Abstract
Background
Breast cancer is the commonest malignancy of women in Nigeria. Change in serum levels of some biochemical parameters could assist diagnosis and follow-up of breast cancer.
Objective
To determine serum levels of calcium, inorganic phosphates, alkaline phosphatase (ALP) and acid phosphatase (ACP) activities in patients with breast cancer, and change in the serum levels over time.
Methods
Total serum calcium and inorganic phosphates, and serum ALP and ACP activities were determined in 25 women with breast cancer and 25 age-matched controls using colorimetric and enzymatic methods, over 6 months with bimonthly analysis.
Results
The serum calcium level, ALP and ACP activities were significantly higher (p<0.05) in the study group than in the control group. No significant difference was seen in the inorganic phosphate levels of both groups. There were significant increases in serum calcium levels, ALP and ACP activities in the study group with time (p<0.05), whereas no significant increase was observed in the control group.
Conclusion
Breast cancer patients have higher calcium levels and higher ALP and ACP activities. The increase in the levelsof these parameters with time shows that they could be of importance in monitoring treatment and disease progress in a resource-poor setting.
Key words: Breast cancer, serum calcium, inorganic phosphate, alkaline phosphatase, acid phosphatase, tumor markers
Introduction
Breast cancer is the most frequently diagnosed malignancy and the second principal cause of death among women world wide as well as in Nigeria.1, 2, 3 The incidences of the disease appear to be on the increase in population groups that hitherto enjoyed low incidence.4 The increasing prevalence of breast cancer in our society has been attributed to changing life style and dietary pattern from the traditional African pattern to the western pattern.
Breast cancer like any other disease condition is associated with derangement in the body's physiological functions, alterations in the homeostasis and production of some biochemical metabolites. Production of tumor markers has been utilized as a parameter for diagnosis and monitoring disease progress. Screening for markers such as CA 15-3, CA 549, CA 27–29 and mucin-like carcinomaassociated antigen (MCA) has been utilized for early disease diagnosis and intervention.5, 6 Some studies have shown increased serum levels of calcium and elevated activities of alkaline phosphatase (ALP) and acid phosphatase (ACP) in patients with malignancies including breast cancer.7, 8, 9, 10 The mechanisms postulated for the increased levels of these parameters in malignancy includes humoral mechanisms mainly mediated by parathyroid hormone related peptide (PTH-rp), osteolytic bone metastasis11 and increase in concentration of ACP particles which characterize lysosomes in breast tumor cells.7
Assessment of the serum levels of these biochemical parameters could substitute for the classical tumor markers in areas where facilities for these are not readily available. Calabar is one of such areas; hence this study is aimed at assessment of serum calcium, inorganic phosphates, ALP and ACP activities in breast cancer patients and change in the serum levels over time.
Methods
Patients and Study Design
This case-controlled comparative study was carried out in the department of surgery and department of chemical pathology, University of Calabar Teaching Hospital, Nigeria. The patient's consent was sought before recruitment into the study. Twenty-five female breast cancer patients confirmed by biopsy were selected by convenience sampling. Twenty-five age-matched breast cancer-free females were used as controls after exclusion of the disease by history and clinical examination. None of the patients or control subjects was on calcium supplements. A follow up study was carried out on the women for six months with bi-monthly analysis of serum calcium, inorganic phosphates, ALP and ACP activities.
Sample Collection
Five milliliters of whole blood samples were collected from each subject into plain tubes with precautions to avoid pre-analytical errors, avoiding the use of tourniquet and fist clenching.12 Serum was extracted for analysis of total calcium and inorganic phosphate, and ALP and ACP activities. Total calcium and inorganic phosphate was estimated using colorimetric methods,13, 14 while ALP and ACP activities were determined using the enzymatic method.15, 16
Statistical Analysis
Data was analyzed using the paired t-test analysis and analysis of variance (ANOVA) for inferential statistics between study group and control subjects.
