Abstract
Background
Pyogenic osteomyelitis is still frequently seen in the developing world and the treatment of chronic osteomyelitis presents a considerable challenge despite advances in microbiological techniques, antibiotics and surgical techniques. Acute haematogenous osteomyelitis is commoner in children.
Results
In the pre-antibiotic era, mortality rate was high and progression to chronic osteomyelitis was common. A near similar scenario still exists in many developing countries due to the combination of inappropriate and/or inadequate antibiotic therapy, delayed presentation and unorthodox interventions by traditional healers.
Discussion
Chronic osteomyelitis may result from poorly treated or untreated acute osteomyelitis, open fractures, surgery for an array of orthopaedic conditions and from contiguous spread from infected soft tissue as may occur in diabetic foot infections. A large array of treatment techniques hinged on sequestrectomy/ debridement, management of dead space, improvement of oxygenation and perfusion to ischaemic tissue exist. Despite these, total eradication of disease is difficult.
Conclusion
This article summarizes the pathology and methods of management available for pyogenic osteomyelitis. In its acute and chronic forms, the disease is likely to remain prevalent in the developing world until issues of ignorance, poverty and prompt access to appropriate and efficacious medical care are addressed.
Introduction
Osteomyelitis is the progressive infection of bone and bone marrow by micro-organisms, resulting in inflammatory destruction of bone, bone necrosis and new bone formation.1,2 Bone infections differ with regard to duration, etiology, pathogenesis, extent of bone involvement and class of host1,3,4 and may result from bone infection by pus-forming bacteria or other organisms such as mycobacteria. This discourse will be limited to pyogenic osteomyelitis. Acute osteomyelitis is a suppurative infection of bone and bone marrow accompanied by oedema, vascular congestion and small vessel thrombosis.2 It is mainly a disease of children2,3,4. Chronic osteomyelitis exists in the presence of any of the following conditions:5
Infection lasting 6 weeks or more (with radiologic evidence)
Sequestrum formation or sclerosis
Relapse or persistence after initial treatment of acute osteomyelitis
Osteomyelitis associated with foreign bodies
Osteomyelitis associated with peripheral vascular disease
Osteomyelitis from organisms that produce chronic, indolent disease e.g Mycobacterium tuberculosis.
In the pre-antibiotic era, the mortality rate of acute haematogenous osteomyelitis in children was about 50%4,6,7 due to overwhelming sepsis and metastatic abscesses7. Chronic osteomyelitis was the common sequelae in those who survived. Despite advances in antibiotics and operative techniques, osteomyelitis remains an Orthopaedic challenge and expensive to treat especially in developing countries.
Pathology of bone infections
Micro-organisms reach the bone by haematogenous spread, direct inoculation or from a contiguous focus of infection.1 The bloodstream may be invaded from a skin abrasion, boil or infected umbilical cord.8 Causes of direct inoculation include penetrating injuries, open fractures and surgical contamination. Contiguous focus osteomyelitis may occur in diabetics with spread from soft tissues to bone.
The commonest causative organism is Staphylococcus aureus. In acute osteomyelitis, different organisms predominate in different age groups. Staphylococcus aureus, Streptococcus agalactiae and Escherichia coli are the most frequent causes ofacute haematogenous osteomyelitis in infants. Staphylococcus aureus, Streptococcus pyogenes and Haemophilus influenzae are common in children below the age of four years. In those regions where the Haemophilus influenzae vaccine is given to children, the incidence of Haemophilus influenzae as a cause of osteomyelitis has decreased.1 In patients with immunocompromise, unusual organisms are commonly isolated. Patients with sickle cell disease are prone to Salmonella infection. However Staphylococcus aureus remains the commonest causative organism of acute and chronic osteomyelitis in these patients in Africa and the Middle East.9 When Osteomyelitis results from direct inoculation or contiguous spread, multiple organisms are usually isolated.1
In haematogenous Osteomyelitis, organisms usually settle in the metaphysis. This predilection has been attributed to the arrangement of blood vessels in the metaphysis where non-anastomosing terminal branches of the nutrient artery twist back in hairpin bends and flow into a large network of sinusoidal veins.8 The resultant slowing of blood flow favours bacterial colonization. However, in sickle cell disease, long bone diaphysis is a common site for osteomyelitis.
