Abstract
Drug resistance is a central challenge of cancer therapy that ultimately leads to treatment failure. In this study, we characterized a mechanism of drug resistance that arises to AZD6244, an established mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) 1/2 inhibitor currently being evaluated in cancer clinical trials. AZD6244 enhanced the expression of transcription factor FOXO3a, which suppressed cancer cell proliferation. In AZD6244-resistant cancer cells, we observed the impaired nuclear localization of FOXO3a, reduced FOXO3a-mediated transcriptional activity, and decreased the expression of FOXO3a target gene Bim after cell treatment with AZD6244. Resistant cells could be sensitized by phosphoinositide 3-kinase (PI3K)/AKT inhibitors, which are known to enhance FOXO3a nuclear translocation. Our findings define FOXO3a as candidate marker to predict the clinical efficacy of AZD6244. Furthermore, they suggest a mechanism of resistance to MEK inhibitors that may arise in the clinic yet can be overcome by cotreatment with PI3K/AKT inhibitors.
Introduction
Constitutive activation of certain signal transduction cascades leads to the development of tumors and the resistance of tumors to clinical therapy (1, 2). Approximately 30% of tumors carry an activating mutation in the RAS oncoprotein (3–5). Mitogen-activated protein (MAP) kinase kinase 5 is an important effecter in the RAS/extracellular signal-regulated kinase (ERK) pathway where activation of RAS/ERK signaling is known to result in tumor proliferation, angiogenesis, and metastasis (3, 6). Thus, developing chemical inhibitors targeting the RAS pathway has become an important cancer therapeutic strategy (3, 6). AZD6244/ARRY-142886, a novel, orally active, potent, selective, and ATP-uncompetitive MAP/ERK kinase (MEK)1/2 inhibitor, targets the crucial MEK kinase in the RAS/ERK signaling pathway (7). A phase I clinical trial of AZD6244 showed promising results in solid tumors with the best clinical response in several heavily pretreated cancer patients (8). AZD6244 phase II clinical trials in various cancers, such as breast, lung, colorectal, liver, pancreatic cancers, and melanoma are either currently ongoing or recently completed (from the NIH Web site: http://www.Clinicaltrials.gov).
FOXO3a, a transcription factor in the FOXO family, is a crucial tumor suppressor. FOXOs are deregulated in several tumor types, including breast cancer, prostate cancer, glioblastoma, rhabdomyosarcoma, and leukemia (9, 10). As a transcription factor, FOXOs activate or repress multiple target genes, such as p27kip1 and cyclin D for cell cycle regulation, and Bim and FasL for inducing apoptosis (11–13). Loss of FOXO1a through chromosomal deletion (13q14) was shown to promote androgen-independent prostate cancers (14). In addition, cytoplasmic localization or downregulation of FOXOs through AKT, IKK, and ERK-mediated phosphorylation was observed in breast cancers (12, 13). Inhibition of FOXO3a expression and activity is critical to promote cell transformation, tumor progression, and angiogenesis (12, 13, 15). Therefore, FOXO family members have been proposed to be important factors influencing the efficacy of a variety of chemotherapeutic drugs. For example, the chemotherapeutic drugs paclitaxel (16, 17) and Akt/protein kinase B signaling inhibitor-2 (API-2)/Triciribine (AKT inhibitor; ref. 18), which are clinically used for the treatment of breast carcinoma and acute myeloid leukemia, can activate FOXO3a by reducing AKT activity.
Based on our previous finding of FOXO3a downregulation by ERK, we were intrigued to ask whether FOXO3a is an essential target for AZD6244-mediated cell cycle arrest and apoptosis. Indeed, we found that AZD6244 enhances G1 growth arrest and cell apoptosis through the downregulation of ERK phosphorylation and stabilization of FOXO3a in AZD6244-treated cancer cell lines and xenograft tumors in mice. In addition, knocking down FOXO3a and its downstream apoptotic gene Bim impaired AZD6244-induced growth suppression, suggesting that FOXO3a and Bim are essential targets of AZD6244. Furthermore, AZD6244-resistant cancer cells showed impaired endogenous FOXO3a nuclear translocation and reduced Bim activation. LY294002 and API-2, through restoring FOXO3a nuclear translocation and Bim activation, synergize with AZD6244 in suppressing proliferation and colony formation in AZD6244-resistant cells.