Results
Serum calcium levels, ALP and ACP activities were significantly higher (p<0.05) in female breast cancer patients when compared with those of the control subjects. No significant difference (p>0.05) was seen in the inorganic phosphate levels of both groups as shown in Table 1.
Table 1.
Mean serum calcium (Ca), Inorganic phosphate (PO4−) alkaline phosphatase (ALP) and acid phosphatase (ACP) activities in female breast cancer patients and controls
| Subjects | Ca (mmol/l) |
PO4− (mmol/l) |
ALP (iu/l) | ACP (iu/l) |
| Breast cancer | ||||
| n = 25 | 2.75 ±1.32 | 0.94 ±0.36 | 150.26 ±58.40 | 8.72 ± 2.96 |
| Controls | ||||
| n = 25 | 2.22 ±0.41 | 1.48 ±0.62 | 48.10 ±16.70 | 1.89 ±0.53 |
| p-value | p<0.05 | p>0.05 | p<0.05 | p<0.05 |
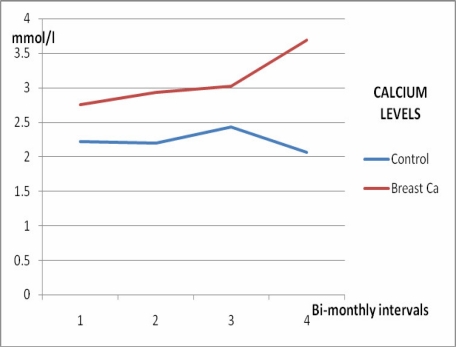
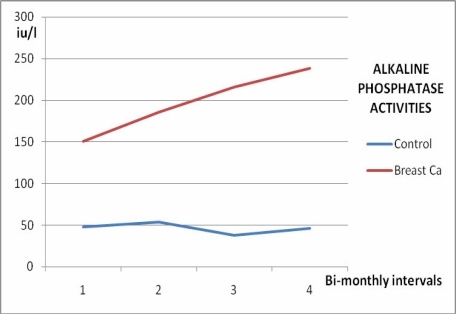
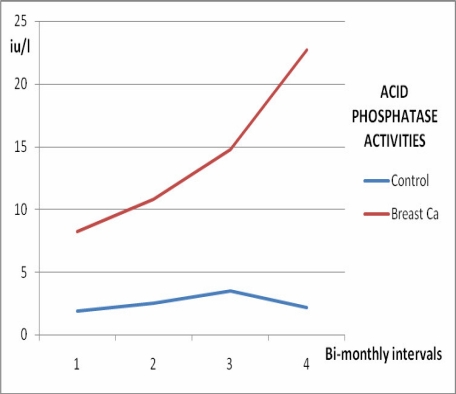
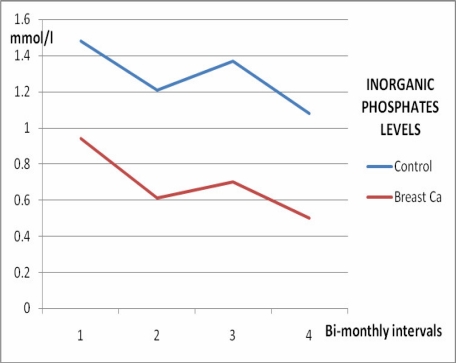
Serum calcium levels, ALP and ACP activities increased significantly in women with breast cancer with time (p<0.05) as shown in Figures 1, 2, and 3, and Table 2, whereas no significant variation (p>0.05) was observed in the inorganic phosphate levels with time (Figure 4 and Table 2).
Figure 1.
Calcium levels in female breast cancer patients and controls over 6 months
Figure 2.
Alkaline phosphatase activities in female breast cancer patients and controls over 6 months
Figure 3.
Acid phosphatase activities in female breast cancer patients and controls over 6 months
Table 2.
