Abstract
We report the first electrochemiluminescent immunosensor combining single-wall carbon nanotube forests with RuBPY– silica–secondary antibody nanoparticles for sensitive detection of cancer biomarker prostate specific antigen.
The measurement of protein biomarkers holds significant promise for early cancer detection.1,2 Detection of multiple protein biomarkers provides more reliable diagnostics than single biomarker measurements.1–3 Proteins in serum can be measured with enzyme-linked immunosorbent assays (ELISA),4 radioimmunoassay (RIA),5 electrophoretic immunoassay6 and mass spectrometry-based proteomics.7 However, there is a clear need for simple, rapid, sensitive, accurate and low cost protein detection suitable for point of care use.
Electrochemiluminescence (ECL) is initiated when tris (2,2′-bipyridyl)ruthenium(ii), [Ru-(bpy)3]2+, or RuBPY, in its oxidized form reacts with a suitable sacrificial reductant, and has been used for detection of DNA and proteins.8–10 Magnetic bead ECL methods have been commercialized.11,12
Dye doped silica nanoparticles show promise as labels in ultrasensitive ECL bioassays.13 RuBPY can be trapped inside mesoporous silica nanoparticles synthesized in water-in-oil (W/O) microemulsions.14 A single nanoparticle can encapsulate thousands of RuBPY to provide large signal amplification. Surfaces of the particles can be decorated with attached antibodies or proteins and arrays utilizing ECL can be fabricated using analytical spots on a simple conductive chip with a single connection to a power source and readout with a CCD camera, as we demonstrated with DNA-based toxicity screening arrays.9 This approach is simpler than the commercial bead-based ECL assays, which require sophisticated and expensive sample manipulation and measurement systems.15
In this communication, we combine ECL nanoparticle labels with a single-wall carbon nanotube (SWCNT) forest platform in a sandwich immunoassay procedure for protein detection. The SWCNT forests feature self-assembled 20–30 nm long terminally carboxylated SWCNT standing in upright bundles on a thin Nafion–iron oxide layer on a pyrolytic graphite surface,16 and provide a large conductive, functionalized surface area for attachment of capture antibodies in immunoassays.16b We have used SWCNT forests to build ultrasensitive amperometric immunosensors and detected PSA in serum using multiple enzyme labels.17 However, arrays using this strategy involve microfabricated multi-electrode chips as well as multi-electrode potentiostats, which are not necessary in ECL arrays.9