When infection has occurred, inflammation, suppuration, necrosis, reactive new bone formation, resolution and healing are the usual pattern of progress. Variations of the pathological picture are influenced by patient's age, site of infection, virulence and host defence responses. Underlying the pathologic processes are exudation, vascular congestion and intraosseous hypertension, intravascular thrombosis with occlusion of blood flow.
In acute haematogenous osteomyelitis, inflammation is followed by suppuration which may be evident by the 2nd or 3rd day8. Pus tracks within the Volkmann canals to subsequently form a subperiosteal abscess with periosteal stripping. The combination of intraosseous hypertension, vascular stasis, thrombosis and periosteal stripping lead to necrosis of a bone segment (Sequestrum). Reactive new bone forms from the cambial layer of the stripped periosteum and is radiologically obvious by 10–14 days. If the process is aborted by appropriate antibiotics and surgical drainage, resolution and healing will occur.1 If infection persists, cloacae will result to allow egress of pus from the bone and chronic osteomyelitis is established characterized by the presence of a Sequestrum (dead bone) surrounded by an involucrum (new bone formation) with cloacae (perforations in the new bone). It may also be characterized by sclerosis without the classical sequestrum, involucrum and cloacae.
In osteomyelitis following inoculation, bacteria adhere to bone extracellular matrix and surgical implants. Staphylococci possess a large variety of adhesive proteins and glycoproteins which mediate binding to bone via receptors to fibronectin and other structural proteins.5,6 They cause osteolysis by interaction of bacterial surface components with immune system cells leading to cytokine production. Bacteria elude antibiotics and host defences by lowering their metabolic rates, formation of a glycocalyx coat and “hiding” intracellularly7.
There is evidence that the presence of Orthopaedic implants induce a local polymorphonuclear cell defect7, and a surface negative charge of devitalized bone or metal implant promotes bacterial adherence and glycocalyx formation. With progression, the site becomes surrounded by dense fibrous tissue. This lowers perfusion, oxygen tension and antibiotic bioavailability to the area and perpetuates the infection
Classification
Two classification systems are commonly used.1,3 The Waldvogel classification is based on aetiology and duration, and has little clinical use.1 The Cierny-Mader classification is based on two criteria: 1) Anatomy of the bone infection: 2) the physiologic class of the host.1,3
Waldvogel classification
Haematogenous osteomyelitis
Osteomyelitis secondary to contigous focus of infection
No generalized vascular disease
Generalised vascular disease
Chronic osteomyelitis (Necrotic bone)
Cierny-Mader classification
Anatomic types
Type I: Endosteal or medullary osteomyelitis
Type II: Superficial osteomyelitis limited to the surface
Type III:Localized, well marked lesion involving the entire cortical thickness with sequestration and cavity formation
Type IV: Diffuse Osteomyelitis
Physiologic classes of host
Class A: Normal, otherwise healthy host
Class B: Compromised host with local, systemic or combined deficiencies
Class C: Severely compromised host such that the radical treatment necessary will have an unacceptable risk — benefit ratio.
Clinical presentation
Acute Haematogenous osteomyelitis
Fever, irritability, lethargy, and signs of local inflammation — swelling, warmth, redness, pain as well as refusal to move the limb or allow the limb to be handled are the presenting features in children with acute haematogenous osteomyelitis.8 However, when modified by antibiotics, symptoms may be vague. Adults with primary or recurrent haematogenous osteomyelitis may present with nonspecific pain and low-grade fever. Occasionally, acute features of fever, chills, swelling and local redness and warmth may be present.
Chronic osteomyelitis
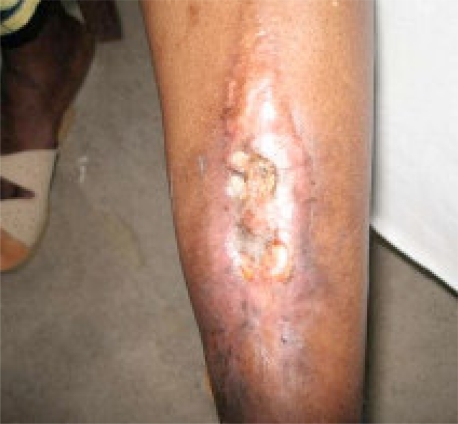
The main presenting features are long standing (chronic) discharging sinuses (Fig. 1) and/or chronic bone pain. There is usually a history of acute osteomyelitis in the past, trauma or surgery for fracture treatment. Occasionally there may be a “flare phenomenon” characterized by acute exacerbations of pain, swelling and redness of the region involved. Other associated features in long-standing cases include muscle wasting and joint contractures. A deep boring bone pain may be the only presentation in Brodie's abscess.
