal lobes, and parietal lobes (Sowell et al., 2001). Surface mapping techniques show decreased growth in the inferior parietal, perisylvian, and frontal cortex in heavy prenatal exposure (Sowell et al., 2002a).
Prenatal alcohol exposure disproportionately affects midline craniofacial and brain structures. Magnetic resonance imaging studies show a variety of abnormalities in the corpus callosum including complete and partial agenesis (Clark et al., 2000; Riley et al., 1995; Swayze et al., 1997). Riley et al. (1995) showed smaller callosal volumes, even after correcting for smaller brain size. Sowell et al. (2002b) reported no callosal volume differences after adjusting for brain size, but did report alterations in callosal shape, especially in posterior regions (isthmus and splenium). Other studies have reported similar findings in both FAS and fetal alcohol effects (FAE) (Bookstein et al., 2001; 2002a; 2002b; Roebuck et al., 2002).
Diffusion tensor imaging provides a measure of tissue microstructural integrity that is useful in characterizing white matter (Le Bihan, 1995). Diffusion tensor imaging contrast depends on the diffusion of water molecules; in white matter, diffusion occurs more freely parallel to, rather than perpendicular to, axons because of restriction by cell membranes and myelin (Moseley et al., 1990). Collecting a set of diffusion-weighted images and, subsequently, computing the diffusion tensor matrix quantifies water diffusion in 3 dimensions (Basser et al., 1994; Basser and Pierpaoli, 1998). The scalar measure fractional anisotropy (FA) represents the directional portion of the diffusion. Fractional anisotropy is the highest in organized white matter such as the corpus callosum. Mean diffusivity (MD) represents average diffusion in all directions. It is low in tissues that restrict diffusion, such as organized white matter. Conditions that affect axon development or damage axons (e.g., demyelination) are reflected in lower FA and higher MD (Neil et al., 2002). Both measures reflect microstructural changes occuring normally during development and aging (Neil et al., 1998; Schmithorst et al., 2002; Sullivan et al., 2001; Sullivan and Pfefferbaum, 2003). Mean diffusivity and FA are inversely related to one another, but developmental and pathological factors can result in more significant changes in one versus the other.
Diffusion tensor imaging may be useful in studying neurodevelopmental disorders, including FASD, because it is sensitive to abnormalities against the “background” changes of normal development (see Wozniak and Lim, 2006, for a review). Diffusion tensor imaging microstructural abnormalities are seen in neurological and neuropsychiatric conditions including neuro-HIV, multiple sclerosis, epilepsy, schizophrenia, and depression (Ciccarelli et al., 2003; Gupta et al., 2005; Lim and Helpern, 2002), as well as in alcohol and other drug abuse (Lim et al., 2002; Pfefferbaum and Sullivan, 2005), even in the absence of macrostructural changes (Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2006).
One published study used DTI to examine brain integrity in FASD (Ma et al., 2005). This study examined the corpus callosum in adults who met criteria for FAS with the exception of growth deficiency. The subjects had intellectual impairment (IQ range: 44–74). Lower FA and higher MD were observed in splenium and genu of the corpus callosum in the alcohol-exposed group versus controls. No associations were found between the DTI measures and dysmorphia score, IQ, or processing speed.
The current study used a similar methodology, but examined children rather than adults and studied a less severely affected group by excluding subjects with full-criteria FAS and those with mental retardation. Full-criteria FAS likely represents a minority of cases of individuals impacted by prenatal alcohol exposure. The main hypotheses were that children with FASD would show lower FA and higher MD than control subjects in the corpus callosum and that measures of microstructural integrity would correlate with facial dysmorphology.
MATERIALS AND METHODS
Subjects
Subjects were ages 10 to 13. Fifteen subjects with FASD were selected from a database of patients seen for evaluations at a university FASD clinic. Patients were seen by a pediatric psychologist and pediatrician with formal training and experience with Astley and Clarren’s 4-Digit Diagnostic System (Astley and Clarren, 2000). The demographic, developmental, and medical data were collected during the clinic evaluation and reviewed with the care-giver at the time of MRI.