In related work, [Ru-(bpy)3]2+ immobilized on composite films of disordered CNTs in combination with Nafion,18a partially sulfonated polystyrene18b and Eastman-AQ polymers18c were used for ECL determination of tripropylamine18 with a detection limit of 3 pM.18c SWCNT forests with large carboxylated surface areas combined with immunoassays using RuBPY–silica–secondary antibody (Ab2) nanoparticles as labels might provide excellent sensitivity for proteins with a simplicity of approach amenable to future immunosensor array construction. Herein, we provide proof-of-concept for this hypothesis.
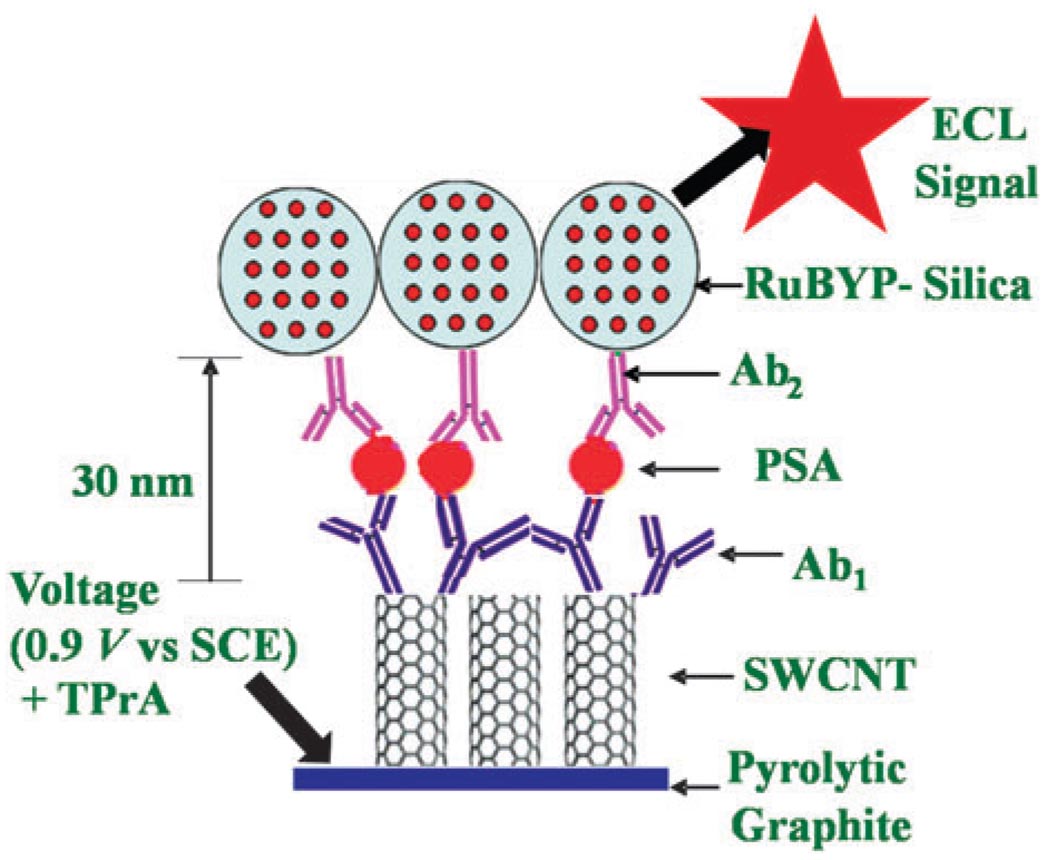
We chose prostate specific antigen (PSA), a biomarker for prostate cancer,19 as a test protein. Detection of PSA in human serum at 4 to 10 ng mL−1 is indicative of prostate cancer, and normal levels are below 3 ng mL−1. We designed a sandwich immunoassay for PSA by first chemically attaching capture antibodies (Ab1) for PSA on SWCNT forests on pyrolytic graphite (PG, 4 mm diam.) disks (Scheme 1) (see ESI† for immunosensor fabrication). This immunosensor was then incubated with 10 µL serum or a standard in calf serum, and PSA was captured on the sensor surface. After washing with non-specific binding blockers, the RuBPY–silica–Ab2 nanoparticle bioconjugate was added, followed by a second washing cycle.
Scheme 1.
Representation of ECL-based SWCNT immunosensors after addition of PSA and the RuBPY–silica–Ab2 nanoparticles.
The sensor was then transferred to a flat-bottomed electro-chemical cell and a voltage applied. ECL was measured via an optical fiber placed external to the cell directly underneath the sensor and connected to a photomultiplier tube (PMT).20 Amperometry or voltammetry can be recorded simultaneously with ECL. The electrolyte included 100 mM tripropylamine (TprA), 0.05% Tween 20 and 0.05% Triton X-100 in pH 7.5 buffer. The cell was covered with a black cloth to avoid external light and photodecomposition of the dye.