Figure 1.
Chronic osteomyelitis of the leg with discharging sinuses
Radiological investigations
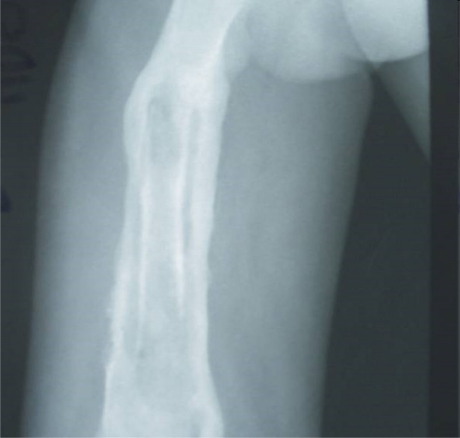
1. Plain X-Rays: In acute osteomyelitis, plain X-ray features are often non-specific in the early stages. By the 10th – 14th day, an extra-cortical outline denoting periosteal new bone formation is usually visible. In chronic osteomyelitis, a sequestrum surrounded by an involucrum gives a typical “bone-in-bone” picture (Fig. 2). Perforations through the involucrum envelop may be visible. In some instances, the typical “bone-in-bone” picture may be absent and the infected area merely appears densely sclerotic with surrounding regional osteoporosis.
Figure 2.
X-Ray of chronic osteomyelitis of the femur with “bone-in-bone” appearance
2. Computerized Tomography (CT) is more useful in chronic osteomyelitis for identification of sequestra. In acute and chronic osteomyelitis it is useful for image guided biopsy. CT shows changes earlier than conventional X-rays and can show lesions in the medullarycavity.
3. Magnetic Resonance Imaging (MRI) is very sensitive even in the early phase of bone infections. It can differentiate between soft tissue infections and osteomyelitis. MRI findings are due to the replacement of marrow fat by water. The typical finding is a reduced signal intensity on T1 and T2 weighted images. It may also show a well-defined rim of high signal intensity surrounding the focus of active disease (rim sign).10
4. Scintigraphy involves the use of radioactive chemicals. The commonest agent used is Technetium-99m methylene diphosphonate. Technetium-99 scintigraphy demonstrates increased intensity of uptake in affected sites. Other agents used include Gallium-67 and Indium-III labeled leucocytes, 2-(Fluorine 18)-Fluro-2 deoxy — D — glucose positron emission tomography (FDG PET) and 99 Technetium sulphur — colloid scintigraphy. Technitium-99 bone scintigraphy can be positive within 24–48 hours of onset of symptoms of acute osteomyelitis.6 Decreased uptake (“cold” scans) can occur in the early stages of infection. Such “cold” scans reportedly have a positive predictive value of 100% compared with 83% for hot scans11,12, and are due to the relative ischaemia caused by increased pressure from the presence of purulent material.6 Gallium — 67 scanning is probably more useful because it has a proven role in monitoring treatment after surgery.9
5. Ultrasonography is cheap, non-invasive, relatively available and has no risk for radiation exposure. It can localize subperiosteal fluid4 collections prior to their appearance on plain X-rays. However, it lacks specificity, cannot image the marrow or show cortical detail and is operator dependent. It has very limited use in bone infections.