Diagnoses were made by Astley and Clarren’s 4-Digit Diagnostic system (2000), which assesses 4 criteria: (1) growth deficiency, (2) facial phenotype (palpebral fissure length, lip flattening, philtrum definition), (3) brain status (including cognitive dysfunction), and (4) alcohol exposure. Patients with full-critera FAS, growth deficiency (<3rd percentile height or weight), microcephaly, or mental retardation were excluded. Table 1 contains the diagnoses. Partial FAS consists of most FAS features, but not full criteria (typically, facial stigmata, and significant cognitive deficit are present). Astley and Clarren’s definition of “significant cognitive deficit” as performance 2 or more standard deviations below average on 3 psychometric measures was used. Static encephalopathy (alcoholexposed) consists of significant cognitive deficit with or without facial features. Neurobehavioral disorder (alcohol-exposed) consists of mild cognitive deficit with or without facial features. Patients with “neurobehavioral disorder” have cognitive deficits on testing, but not “significant cognitive deficit” (< 2 SD on 3 measures) as described. These diagnostic distinctions are for descriptive purposes only. Because the sample size did not allow for separate analyses, all 3 are included in the FASD group.
Table 1.
Subject Characteristics for FASD and Control Groups
| N(%) or mean ± SD | FASD (n = 14) | Control (n = 13) | Statistical test (N = 27) |
|---|---|---|---|
| Age at MRI scan | 12.3 ± 0.97 years | 12.4 ± 1.2 years | t = 0.034, p = 0.97 |
| Gender | |||
| Male | 7 (50%) | 6 (46.2%) | χ2 = 0.040, p = 0.84 |
| Female | 7 (50%) | 7 (53.8%) | |
| Growth measures | |||
| Gestation (wk) | 39.1 ± 2.3 | 39.2 ± 1.6 | t = 0.162, p = 0.873 |
| Birth weight (pounds) | 6.0 ± 1.4 | 7.2 ± 0.83 | t = 2.57, p = 0.057 |
| Birth length (in) | 18.5 ± 4.9 | 20.6 ± 0.70 | t = 1.50, p = 0.153 |
| Current weight (%ile) | 59.3 ± 29.4 | 75.0 ± 20.0 | t = 1.56, p = 0.132 |
| Current height %ile | 47.9 ± 32.0 | 71.54 ± 31.8 | t = 1.93, p = 0.065 |
| Craniofacial measures (FASD only) | |||
| Current head circumference (cm) | 52.84 ± 2.1 | – | – |
| Current head circumference (%ile) | 47.5 ± 32.4 | – | – |
| Palpebral tissue length (mm) | 25.4 ± 0.20 | – | – |
| Palpebral tissue length (%ile) | 5.2 ± 5.3 | – | – |
| Facial phenotype features | – | – | |
| None | 5 (35.8%) | – | – |
| Mild | 3 (21.4%) | – | – |
| Moderate | 3 (21.4%) | – | – |
| Severe | 3 (21.4%) | – | – |
| Diagnosis (FASD only) | – | – | |
| Partial fetal alcohol syndrome | 5 (35.8%) | – | – |
| Neurobehavioral disorder | 4 (28.4%) | – | – |
| Static encephalopathy | 5 (35.8%) | – | – |
FASD, fetal alcohol spectrum disorder.
All FASD subjects had documentation of alcohol exposure, either by the biological parent or by adoption/social service records. In 11 of 14 cases, exposure was high (Astley and Clarren rating = 4). Alcohol use was heavy, extensive, involved frequent intoxication, and often persisted throughout pregnancy. Alcohol exposure was confirmed for the remaining 3 cases, but the level was lower or not specified (Astley and Clarren rating = 3). In one case, consumption was “3 to 4 beers per day, 3 or 4 times per week” until the mother became aware of pregnancy. In another case, the adoptive parents had observed the biological mother using alcohol to intoxication during pregnancy, but could not specify her frequency of use. Based on detailed histories collected during clinical evaluation, subjects with reported prenatal exposure to any other drugs of abuse, except for tobacco, were excluded.
Fifteen age-matched, gender-matched, controls were recruited through advertising in low-income, ethnically diverse, urban neighborhoods in which many of the FASD subjects reside. Parents completed a medical history and interview, including prenatal drug and alcohol questions. No controls had prenatal alcohol exposure. Controls were excluded if even minimal alcohol consumption was reported any time during pregnancy. Controls received the same procedures as FASD subjects, except for the facial examination.
Exclusion criteria for both groups included neurological disease or injury, major psychiatric condition (Major Depression, Anxiety Disorder, Schizophrenia, Bipolar Disorder, Obsessive-Compulsive Disorder, etc.), and MRI contraindications (such as metal implants). Fetal alcohol spectrum disorders subjects were not excluded for attention-deficit hyperactivity disorder, oppositional defiant disorder, or learning disabilities, as these are very common comorbid disorders. Controls were excluded for any psychiatric condition, as determined by a screening diagnostic interview.