Development of cancer cell resistance to cancer therapeutics is a problem of clinical concern; therefore, it is of importance to understand the molecular mechanisms that contribute to drug resistance and to further identify the molecular targets for novel therapeutics that can overcome resistance. Previous reports suggested that cancer cells resistant to MEK inhibitors exhibit the activation of phosphoinositide 3-kinase (PI3K)/AKT signaling (19–21). These data are in concert with our results showing that FOXO3a is inactivated in AZD6244-resistant cells, which likely results from AKT activation. Our data shows that the combination therapy of AZD6244 with pharmacologic agents that enhance FOXO3a activity may effectively treat AZD6244-resistant cells by modulating FOXO3a activation and thereby converting an AZD6244-resistant cancer into an AZD6244-sensitive one. Ultimately, our study implicates that FOXO3a activation may be an essential pharmacologic indicator to predict AZD6244 efficacy in clinical use.
Materials and Methods
Reagents and plasmids
AZD6244 was provided by AstraZeneca as well as purchased from Selleck Chemicals. API-2 was purchased from Calbiochem. NVP-BEZ235 was purchased from Selleck Chemicals. Taxol was ordered from the Bristol-Myers Squibb Company through our institution. LY294002 was purchased from Sigma. We generated the green fluorescent protein (GFP)-FOXO3a construct in our previous study (12). The pSuper-FOXO3a vector was a gift from Dr. Alex Toker (Harvard Medical School, Boston, MA).
Cell culture, cell growth, MTT assay, and colony formation assay
All cell cultures were kept in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) at 5% CO2. The cell growth rate was determined with the MTT assay. Cells (3 × 103/well) were plated in 96-well culture plates in 0.2 mL of culture medium and allowed to adhere for 2 hours; 20 μL of MTT were then added to each well. Cells were cultured for an additional 2 hours and 100 μL of lysis buffer [(20% SDS in 50% N, N-dimethylformamide (pH 4.7)] was added. The cells were incubated for 4 hours and absorbance at 570 nm was measured. For the soft agar colony formation assay, 2 × 104 cells were placed in 1.5 mL of DMEM with 10% FBS and 0.3% agarose, and overlaid onto 3 mL DMEM with 10% FBS and 0.6% agarose in each well of a six-well plate. After 2 weeks, colonies larger than 20 μm in diameter were counted.
Immunohistochemical staining and immunoblotting
Immunohistochemical staining and immunoblotting were performed as previously described (13) with the following antibodies: FOXO3a (Santa Cruz Biotechnology), ERK (Upstate Biotechnology), p-ERK (Cell Signaling), p27, and Bim (Stressgen).
Real-time PCR
Total RNAs were extracted from cells by using RNeasy kit (Qiagen). RNAs were reverse transcribed by using SuperScript II kit (Invitrogen). Results were analyzed by the iCycler (Bio-Rad) real-time PCR and relative quantification of RNA levels normalized to glyceraldehyde-3-phosphate dehydrogenase as difference of cycling threshold (ΔCT) = CT (target) – CT (control). Higher CT values indicate relatively lower expression RNA levels. Bim primer was showed as previously described (13).
Chromatin immunoprecipitation analysis
Chromatin immunoprecipitations were modified from the EZ-CHIP (Upstate) protocol using antibody FOXO3a (Santa Cruz).
Cell cycle analysis
Cells were dissociated with trypsin, washed, and resuspended in PBS as a single-cell suspension. Cells were fixed in 70% ethanol overnight, stained with propidium iodide (25 μg/mL; Sigma), and incubated for 30 minutes at 37°C with RNase A (20 μg/mL). The DNA content of the cells was then evaluated by FACSCalibur (BD Immunocytometry Systems). Linear red fluorescence FL2 was analyzed.
Statistical analysis
All data were presented as means ± the SD of the mean. Statistical calculations were performed with Microsoft Excel analysis tools. Differences between individual groups were analyzed by paired t test. P values of <0.05 were considered statistically significant.