RuBPY–silica nanoparticles were synthesized in W/O reverse microemulsions14 and had a diameter of 97 ± 8 nm as measured by TEM (ESI† Fig. S1). This value was confirmed by atomic force microscopy (ESI† Fig. S2). Each RuBPY–silica particle contains 2.47 × 105 [Ru-(bpy)3]2+ ions (see ESI†). RuBPY–silica nanoparticles were coated with successive layers of poly-diallyldimethylammonium chloride (PDDA) and poly(acrylic acid) (PAA) and linked to Ab2 using EDC–NHSS at a high Ab2 : RuBPY–silica ratio (16 : 1) to ensure that each nano-particle is linked to at least one Ab2 (ESI† Fig. S3, S4, S5).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
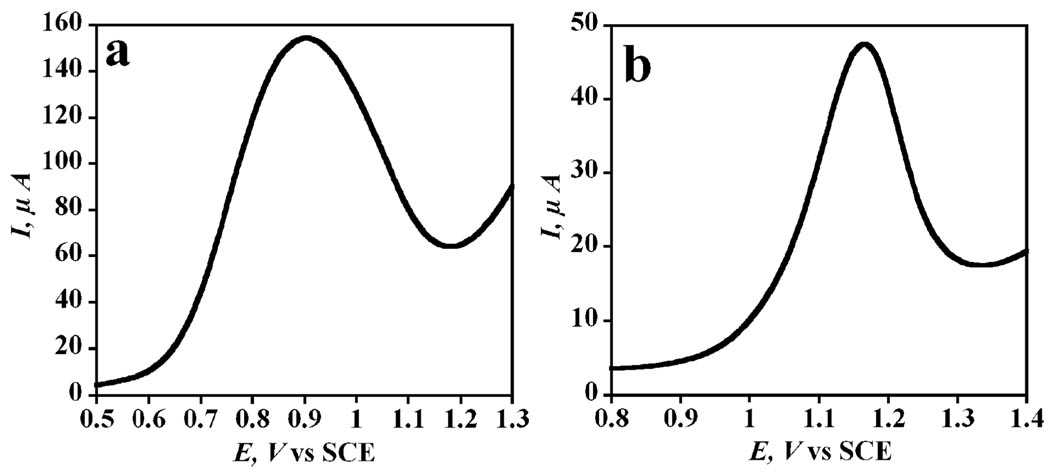
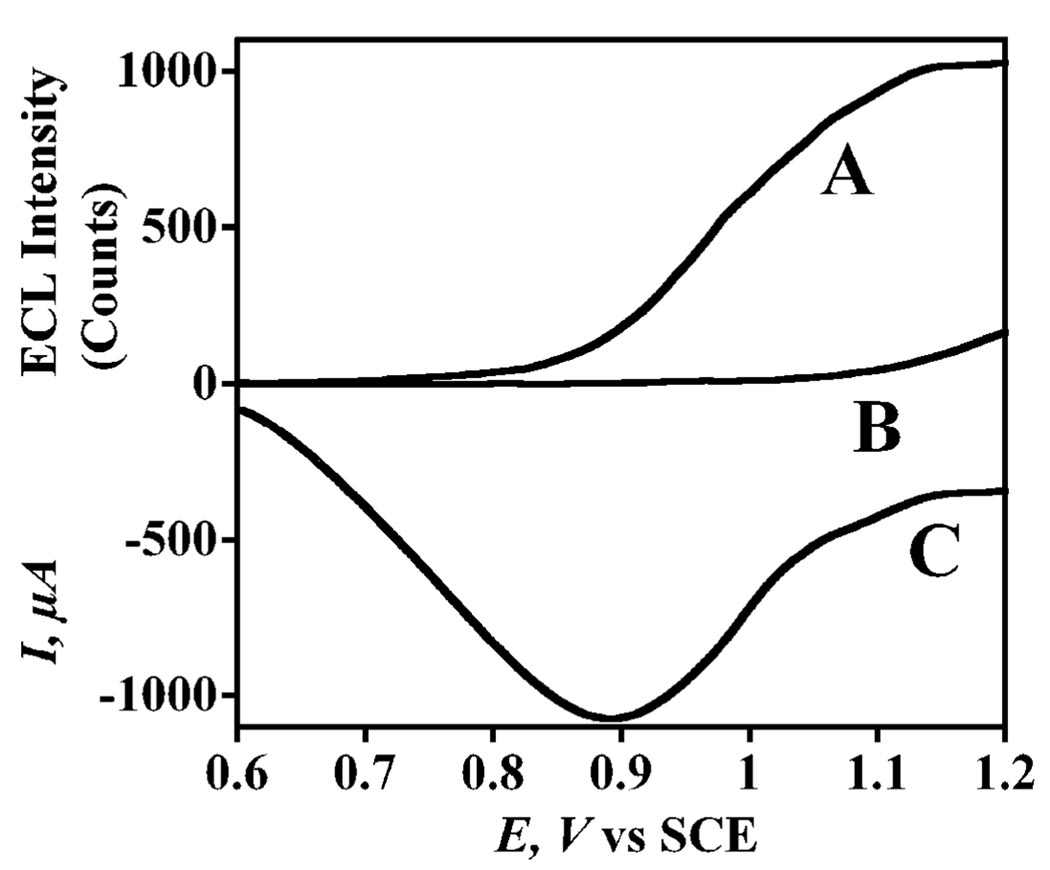
The pathway for ECL generation is shown in eqn (1)–(5).21 In the measuring cell, ECL is generated due to reduction of [Ru-(bpy)3]2+ mediated by direct oxidation of TPrA on the sensor surface and is directly proportional to the concentration of PSA. A competing pathway involves generation of ECL by direct electrochemical oxidation of [Ru-(bpy)3]2+ to [Ru-(bpy)3]3+, which can then react with TPrA radical (TPrA•) to give ECL.21 Such a scenario might result from non-specific binding (NSB) of the label, since the distance between the RuBPY–silica label and the electrode for a label properly bound via Ab2 to PSA is too large (~30 nm) for direct oxidation of [Ru-(bpy)3]2+ in the particles, as predicted by Marcus theory.22 To avoid direct oxidation of the label, a potential causing indirect oxidation of [Ru-(bpy)3]2+ by TprA• on the SWCNT immunosensor was chosen. The peak for indirect reduction of [Ru-(bpy)3]2+ was observed using square wave voltammetry (SWV) (Fig. 1), which showed that TPrA was oxidized at 0.9 V vs. SCE forming TPrA•+, which reacted with [Ru-(bpy)3]2+ to form [Ru-(bpy)3]+ (Fig. 1a and eqn (1)–(3)). Sequential reactions of [Ru-(bpy)3]+ produce ECL that is monitored