One FASD subject and 2 controls were excluded for MRI motion. The final sample included 14 FASD subjects and 13 controls. The FASD group was 64% White, 29% Native American, and 7% Black. Controls were 62% White, 15% Native American, 15% Black, and 8% Asian.
Procedure
Subjects were administered the Wechsler Intelligence Scale for Children (WISC-III; Wechsler, 1991 or WISC-IV; Wechsler, 2003) as part of their visit to the FASD clinic.
MR Image Acquisition
The MRI, completed within 12 months of the clinic visit, was performed on a 3-T Trio scanner (Siemens, Erlangen, Germany) using an 8-channel array head coil. A 3-plane localizer sequence was acquired to position subsequent scans. Scans with T1 and PD contrasts were collected for tissue segmentation. T1 images were acquired coronally, using a 3D MPRAGE sequence (TR = 2,530 ms, TE = 3.65 ms, TI = 1,100 ms, 240 slices, 1 mm isotropic voxels, flip angle = 7°, FOV = 256 mm, acquisition matrix of 256×256). Proton density (PD)-weighted images were acquired axially using a hyperecho turbo spin echo (TSE) sequence (TR = 8,550 ms, TE = 14 ms, 80 slices, voxel size = 1.0× 1.0×2.0 mm, flip angle = 120, FOV = 256 mm, acquisition matrix = 256×256). Field maps were acquired and used to correct the DTI data for geometric distortion (TR = 700 ms, TE = 4.62 ms/7.08 ms, flip angle = 90°, 64 slices, 2 mm isotropic voxels, 0 skip, FOV = 256 mm, matrix = 128×128 matrix, magnitude and phase difference contrasts). Diffusion tensor imaging data were acquired axially, aligned with the TSE images, using a dual spin echo, single shot, pulsed gradient, echo planar imaging sequence (TR = 8,000 ms, TE = 83 ms, 64 slices, voxel size = 2.5×2.5×2.0 mm, 0 skip, FOV= 320 mm, matrix = 128× 128, 3 averages, 6/8 partial Fourier, GRAPPA with acceleration factor = 2, b value = 1,000 s/mm2). Thirteen unique volumes were collected to compute the tensor: a b = 0 s/mm2 image and 12 images with diffusion gradients applied in 12 noncollinear directions: (Gx, Gy, Gz) = [1.0, 0.0, 0.5], [0.0, 0.5, 1.0], [0.5, 1.0, 0.0], [1.0, 0.5, 0.0], [0.0, 1.0, 0.5], [0.5, 0.0, 1.0], [1.0, 0.0, −0.5], [0.0, −0.5, 1.0], [−0.5, 1.0, 0.0], [1.0, −0.5, 0.0], [0.0, 1.0, −0.5], [−0.5, 0.0, 1.0].
Anatomical Image Processing
The brain was extracted from the T1 and PD acquisitions using the Brain Extraction Tool (BET), which is part of the FMRIB Software Library (http://www.fmrib.ox.ac.uk/). The PD brain was aligned to the T1 brain using the FMRIB Linear Registration Tool (FLIRT), allowing for translations and rotations but no scaling or shear [6 degrees of freedom (df) fit]. Dual-channel segmentation was performed on T1 and aligned PD brains using FMRIB’s Automated Segmentation Tool (FAST), producing 4 tissue classes (CSF, white, gray, and blood). The cerebrum mask was created by first registering the T1 brain to the FSL template brain using a 12 df. The inverse transform from the T1 to template registration was computed, and the FSL template segmentation mask was transformed back to the subject T1 brain. The cerebrum mask was the transformed segmentation mask, modified by hand to fully exclude the cerebellum and the brainstem.
DTI Processing
Diffusion tensor imaging data were processed with FMRIB’s Diffusion Toolbox (FDT). Warping was applied to the diffusion-weighted images to correct for eddy current distortions (Haselgrove and Moore, 1996), and the apparent diffusion coefficient (ADC) maps were computed. Twelve eddy current–corrected images were used to compute the diffusion tensor. Mean Diffusivity (mean of the 3 eigenvalues) and FA were derived. Fractional Anisotropy, the anisotropic component of the tensor (Basser, 1995), ranges between 0 (perfectly isotropic) and 1 (hypothetical cylinder, infinitely long and thin).