Results
Activation of FOXO3a by AZD6244 is essential for AZD6244-induced suppression of cancer cell proliferation
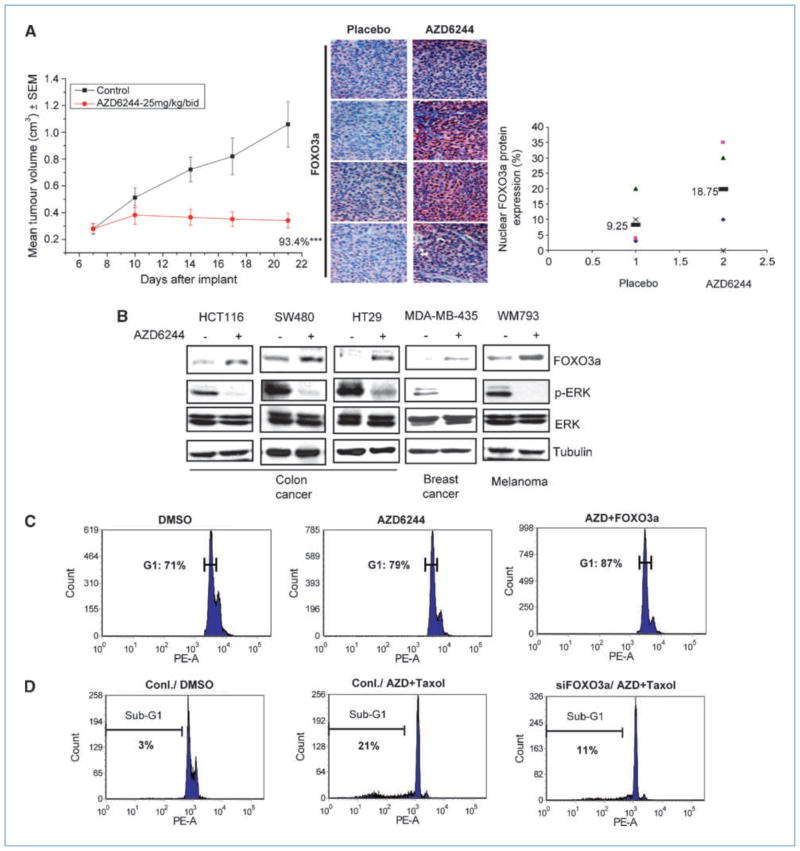
AZD6244 is known to promote cell cycle arrest and apoptosis through inhibiting ERK activation and testing in multiple clinical trials (from the NIH Web site: http://www.Clinicaltrials.gov). It is therefore critical to understand the detailed molecular mechanisms and downstream target genes responsible for its tumor suppression activity. Recently, inhibition of FOXO3a by ERK showed enhanced cell proliferation and tumorigenesis (13). Thus, we sought to determine whether AZD6244 might suppress tumor growth through restoring FOXO3a activity. We found that AZD6244 significantly suppresses HCT116 colon cancer xenograft tumor growth in vivo (Fig. 1A) and these AZD6244-treated colon cancer xenografts showed ~2-fold increased nuclear FOXO3a expression by immunohistochemistry staining (Fig. 1A). To further examine the effect of MEK inhibition on FOXO3a expression in vitro, we tested five different human cancer cell lines from three cancer types in which AZD6244 is currently used in phase I/II clinical trials. We found that AZD6244 significantly inhibits ERK activation and increases FOXO3a expression in all these cancer cell lines (Fig. 1B), in which cell cycle arrest and apoptosis are concurrently enhanced.
Figure 1.
AZD6244 enhanced FOXO3a expression and induced suppression of cancer cell proliferation. A, tumor volume of the HCT116 xenografts treated with Placebo or AZD6244 was measured for 21 d. The tumor sections of four individual DMSO or AZD6244-treated HCT116 xenografts were subjected to immunohistochemistry with a FOXO3a antibody. Relative percentages of nuclear FOXO3a expression of individual xenograft tumors from B were analyzed and the mean values of FOXO3a expression in Placebo or AZD6244-treated group were indicated as bars. B, lysates from various cancer cell lines: breast cancer (MDA-MB-435), colon cancer (HCT116, SW620, and HT29), and melanoma (WM793) treated with DMSO or AZD6244 (10 μmol/L) for 4 h were subjected to immunoblotting with the indicated antibodies. C, after transfection with a GFP vector or GFP-FOXO3a, MDA-MB-435 cells were treated with DMSO or AZD6244 (10 μmol/L) for 24 h and subjected to cell cycle analysis. D, MDA-MB-435 cells transfected with control siRNA or FOXO3a siRNA were treated with AZD6244 (10 μmol/L) for 24 h with or without Taxol and subjected to cell cycle analysis.
To further validate the effects of AZD6244 on cell cycle and apoptosis mediated through FOXO3a, we first ectopically expressed FOXO3a and found that AZD6244 enhances G1 cell cycle arrest (79% versus 71%), which was further increased by FOXO3a expression (79% increased to 87%; Fig. 1C). In addition to RAS/MEK/ERK, the PI3K/AKT pathway is also known to inhibit FOXO3a expression and transcriptional activity (11–13). We tested whether combining AZD6244 with PI3K/AKT pathway inhibitor LY294002 could sensitize cancer cells to growth suppression and apoptosis. Indeed, AZD6244 synergized with LY294002, leading to growth suppression (Supplementary Fig. S2A). In addition, Taxol is the first-line therapeutic drug for breast cancer patient treatment and has been shown to inhibit AKT, which results in FOXO3a activation (17). Thus, we also tested the killing effect with the combination of AZD6244 and Taxol. We found that AZD6244 also synergized with Taxol in apoptosis induction (Supplementary Fig. S1) and growth suppression (Supplementary Fig. S2B). In addition, FOXO3a was shown to be required for the AZD/Taxol-induced cell death as measured in the sub-G1 phase by knocking down FOXO3a (21% reduced to 11%; Fig. 1D). In addition, the ectopic expression of FOXO3a in FOXO3a −/− murine embryonic fibroblast cell led to a 5-fold increase in apoptosis by AZD6244/Taxol treatment (Supplementary Fig. S3). Because Bim is a proapoptotic molecule that is turned on by FOXO3a, we examined the roles of FOXO3a and Bim in AZD6244/LY294002- and AZD6244/Taxol-mediated growth suppression and apoptosis by knocking down FOXO3a and Bim using small interfering RNAs (siRNA; Supplementary Fig. S4A). Knocking down both FOXO3a and Bim substantially diminished their growth suppression effects with either single or combination agents of AZD6244/LY294002/Taxol (Supplementary Fig. S4B). Together, our data suggest that enhanced FOXO3a expression is essential for the sensitization of cancer cells to AZD6244-, AZD6244/Taxol-, and AZD6244/LY294002-induced growth suppression and apoptosis.
