Abstract
AMPK (AMP-activated protein kinase) signalling plays a key role in whole-body energy homoeostasis, although its precise role in pancreatic β-cell function remains unclear. In the present stusy, we therefore investigated whether AMPK plays a critical function in β-cell glucose sensing and is required for the maintenance of normal glucose homoeostasis. Mice lacking AMPKα2 in β-cells and a population of hypothalamic neurons (RIPCreα2KO mice) and RIPCreα2KO mice lacking AMPKα1 (α1KORIPCreα2KO) globally were assessed for whole-body glucose homoeostasis and insulin secretion. Isolated pancreatic islets from these mice were assessed for glucose-stimulated insulin secretion and gene expression changes. Cultured β-cells were examined electrophysiologically for their electrical responsiveness to hypoglycaemia. RIPCreα2KO mice exhibited glucose intolerance and impaired GSIS (glucose-stimulated insulin secretion) and this was exacerbated in α1KORIPCreα2KO mice. Reduced glucose concentrations failed to completely suppress insulin secretion in islets from RIPCreα2KO and α1KORIPCreα2KO mice, and conversely GSIS was impaired. β-Cells lacking AMPKα2 or expressing a kinase-dead AMPKα2 failed to hyperpolarize in response to low glucose, although KATP (ATP-sensitive potassium) channel function was intact. We could detect no alteration of GLUT2 (glucose transporter 2), glucose uptake or glucokinase that could explain this glucose insensitivity. UCP2 (uncoupling protein 2) expression was reduced in RIPCreα2KO islets and the UCP2 inhibitor genipin suppressed low-glucose-mediated wild-type mouse β-cell hyperpolarization, mimicking the effect of AMPKα2 loss. These results show that AMPKα2 activity is necessary to maintain normal pancreatic β-cell glucose sensing, possibly by maintaining high β-cell levels of UCP2.
Keywords: AMP-activated protein kinase (AMPK), ATP-sensitive potassium channel (KATP), β-cell, glucokinase, pancreas, uncoupling protein 2 (UCP2)
Abbreviations: AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; AMPK, AMP-activated protein kinase; CM-H2DCFDA, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester; CRI-G1, Cambridge rat insulinoma-G1; Gck, glucokinase gene; GFP, green fluorescent protein; GK, glucokinase; GLUT2, glucose transporter 2; GSIS, glucose-stimulated insulin secretion; KATP channel, ATP-sensitive potassium channel; NPY, neuropeptide Y; Nrf1, nuclear respiratory factor 1; Pgc1α, peroxisome proliferator-activated receptor γ coactivator 1-α; POMC, pro-opiomelanocortin; ROS, reactive oxygen species; Slc2a2, solute carrier family 2 (facilitated glucose transporter) member 2; SUR1, sulfonylurea receptor 1; Tfam, transcription factor A, mitochondrial; UCP2, uncoupling protein 2; WT, wild-type
INTRODUCTION
Raised plasma glucose increases the amount of glucose taken up by pancreatic β-cells predominantly through the glucose transporter, GLUT2 [1]. This increase in glucose metabolism occurs over the physiological range of glucose concentrations by the action of the rate-limiting enzyme GK (glucokinase). The raised β-cell metabolic flux increases the ratio of cellular [ATP]/[ADP], which closes KATP (ATP-sensitive potassium) channels, leading to membrane depolarization, opening of voltage-gated calcium channels, raised intracellular calcium and insulin secretion [2]. Thus temporal fluctuations of blood glucose levels are directly linked to variations in insulin secretion by the concerted activity of these key pancreatic β-cell proteins. Therefore these proteins are essential for pancreatic β-cells to mount an initial rapid insulin secretory response, which is followed by a slower KATP-independent release of insulin. There is strong evidence that dysfunctional pancreatic β-cells are central to the development of Type 2 diabetes [3,4]. A progressive increase in basal insulin secretion (during fasting) along with a much reduced or absent first-phase of GSIS (glucose-stimulated insulin secretion) are considered early and common defects in Type 2 diabetes. Indeed, the loss or reduction of β-cell secretory responsiveness to glucose, when imposed on a background of peripheral insulin resistance, is likely to precipitate early-impaired glucose tolerance leading rapidly to Type 2 diabetes.
Oral anti-diabetic drug treatment has evolved in recent years to encompass the widespread use of metformin as a first-line drug for treatment of Type 2 diabetes [5]. Current evidence suggests that metformin reduces blood glucose levels by decreasing liver gluconeogenesis and increasing peripheral glucose uptake. The mechanisms by which metformin performs these actions have been linked to increased insulin action and the activation of AMPK (AMP-activated protein kinase). Consequently, AMPK has attracted considerable attention as a potential target for the treatment of Type 2 diabetes [6].
AMPK acts as a cellular integration node for various nutrient and hormone signals, and subsequent changes in AMPK activity regulates multiple metabolic pathways of glucose metabolism [7]. Thus there may be an expectation for AMPK to be critically involved in the regulation of insulin secretion by glucose. Indeed, diminished AMPK activity has been reported in islets from Type 2 diabetic patients [8]. However, the precise role of AMPK in the regulation of insulin secretion from β-cells is presently unclear and controversial. Activation of AMPK by AICAR (5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside), metformin or overexpression of a constitutively active form of AMPK reduces GSIS in rodent and human islets [9–12]. In contrast, overexpression of a dominant-negative form of AMPK is reported to increase insulin release at low glucose concentrations [10]. However, others have failed to replicate these findings [13,14], generating uncertainty over the role of AMPK in insulin secretion. Therefore to define the role of AMPK in β-cell function, we generated mice deficient in AMPKα2 (with or without global deletion of AMPKα1) in β-cells and examined the relationship between AMPK activity and the mechanisms thought to mediate normal glucose sensing.
MATERIALS AND METHODS
Animals
Floxed AMPKα2 allele RIPCre mice (The Jackson Laboratory) were crossed to generate compound heterozygote mice. Double-heterozygote mice were crossed with AMPKα2+/fl mice to obtain WT (wild-type), flox+/+, Cre and Creflox+/+. Mice lacking AMPKα2 in RIPCre-expressing cells were designated RIPCreα2KO mice. RIPCreα2KO and AMPKα1KO mice were crossed to generate mice lacking AMPKα1 globally (α1KO) and AMPKα2 in RIPCre-expressing cells (α1KORIPCreα2KO mice). All mice were maintained on a 12-h light/12-h dark cycle with free access to water and standard mouse chow (4% fat, RM1; Special Diet Services) and housed in pathogen-free barrier facilities. All procedures were in accordance with the UK Home Office Animal Procedures Act of 1986 and approved by the University of Dundee and University College London Animal Ethics Committees. All mice were studied with appropriate littermate controls. Genotyping of mice was performed by PCR amplification of tail DNA as described previously [15,16].
Physiological measurements
Body weight and feeding measurements, and tolerance tests were performed as described previously [15,17]. Blood glucose and plasma insulin levels were determined using mouse ELISAs (Linco Research).
Gene expression studies
Quantitative PCR was performed as described previously [15,16]. Expression levels of AMPKα2, Gck (glucokinase gene), HMOX1 (haem oxygenase 1), GPX4 (glutathione peroxidase 4), NPY (neuropeptide Y), Nrf1 (nuclear respiratory factor 1), Pgc1α (peroxisome proliferator-activated receptor γ coactivator 1-α), POMC (pro-opiomelanocortin), Slc2a2 [solute carrier family 2 (facilitated glucose transporter) member 2; previously known as Glut2], SOD2 (superoxide dismutase 2), SUR1 (sulfonylurea receptor 1), Tfam (transcription factor A, mitochondrial) and UCP2 (uncoupling protein 2) were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) or HPRT (hypoxanthine phosphoribosyltransferase 1) and data were analysed using the 2−ΔCt method [15].