Mean serum calcium (Ca), inorganic phosphate (PO4−) alkaline phosphatase (ALP) and acid phosphatase (ACP) activities in female breast cancer patients over 6 months
| Sample Intervals |
Ca mmol/l |
PO4− mmol/l |
ALP IU/L |
ACP IU/L |
| 1st assay | 2.75 ±0.98 | 0.94 ±0.36 | 150.20 ±58.40 | 8.72 ±3.96 |
| 2 months | 2.93 ±1.22 | 0.61 ±0.44 | 185.60 ±72.60 | 10.84 ±4.88 |
| 4 months | 3.02 ±1.07 | 0.70 ±0.28 | 215.80 ±62.30 | 14.80 ±8.90 |
| 6 months | 3.69 ±1.32 | 0.50 ±0.21 | 238.56 ±88.50 | 22.74 ±12.60 |
| p-value | p<0.05 | p>0.05 | p<0.05 | p<0.05 |
Figure 4.
Inorganic phosphates levels in female breast cancer patients and controls over 6 months
There was no significant variation (p>0.05) in the levels of serum calcium, inorganic phosphate, ALP and ACP activities in the control group during the 6 months follow up (Figures 1–4 and Table 3).
Table 3.
Mean serum calcium (Ca), inorganic phosphate (PO4−) alkaline phosphatase (ALP) and acid phosphatase (ACP) activities in female age-matched controls over 6 months
| Sample Intervals |
Ca mmol/l |
PO4− mmol/l |
ALP IU/L |
ACP IU/L |
| 1st assay | 2.22 ±0.41 | 1.48 ±0.62 | 48.10 ±16.70 | 1.89 ±0.53 |
| 2 months | 2.20 ±0.62 | 1.21 ±0.73 | 54.00 ±26.78 | 2.50 ±1.03 |
| 4 months | 2.43 ±0.03 | 1.37 ±0.94 | 38.20 ±14.52 | 3.47 ±1.98 |
| 6 months | 2.06 ±1.32 | 1.08 ±0.51 | 46.32 ±18.30 | 2.14 ±1.22 |
| p-value | p>0.05 | p>0.05 | p>0.05 | p>0.05 |
Discussion
The results of this study have shown that women with breast cancer have higher total serum calcium levels and higher ALP and ACP activities when compared with the control subjects. Hypercalcemia and high ALP and ACP activities have also been reported in other malignancies.9, 17, 18, 19, 7, 10
The hypercalcemia in breast cancer has been attributed in part to osteolytic bone metastases and this account for 20–30% of the hypercalcemia cases in oncology patients. The skeletal invasion and destruction by tumor induced by tumor-production of various cytokines such as transforming growth factor-á (TGF-á), tumor necrosis factor-á (TNF-á), TNF-â, interleukin-1 and interleukin-2, leads to increasing bone osteolysis20 and modification of the reabsorption, excretion and resorption of calcium and phosphate ion.21
Alteration in humoral regulation of calcium resulting from production of parathyroid hormone related protein (PTH-rp) has also been implicated in tumor associated hypercalcemia.22, 23 Plasma concentration of PTH-rp is rarely elevated in healthy individuals, but elevated concentrations are detectable in about 80% of hypercalcemia patients with solid tumors. PTH-rp interacts with parathyroid hormone receptors on cell membranes, activating adenyl cyclase, which triggers an increase in cyclic AMP production and increases intracellular calcium. These actions are responsible for increasing bone demineralization and elevating serum calcium concentrations, decreasing reabsorption of phosphate in the proximal renal tubules, increasing calcium reabsorption in the distal tubules and increasing cholecalciferol production.17 ACP activity has been used to indicate that lysosomes participate in the execution of cell death in a variety of tissues and the regression of mammary carcinomas.7 Activities of lysosomal acid hydrolases have been demonstrated to be more marked in cancer cells than in homologous normal tissue24, 25 and the histochemical pattern of ACP distribution in the breast tissue showed differences between normal and neoplastic cells.7 Significantly elevated tartrate resistant ACP 5b activity was also reported by Chao & co-workers, (2005).26 ACP has been used as a marker of metastatic bone disease and response to treatment in breast cancer patients.27
ALP has many isoenzymes localized in the liver, bones and in lesser amounts the intestines, placenta kidney and leucocytes.28 Another isoenzyme of ALP termed Regan isoenzyme has also been identified in various malignancies29 and this may be contributing to increased ALP activity seen in breast cancer patients. The increased activity of this enzyme seen in subjects of the study may also be due to osteolytic bone metastases in breast cancer leading to increased osteoclastic activity and bone resorption. Increase in serum ALP levels is however non-specific as it is also frequently associated with a variety of other diseases. Also, the elevation of ALP activity to less than three times the normal level is usually not considered significant.9, 30
Conclusion
Women with breast cancer have higher calcium levels and higher ALP and ACP activities than normal healthy women. The progressive increase in the serum calcium levels and ALP and ACP activities during the six months follow up study is an indication that measurement of these parameters may be useful tools in monitoring treatment and disease progression in areas where facilities for sophisticated studies are not readily available.