Impaired FOXO3a expression and activity contributes to cancer cell resistance in response to AZD6244 treatment
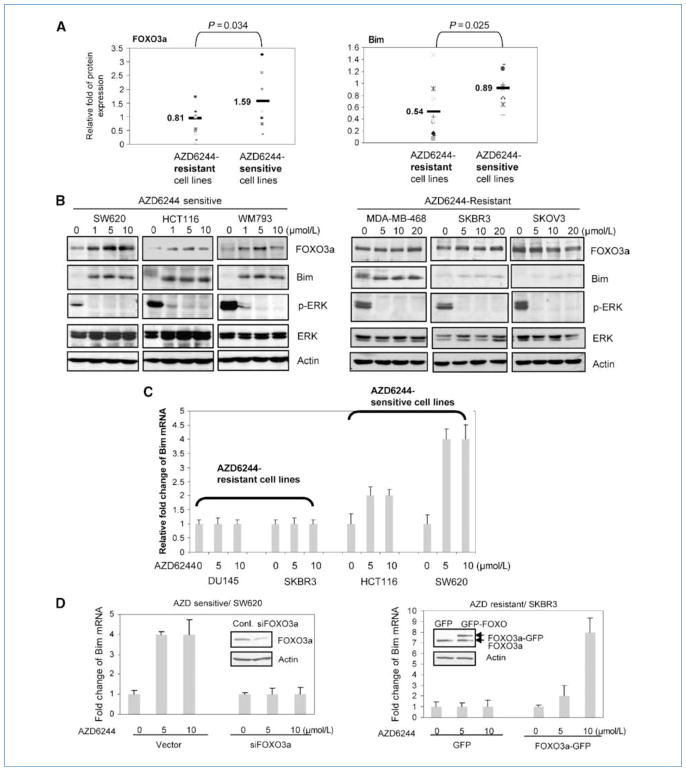
Many human cancer cell lines are resistant to MEK inhibition (7, 22). To further understand resistance to MEK inhibition, we tested whether differential FOXO3a and Bim expression could contribute to the variable sensitivity of human cancer cells toward AZD6244 treatment. We measured the protein expression of FOXO3a and its downstream gene Bim in 19 AZD6244-resistant and AZD6244-sensitive cancer cell lines, which have been described in a previous report (7). We found that AZD6244-sensitive cancer cell lines showed significantly higher FOXO3a and Bim protein levels than the resistant cell lines (FOXO3a P = 0.034 and Bim P = 0.025; Supplementary Fig. S5; Fig. 2A). To further explore whether FOXO3a and Bim expression are modulated by AZD6244, we treated both AZD6244-sensitive and AZD6244-resistant cells with a range of AZD6244 doses. We found that AZD6244 treatment effectively decreased p-ERK levels in AZD6244-sensitive and AZD6244-resistant cells. However, FOXO3a and Bim expression were readily induced in AZD6244-sensitive cells with 1, 5, and 10 μmol/L of AZD6244 (Fig. 2B), in which as AZD6244-resistant cells showed no significant FOXO3a and Bim induction even with up to 20 μmol/L (Fig. 2B). Next, we asked whether FOXO3a transcriptional activity is differently regulated in sensitive and resistant cell lines in response to AZD6244. We found that in AZD6244-sensititive cells, AZD6244 treatment induced up to a 4-fold increase in Bim mRNA but not in AZD6244-resistant cells (Fig. 2C). To further confirm that Bim induction was mediated through FOXO3a, we performed siRNA knockdown of FOXO3a, which significantly impaired Bim induction by AZD6244 in the AZD6244-sensitive SW620 cells (Fig. 2D). Consistently, enforced expression of wild-type FOXO3a restored the sensitivity of Bim induction by AZD6244 in the resistant SKBR3 cells (Fig. 2D). Together, the results suggest that FOXO3a activation is essential to mediate and predict the sensitivity of cancer cells toward AZD6244 treatment (Please also see Fig. 4B).