with the photomultiplier tube (Fig. 2 and eqn (4) and (5)). However, direct oxidation of the [Ru-(bpy)3]2+-label on the SWCNT immunosensor surface in the presence of coreactant oxalate, which does not mediate [Ru-(bpy)3]2+-oxidation,23 occurred at 1.17 V vs. SCE (Fig. 1b). Therefore a potential window for indirect reduction of [Ru-(bpy)3]2+ via TPrA between 0.9 and 1.0 V vs. SCE would avoid direct oxidation of non-specifically bound RuBPY–silica. This was confirmed using SWV simultaneously with ECL. The ratio of the ECL photon count for sample (5 ng mL−1 PSA) to control (0 ng mL−1 PSA) was largest (26.7 : 1) at 0.95 V vs. SCE (Fig. 2). Thus, subsequent ECL was done at 0.95 V vs. SCE.
Fig. 1.
SWV at 5 Hz and 25 mV pulse height of SWCNT forest immunosensors with attached capture antibody incubated with 5 ng mL−1 PSA in 10 µL calf serum for 1.15 h after treating with 20 µL of 2% BSA for 1.15 h, followed by addition of 10 µL RuBPY–silica–Ab2 in 2% BSA and 0.05% Tween 20 for 1.15 h. Every step was followed by washing with 0.05% Tween 20 and PBS for 3 min. (a) Direct oxidation of TPrA in presence of 0.05% Tween 20 + 0.05% Triton X-100 + 100 mM TPrA in 0.2 M buffer (pH 7.5). (b) Direct oxidation of RuBPY–silica–Ab2 in presence of 10 mM NH4C2O4 + 50 mM NaCl (pH 6.0).
Fig. 2.
Simultaneous SWV-ECL at 5 Hz and 25 mV pulse height for SWCNT forest immunosensors with attached capture antibodies incubated with 5 ng mL−1 PSA in 10 µL calf serum for 1.15 h after treating with 20 µL of 2% BSA for 1.15 h, followed by addition of 10 mL RuBPY–silica–Ab2 in 2% BSA and 0.05% Tween 20 for 1.15 h. Every step was followed by washing with 0.05% Tween 20 and PBS buffer for 3 min. The coreactant solution was 0.05% Tween 20 + 0.05% Triton X-100 + 100 mM TPrA in 0.2 M phosphate buffer (pH 7.5). Curves are ECL signals for (A) [PSA] = 5 ng mL−1, (B) [PSA] = 0 ng mL−1, and (C) SWV showing direct oxidation of TPrA.