Western blot analysis
Islet and hypothalamic lysates were prepared as described previously [15–17] and immunoblots probed with polyclonal antibodies against ATP synthase (Abcam), AMPKa2 (supplied by David Carling) and β-actin (Cell Signalling), with ECL (enhanced chemiluminescence; Amersham Biosciences) detection.
Pancreas morphometry
Antibodies used were mouse anti-insulin (I-2018; Sigma–Aldrich), rabbit anti-glucagon (ab9379; Abcam), chicken anti-mouse Alexa Fluor® 594 and chicken anti-rabbit Alexa Fluor® 488 (both Molecular Probes/Invitrogen). Confocal images were captured using a Carl Zeiss LSM 700 laser scanning microscope with 20× air objective.
Islets and CRI-G1 (Cambridge rat insulinoma-G1) experiments
Mouse pancreatic islet isolation, islet insulin secretion studies and β-cell culture were performed as described previously [16,18]. Treatments of CRI-G1 cells with Compound C (Calbiochem), AICAR (Toronto Research Chemicals) and A-769662 were carried out in normal saline±10 mM glucose. Hexokinase activity in CRI-G1 cells was measured as described previously [19]. Cell lysates were prepared for immunoblotting and analysis as described previously [20]. For glucose uptake, the fluorescent glucose analogue 2-NBDG (Molecular Probes) was applied at 100 μM in the presence of glucose±AICAR and±Compound C, and imaged as described previuosly [21]. In control experiments, CRI-G1 cells took-up 2-NBDG in a linear manner for >1 h. For immunostaining, CRI-G1 cells were washed and immediately fixed after treatments and then permeabilized with 0.5% Triton X-100 in PBS. Non-specific-binding was blocked with 10% (w/v) BSA and primary antibodies against GLUT2 and GK (Santa Cruz Biotechnology) were applied at a 1:1000 dilution for 1 h. For visualization, either Alexa Fluor® 488 (Molecular Probes) or Cy3 (indocarbocyanine; Jackson ImmunoResearch)-conjugated secondary antibodies were used according to the manufacturers' instructions. CRI-G1 cell nucleotide determination was performed as described previously [9]. H2O2 levels in individual islets were measured by epifluorescent microscopy using the fluorescent probe CM-H2DCFDA [5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester] at a concentration of 1 μM, with a 60 min preloading time.
Electrophysiological studies
Mouse cultured β-cells were super-fused at room temperature (22–25 °C) with normal saline [135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes and 10 mM glucose (pH 7.4)]. Whole-cell recordings were made using borosilicate glass pipettes (5–10 MΩ) containing 140 mM KCl, 10 mM EGTA, 5 mM MgCl2, 3.8 mM CaCl2 and 10 mM Hepes (pH 7.2)±ATP at 3.0 mM, 1.0 mM, 0.1 mM and 0 mM. For voltage-clamp recordings the membrane potential was held at −70 mV and 20 mV steps of 200 ms duration, with 20 ms between pulses, applied (voltage range from −160 to −40 mV). Voltage-clamp data were analysed using pCLAMP 8.2 software. Perforated patch recordings were achieved by addition of 25–50 μg/ml amphotericin B (Sigma–Aldrich) to the pipette solution. Series resistance was <30 MΩ for all recordings. Following a minimum of 10 min of stable recording, the extracellular glucose concentration was altered or drugs applied by bath superfusion.
Statistics
Data are presented as means±S.E.M. All statistics were performed using GraphPad (Prism 5) software. Paired or Wilcoxon signed-rank sum test and unpaired t tests were performed. P values≤0.05 were considered statistically significant.
RESULTS
Deletion of AMPKα2 in the pancreas and a subset of hypothalamic neurons does not affect body weight or food intake, but impairs glucose homoeostasis and GSIS in mice
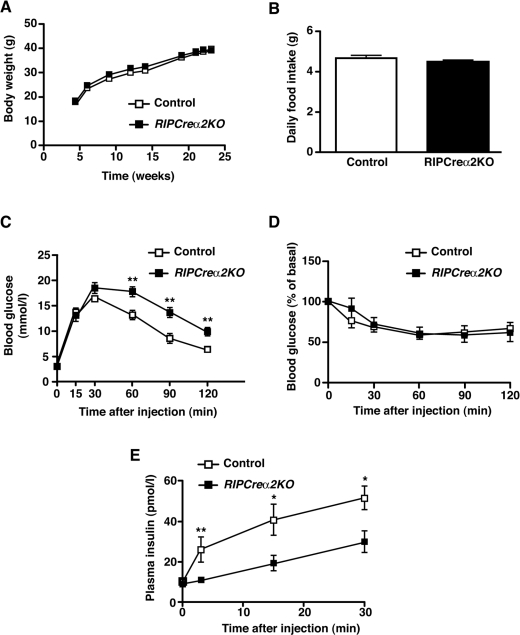
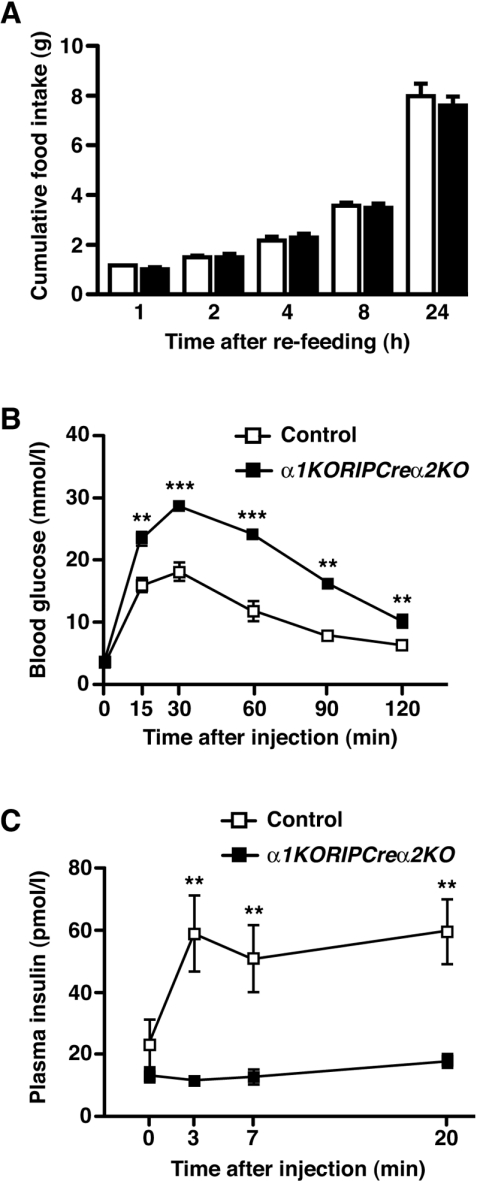
Mice expressing Cre under the control of the rat insulin II promoter and mice with a floxed allele of AMPKα2 were crossed to generate mice lacking AMPKα2 in pancreatic β-cells and a small population [15] of hypothalamic neurons (RIPCreα2KO mice). We detected recombination of the floxed AMPKα2 allele in islets and hypothalamus from RIPCreα2KO mice and protein expression of AMPKα2 was significantly reduced in islets (Figures 1A and 1B). No significant change in NPY or POMC mRNA was detected in fasted RIPCreα2KO mice (Supplementary Figure S1A at http://www.BiochemJ.org/bj/429/bj4290323add.htm). Furthermore, RIPCreα2KO mice exhibited normal body weight and unchanged daily food intake compared with littermate controls (Figures 2A and 2B), suggesting no significant hypothalamic phenotype. RIPCreα2KO mice displayed normal fasted and fed blood glucose levels, and fasted plasma insulin as compared with control mice (Supplementary Figures S1B–S1D). RIPCreα2KO mice displayed mild glucose intolerance compared with littermate controls (Figure 2C), but no reduction in peripheral insulin sensitivity (Figure 2D). However, in vivo GSIS was depressed in RIPCreα2KO mice (Figure 2E), potentially explaining their mild glucose intolerance. To address the possibility of compensatory up-regulation of AMPKα1 expression in cells deleted for AMPKα2, we used mice with global deletion of AMPKα1 (α1KO), which exhibit a normal metabolic phenotype [22] and α1KORIPCreα2KO mice (lacking AMPKα2 in pancreatic β-cells and some hypothalamic neurons and AMPKα1 in all tissues). The α1KORIPCreα2KO mice exhibited normal body weight (results not shown) and food intake compared with controls (Figure 3A), again indicating no hypothalamic disturbance. However, the α1KORIPCreα2KO mice displayed profound impairment of glucose tolerance and in vivo GSIS was markedly depressed in α1KORIPCreα2KO mice compared with controls (Figures 3B and 3C).