Figure 2.
AZD6244-resistant cancer cells show impaired FOXO3a activity and decreased Bim expression in response to AZD6244 treatment. A, lysates from AZD6244-sensitive cell lines and AZD6244-resistant cell lines were subjected to immunoblotting with FOXO3a and Bim antibodies. Relative fold of protein expressions were normalized by actin and the mean values of AZD-sensitive and AZD-resistant cells were indicated as bars. B, AZD6244-sensitive cell lines (HCT116, SW620, and WM793) and AZD6244-resistant cell lines (MDA-MB-468, SKOV3, and SKBR3) were treated with AZD6244 of indicated concentrations and the lysates were subjected to immunoblotting with indicated antibodies. C, AZD6244-sensitive cell lines (HCT116 and SW620) and AZD6244-resistant cell lines (DU145 and SKBR3) were treated with indicated concentrations of AZD6244 and the Bim mRNAs were subjected to real-time PCR analysis. D, after transfection with control siRNA or FOXO3a siRNA, SW620 cells were treated with AZD6244 of indicated concentrations for 6 h and subjected to real-time PCR analysis. After being transfected with GFP vector or GFP-FOXO3a, SKBR3 cells were treated with AZD6244 of indicated concentrations for 6 h and subjected to real-time PCR analysis. Bar graphs, mean values of the representative results from two experiments conducted in triplicates for each.
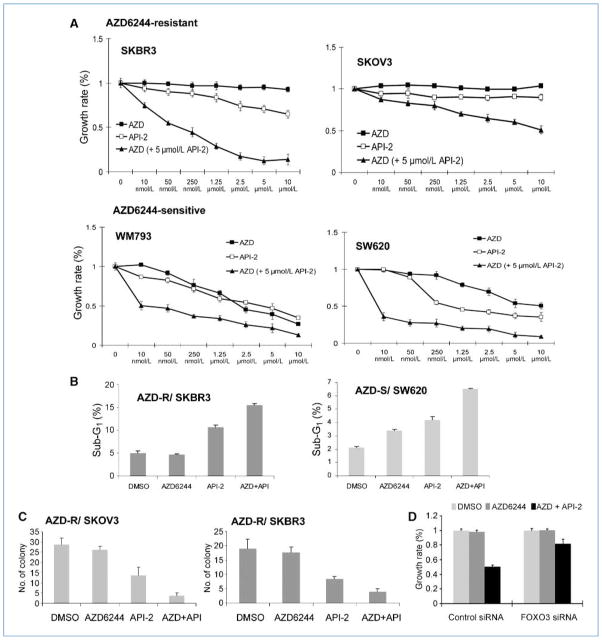
Figure 4.
API-2 synergizes with AZD6244, suppressing cell proliferation and colony formation in AZD6244-resistant cancer cells. AZD6244-resistant cells (A) SKBR3 and SKOV3, and AZD6244-sensitive cells WM793 and SW620, were treated with DMSO, AZD6244, API-2, or AZD6244 along with API-2 for 48 h and then subjected to MTT assays. B, SKBR3 and SW620 cells were treated with DMSO, AZD6244 (5 μmol/L), API-2 (5 μmol/L), or AZD6244 (5 μmol/L) along with API-2 (5 μmol/L) for 48 h and then subjected to propium iodide staining and analyzed for sub-G1 percentage. AZD6244-resistant cells (C) SKOV3 and SKBR3 cells were treated with DMSO, AZD6244 (10 μmol/L), API-2 (10 μmol/L), or AZD6244 (10 μmol/L), along with API-2 (10 μmol/L), while subjected to colony formation assays. Bar graphs, the mean values of the representative triplicate results from two experiments. D, MDA-MB-231 cells transfected with control or FOXO3a siRNA were treated with DMSO, AZD6244 (5 μmol/L), or AZD6244 (5 μmol/L), along with API-2 (5 μmol/L) for 48 h and then subjected to MTT assays. Bar graphs, the mean values of the representative triplicate results from two experiments.