ECL depends on radicals derived from TPrA (eqn (1)–(5)). Production and deprotonation of TPrA•+ is necessary to give TPrA• to reduce [Ru-(bpy)3]2+ to [Ru-(bpy)3]+ (eqn (3)). TPrA•+ oxidizes [Ru-(bpy)3+] to [[Ru-(bpy)3]2+]* (eqn (4)). The amounts of these TPrA radicals are likely to be controlled by diffusion of TPrA to the SWCNT surface. Therefore the capture antibody concentration [Ab1] was optimized to increase the rate of direct oxidation of TPrA at the electrode (ESI† Fig. S6a). Results suggested that permeation of TPrA through the protein film to the sensor surface may be controlled by steric hindrance of excess Ab1 on the surface. Also, ECL may be suppressed by deprotonation of TPrA•+, possibly facilitated by carboxylates on SWCNT ends acting as proton acceptors.24 Increasing the hydrophobicity of the sensor surface by using detergent has also been shown to increase ECL by preconcentrating TPrA.25 Thus, inclusion of surfactants Triton X-100 and Tween 20 in the electrolyte with TPrA resulted in a 10-fold increase in ECL relative to the use of TPrA alone (ESI† Fig. S6b). Since it is well known that SWCNTs adsorb surfactants,16b it is likely that they increase the hydrophobicity of the sensor surface via an adsorbed hydrophobic layer which facilitates oxidation of TPA.
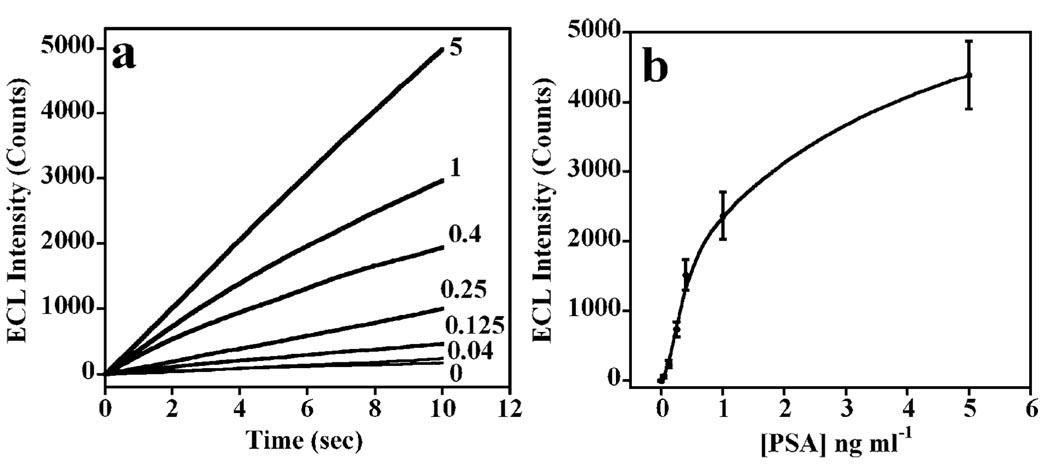
The optimized immunosensor procedure was applied using ECL at 0.95 V vs. SCE after incubating sensors with PSA standards in calf serum, which we previously showed to be a reasonable approximation to human serum in PSA immunoassays.17 ECL accumulated over 10 s was plotted for the relevant [PSA] range 0.04–5 ng mL−1. ECL increased nearly linearly with time (Fig. 3a). The sensor ECL increased with increasing [PSA] to achieve a sensitivity of 2864 (photons) mL ng−1 (Fig. 3b). A detection limit (DL) of 40 pg mL−1 was obtained by measuring ECL at 3 × standard deviation plus ECL counts for the PSA-free control (see ESI† Table S1). SWCNT immunosensors had a 34-fold better sensitivity and 10-fold lower DL compared to control immunosensors made on bare PG without SWCNT, which had a sensitivity of 85.4 (photons) mL ng−1 and DL 400 pg mL−1 (ESI† Fig. S7).
Fig. 3.
ECL obtained at 0.95 V vs. SCE with a [PSA] range of 0.4–5 ng mL−1 in the presence of 0.05% Tween 20 + 0.05% Triton X-100 + 100 mM TPrA in 0.2 M phosphate buffer (pH 7.5). SWCNT forest immunosensors with attached capture antibody were incubated with 5 ng mL−1 PSA in 10 µL undiluted calf serum for 1.15 h after treating with 20 µL of 2% BSA for 1.15 h, followed by addition of 10 µL RuBPY–silica–Ab2 in 2% BSA and 0.05% Tween 20 for 1.15 h. Every step was followed by washing with 0.05% Tween 20 and PBS buffer for 3 min each. (a) Influence of time on cumulative ECL counts at 0.95 V over 10 s. Control is immunoassay omitting addition of PSA. (b) Influence of PSA concentration on ECL signal at 10 s. Error bars show standard deviations (n = 4).