Non-radiological investigations
These can be grouped as specific and non-specific modalities. The specific modalities are tissue and/or swab specimens obtained from surgery, aspirates or discharging sinuses for microbiological culture and sensitivity assays. The non-specific modalities include a Full Blood Count, (FBC) Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Blood culture and sinus tract cultures for microscopy, culture and sensitivity.4
The leucocyte count is elevated in acute osteomyelitis but is often normal in chronic cases.1 The ESR and CRP are non-specific inflammatory indices which are raised in acute and chronic bone infections. A serial drop in these indices denotes a positive response to treatment. The CRP is a more sensitive parameter than the ESR.1
Diagnosis and identification of aetiologic organisms in bone infection is dependent on microbiology. Culture specimens are bone (currettings of marrow or sequestrum), blood, aspirate from the bone in acute cases and sinus tract swabs/currettings in chronic infections. Ideally, culture specimens should be taken before antibiotics are commenced. In practice however, empirical antibiotics may be commenced before culture specimens are taken. In acute osteomyelitis, an aspirate microscopy and Gram-stain alone is a useful guide to the best-guess choice of antibiotics when empirical therapy is started. Conventional microbiological techniques are usually used for diagnosis. Standard bacterial cultures may however give low yield and improved molecular techniques are useful for detection and speciation of micro-organisms.1 These techniques include lysis-centrifrigation techniques, polymerase chain reaction and labeled oligonucleotide probe/monoclonal antibody binding followed by detection.1,6 These molecular methods are more sensitive than standard culture techniques.6
Other laboratory tests useful when indicated include serum creatinine level and liver function test (to monitor drug toxicity), blood glucose levels and other tests for co-morbidities and nutritional assessment (Serum albumin level and total iron binding capacity).
Treatment
Acute haematogenous osteomyelitis
The treatment of acute osteomyelitis is mainly nonoperative. Surgery is indicated only for drainage of pus. Treatment may be considered under 4 aspects: supportive treatment for pain and dehydration, splintage, antibiotics therapy and surgical decompression.8
Analgesics and fluids are used for pain and dehydration, the limb is splinted for comfort andto prevent contractures and antibiotics are commenced empirically. Drugs can be changed when culture and sensitivity results become available. The duration and routes of antibiotic therapy have traditionally been 1 to 2 weeks intravenously followed by 3 to 6 weeks of oral therapy. Some literature suggest a shorter duration of therapy is efficacious.13 Generally however, sequential intravenous — oral therapy is the accepted standard. Appropriate intravenous therapy should be continued until there is clinical improvement and the CRP levels approach normal. Oral therapy is then commenced and continued until the ESR normalizes.6
Ideally, antibiotic therapy is based on culture and sensitivity results. Treatment must however be started before the results become available. The choice of drugs is based on the organisms that predominate within the age of the patient in the locality. Flucloxacillin, Fusidic acid and Cefazolin have activity against Staphylococcus aureus and are used in older children and adults with no pre-morbid medical conditions.3,4,8 Other drugs active against Staphylococcus aureus and with good bone penetration can also be used. Children under 4 years of age will benefit from Cephalosporins or Amoxycillin and Clavulanic acid combinations to cover Staph, Haemophilus and Gram negative infections. Patients with sickle cell disease should be offered antibiotics that are active against Salmonella organisms. Such agents include Chloramphenicol, Co-trimoxazole or Amoxycillin-Clavulanic acid combination. A wide range of organisms have been reported as causative agents for osteomyelitis in drug addicts and patients with the Human Immunodeficiency Virus(HIV) infection. These individuals should be started with broad spectrum agents such as newer generation Cephalosporins or Gentamycin and Flucloxacillin combination therapy. Neonates with osteomyelitis are not treated with the sequential intravenous-oral regimen. Serious permanent complications occur in 6–50% of affected children in this age group due to multiple sites of involvement (20–50% of cases) and concomitant septic arthritis. Neonates are also more prone to generalized sepsis, have less consistent oral antibiotic absorption and less predictable radiologic and serologic responses to treatment6. In neonates therefore, it is generally recommended that the entire course of treatment be intravenous.6
Early appropriate antibiotic therapy usually makes surgery unnecessary. If clinical features do not improve within 36 hours of commencement of antibiotic therapy or in the presence of signs of deep pus formation and pus aspiration, surgical drainage is indicated under appropriate anaesthesia.8 When pus is found and released, it is unnecessary to drill into the medullary cavity; if no pus is found, a few holes are drilled into the medullary cavity8 for decompression. The presence of an intramedullary abscess upon medullary drilling is an indication for a corticotomy to allow effective drainage. Splints are useful post operatively. When signs of infection abet, movements are encouraged and protected weight bearing allowed. Full weight bearing is allowed after 3–4 weeks.8
Chronic osteomyelitis
Surgery is the mainstay of treatment. The goal is eradication of infection with achievement of a viable and vascular environment. The principles of surgical treatment are:
1. Adequate debridement/sequestrectomy
2. Management of ensuing dead space
3. Management of bone loss and possible instability post-sequestrectomy.
4. Reconstruction of soft tissue defects.
5. Adjunctive antibiotic therapy.
Adequate sequestrectomy and curettage offers the only real chance for disease eradication. Different options for managing ensuing dead space include open bone grafting (Papineau technique), antibiotic impregnated beads, closed suction drains (Lautenbach technique), biodegradable antibiotic delivery systems such as ceramics, soft tissue (muscle flap) transfer and continuous irrigation. Antibiotic-impregnated bone grafts have also been described14,15,16 and Alendronate (a Bisphosphonate) has been reported to enhance antibiotic impregnated bone grafts in the treatment of experimental osteomyelitis. Alendronate acts by stimulation of the proliferation and maturation of osteoblasts at the local infection site.14 Clinical reports on biodegradable antibiotic delivery vehicles are few16. The Ilizarov external fixator is indicated in refractory disease and allows radical resection of infected bone and subsequent bone transport in the management of instability and bone loss. Adjunctive treatment for chronic osteomyelitis includes the use of hyperbaric oxygen at 2 atmospheres for two hours daily for six days of each week, silver ion dressings and antibiotics.
The duration of post-operative antibiotic therapy in chronic osteomyelitis is unsettled. Traditionally, treatment recommendations are six weeks parenteral (induction) followed by 2–3 months of appropriate oral therapy (consolidation).1,4,10 Some workers have recommended a 4–6 weeks total duration of treatment arguing that there are no advantages with prolonged treatment.1 The choice of antibiotic is best based on the outcome of marrow currettings/sequestral culture and sensitivity results. The duration of treatment is based on the response of the C-reactive protein and Erythrocyte sedimentation rates. When operative treatment of chronic osteomyelitis is not feasible, suppressive antibiotic therapy to control disease and prevent flare-ups is indicated.
Suppressive therapy should be culture and sensitivity directed, usually administered orally for 6 months but may become life long. Ideal drugs must possess good bioavailability, low toxicity and adequate penetration of bone. Rifampicin in combination with other drugs is commonly used. Suppressive therapy has been found useful only in chronic osteomyelitis with implants in-situ, its place in chronic osteomyelitis without implants in-situ has not been determined1. Amputation though generally unaccepted in many cultures17 is reserved for chronic osteomyelitis with severe deformities incompatible with reconstruction, useful or cosmetic function. It may be the only option followed by prosthetic fitting to return the patient to a productive life.
Special considerations
1. Implant related infection
Bone infections with Orthopaedic implants in situ present special considerations. In prosthesis-related bone infections, micro-organisms have been reported to grow in a glycocalyx biofilm adherent to the surface of biomaterials and may be more resistant to host defences and antimicrobials.16 The question is if the implant should be removed or left in situ. The decision is guided by factors like stage of fracture healing, stability of the construct, location of fracture, duration of time post surgery,19 and type of implant. Generally, if the fracture has healed, the implant is removed. When the fracture has not healed but the construct is stable, the implant is left in-situ and antibiotic treatment commenced to allow for healing. When the construct is unstable, the implant is removed, debridement performed and restabilization secured either with another implant or external fixation. When osteomyelitis has followed plate and screw osteosynthesis and the implants have to be removed, replacement is preferably done using intramedullary devices or external fixation. External fixators however run the risk of pin loosening, infection and muscle transfixation.
When osteomyelitis follows an intramedullary device, exchange nailing can be performed either immediately post-debridement or as a delayed procedure following debridement of intramedullary infection, a period of traction and parenteral and/or local antibiotic therapy.