Acknowledgement
The assistance of Mr Henry Efobi of Chemical Pathology Department, University of Calabar Teaching Hospital, Nigeria, in respect of a citation is appreciated.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Adebamowo CA, Ajayi OO. Breast cancer in Nigeria. West Afr J Med. 2000;19:179–191. [PubMed] [Google Scholar]
- 3.Mcpherson K, Steel CM, Dixon JM. ABC of breast disease: Breast cancer epidemiological risk factors and genetics. BMJ. 2000;321:624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okobia MN, Bunker CH, Okonofua FE, Osime U. Knowledge, attitude and practice of Nigeria women towards cancer: A cross section study. World J Surg Oncol. 2006;4:11–20. doi: 10.1186/1477-7819-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan DW, Stewart S. Tumor markers. In: Burtis CA, Ashwood ER, editors. Tietz text book of Clinical Chemistry. Philadelphia: W B Saunders Company; 2005. pp. 897–927. [Google Scholar]
- 6.Hilken J. Biochemistry and functions of mucins in malignant diseases. Cancer Rev. 1988;11–12:24–55. [Google Scholar]
- 7.Halabay R, Abdollahi J, Martinez ML. Acid phosphatase in human breast tumors. Breast Cancer Res. 2001;3:E002. [Google Scholar]
- 8.Solimando DA. Overview of hypercalcemia of malignancy. Am J Health-Syst Pharm. 2001 Nov 15;58(Suppl 3)(S4–7) doi: 10.1093/ajhp/58.suppl_3.S4. [DOI] [PubMed] [Google Scholar]
- 9.Wiwanitkit V. High Serum alkaline phosphatase level, a study in 181 Thai adult hospitalized patients. BMC Fam Pract. 2001;2:2. doi: 10.1186/1471-2296-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ijaz A, Mehmood T, Qureshi AH, Anwar M, Dilawar M, Hussain I, Khan FA, Khan DA, Hussain S, Khan IA. Estimation of ionized calcium, total calcium and albumin corrected calcium for the diagnosis of hypercalcemia of malignancy. J Coll Physicians Surg Pak. 2006;16(1):49–52. [PubMed] [Google Scholar]
- 11.Ohsako T, Yamamoto Y, Kawazoe T, Inoue K, Nagamoto N, Yoshida Y, Nakahara O, Sakamoto N, Iwase H. Two cases of stage IV breast cancer with severe hypercalcemia. Gan To Kagaku Ryoho. 2006;33(2):223–226. [PubMed] [Google Scholar]
- 12.Toffaletti JG, Wildermann RF. The effects of heparin anticoagulants and fill volume in blood gas syringes on ionized calcium and magnesium measurements. Clin Chim Acta. 2001;304:147–151. doi: 10.1016/s0009-8981(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 13.Baginski ES, Marie SS, Clark WL, Zak B. Direct microdetermination of serum calcium. Clinica Chimica Acta. 1973;46(1):49–54. doi: 10.1016/0009-8981(73)90101-0. [DOI] [PubMed] [Google Scholar]
- 14.King EJ. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King EJ. Microanalysis in medical biochemistry. London: Churchill; 1946. pp. 336–338. [Google Scholar]
- 16.Fishman WH, Lerner F. A method for estimating serum acid phosphatase of prostatic origin. J Biol Chem. 1953;200(1):89–97. [PubMed] [Google Scholar]