Retarded endogenous FOXO3a nuclear translocation and reduced FOXO3a-Bim promoter association lead to impaired sensitivity to AZD6244 treatment
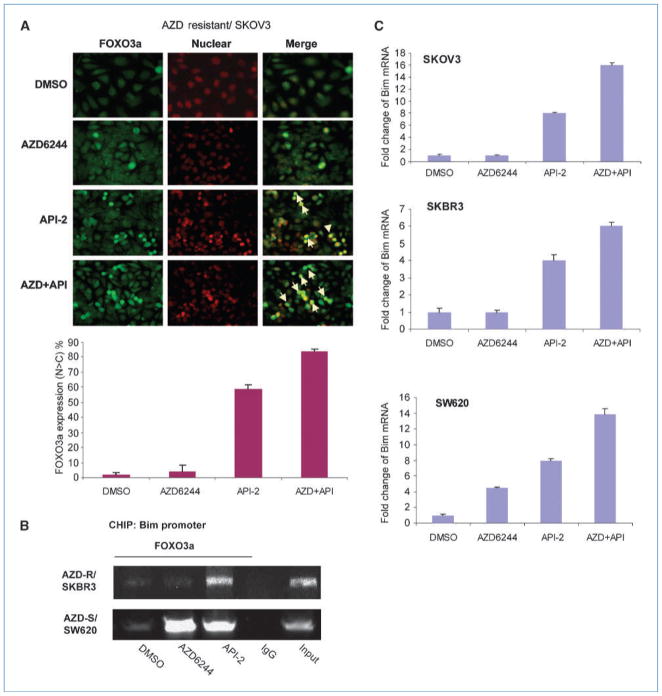
To further understand the molecular mechanism of the impaired FOXO3a activation in AZD6244-resistant cells in response to AZD6244, we examined FOXO3a cellular localization under fluoresence microscopy. We found that FOXO3a was primarily localized in the cytoplasm when treated with AZD6244 in the AZD6244-resistant SKOV3 (Fig. 3A), in which FOXO3a was not able to associate with the Bim promoter by chromatin immunoprecipitation analysis (Fig. 3B, lane 2) nor was Bim mRNA induced following AZD6244 treatment (Fig. 3B). These results also correspond to previous data and may explain why FOXO3a activity was impaired in AZD6244-resistant cells as shown in Fig. 2B and C (Bim protein and mRNA expression). Interestingly, FOXO3a nuclear localization in AZD6244-resistant cells was increased under the treatment of LY294002 (PI3K inhibitor; Supplementary Fig. S6). A similar result was also observed by treating AZD6244-resistant cells with API-2, an AKT inhibitor currently used in clinical trials (Fig. 3A and B). API-2 also significantly enhanced the binding of FOXO3a to the Bim promoter in AZD6244-resistant cells (Fig. 3C, lane 3). Thus, AZD6244 is not able to induce FOXO3a nuclear localization and activate FOXO3a in AZD6244-resistant cells. However, PI3K/AKT inhibitors can still activate FOXO3a by increasing its nuclear localization. As expected, in the AZD6244-sensititive SW620 cells, FOXO3a expression was readily increased in the nuclear fraction (Supplementary Fig. S7) and bound to Bim promoter under either AZD6244 or API-2 treatment (Fig. 3B). It is worthy to note that AZD6244 treatment increased Bim mRNA up to 4-fold in the AZD6244-sensitive SW620 cell line but had no effect on Bim mRNA expression in the two resistant cell lines, SKBR3 and SKOV3 (Fig. 3C). Moreover, combination of API-2 and AZD6244 was able to increase FOXO3a nuclear relocalization (Fig. 3A), and thus, Bim mRNA induction was enhanced in both AZD6244-sensitive/resistant cells (Fig. 3C). These data suggest that FOXO3a failing to translocate to the nucleus may contribute to impaired Bim activation and AZD6244 resistance. Pharmacologic agents, such as API-2, which are able to relocalize FOXO3a to the nucleus and thereby restore FOXO3a activity, could reverse AZD6244 resistance and promote the efficacy of AZD6244 treatment.
Figure 3.
AZD6244-resistant cancer cells show retarded endogenous FOXO3a nuclear translocation and reduced Bim promoter association in response to AZD6244 treatment. A, AZD6244-resistant SKOV3 cells were treated with DMSO, AZD6244 (10 μmol/L), API-2 (10 μmol/L), or AZD6244 (10 μmol/L) along with API-2 (10 μmol/L) for 24 h and then subjected to immunoflourescence analysis. Bar graphs, the percentages of cells with high nuclear FOXO3a expression from two independent immunoflourescence experiments. B, AZD6244-sensitive SW620 cells and AZD6244-resistant SKBR3 cells were treated with DMSO, AZD6244 (10 μmol/L), or API-2 (10 μmol/L) for 6 h and then subjected to chromatin immunoprecipitation analysis. C, SKOV3, SKBR3, and SW620 were treated with DMSO, AZD6244 (10 μmol/L), and API-2 (10 μmol/L) for 6 h and subjected to real-time PCR analysis. Bar graphs, the mean values of the representative results from two experiments conducted in triplicates for each.