Fractional anisotropy and MD maps were aligned to the AC-PC plane in the following manner. FMRIB Linear Registration Tool was used to align the PD brain to the FSL template brain (6 df fit). The dewarped DTI b =0 image was aligned to the PD image using FLIRT and was aligned into the FSL template brain by applying the transform that aligned the PD brain to the FSL template. Mean diffusivity and FA maps were aligned to the template brain by applying the transformations determined from aligning the DTI b = 0 to PD image and aligning the PD image to the template image.
Regions of Interest
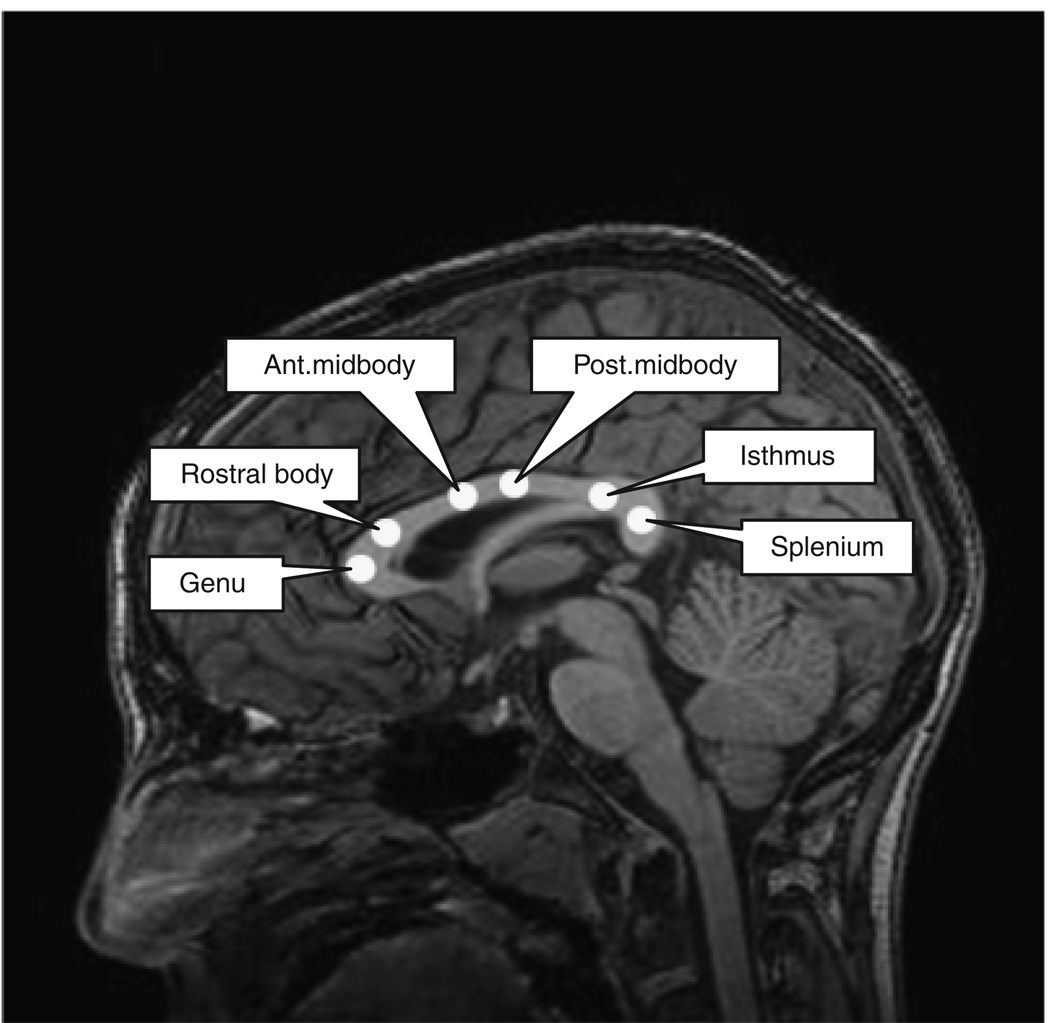
Circular regions of interest (ROIs) (13-mm2 area) were manually defined on reconstructed sagittal DTI b = 0 images by a trained technician (R.L.M.), who was blind to subject group. Regions of interest were applied to FA and MD maps using a custom IDL program. Six regions of the corpus callosum were identified: genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium (Witelson, 1989) (Fig. 1). Regions of interest were placed within the bulk of callosal white matter, avoiding edges. The Reliability of this ROI placement method has been examined in our laboratory in another sample (n = 20) and found to be high (r = 0.867).