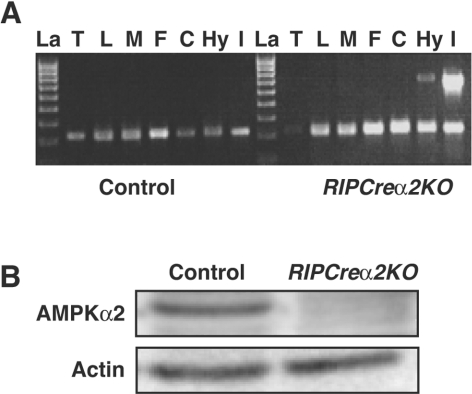
Figure 1. Reduction in islet and hypothalamic AMPKα2 in RIPCreα2KO mice.
(A) Detection of the deletion of the AMPKα2 allele in RIPCreα2KO mice. DNA was extracted from different tissues (T, tail; L, liver; M, skeletal muscle; F, fat; C, cerebral cortex; Hy, hypothalamus; I, islets of Langerhans) and recombination of the floxed AMPKα2 allele was detected by PCR. The presence of the 750 bp band indicates deletion of AMPKα2. Recombination was only detected in islets of Langerhans and the hypothalamus from RIPCreα2KO mice. A PCR with IL2 (interleukin-2) as an internal control shows the presence of DNA template in all of the samples. La, DNA ladder. (B) Representative immunoblot for AMPKα2 from control and RIPCre2αKO islets.
Figure 2. Glucose homoeostasis in RIPCreα2KO mice.
(A) Weight curves of male control and RIPCreα2KO mice on a chow diet (n=5–8). (B) Daily food intake of RIPCreα2KO and control mice (n=7–9). (C) Intraperitoneal glucose tolerance test performed on 16-week-old male RIPCreα2KO (■) and control (□) mice (n=8). (D) Insulin tolerance test performed on 20-week-old male RIPCreα2KO (■) and control (□) mice (n=7). (E) Plasma insulin levels before and after intraperitoneal injection of glucose (2 g/kg of body weight) in 10-week-old male RIPCreα2KO (■) and control (□) mice (n=6–7). Values are means±S.E.M. *P<0.05, **P<0.01.
Figure 3. Mice lacking AMPKα1 globally and AMPKα2 in β-cells and a population of hypothalamic neurons exhibit glucose intolerance and defective GSIS.
(A) Cumulative 24-h food intake in 20-week-old male control (open bars) and α1KORIPCreα2KO (solid bars) mice in response to an overnight fast (n=5). (B) Intraperitoneal glucose tolerance test performed on 5-week-old male α1KORIPCreα2KO (■) and control (□) mice (n=8–11). (C) Plasma insulin levels before and after intraperitoneal injection of glucose (2 g/kg of body weight) in 10-week-old male α1KORIPCreα2KO (■) and control (□) mice (n=6–7). Values are means±S.E.M. **P<0.01, ***P<0.001.
AMPK-deleted islets in vitro exhibit increased insulin release at basal glucose and decreased GSIS
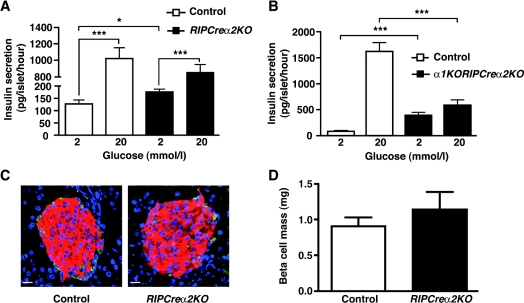
Because AMPKα2 was also deleted in some hypothalamic neurons, we were concerned that altered autonomic activity to peripheral organs, as described for the global AMPKα2KO mouse [22], was influencing β-cell function. We therefore undertook further in vitro analysis of β-cell function using RIPCreα2KO and α1KORIPCreα2KO isolated islets. Static incubation of RIPCreα2KO islets showed enhanced insulin secretion under basal (2 mM) glucose conditions compared with control islets, whereas GSIS (glucose raised to 20 mM) displayed a non-significant trend towards decreased secretion (Figure 4A). Furthermore, insulin secretion from α1KORIPCreα2KO islets at 2 mM glucose was enhanced and GSIS significantly impaired compared with control animals (Figure 4B). The increased basal insulin secretion is consistent with that reported previously with adenoviral overexpression of a dominant-negative form of AMPK in β-cells [10]. Taken together, these findings indicate that appropriate expression of AMPK α subunits is required for normal glucose sensing and GSIS in pancreatic β-cells.
Figure 4. Defective GSIS in isolated islets from mice lacking AMPK catalytic α subunits.
(A) Insulin secretion from isolated control (open bars) and RIPCreα2KO (solid bars) islets in static cultures in response to 2 and 20 mM glucose. (B) Insulin secretion from isolated control (open bars) and α1KORIPCreα2KO (solid bars) islets in static cultures in response to 2 and 20 mM glucose. (C) Pancreatic sections from control and α1KORIPCreα2KO islets, from 10-week-old male mice, co-stained for insulin (red), glucagon (green) and DAPI (4′,6-diamidino-2-phenylindole; blue). (D) Mean β-cell mass in islets of control and α1KORIPCreα2KO mice (n=4). Values are means±S.E.M. *P<0.05, ***P<0.001.
Deletion of AMPKα2 does not alter β-cell mass
Recent studies show that loss of LKB1, an AMPK kinase, increases β-cell size and enhances insulin secretion [23,24]. Therefore we undertook islet morphometric analysis in RIPCreα2KO and control mice to exclude increased β-cell mass as a contributing factor. Absolute β-cell mass was unaltered in RIPCreα2KO mice compared with control animals (Figures 4C and 4D), which indicated that altered β-cell function underlies the changes observed in basal insulin secretion and GSIS.