2. Spondylitis
Pyogenic spondylitis is the infection of the extradural components of the spine with involvement of the vertebral body and intervertebral disc.20 Pyogenic spinal infection may be considered as a spectrum of diseases including spondylitis, discitis, spondylodiscitis, pyogenic facet joint arthropathy and epidural abscess.21 The vertebrae are involved in 0.15–3.9% of all osteomyelitic infections with pyogenic infections accounting for 1%.22,23 The condition is commoner in adults with peak ages between 30–65 years; commoner in men(male :female ratio 3.3:1.5) and the sites of predilection are thoracolumbar region, lumbar spine, thoracic spine and cervical spine in that order.22,23,24 The route of infection is commonly haematogenous and infection usually begins from the vertebral body and subsequently spreads to the disc because the disc is avascular. Disc involvement and destruction is the distinguishing feature between spinal infection and (metastatic) spinal tumours;25 and aids in the differentiation of both conditions in diagnostic imaging. Other routes of infection are contiguous spread, iatrogenic and post-traumatic causes.20,23 The risk factors for spondylitis include spine surgery, invasive manipulations in the urinary and digestive tracts, intravenous drug use and intravenous catheters, diabetes mellitus, malnutrition and end stage renal failure.23,24
The presenting features are pain, spinal tenderness, (low grade) fever, elevated ESR and CRP. MRI is the most accurate diagnostic imaging modality. Plain x-rays usually show a destructive lesion involving the vertebral body and intervertebral disc. A histobacteriological diagnosis can be obtained by transpedicular needle biopsy.20,23,24 Treatment consists of antibiotics for a minimum of 12 weeks, laparoscopic drainage and biopsy, bed rest and bracing.22,23 In late cases with vertebral destruction, progressive neurologic deficits and abscess formation, surgical debridement and stabilization of the spine is indicated.21 The commonest complication is paralysis.22
Complications
Acute haematogenous osteomyelitis
Septic arthritis, septicaemia, metastatic infection, altered bone growth and limb-length discrepancy, recurrent infection, pathologic fractures, angular deformity and chronic osteomyelitis.
Chronic Osteomyelitis
Limb deformities, limb-length inequality, pathologic fractures, malignant transformation (relatively rare), compartment syndrome and Volkmann contracture, chronic renal failure, loss of self esteem and depression, secondary amyloidosis leading to nephrotic syndrome.
Conclusion
Bone infections are severe disorders. Appropriate antibiotic therapy can prevent progression from acute to chronic disease. Late presentation, open fractures, surgery and injudicious antibiotic therapy for acute osteomyelitis predispose to chronic osteomyelitis which remains a continuing orthopaedic challenge in developing countries25,26. Underpinning these factors in the developing world are poverty, ignorance and lack of access to appropriate medical care.
References
- 1.Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg (Am) 2004;86:2305–2318. doi: 10.2106/00004623-200410000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Mader J, Mohan D, Calhoun J. A practical guide to the diagnosis and management of bone and joint infections. Drugs. 1997 Aug;54(2):253–264. doi: 10.2165/00003495-199754020-00004. [DOI] [PubMed] [Google Scholar]
- 3.Carek PJ, Dickerson LM, Sack JL. Diagnosis and management of Osteomyelitis. Am Fam Physician. 2001;63:2413–2420. PubMed. [PubMed] [Google Scholar]
- 4.Mousa H. BoneInfection. Review. [14 February, 2009];East Med Health J. 2003 9(1/2) URL: http://www.emro.who.int/publications/emhj/0901_2/bone.htm. [Google Scholar]
- 5.Sexton D, McDonald M. Osteomyelitis: approaching the 1990s. Med J of Australia. 1990;153:91–96. [PubMed] [Google Scholar]