AZD6244 synergizes with API-2, which sensitizes AZD6244-resistant cells to growth suppression and apoptosis mediated by FOXO3a
We have shown that AZD6244 synergizes with PI3K/AKT inhibitors, such as LY294002 or cytotoxic drugs like Taxol, to suppress cancer cell proliferation (Supplementary Fig. S2). We further asked if the synergism between AZD6244 and PI3K/AKT inhibitors could functionally sensitize AZD6244-resistant cancer cells. Consistent with the previous data showing the re localization of FOXO3a to the nucleus and enhancement of Bim mRNA expression by API-2 (Fig. 3C), AZD6244 combined with API-2 led to significant growth suppression (Fig. 4A) and cell death (Fig. 4B) in multiple AZD6244-resistant cells (SKOV3, SKBR3; MDA-MB-468 and MDA-MB-231 in Supplementary Fig. S8). The enhanced killing effects by the combined treatment of AZD6244 and API-2 were also observed in AZD6244-sensitive cells (WM793 and SW620; Fig. 4A and B). Additionally, the sensitization effect of AZD6244 and API-2 in the AZD6244-resistant cells was detected by colony formation assay (Fig. 4C). Furthermore, knocking down FOXO3a reversed the suppression of proliferation (Fig. 4D) by AZD6244/API-2 combination in an AZD6244-resistant cell line, indicating that FOXO3a is a key target for sensitizing AZD6244 treatment. In addition, to test pharmacologic toxicity compared between cancer and normal cells, a panel of cancer cell lines and normal epithelial cell lines (two from breast and one from lung) were treated with the above-mentioned condition simultaneously (Fig. 5A). Consistent with Fig. 4A and B, AZD6244 combined with API-2 effectively killed the cancer cells, whereas the same treatment caused little toxicity in the normal epithelial cells. Together, our findings suggest that combining AZD6244 with other clinical pharmacologic agents that enhance FOXO3a activity, such as API-2, can promote the efficacy of AZD6244 treatment and even sensitize AZD6244-resistant cells to growth suppression. Given the results (Figs. 3 and 4) that the combination of AZD6244 and API-2 increased FOXO3a nuclear translocation, enhanced Bim promoter association, rescued Bim transcriptional activation, and sensitized AZD6244-resistant cancer cells to growth suppression and cell death, we believe that FOXO3a activation is an important factor in reversing AZD6244 resistance. The preferential killing effect in cancer cells versus normal cells (Fig. 5A) may also benefit AZD6244 treatment by preventing potential side effects in normal cells. A model depicting molecular responses toward AZD-resistant and AZD-sensitive cancer cells is proposed in Fig. 5B.
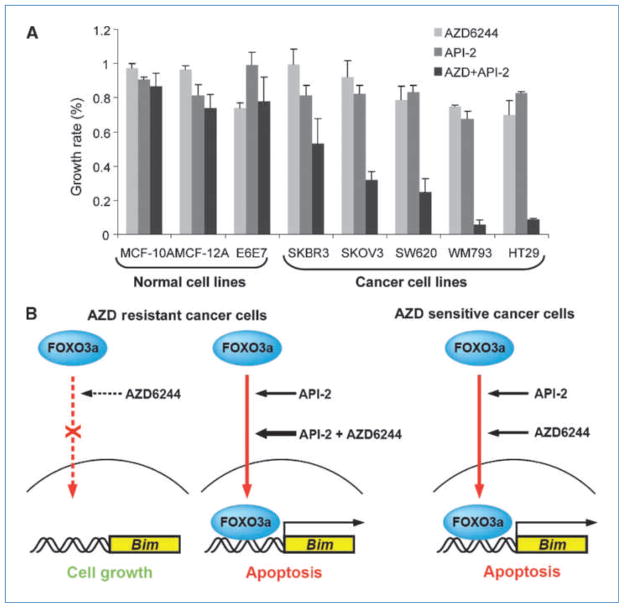
Figure 5.
AZD6244 combined with API-2 specifically kill cancer cells but not normal cells. A, a panel of normal breast and lung epithelial cell lines (MCF-10A, MCF-12A, and HBE4-E6E7) and several cancer lines were treated with DMSO, AZD6244 (5 μmol/L), API-2 (5 μmol/L), or AZD6244 (5 μmol/L) along with API-2 (5 μmol/L) for 48 h and then subjected to MTT assays. Bar graphs, the mean values of the representative results from two experiments conducted in triplicates for each. B, AZD6244-resistant cancer cells showed impaired endogenous FOXO3a nuclear translocation, reduced FOXO3a-Bim promoter association, and significantly decreased Bim expression in response to AZD6244. However, API-2 (bold arrow, AKT inhibitor) enhanced FOXO3a nuclear translocation, increased FOXO3a-Bim promoter association and enhanced Bim expression, and induced cell apoptosis. In AZD6244-sensitive cancer cells, both AZD6244 and API-2 were shown to induce FOXO3a activity and induced cell apoptosis.