AMPKα2 is required for low-glucose-induced hyperpolarization of pancreatic β-cells
We next examined whether the high basal insulin secretion was associated with altered β-cell electrical responsiveness to glucose. Perforated patch recordings from single cultured isolated β-cells showed that control (WT and RIPCre) β-cells responded to low glucose (10 mM to 2 mM) by membrane potential hyperpolarization causing cessation of action potential firing, actions reversed on returning to 10 mM glucose (Figures 5A and 5B). In contrast, the majority (19 out of 24) of β-cells from RIPCreα2KO islets displayed no hyperpolarizing response to low glucose, maintaining an unchanged membrane potential in 2 mM and 10 mM glucose (Figure 5C). The remaining five cells responded normally to hypoglycaemia (results not shown), consistent with lack of deletion of the floxed allele in all β-cells [15].
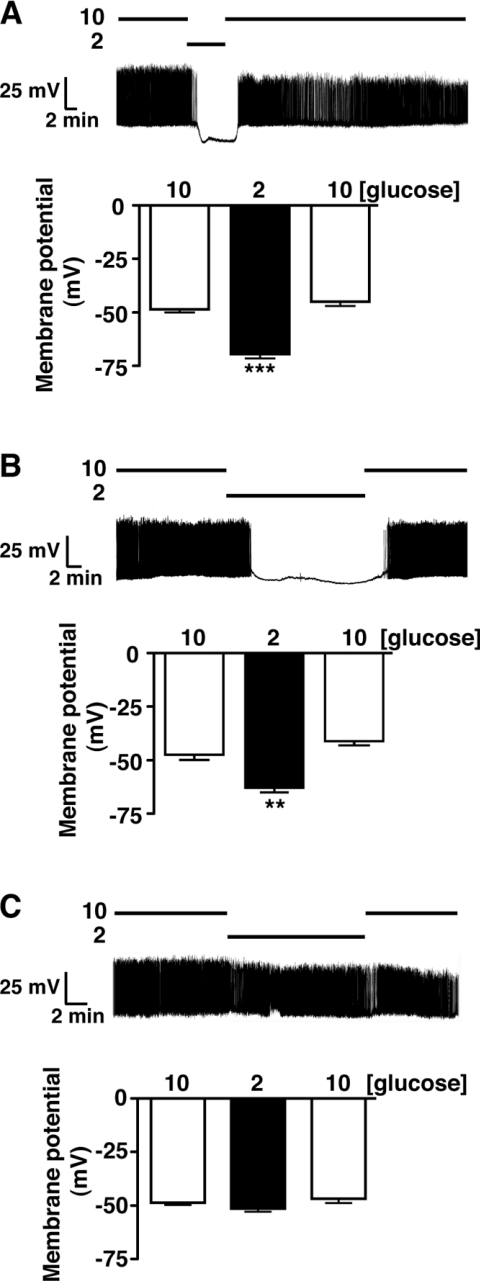
Figure 5. Loss of hypoglycaemic sensing in isolated β-cells from RIPCreα2KO mice.
WT (A) and RIPCre (B) mouse cultured β-cells respond, reversibly, to the reduction of glucose from 10 to 2 mM by hyperpolarization and cessation of firing. (C) RIPCreα2KO β-cells are unresponsive to a reduction of glucose concentration from 10 to 2 mM. The histograms in (A–C) are the mean values for membrane potential in β-cells exposed to 10, 2 and 10 mM glucose for each condition. Values were derived from seven to 19 individual β-cell recordings per group and are shown as means±S.E.M. **P<0.01, ***P<0.001.
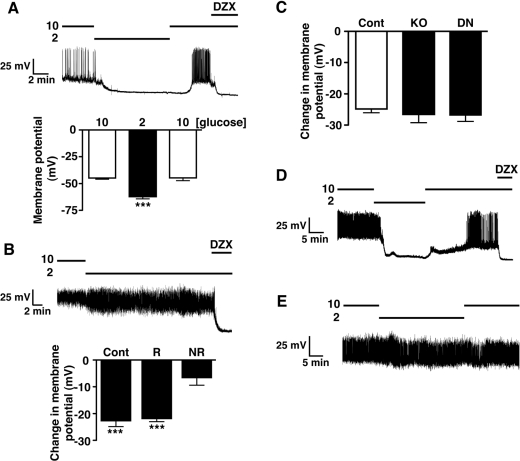
One potential issue with this type of genetic manipulation is that developmental compensation for loss of the target gene may occur and modify the observed phenotype. In an attempt to preclude this possibility, we treated WT mouse cultured β-cells with adenovirus containing GFP (green fluorescent protein) as a control or a kinase-dead mutant form of AMPKα2, which acts as a dominant-negative towards native AMPK (DNAMPKα2). Control viral infected β-cells were electrically active and responded, reversibly, to low glucose by hyperpolarization and inhibition of action potential firing (Figure 6A), identical with control β-cells. Cells treated with DNAMPKα2 adenovirus were also electrically active in 10 mM glucose and, on reduction to 2 mM glucose, a significant proportion of the infected cells (n=11/33) displayed no response to hypoglycaemic challenge (Figure 6B), with the remainder behaving as controls. The low proportion of β-cells unresponsive to hypoglycaemia is probably related to infection efficiency, as obvious GFP expression was observed in <50% of β-cells. Attempts to increase the MOI (multiplicity of infection) to achieve higher expression of DNAMPKα2 resulted in cell toxicity. The non-responsive RIPCreα2KO and DNAMPKα2 β-cells were demonstrated to be capable of hyperpolarization by their responsiveness to the KATP activator, diazoxide, as compared with control β-cells (Figure 6C).
Figure 6. Loss of hypoglycaemic sensing in isolated β-cells lacking AMPK activity.
(A) WT mouse cultured β-cells treated with GFP adenovirus display a normal reversible response to reduction of glucose from 10 to 2 mM. (B) WT mouse cultured β-cells treated with DNAMPKα2 adenovirus exhibit loss of electrical responsiveness to hypoglycaemia. The histograms are the mean values for the change in membrane potential associated with reduction in glucose from 10 to 2 mM in responding (R, n=22) and non-responding (NR, n=11) β-cells, in comparison with control (WT) β-cells (Cont, n=7). (C) Mean change in membrane potential of control (n=12), RIPCreα2KO (KO, n=11) and glucose-unresponsive DNAMPKα2-treated (DN, n=11) β-cells in response to application of diazoxide (250 μM). (D) α1KO mouse β-cells respond normally to reduction of glucose from 10 to 2 mM, showing typical hyperpolarization and inhibition of action potential activity. (E) α1KORIPCreα2KO β-cells are unresponsive, electrically, to hypoglycaemic challenge. Values are means±S.E.M. ***P<0.001. DZX, diazoxide.
Isolated islets from α1KORIPCreα2KO mice also displayed significantly higher insulin secretion at 2 mM glucose in comparison with control mice. Thus to confirm the role of AMPKα2 in regulating β-cell sensitivity to hypoglycaemia, we made recordings from β-cells of α1KO and α1KORIPCreα2KO mice. All α1KO β-cells displayed normal responses to hypoglycaemic challenge, with rapid hyperpolarization and cessation of firing, actions reversed on re-addition of 10 mM glucose (Figure 6D). In contrast, the majority (7 out of 11) of α1KORIPCreα2KO β-cells displayed little or no sensitivity to hypoglycaemic challenge (Figure 6E), with the remaining cells responding like controls (results not shown), as described for RIPCreα2KO β-cells. Taken together, these findings suggest that AMPKα2 contributes to the normal glucose sensing behaviour of pancreatic β-cells.