Fig. 1.
Locations of 6 regions of interest placed in corpus callosum illustrated on a T1-weighted anatomical image.
RESULTS
Intellectual Findings
An ANOVA tested for group differences (FASD vs Control) on the IQ measure. The ANOVA was significant [F(1,25) = 9.317, p = 0.005]. Control subjects had significantly higher IQ scores (mean = 108, SD = 15.3) than FASD subjects (mean = 88.6, SD = 17.5).
Volumetric Findings
Planned volumetric analyses were carried out using segmentation results for gray matter, white matter, CSF, and blood. Total cerebral volume (sum of all 4 tissue types) was compared. An ANOVA [F(1, 25) = 3.97, p = 0.057] indicated a trend toward smaller cerebral volume in the FASD group (Cohen’s d effect size = 0.73). Separate comparisons were carried out for gray matter, white matter, and CSF. ANOVAs revealed that the FASD group had significantly smaller gray matter volume than controls [F(1,25) = 5.08, p = 0.033] (effect size = 0.81) but there was no significant group difference in white matter volume [F(1, 25) = 0.82, p = 0.375]. CSF volume was also significantly lower in the FASD group [F(1,25) = 4.30, p = 0.049] (effect size= 0.75). After adjusting gray and white matter volumes for cerebral volume, there was only a trend-level difference in gray matter volume between groups [F(1,25) = 3.19, p = 0.086].
DTI Findings
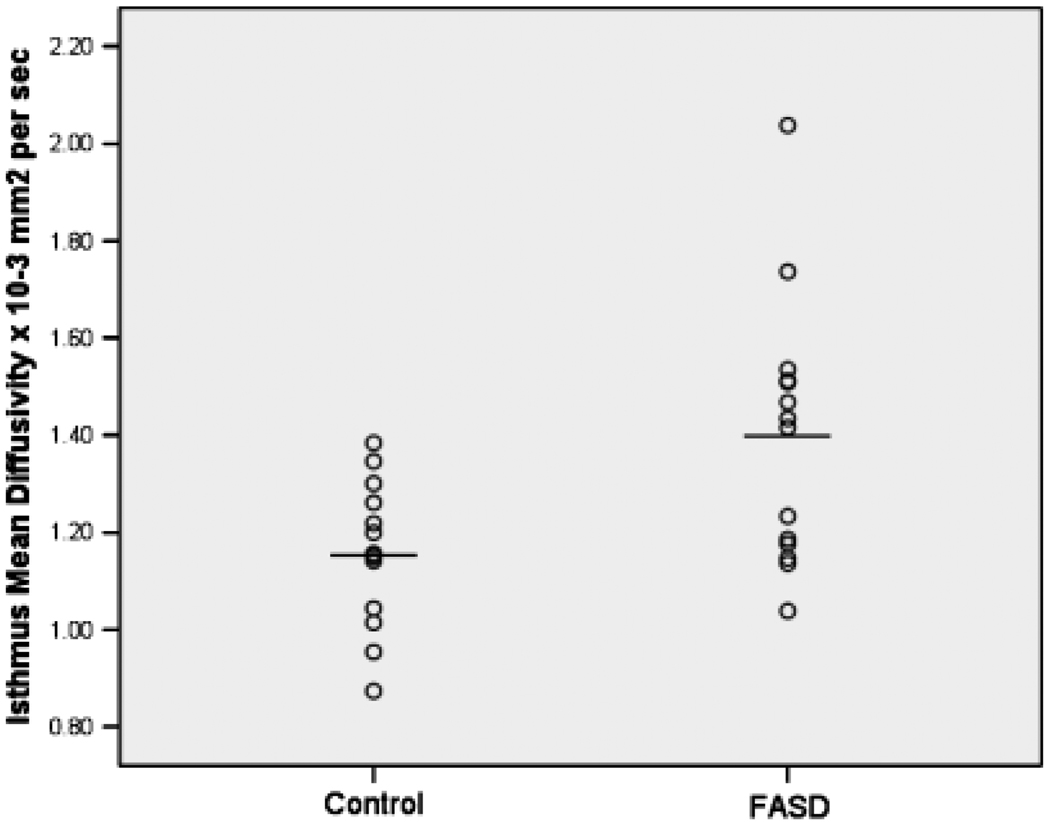
The DTI data set from 1 FASD subject was unusable because of artifact, reducing the sample size to 13 FASD and 13 controls. Diffusion tensor imaging data from 6 corpus callosum ROIs were compared using 2 planned MANOVAs. The first showed no significant group differences in FA in any region [Wilks’ λ = 0.785, F(6,19) = 0.868, p = 0.536]. The largest FA effect (effect size = 0.42) was in the isthmus (Table 2). A second MANOVA, testing MD in 6 ROIs, was significant [Wilks’ λ = 0.530, F(6,19) = 2.81, p = 0.040]. Univariate tests showed a significant difference only in isthmus, with FASD subjects showing higher MD (effect size = 1.05) (Fig. 2).