- 6.Song KM, Slobada JF. Acute haematogenous osteomyelitis in children. J Am Acad Orthop Surg. 2001 May–Jun;9(3):166–175. doi: 10.5435/00124635-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ciampolini J, Harding JG. Pathophysiology of chronic bacterial osteomyelitis. Why do antibiotics fail so often? Postgrad Med J. 2000 Aug;76:479–483. doi: 10.1136/pmj.76.898.479. [MEDLINE] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon L, Warwick D, Nayagam S, editors. Apley's system of Orthopaedics and fractures. 8. London: ARNOLD Publishers; 2001. pp. 27–49. [Google Scholar]
- 9.Thanni LOA. Bacterial osteomyelitis in major sickling haemoglobinopathies: geographic difference in pathogen prevalence. Afr Health Sciences. 2006;6(4):236–239. doi: 10.5555/afhs.2006.6.4.236. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan AN, Garimella V, Macdonald S. Osteomyelitis, Chronic. 2004. May 25, MEDLINE continuing Medical Education, section 1–12. [Google Scholar]
- 11.Mardell GA. Imaging in the diagnosis of musculoskeletal infections in children. Curr Probl Padiatr. 1996;26:218–237. doi: 10.1016/s0045-9380(06)80060-5. PubMed (MEDLINE) [DOI] [PubMed] [Google Scholar]
- 12.Tuson CE, Hoffman EB, Mann MD. Isotope bone scanning for acute osteomyelitis and septic arthritis in children. J Bone Joint Surg Br. 1994;76:306–310. [MEDLINE] [PubMed] [Google Scholar]
- 13.Peltola H, Unkila-Kallio L, Kalio MJT, Finnish study Group Simplified treatment of acute staphylococcal osteomyelitis of childhood. Pediatrics. 1997;99:846–850. doi: 10.1542/peds.99.6.846. PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Ozturk AM, Tabak AY, Aktekin CN, Altay M, Erdemli E, Karahuseyinoglu S, Korkusuz F. Alendronate enhances antibiotic-impregnated bone grafts in the treatment of osteomyelitis. Int Orthop(SICOT) 2008;32:821–827. doi: 10.1007/s00264-007-0396-8. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CE, Ko JY, Pan CC. Results of vancomycin-impregnated cancellous bone grafting for infected tibial non-union. Arch Orthop Trauma Surg. 2005;125(6):369–375. doi: 10.1007/s00402-005-0794-6. [DOI] [PubMed] [Google Scholar]
- 16.Dabov GD. Osteomyelitis. In: Canale ST, Beaty JH, editors. Campbell's Operative Orthopaedics. 11th Edition. PHILADELPHIA: MOSBY ELSEVIER; 2008. pp. 695–722. Chapter 16. [Google Scholar]
- 17.Udosen AM, Ikpeme IA, Etiuma A, Egor S. Major Amputation at the University of Calabar Teaching Hospital. Nig J Surg Sciences. 2004;14(2):60–63. PubMed. [Google Scholar]
- 18.Patzakis MJ. Management of Acute and Chronic Bone infections. In: Chapman MW, editor. Chapman's Orthopaedic Surgery. 3rd Edition. CD Lippincott Williams and Wilkins; 2001. Chapter 133, ONLINE. [Google Scholar]
- 19.Ben Taarit CH, Turki S, Maiz HB. Infectious spondylitis: a review of 151 cases. Acta Orthop Belgica. 2002;68(4):381–386. PubMed. [PubMed] [Google Scholar]
- 20.Lai P, Leu H, Niu C, Chen W, Chen L. Pyogenic spondylitis presenting with skip lesions. Chang Gung Med J. 2005;28:651–656. [PubMed] [Google Scholar]
- 21.Camilo FX. Infections of the Spine. In: Canale ST, Beaty JH, editors. Campbell's Operative Orthopaedics. 11th Edition. PHILADELPHIA: MOSBY ELSEVIER; 2008. pp. 2237–2272. Chapter 40. [Google Scholar]
- 22.Ahn JS, Lee JK. Diagnosis and treatment of Tuberculous spondylitis and Pyogenic spondylitis in Atypical cases. Asian Spine J (ASJ) 2007;1(2):75–79. doi: 10.4184/asj.2007.1.2.75. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Nammari SS, Lucas JD, Lam KS. Haematogenous Methicillin-Resistant Staphylococcus Aureus Spondylodiscitis. Spine. 2007;32(22):2480–2486. doi: 10.1097/BRS.0b013e318157393e. PubMed. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Lee K, Park E, Riew KD. Fas/FasL interaction of nucleus pulposus and cancer cells with the activation of caspases. Int Orthop(SICOT) 2008;32:835–840. doi: 10.1007/s00264-007-0410-1. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Museru LM, Mcharo CN. Chronic Osteomyelitis: A continuing Orthopaedic challenge in developing countries. Int Orthop. 2001;25(2):127–131. doi: 10.1007/s002640100239. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onuba O. Coping with osteomyelitis. Africa Health. 1992 Nov;15(1):27–28. PubMed. [PubMed] [Google Scholar]