DL of the SWCNT immunosensor was slightly larger than that of 10 pg mL−1 PSA on ELECSYS immunoassay analyzers12 and of 4 pg mL−1 from multi-enzyme label detection with amperometric SWNT immunosensors.17 However, the ECL DL is still well below the normal serum levels of PSA, and the ECL sensor presents a clinically viable concentration range.
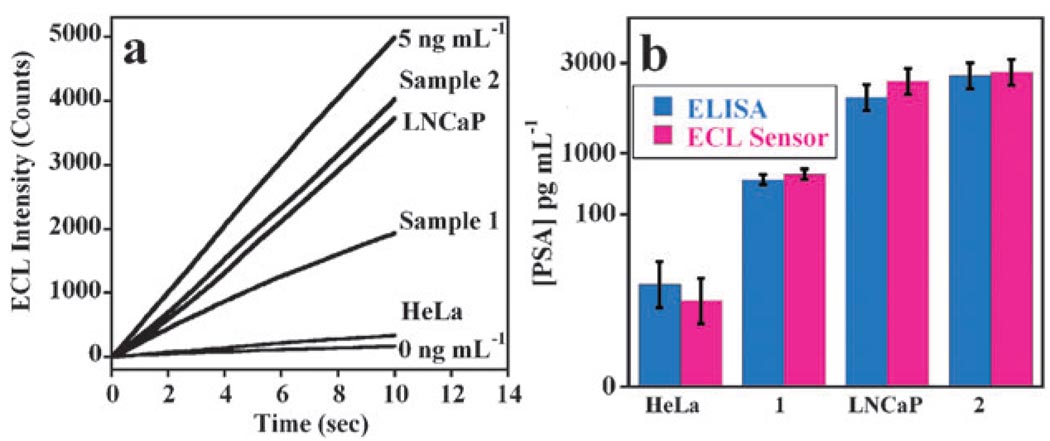
SWCNT immunosensors were used to determine PSA in 2 cell lysates (HeLa, LNCaP) and two human serum samples, and showed a very good correlation with ELISA (Fig. 4). There was no significant difference in PSA values between the two methods at the 95% confidence level as shown by t-tests. In addition, the HeLa cell lysate as a low PSA control gave 0.05 ng mL−1 PSA, while the positive control LNCaP cell lysates gave 2.6 ng mL−1, consistent with the known PSA expressions of these cell lines (Fig. 4b). These data confirm a high selectivity for PSA in the presence of large numbers of other proteins in serum and lysate samples.
Fig. 4.
SWCNT immunosensor results for real samples assayed as in Fig. 3. (a) Influence of time on ECL counts at 0.95 V for cell lysates (HeLa and LNCaP cells), human serum samples (1–2) and PSA standards in calf serum. (b) Comparison of SWCNT immunosensor and standard ELISA assay. Error bars show standard deviations (n = 4).
In summary, results demonstrate sensitive and selective ECL detection of a protein cancer biomarker using SWCNT forest immunosensors and ECL nanoparticle labels, as well as reliable accuracy for PSA in cell lysates and human serum. It is likely that sensitivity can be improved by further optimization of ECL particle properties and further decreasing NSB. A major advantage is simplicity of approach, which could lead to simple, sensitive, array methods for multiple protein detection.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge financial support from the National Institute of Environmental Health Sciences (NIEHS), NIH through US PHS grant No. ES013557, and thank Gary Jensen and Michael Duff for assistance.