Table 2.
Means, Standard Deviations (SD), and Univariate Test Results (Following MANOVA) for Fractional Anisotropy (FA) and Mean Diffusion (MD) in 6 Corpus Callosum Regions
| FASD (n = 13) | Control (n = 13) | Univariate tests following MANOVA | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | p | Effect size (Cohen’s d) | |
| Fractional anisotropy (FA) | |||||||
| Genu | 0.723 | 0.053 | 0.707 | 0.075 | 0.368 | 0.550 | 0.24 |
| Rostral body | 0.567 | 0.063 | 0.552 | 0.058 | 0.425 | 0.521 | 0.26 |
| Anterior midbody | 0.499 | 0.067 | 0.479 | 0.058 | 0.658 | 0.425 | 0.32 |
| Posterior midbody | 0.504 | 0.057 | 0.507 | 0.065 | 0.019 | 0.892 | 0.05 |
| Isthmus | 0.471 | 0.079 | 0.503 | 0.072 | 1.140 | 0.296 | 0.42 |
| Splenium | 0.734 | 0.078 | 0.713 | 0.058 | 0.609 | 0.443 | 0.30 |
| Mean diffusivity (MD) × 10−3 | |||||||
| Genu | 0.919 | 0.082 | 0.935 | 0.069 | 0.268 | 0.609 | 0.20 |
| Rostral body | 0.964 | 0.100 | 0.994 | 0.104 | 0.593 | 0.449 | 0.30 |
| Anterior midbody | 1.106 | 0.156 | 1.156 | 0.187 | 0.568 | 0.458 | 0.30 |
| Posterior midbody | 1.130 | 0.123 | 1.164 | 0.143 | 0.429 | 0.519 | 0.26 |
| Isthmus | 1.348 | 0.207 | 1.157 | 0.153 | 7.17 | 0.013* | 1.05 |
| Splenium | 0.983 | 0.142 | 0.974 | 0.132 | 0.031 | 0.862 | 0.07 |
p<.05.
Mean diffusivity values are ×10−3 mm2/s.
Fig. 2.
Mean diffusivity in the isthmus of the corpus callosum in controls and patients with fetal alcohol spectrum disorder.
Dysmorphology
The association between facial dysmorphology and cerebral volume was examined using a planned nonparametric correlational analysis. The metric of facial dysmorphology was the ordinal scale described by Astley and Clarren (2000), which is a composite of palpebral fissure length and ratings of philtrum and upper lip. The scale is: 1 = None; 2 = Mild; 3 = Moderate; and 4 = Severe. Total cerebral volume was not correlated with facial score (Spearman’s ρ = 0.13, p = 0.66). Because of the group difference in isthmus MD, a post hoc correlational analysis was performed with facial dysmorphology. This was nonsignificant (ρ = 0.176, p = 0.55).
DISCUSSION
Diffusion tensor imaging was used to investigate the microstructural integrity of the corpus callosum in children with prenatal alcohol exposure. Children with partial FAS, neurobehavioral disorder, and static encephalopathy were studied because previous data suggest that structural abnormalities may be present and because these diagnostic groups represent a large proportion of the overall FASD population.
A key finding was evidence of microstructural abnormality in the isthmus of the corpus callosum in FASD. Mean diffusivity is normally low in the corpus callosum compared with other brain structures because of the highly organized, parallel fibers. A higher MD suggests an alteration of tissue in that region in FASD. This finding in a posterior region of the callosum is consistent with previous macrostructural findings, including evidence of callosal displacement in the isthmus and splenium (Sowell et al., 2002b). It should be noted that the current study utilized small ROIs, placed by hand in the bulk of the corpus callosum, reducing the probability of biased results due to callosal displacement or other macrostructural abnormality that could contribute to partial voluming (capturing both white matter and surrounding tissue in the same voxels).