Altered AMPK levels or activity do not modify ATP levels, glucose uptake or GK activity
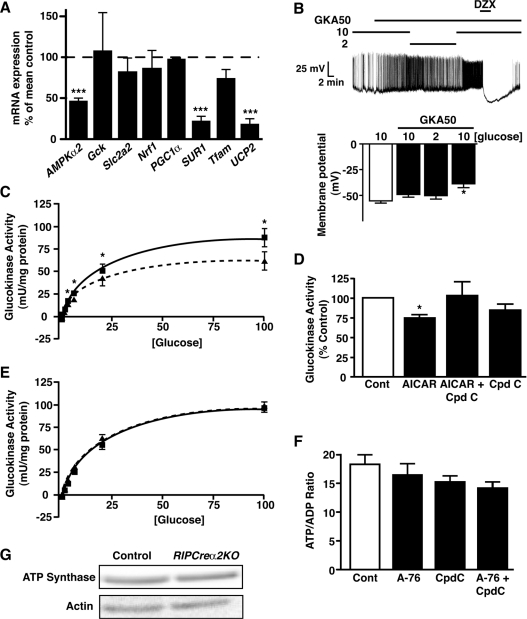
Changing AMPK activity may regulate the expression, localization or activity of GK and GLUT2 [25–28]. Therefore we explored whether altered AMPK activity modified glucose uptake or metabolism in β-cells, using RIPCreα2KO islets and a rat β-cell line. Although AMPKα2 mRNA was significantly reduced in RIPCreα2KO compared with control islets, levels of GLUT2 or GK mRNA were unchanged (Figure 7A and Supplementary Figure S2D at http://www.BiochemJ.org/bj/429/bj4290323add.htm). Additionally, there was no change in the levels of mRNA for the low-Km hexokinase isoforms (HK I–III) in RIPCreα2KO islets compared with control islets (Supplementary Figures S2A–S2C). The CRI-G1 cell line was used to examine the actions of AMPK activity modification because it demonstrated significant GK expression and an innate hypersensitivity to glucose, whereby reducing glucose from 10 mM to 2 mM did not affect cell membrane potential or firing frequency, although lower glucose (<1 mM) levels caused hyperpolarization and inhibition of firing (Supplementary Figures S3A and S3B at http://www.BiochemJ.org/bj/429/bj4290323add.htm). CRI-G1 cells exhibited unchanged GLUT2 or GK protein expression, GK localization (results not shown) and glucose uptake (Supplementary Figure S3C) following stimulation or inhibition of AMPK activity with AICAR (1 mM) or compound C (40 μM) respectively. We next considered the possibility that AMPKα2 acts as a negative regulator of GK activity. GK is the primary glucose sensor of β-cells [29] and pharmacological activation of β-cell GK with GKA50 increases insulin secretion at 2 mM glucose [30], mimicking the RIPCreα2KO β-cell phenotype. Indeed, exposure of WT β-cells to 100 nM GKA50 prevented hyperpolarization and inhibition of electrical activity by hypoglycaemic challenge (Figure 7B). Furthermore, CRI-G1 cells displayed a glucose concentration-dependent increase in GK activity (Figure 7C), significantly higher than for INS-1 cells (CRI-G1 cells, ~90–100 milli-units/mg of protein; INS-1 cells, 8–10 milli-units/mg of protein at 100 mM glucose [31]). This is consistent with CRI-G1 insensitivity to low glucose as high GK activity should raise cellular ATP and maintain KATP in the closed state, analogous to activating GK mutations, which are also characterized by hypersecretion of insulin at basal glucose levels [32]. Our initial studies appeared to corroborate this hypothesis, as AICAR (1 mM) caused significant inhibition (~30%) of GK activity in CRI-G1 cells, at glucose concentrations above 0.5 mM, and compound C prevented this effect (Figures 7C and 7D). Although an attractive explanation for modification of glucose sensing by AMPK, the inhibition of GK activity was not replicated when AMPK activity was stimulated with the direct AMPK activator [33], A-769662 (Figure 7E). Additionally, the [ATP]/[ADP] ratio of CRI-G1 cells following stimulation or inhibition of AMPK was unaffected (Figure 7F).
Figure 7. AMPK activity does not correlate with altered GK activity, nucleotide ratios or mitochondrial mass.
(A) Relative islet mRNA expression, from 20-week-old male control and RIPCreα2KO mice, of AMPKα2, Gck, Slc2a2, Nrf1, PGC1a, SUR1, Tfam and UCP2 (n=6). (B) WT mouse cultured β-cells treated with 100 nM GKA50 fail to respond electrically to hypoglycaemic challenge, but respond normally to diazoxide (250 μM; DZX). Histograms are the mean values for membrane potential in β-cells exposed to 10 mM glucose alone, and 10, 2 and 10 mM glucose in the presence of GKA. (C) Activity of GK in CRI-G1 β-cells as a function of glucose concentration in the absence (■) and presence (▲) of AICAR (1 mM). The curved lines (Control, solid; AICAR, broken) represent lines of best fit to the data points (n=5–8 determinations per point). (D) GK activity, expressed relative to control conditions (6 mM glucose), in CRI-G1 β-cells exposed to 1 mM AICAR ±40 μM Compound C in 6 mM glucose (n=5–8 determinations for each condition). (E) Activity of GK in CRI-G1 β-cells as a function of glucose concentration in the absence (■) and presence (▲) of A-769662 (10 μM). The curved lines (Control, solid; A-769662, broken) represent lines of best fit to the data points (n=6 determinations per point). (F) Mean values for the [ATP]/[ADP] ratio of CRI-G1 β-cells in control conditions and treated with 10 μM A-769662±40 μM Compound C (n=3–6 determinations for each condition). (G) Representative immunoblot for ATP synthase from control and RIPCre2αKO islets. A-76, A-769662; cont, control; Cpdc, compound C.
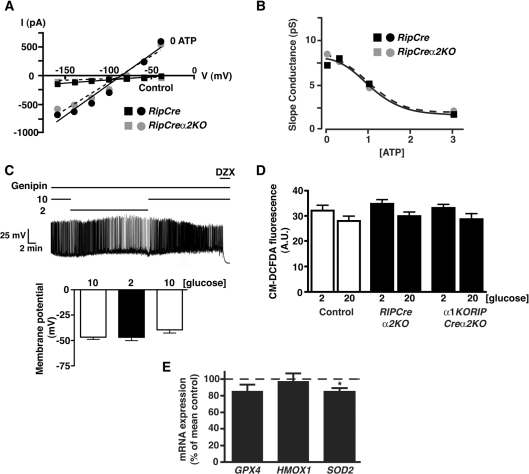
RIPCreα2KO islets also displayed unaltered mitochondrial mass reflected by unchanged levels of ATP synthase (Figure 7G) and mRNA levels of transcription factors involved in mitochondrial energy metabolism, PGC1α, Tfam and Nrf were unaltered (Figure 7A). In contrast, the mRNA level for the KATP channel subunit, SUR1 (Figure 7A) was reduced, raising the possibility that altered KATP current levels or ATP sensitivity may underlie the lack of responsiveness to hypoglycaemia by AMPKα2 deletion. However, the maximal KATP current and ATP-sensitivity (Figures 8A and 8B) were unaltered between RIPCreα2KO and RIPCre control β-cells. Overall, we could not identify any alteration to glucose uptake, coupling of glucose metabolism to mitochondrial ATP production or deficiency in KATP channel function, which would explain the hypersecretion of insulin at low glucose or the reduction in GSIS in AMPKα2-deficient β-cells.
Figure 8. Loss of AMPKα2 does not alter β-cell KATP current or ATP-sensitivity or islet ROS response.