- 17.Heys SD, Smith IC, Eremin O. Hypercalcemia in patients with cancer: aetiolgy and treatment. Eur J Surg Oncol. 1998;24(2):139–142. doi: 10.1016/s0748-7983(98)91589-x. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KA, Walsh D, Abdullah O, McDonnel F, Homsi J, Komurcu S, LeGrand SB, Zhukovsky DS. Common complications of advanced cancer. Semin Oncol. 2000;27(1):34–44. [PubMed] [Google Scholar]
- 19.Fierabracci P, Pinchera A, Miccoli P, Conte PF, Vignali E, Zaccagnini M, Mancocci C, Giani C. Increased prevalence of primary hyperparathyroidism in treated breast cancer. J Endocrinol Invest. 2001;24(5):315–320. doi: 10.1007/BF03343867. [DOI] [PubMed] [Google Scholar]
- 20.Esbrit P. Hypercalcemia of malignancy: New insights into an old syndrome. Clin Lab. 2001;47(1–2):67–71. [PubMed] [Google Scholar]
- 21.Francini G, Petrioli R, Maioli E, Gonnelli S, Marsili S, Aquino A, Bruni S. Hypercalcemia in breast cancer. Clin Exp Metastasis. 1993;11(5):359–367. doi: 10.1007/BF00132979. [DOI] [PubMed] [Google Scholar]
- 22.Godsall JW, Burtis WJ, Insogna KL. Nephrogenous cyclic AMP, adenylate cyclasestimulating activity, and the humoral hypercalcemia of malignancy. Recent Prog Horm Res. 1986;42:705–750. doi: 10.1016/b978-0-12-571142-5.50021-5. [DOI] [PubMed] [Google Scholar]
- 23.Budayr AA, Nissenson RA, Klein RE. Increased serum levels of parathyroid hormone like protein in malignancy associated hypercalcemia. Ann Inter Med. 1989;11:807–812. doi: 10.7326/0003-4819-111-10-807. [DOI] [PubMed] [Google Scholar]
- 24.Isidoro C, Démoz M, De Stefanis D, Baccino FM, Bonelli G. High levels of proteolytic enzymes in the ascitic fluid and plasma of rats bearing the Yoshida AH-130 hepatoma. Invasion Metastasis. 1995;15:116–124. [PubMed] [Google Scholar]
- 25.Sinadinoviæ J, Cvejiæ D, Savin S, Miæiæ JV, Janæiæ-Zguricas M. Enhanced acid protease activity of lysosomes from papillary thyroid carcinoma. Cancer. 1989;63(6):1117–1182. doi: 10.1002/1097-0142(19890315)63:6<1179::aid-cncr2820630623>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Chao TY, Yu JC, Ku CH, Chen MM, Lee SH, Janckila AJ, Yam LT. Tartarate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res. 2005;11:544–550. [PubMed] [Google Scholar]
- 27.Harris JR, Lipmann ME, Veronesi U, Willett W. Breast cancer (1) N Engl J Med. 1992;327:319–328. doi: 10.1056/NEJM199207303270505. [DOI] [PubMed] [Google Scholar]
- 28.Friedman LS, Martin P, Munoz SJ. Liver Function tests and the objective evaluation of the patient with liver disease. In: Zakim D, Boyer T D, editors. Hepatology: a Textbook of liver diseases. Philadelphia: WB Saunders; 1996. pp. 791–833. [Google Scholar]
- 29.Fishman WH, Inglis NI, Stolbach LL, Krant MJ. A serum alkaline phosphatase isoenzyme of neoplastic cell origin. Cancer Research. 1968;28:150–154. [PubMed] [Google Scholar]