Contrary to Ma et al. (2005), this study did not find group differences in genu or splenium. The current data differ in several respects from that study: (1) the subjects in this study were all children, whereas the Ma et al. study examined adults; (2) the subjects in this study did not have full-criteria FAS; and (3) the subjects in this study were less cognitively impaired (mean FSIQ = 88.6 vs 58.3 in the Ma et al. study). Despite the differences, these 2 studies together suggest that corpus callosum microstructural abnormalities may be associated with prenatal alcohol exposure.
The data did not reveal a relationship between MD in the isthmus and facial dysmorphology. Although both of these “markers” of prenatal alcohol exposure may be related to severity, they may change independently with age and development. In fact, FASD facial features become less apparent in adolescence and adulthood (Lemoine and Lemoine, 1992; Loser et al., 1999), suggesting that the relationship between facial and brain abnormalities may be more apparent in younger children.
The current finding of a microstructural abnormality in one region of the callosum, as opposed to the whole structure, highlights the fact that the callosum is a complex, heterogeneous structure. The various portions of the callosum project to distinct cortical regions, and its topography corresponds to functionally specialized cortical regions (Innocenti, 1986). The microstructure of the callosum also varies significantly, with proportionally more high-density, small-diameter fibers in the genu and more large-diameter fibers in the midbody, as seen by light microscopy (Aboitiz et al., 1992). This regional variance was, in fact, reflected in the current data in high FA/low MD in the genu and relatively lower FA/higher MD in the midbody.
Several limitations to the current study must be acknowledged. The results show a group difference in only one region (isthmus) and one measure (MD but not FA). The lack of an FA finding may be related to the small sample size, but clearly the current findings should be viewed as preliminary and in need of replication. Second, because a narrow age range was included (10–13 years), the generalizability to other ages is unknown. The corpus callosum develops primarily during the prenatal period, but clearly continues to change throughout childhood and into adulthood (Giedd et al., 1996; Giedd et al., 1999; Pujol et al., 1993; Rauch and Jinkins, 1994). Diffusion tensor imaging may be differentially sensitive to abnormalities in collosal integrity at different points in development. A related limitation of this study is the lack of specificity of the measure. The observed DTI differences could be related to differences in axon size, density, distribution, to integrity or to variations in myelination. Although the finding of a higher MD in FASD may suggest lower density of axons, at least 1 study of nonhuman primates has shown that prenatal alcohol exposure is associated with an overabundance of axons in the corpus callosum, perhaps because of disruption in the pruning process (Miller et al., 1999). Clearly, there is much to be learned about the microstructural-level changes involved in FASD. Lastly, the inclusion of a group of children with full-criteria FAS would have helped place the current findings in a larger context. These data are currently being collected.
In conclusion, the current data suggest that there may be specific regional callosal abnormalities in FASD. The findings suggest that DTI may provide useful markers for the study of prenatal alcohol exposure and may help advance understanding of the neurodevelopmental underpinnings of FASD. Future studies using this methodology across a wider age range and a broader diagnostic group (including FAS) will be especially important in systematically documenting the downstream effects of prenatal alcohol exposure at critical points in brain development.
ACKNOWLEDGMENTS
We are grateful for Gabrielle Mauren for her invaluable contributions to subject recruitment, neurocognitive data collection, and data management. She was instrumental in completing this project. We thank the Archie D. and Bertha H. Walker Foundation (#555911785), the Minnesota Medical Foundation, the National Institutes of Health (5P41RR008079-13), and the MIND Institute for supporting this research. Lastly, we thank the children and families who gave of their time to participate in this research.
This work was supported by the Archie D. and Bertha H. Walker Foundation (#555911785), the Minnesota Medical Foundation, National Institutes of Health (5P41RR008079-13 & MO1-RR00400), and the MIND Institute.