Footnotes
Electronic supplementary information (ESI) available: Experimental details, Fig. S1–S5. See DOI: 10.1039/b909220j
Notes and references
- 1.Xiao Z, Prieto D, Conrads TP, Veenstra TD, Issaq HJ. Mol. Cell. Endocrinol. 2005;230:95–106. doi: 10.1016/j.mce.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Weston AD, Hood L. J. Proteome Res. 2004;3:179–196. doi: 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- 3.Wagner PD, Verma M, Srivastava S. Ann. N. Y. Acad. Sci. 2004;1022:9–16. doi: 10.1196/annals.1318.003. [DOI] [PubMed] [Google Scholar]
- 4.Voller A, Bartlett A, Bidwell DE. J. Clin. Pathol. 1978;31:507–520. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldsmith SJ. Semin. Nucl. Med. 1975;5:125–152. doi: 10.1016/s0001-2998(75)80028-6. [DOI] [PubMed] [Google Scholar]
- 6.Schmalzing D, Nashabeh W. Electrophoresis. 1997;18:2184–2193. doi: 10.1002/elps.1150181209. [DOI] [PubMed] [Google Scholar]
- 7.Aebersold R, Mann M. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 8.Debad JB, Glezer EN, Leland JK, Sigal GB, Wholstadter J. In: Electrogenerated Chemiluminescence. Bard AJ, editor. NY: Marcel Dekker; 2004. pp. 359–396. [Google Scholar]
- 9.Hvastkovs EG, So M, Krishnan S, Bajrami B, Tarun M, Jansson I, Schenkman JB, Rusling JF. Anal. Chem. 2007;79:1897–1906. doi: 10.1021/ac061975q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao W, Bard AJ. Anal. Chem. 2004;76:5379–5386. doi: 10.1021/ac0495236. [DOI] [PubMed] [Google Scholar]
- 11. http://rochediagnostics.ca/lab/solutions/e2010.php.
- 12. http://www.meso-scale.com/CatalogSystemWeb/WebRoot/technology/ecl.htm.
- 13.Yan JL, Estévez MC, Smith JE, Wang KM, He XX, Wang L, Tan WH. Nano Today. 2007;2(3):44–50. [Google Scholar]
- 14.Santra S, Zhang P, Wang KM, Tapec R, Tan WH. Anal. Chem. 2001;73:4988–4993. doi: 10.1021/ac010406+. [DOI] [PubMed] [Google Scholar]
- 15.(a) US Pat. 2006 7015046.; (b) Stockmann W, Bablok W, Luppa P. Wienerklinische Wochenschrift. 1998;110:10–21. [PubMed] [Google Scholar]
- 16.(a) Chattopadhyay D, Galeska I, Papadimitrakopoulos F. J. Am. Chem. Soc. 2001;123:9451–9452. doi: 10.1021/ja0160243. [DOI] [PubMed] [Google Scholar]; (b) Kim SN, Rusling JF, Papadimitrakopolous F. Adv. Mater. 2007;19:3214–3228. doi: 10.1002/adma.200700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X, Munge B, Patel V, Jensen G, Bhirde A, Gong JD, Kim SN, Gillespie J, Gutkind JS, Papadimitrakopoulos F, Rusling JF. J. Am. Chem. Soc. 2006;128:11199–11205. doi: 10.1021/ja062117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Guo Z, Dong S. Anal. Chem. 2004;76:2683–2688. doi: 10.1021/ac035276e. [DOI] [PubMed] [Google Scholar]; (b) Li J, Xu Y, Wei H, Huo T, Wang E. Anal. Chem. 2007;79:5439–5443. doi: 10.1021/ac0706224. [DOI] [PubMed] [Google Scholar]; (c) Zhang L, Guo Z, Xu Z, Dong S. J. Electroanal. Chem. 2006;592:63–67. [Google Scholar]
- 19.Lilja H, Ulmert D, Vickers AJ. Nature Rev. Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 20.So M, Hvastkovs EG, Schenkman JB, Rusling JF. Biosens Bioelectron. 2007;23(4):492–498. doi: 10.1016/j.bios.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao W, Choi JP, Bard AJ. J. Am. Chem. Soc. 2002;124:14478–14485. doi: 10.1021/ja027532v. [DOI] [PubMed] [Google Scholar]
- 22.Marcus AR, Sutin N. Biochim. Biophys. Acta. 1985;811:265–322. [Google Scholar]
- 23.Forster RJ, Hogan CF. Anal. Chem. 2000;72:5576–5582. doi: 10.1021/ac000605d. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Zu Y. J. Phys. Chem. C. 2008;112:16663–16667. [Google Scholar]
- 25.Zu Y, Bard AJ. Anal. Chem. 2001;73:3960–3964. doi: 10.1021/ac010230b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.