(A) Representative current–voltage relationships for voltage-clamped currents of RIPCre control (black symbols) and a RIPCreα2KO (grey symbols) cultured β-cells. Mean currents were measured at various membrane potentials shortly after attaining whole-cell recording (i.e. prior to significant washout of cell ATP; filled squares) and 20 min later (following maximal washout of cellular ATP; filled circles). (B) Mean slope conductance values for current–voltage relationships as shown in (A), as a function of [ATP] (0, 0.5, 1.0 or 3.0 mM in the pipette solution). Lines of best fit (dose–response) are shown for RIPCre control (black filled squares) and RIPCreα2KO (grey filled circles) β-cells (n=3–6). (C) WT β-cells exposed to genipin (100 nM) are electrically unresponsive to the reduction of glucose from 10 mM to 2 mM. Note that 250 μM diazoxide (DZX) hyperpolarizes the cell in the presence of genipin. Histograms are the mean values for membrane potential for β-cells, in the presence of genipin exposed to 10, 2 and 10 mM glucose respectively (n=6). (D) H2O2-derived fluorescence in isolated islets of control, RIPCreα2KO and α1KORIPCreα2KO mice (n=16–23 islets each) at 2 and 20 mM glucose. A. U., arbitray units. (E) Expression of antioxidant enzymes in isolated islets of RIPCreα2KO mice, normalized to the levels expressed in control WT mice (n=8–10). *P<0.05.
Islets lacking AMPKα2 have reduced UCP2, and UCP2 inhibition causes glucose insensitivity of β-cells
Increased UCP2 activity has been reported to impair GSIS in islets [34,35]. Conversely, UCP2 reduction increases GSIS [36,37], and pharmacological inhibition of UCP2 with genipin stimulates insulin secretion [38]. UCP2 mRNA was markedly suppressed in RIPCreα2KO islets (Figure 7A). Furthermore, exposure of WT β-cells to genipin prevented the hyperpolarization associated with hypoglycaemic challenge (Figure 8C), but did not influence the response of the cells to diazoxide (ΔV control = −19.6±1.3 mV; n=5; ΔV genipin −19.0±3.7 mV; n=6). Although genipin has been reported to have a variety of activities, at the concentration used in the present study it is considered to act relatively specifically [38]. In addition, genipin excites mouse arcuate POMC neurons by closure of KATP channels only when UCP2 is present in the neurons [39]. Consistent with our β-cell data, hyperpolarization of POMC neurons in response to reduced glucose is also ablated in AMPKα2KO POMC neurons [17] and is reversibly occluded by genipin in WT mouse POMC neurons (Supplementary Figure S4 at http://www.BiochemJ.org/bj/429/bj4290323add.htm). However, UCP2 reduction itself, would be predicted to increase GSIS, whereas the loss of AMPKα2 in β-cells resulted in diminished GSIS. Thus we sought an alternative explanation. UCP2 activity plays a key role in the control of mitochondrial-driven ROS (reactive oxygen species) production [40] and a lack or reduction of UCP2 in β-cells increases ROS and impairs GSIS from islets [41]. However, we detected no increase in H2O2 production in WT, RIPCreα2KO or α1KORIPCreα2KO islets at 2 or 20 mM glucose, by CM-H2DCFDA-derived fluorescence (Figure 8D). Furthermore, we did not detect any increase in mRNA level for antioxidant enzymes in RIPCreα2KO islets (Figure 8E), indicative of an increased innate adaptive response to oxidative stress, which has also been reported to impair GSIS [41].
DISCUSSION
A previous study has shown that global AMPKα2KO mice demonstrate impaired glucose homoeostasis, which was ascribed to increased catecholamine secretion [22]. In contrast, we show that RIPCreα2KO and α1KORIPCreα2KO mice exhibit impaired glucose tolerance associated with dysfunctional GSIS in vivo and in isolated islets. Electrophysiological examination of AMPKα2-depleted isolated β-cells showed that the normal inhibitory electrical response to low glucose was ablated in the majority of these cells compared with control β-cells, in accordance with the observed enhanced basal insulin secretion. This outcome indicates that the KATP channels of AMPKα2-depleted β-cells, in contrast with control β-cells, remain closed under hypoglycaemic conditions. Elevated electrical activity and increased insulin release at low-glucose concentrations have been reported for β-cells with reduced levels of functional KATP channels in the plasma membrane [42,43], or which have loss of function mutations in one of the KATP channel subunits, KIR6.2 or SUR1 [44]. It has also been suggested that AMPK can modify β-cell KATP activity by phosphorylation of KIR6.2 [45] or altered plasma membrane channel numbers [46]. However, this is unlikely to explain AMPKα2KO β-cell electrical insensitivity to hypoglycaemia because the cells display normal hyperpolarizing responses to diazoxide and exhibit an unaltered maximal KATP current compared with control cells. An alternative explanation is that lack of AMPKα2 activity decreases KATP current by increased channel ATP sensitivity causing maintained KATP closure in the face of reduced cell ATP levels during the hypoglycaemic challenge. However, we could detect no difference in the ATP-sensitivity of KATP comparing AMPKα2-null and control β-cells.
Reduced expression of GLUT2 in rodents and humans correlates with β-cell glucose insensitivity [8,47]. However, as we could detect no evidence to link altered AMPK levels or activity to a change in GLUT2 mRNA or protein levels or to glucose uptake, this component of β-cell glucose sensing is unlikely to be responsible for the defective control of insulin secretion described in the present study. Deletion of the upstream AMPK regulator LKB1 results in improved glucose tolerance, β-cell hypertrophy, higher insulin content and increased secretion in vivo and in vitro [23,24]. These results have been interpreted as LKB1, by increasing AMPK activity, acting to restrain insulin secretion. However, we observe no change in β-cell mass, and GSIS in AMPKα2-deleted islets is impaired not enhanced, indicating that AMPKα2 β-cell deletion is unlikely to be associated with reduced LKB1 activity. Our results also indicate that the outcome of LKB1 deletion in β-cells described in these reports may not be due simply to altered AMPK activity.
GK plays a central role in the control of GSIS because it allows glucose phosphorylation at physiological glucose concentrations. Inactivating mutations of GK result in reduced insulin secretion and Type 2 diabetes, whereas GK-activating mutations cause insulin hypersecretion and hypoglycaemia [48]. Thus high GK activity should result in high levels of ATP at low-glucose concentrations and keep KATP channels closed. Indeed, stimulation of GK with GKA50 in control β-cells resulted in the loss of electrical sensitivity to low glucose. In support of this notion, CRI-G1 cells have reduced sensitivity to hypoglycaemic challenge and express high GK activity. Furthermore, stimulation of AMPK activity with AICAR significantly reduced GK activity, which was prevented by co-application of the AMPK inhibitor compound C. Although these results encouraged the view that loss of AMPKα2 up-regulated GK activity in β-cells, this effect was not replicated using the direct AMPK activator A-769662. In addition, we could detect no change in the levels of low-Km hexokinases in AMPKα2-deleted islets, abrogating an induction of one or more of these hexokinases as a potential loss-of-function mediator. Taken together with the lack of change in bulk [ATP]/[ADP] ratio in CRI-G1 cells, we think it unlikely that modification of GK activity explains the insensitivity of β-cell electrical activity to low glucose in AMPKα2-deficient cells.