REFERENCES
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Barr HM, Streissguth AP. Identifying maternal self-reported alcohol use associated with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2001;25:283–287. [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec. 2002a;269:162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 2001;64:4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002b;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Guidelines for identifying and referring persons with fetal alcohol syndrome. Morb Mortal Wkly Rep. 2005;54:1–15. [PubMed] [Google Scholar]
- Ciccarelli O, Werring DJ, Barker GJ, Griffin CM, Wheeler-Kingshott CA, Miller DH, Thompson AJ. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging—evidence of Wallerian degeneration. J Neurol. 2003;250:287–292. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- Clark CM, Li D, Conry J, Conry R, Loock C. Structural and functional brain integrity of fetal alcohol syndrome in nonretarded cases. Pediatrics. 2000;105:1096–1099. doi: 10.1542/peds.105.5.1096. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss YC, Hamburger SD, Rapoport JL. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Res Dev Brain Res. 1996;91:274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Saksena S, Agarwal A, Hasan KM, Husain M, Gupta V, Narayana PA. Diffusion tensor imaging in late posttraumatic epilepsy. Epilepsia. 2005;46:1465–1471. doi: 10.1111/j.1528-1167.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- Haselgrove JC, Moore JR. Correction for distortion of echoplanar images used to calculate the apparent diffusion coefficient. Magn Reson Med. 1996;36:960–964. doi: 10.1002/mrm.1910360620. [DOI] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol. 2006;31:116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. General ogranization of callosal connections in cerebral cortex. In: Jones EG, editor. Cereb Cortex. New York: Plenum; 1986. pp. 291–354. [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Don A, Mateer CA, Streissguth AP. Cognitive deficits in nonretarded adults with fetal alcohol syndrome. J Learn Disabil. 1997;30:685–693. doi: 10.1177/002221949703000612. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kettunen S, Autti-Ramo I. Neurocognitive impairment in early adolescence following prenatal alcohol exposure of varying duration. Neuropsychol Dev Cogn C Child Neuropsychol. 2003;9:117–128. doi: 10.1076/chin.9.2.117.14503. [DOI] [PubMed] [Google Scholar]
- Laforce R, Jr, Hayward S, Cox LV. Impaired skill learning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc. 2001;7:112–114. doi: 10.1017/s1355617701711113. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995;8:375–386. doi: 10.1002/nbm.1940080711. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Lemoine P. Outcome of children of alcoholic mothers (study of 105 cases followed to adult age) and various prophylactic findings. Ann Pediatr (Paris) 1992;39:226–235. [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Helpern JA. Neuropsychiatric applications of DTI—a review. NMR Biomed. 2002;15:587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- Loser H, Bierstedt T, Blum A. Fetal alcohol syndrome in adulthood. A long-term study. Dtsch Med Wochenschr. 1999;124:412–418. doi: 10.1055/s-2007-1024327. [DOI] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, Laconte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc. 1999;5:462–471. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Ehlers CL, Delis DC, Jones KL, Stern C, Johnson KA, Hesselink JR, Bellugi U. Fetal alcohol syndrome: a case report of neuropsychological, MRI and EEG assessment of two children. Alcohol Clin Exp Res. 1992;16:1001–1003. doi: 10.1111/j.1530-0277.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, Jones KL. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol Teratol. 1994;16:283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Miller MW, Astley SJ, Clarren SK. Number of axons in the corpus callosum of the Mature macaca nemestrina: increases caused by prenatal exposure to ethanol. J Comp Neurol. 1999;412:123–131. [PubMed] [Google Scholar]
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain—a technical review. NMR Biomed. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Neil J, Shiran S, McKinstry R, Schefft G, Snyder A, Almli C, Akbudak E, Aronovitz J, Miller J, Lee B, Conturo T. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Nokelainen P, Valkonen K, Kolehmainen AI, Kumpulainen KI, Kononen M, Vanninen RL, Kuikka JT. Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-beta-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol Psychiatry. 2005;57:1565–1572. doi: 10.1016/j.biopsych.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Dev Med Child Neurol. 1999;41:652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: a decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127:35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Interhemispheric transfer in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:1863–1871. doi: 10.1097/01.ALC.0000042219.73648.46. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Barr HM. On categorizations in analyses of alcohol teratogenesis. Environ Health Perspect. 2000;108 suppl 3:421–428. doi: 10.1289/ehp.00108s3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. J Stud Alcohol. 2001;62:239–246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002a;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002b;17:1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Streissguth AP. The behavioral teratology of alcohol: performance, behavioral, and intellectual deficits in prenatally exposed children: In: West JR, editor. Alcohol and Brain Development. New York: Oxford University Press; 1986. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA. 1991a;265:1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Randels SP, Smith DF. A test-retest study of intelligence in patients with fetal alcohol syndrome: implications for care. J Am Acad Child Adolesc Psychiatry. 1991b;30:584–587. doi: 10.1097/00004583-199107000-00009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Swayze VW, II, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Wass TS, Simmons RW, Thomas JD, Riley EP. Timing accuracy and variability in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 2002;26:1887–1896. doi: 10.1097/01.ALC.0000042221.73478.4F. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Third Edition. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Fourth Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Witelson SF. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev. 2006 doi: 10.1016/j.neubiorev.2006.06.003. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]