β-cells have a relatively modest endogenous antioxidant defence system and are very susceptible to toxicity induced by ROS [49]. Increased respiratory energy production raises mitochondrial electron transport, which drives the generation of ROS. To counter this, pancreatic β-cells exhibit high UCP2 levels and activity, which uncouples respiratory energy production, reduces the mitochondrial membrane potential and attenuates ROS production [37]. UCP2 may be a crucial component of β-cell function as inappropriately high UCP2 levels (e.g. associated with chronic hyperglycaemia or obesity) or UCP2 overexpression causes β-cell dysfunction by lowering ATP levels and reducing GSIS [36]. Conversely, knockdown of UCP2 in islets or global UCP2-deficient mice exhibit raised islet ATP levels and increased insulin secretion [34]. Our finding that AMPKα2 deletion reduces UCP2 mRNA levels in islets might therefore have led to the prediction that insulin secretion would be increased. Clearly, this was not the case when GSIS was examined in vivo or in vitro in RIPCreα2KO and α1KORIPCreα2KO mice. Indeed, in isolated islets although basal insulin secretion in the presence of low glucose was enhanced, GSIS was either relatively unaffected (RIPCreα2KO mice) or significantly reduced (α1KORIPCreα2KO mice) compared with controls.
The finding that AMPKα2 deletion also ablates the KATP-mediated hyperpolarization associated with hypoglycaemia in hypothalamic POMC neurons [17] supports the notion that AMPKα2 deletion alters β-cell glucose sensing capability. Furthermore, KATP activation by low glucose is occluded in both cell types by genipin, supporting the idea that UCP2 activity is a key component of hypoglycaemic sensing in these specialized cells. So how might we explain the reduced GSIS associated with loss of AMPKα2 and reduced UCP2 in β-cells? There is a growing body of evidence to indicate that β-cell ROS production contributes to glucose-sensing behaviour and the regulation of insulin secretion, either as a signal for insulin secretion or as a mediator of the adaptive antioxidant response [41]. However, the reduction of UCP2 in AMPKα2-deleted islets was not accompanied by any significant alteration in ROS levels at low- or high-glucose concentrations, and we could detect no evidence for an adaptive response causing raised levels of antioxidant enzymes.
The permanently depolarized nature of AMPKα2-null β-cells may be, at least partly, responsible for the overall reduction of GSIS and impaired glucose tolerance we observe in RIPCreα2KO mice. This notion arises from reports that suppression of insulin secretion by pharmacological opening of KATP channels causing cell hyperpolarization and a period of rest for the insulin secretion process improves glucose tolerance and β-cell function in animal models of diabetes and preserves and improves human islet function [50]. Thus maintenance of AMPK activity may be an important component of β-cell protection against chronic overstimulation and loss of islet function.
In conclusion, loss of AMPKα2 appears to cause permanently dampened UCP2 levels in islets, which is associated with dysfunctional β-cell glucose sensing and diminished GSIS. Lack of AMPKα2 may therefore result in disconnection of the normal response to nutrient deprivation whereby raised AMPK activity and UCP2 levels would act to lower insulin secretion at low-glucose levels and maintain secretion at high-glucose levels. Thus we hypothesize that AMPK plays a key role in connecting nutrient fluctuations and UCP2 regulation with maintenance of GSIS in β-cells.
Online data
AUTHOR CONTRIBUTION
Craig Beall researched data, contributed to discussion and reviewed/edited manuscript. Kaisa Piipari researched data, contributed to discussion and reviewed/edited manuscript. Hind Al-Qassab, Mark Smith and Nadeene Parker researched data. David Carling provided materials and contributed to the discussion. Benoit Viollet provided materials. Dominic Withers contributed to the discussion and reviewed/edited the manuscript. Michael Ashford contributed to the discussion, wrote the manuscript and reviewed/edited the manuscript.
ACKNOWLEDGEMENTS
GKA was a kind gift from AstraZeneca. We thank Dr K. Green and Professor D.G. Hardie (Division of Molecular Physiology, University of Dundee, College of Life Sciences, Dundee, Scotland, U.K.) for help with the nucleotide measurements.
FUNDING
This work was supported by the Wellcome Trust [grant numbers 073073, 068692 (to M.L.J.A.)]; and the Medical Research Council [grant numbers G0600316, G0600866 (to D.J.W.)].
References
- 1.Thorens B. GLUT2 in pancreatic and extra-pancreatic gluco-detection. Mol. Membr. Biol. 2001;18:265–273. doi: 10.1080/09687680110100995. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald P. E., Joseph J. W., Rorsman P. Glucose-sensing mechanisms in pancreatic β-cells. Philos. T. Roy. Soc. B. 2005;360:2211–2225. doi: 10.1098/rstb.2005.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchetti P., Dotta F., Lauro D., Purrello F. An overview of pancreatic β-cell defects in human type 2 diabetes: implications for treatment. Regul. Pept. 2008;146:4–11. doi: 10.1016/j.regpep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Khan S. E., Hull R. L., Utzschneider K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 5.Bosi E. Metformin: the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes Obes. Metab. 2009;11(Suppl. 2):3–8. doi: 10.1111/j.1463-1326.2008.01031.x. [DOI] [PubMed] [Google Scholar]
- 6.Viollet B., Lantier L., Devin-Leclerc J., Hebrard S., Amouyal C., Mounier R., Foretz M., Andreelli F. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front. Biosci. 2009;14:3380–3400. doi: 10.2741/3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue B., Khan B. B. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Guerra S., Lupi R., Marselli L., Masini M., Bugliani M., Sbrana S., Pollera M., Boggi U., Mosca F., Del Prato S., Marchetti P. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–735. doi: 10.2337/diabetes.54.3.727. [DOI] [PubMed] [Google Scholar]
- 9.Salt I. P., Johnson G., Ashcroft S. J. H., Hardie D. G. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β-cells, and may regulate insulin secretion. Biochem. J. 1998;335:533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva Xavier G., Leclerc I., Varadi A., Tsuboi T., Moule S. K., Rutter G. A. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem. J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclerc I., Woltersdorf W. W., da Silva Xavier G., Rowe R. L., Cross S. E., Korbutt G. S., Rajotte R. V., Smith R., Rutter G. A. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2004;286:E1023–E1031. doi: 10.1152/ajpendo.00532.2003. [DOI] [PubMed] [Google Scholar]
- 12.Richards S. K., Parton L. E., Leclerc I., Rutter G. A., Smith R. M. Over-expression of AMP-activated protein kinase impairs pancreatic β-cell function in vivo. J. Endocrinol. 2005;187:225–235. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 13.Wang C-Z., Wang Y., Di A., Magnuson M. A., Ye H., Roe M. W., Nelson D. J., Bell G. I., Phillipson L. H. 5-Amino-imidazole carboxamide riboside acutely potentiates glucose-stimulated insulin secretion from mouse pancreatic islets by KATP channel-dependent and -independent pathways. Biochem. Biophy. Res. Commun. 2005;330:1073–1079. doi: 10.1016/j.bbrc.2005.03.093. [DOI] [PubMed] [Google Scholar]
- 14.Gleason C. E., Lu D., Witters L. A., Newgard C. B., Birnbaum M. J. The role of AMPK and mTOR in nutrient sensing in pancreatic β-cells. J. Biol. Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 15.Choudhury A. I., Heffron H., Smith M. A., Al-Quassab H., Xu A. W., Selman C., Simmgen M., Clements M., Claret M., MacColl G., et al. The role of insulin receptor substrate 2 in hypothalamic and β-cell function. J. Clin. Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantley J., Selman C., Shukia D., Abramov A. Y., Forstreuter F., Esteban M. A., Claret M., Lingard S. J., Clements M., Harten S. K., et al. Deletion of the von Hippel-Lindau gene in pancreatic β-cells impairs glucose homeostasis in mice. J. Clin. Invest. 2009;119:125–135. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claret M., Smith M. A., Batterham R. L., Selman C., Choudhury A. I., Fryer L. G., Clements M., Al-Quassab H., Heffron H., Xu A. W., et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning K., Miller L. C., Laidlaw H. A., Burgess L. A., Perera N. M., Downes C. P., Leslie N. R., Ashford M. L. A novel leptin signalling pathway via PTEN inhibition in hypothalamic cell lines and pancreatic β-cells. EMBO J. 2006;25:2377–2387. doi: 10.1038/sj.emboj.7601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meakin P. J., Fowler M. J., Rathbone A. J., Allen L. M., Ransom B. R., Ray D. E., Brown A. M. Fructose metabolism in the adult mouse optic nerve, a central white matter tract. J. Cereb. Blood Flow Metab. 2007;27:86–99. doi: 10.1038/sj.jcbfm.9600322. [DOI] [PubMed] [Google Scholar]
- 20.Mirshamsi S., Laidlaw H. A., Ning K., Anderson E., Burgess L. A., Gray A., Sutherland C., Ashford M. L. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI 3-kinase dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada K., Nakata M., Horimoto N., Saito M., Matsuoka H., Inagaki N. Measurement of glucose uptake and intracellular calcium concentration in single, living pancreatic β-cells. J. Biol. Chem. 2000;275:22278–22283. doi: 10.1074/jbc.M908048199. [DOI] [PubMed] [Google Scholar]
- 22.Viollet B., Andreelli F., Jørgensen S. B., Perrin C., Flamez D., Mu J., Wojtaszewski J. F., Schuit F. C., Birnbaum M., Richter E., et al. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem. Soc. Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 23.Fu A., Ng A. C-H., Depatie C., Wijesekara N., He Y., Wang G. S., Bardeesy N., Scott F. W., Touyz F. W., Wheeler M. B., Screaton R. A. Loss of Lkb1 in adult β-cells increases β-cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10:285–295. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Granot Z., Swisa A., Magenheim J., Stolovich-Rain M., Fujimoto W., Manduchi E., Miki T., Lennerz J. K., Stoeckert C. J., Jr, Meyuhas O., et al. LKB1 regulates pancreatic β-cell size, polarity and function. Cell Metab. 2009;10:296–308. doi: 10.1016/j.cmet.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent M. F., Bontemps F., Van den Berghe G. Inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside in isolated rat hepatocytes. Biochem. J. 1992;281:267–272. doi: 10.1042/bj2810267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker J., Jijon H. B., Diaz H., Salehi P., Churchill T., Madsen K. L. 5-aminoimidazole-4-carboxamine riboside (AICAR) enhances GLUT2dependent jejunal glucose transport: a possible role for AMPK. Biochem. J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhtar M. H., Payne V. A., Arden C., Harbottle A., Khan S., Lange A. J., Agius L. Inhibition of glucokinase translocation by AMP-activated protein kinase is associated with phosphorylation of both GKRP and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R766–R774. doi: 10.1152/ajpregu.00593.2007. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Sun N., Wang L., Guo H., Guan Q., Cui B., Tian L., Gao L., Zhao J. AMP-activated protein kinase and pancreatic/duodenal homeobox-1 involved in insulin secretion under high leucine exposure in rat insulinoma β-cells. J. Cell. Mol. Med. 2009;13:758–770. doi: 10.1111/j.1582-4934.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matschinsky F. M. Regulation of pancreatic β-cell glucokinase: from basics to therapeutics. Diabetes. 2002;51:S394–S404. doi: 10.2337/diabetes.51.2007.s394. [DOI] [PubMed] [Google Scholar]
- 30.Johnson D., Shepherd R. M., Gill D., Gorman T., Smith D. M., Dunne M. J. Glucose-dependent modulation of insulin secretion and intracellular calcium ions by GKA50, a glucokinase activator. Diabetes. 2007;56:1694–1702. doi: 10.2337/db07-0026. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Iynedijian P. B. Modulation of glucose responsiveness of insulinoma β-cells by graded overexpression of glucokinase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4372–4377. doi: 10.1073/pnas.94.9.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gloyn A. L. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum. Mutat. 2003;22:353–362. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- 33.Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C.-Y., Baffy G., Perret P., Krauss S., Peroni O., Grujic D., Hagen T., Vidal-Puig A. J., Boss O., Kim Y. B., et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, β-cell dysfunction and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 35.Chan C. B., De Leo D., Joseph J. W., McQuaid T. S., Ha X. F., Xu F., Tsushima R. G., Pennefather P. S., Salapatek A. M., Wheeler M. B. Increased uncoupling protein-2 levels in β-cells are associated with impaired glucose-stimulated insulin secretion: mechanisms of action. Diabetes. 2001;50:1302–1310. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- 36.Krauss S., Zhang C-Y., Scorrano L., Dalgaard L. T., St-Pierre J., Grey S. T., Lowell B. B. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic β-cell dysfunction. J. Clin. Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Affourtit C., Brand M. D. On the role of uncoupling protein-2 in pancreatic β-cells. Biochim. Biophys. Acta. 2008;1777:973–979. doi: 10.1016/j.bbabio.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C.-Y., Parton L. E., Ye C. P., Krauss S., Shen R., Lin C. T., Porco J. A., Jr, Lowell B. B. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced β-cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Parton L. E., Ye C. P., Coppari R., Enriori P. J., Choi B., Zhang C. Y., Xu C., Vianna C. R., Balthasar N., Lee C. E., et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 40.Brand M. D., Affourtit C., Esteves T. C., Green K., Lambert A. J., Miwa S., Pakay J. L., Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radical Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Pi J., Zhang Q., Fu J., Woods C. G., Hou Y., Corkey B. E., Collins S., Andersen M. E. ROS signalling, oxidative stress and Nrf2 in pancreatic β-cell function. Toxicol. Appl. Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miki T., Nagashima K., Tashiro F., Kotake K., Yoshitomi H., Tamamoto A., Gonoi T., Iwanaga T., Miyazaki J., Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seghers V., Nakazaki M., DeMayo F., Aguilar-Bryan L., Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J. Biol. Chem. 2000;275:9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 44.Ashcroft F. M. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang T. J., Chen W. P., Yang C., Lu P. H., Liang Y. C., Su M. J., Lee S. C., Chuang L. M. Serine-385 phosphorylation of inwardly rectifying K+ channel subunit (Kir6.2) by AMP-dependent protein kinase plays a key role in rosiglitazoneinduced closure of the KATP channel and insulin secretion in rats. Diabetologia. 2009;52:1112–1121. doi: 10.1007/s00125-009-1337-4. [DOI] [PubMed] [Google Scholar]
- 46.Lim A., Park S. H., Sohn J. W., Jeon J. H., Park J. H., Song D. K., Lee S. H., Ho W. K. Glucose deprivation regulated KATP channel trafficking via AMP-activated protein kinase in pancreatic β-cells. Diabetes. 2009;58:2813–2819. doi: 10.2337/db09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostenson C. G., Efendic S. Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans. Diabetes Obes. Metab. 2007;9:180–186. doi: 10.1111/j.1463-1326.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 48.Gloyn A. L., Tribble N. D., van de Bunt M., Barrett A., Johnson P. R. Glucokinase (GCK) and other susceptibility genes for β-cell dysfunction: the candidate approach. Biochem. Soc. Trans. 2008;36:306–311.. doi: 10.1042/BST0360306. [DOI] [PubMed] [Google Scholar]
- 49.Robertson R. P. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr. Opin. Pharmacol. 2006;6:615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Wajchenberg B. L. β-Cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.