Discussion
Until now, AZD6244 has been under evaluation in 21 clinical trials with about 10 different cancer types including breast cancer, colon cancer, lung cancer, melanoma, kidney cancer, hepatocellular carcinoma, pancreatic cancer, ovarian cancer, acute myelogenous leukemia, and thyroid cancer in which AZD6244 has shown promising therapeutic effects especially in cancers with BRAF mutations with lower toxicity. Other MEK inhibitors such as PD-0325901 are also shown to have promising antitumor activity in mouse models but ocular and neurologic toxicity was presented in a phase I clinical study (from the NIH Web site: http://www.Clinicaltrials.gov). In Fig. 5A, the combination of AZD6244 and API-2 results in significant cell death in the five different cancer cell lines but not in the three different normal cell lines, suggesting that AZD6244 selectively targets cancer cells and has relatively low toxicity to normal cells.
AKT and ERK are commonly activated oncogenic kinases in human cancers. Interestingly, both kinases target the same tumor suppressor gene, FOXO3a. It was known that AKT and ERK phosphorylate FOXO3a at different phosphorylation sites (23). Similarly, the phosphorylation of FOXO3a by these oncogenic kinases results in FOXO3a translocation from the nucleus to the cytoplasm and subsequent degradation. Taxol (17), LY2940024, and API-2 were shown to effectively block PI3K-AKT pathway and activate FOXO3a nuclear translocation and activity. In our current study, we showed that inhibition of both RAS/MEK/ERK and PI3K/AKT pathways enhances FOXO3a activity (Fig. 5B). We showed that the activation of FOXO3a and its downstream gene Bim is particularly important for the maximal sensitivity of cancer cells responding to AZD6244 treatment.
It has been proposed that the emergence of resistant tumor cells is partly due to the expansion of preexisting resistant cells or acquired resistance; therefore, the challenges in treating cancer with conventional therapeutics have led to the development of novel molecular therapeutics aimed at resolving chemoresistance. Here, we identify a molecular mechanism for resistance to AZD6244. The AZD6244-resistant cancer cell lines are unable to reactivate FOXO3a in response to AZD6244 treatment and, thereby, have become resistant to AZD6244. We have also shown that further reactivation of FOXO3a by PI3K/AKT inhibitors can sensitize AZD6244-resistant cancer cells, suggesting that AZD6244/API-2 and AZD6244/Taxol combination therapy may overcome AZD6244 resistance to reach maximum therapeutic efficiency. The AZD6244 and Taxol/Docetaxel combination treatment is currently being assessed in clinical trials.
Recently, an application of combining PI3K and MEK inhibitor for synergistically treating lung cancer was published in by Engelman and colleagues (24). In this study, using the clinical PI3K/mammalian target of rapamycin inhibitor NVP-BEZ235 combined with AZD6244 led to marked synergy in shrinking murine KRAS-mutant lung tumors, which, however, did not respond to single-agent NVP-BEZ235. It is known that KRAS mutation can activate both ERK and AKT (25). Thus, it is likely that both KRAS-mediated AKT and ERK activation contribute to resistance to NVP-BEZ235 and AZD6244, respectively, in the lung cancer story. To test whether FOXO3a may be a pivotal regulator for growth suppression in the KRAS mutation lung cancer cells, we investigate nuclear FOXO3a level by immnuohistochemical staining (Supplementary Fig. S9). Indeed, nuclear FOXO3a was only partially elevated in each single-agent treatment. However, AZD6244/BEZ235 combination, which inhibited both AKT and ERK pathways, synergistically enhanced nuclear FOXO3a level (Supplementary Fig. S9). Together, these data support the notion that similar to API-2, NVP-BEZ235 could synergize with AZD6244 in suppressing the growth of AZD6244-resistant cells (Supplementary Fig. S10). Our results suggest that FOXO3a activation might be an essential marker for predicting the efficacy of MEK inhibitors. Ultimately, our study provides a timely therapeutic strategy for AZD6244 application in current cancer treatments, given that FOXO3a is a potential target for therapeutic intervention by MEK inhibitors and other therapeutic agents.
Supplementary Material
Acknowledgments
We thank Dr. Paul D. Smith for the scientific comments and for providing the colon cancer mouse xenograft model, and Jing-Yu Lang for the technical support. AZD6244 was provided by AstraZeneca.
Grant Support
NIH grant P01 CA 099031, the MDACC Specialized Programs of Research Excellence in Breast Cancer CA116199 and Ovarian Cancer CA83639; The University of Texas M.D. Anderson Cancer Center support grant CA16672; partial support from the Sister Institution fund of MD Anderson Cancer Center, China Medical University Hospital, and National Breast Cancer Foundation, Inc; and the Patel Memorial Breast Cancer Research Foundation, Breast Cancer Research Foundation grant, Kadoorie Charitable Foundations, and DOH-TD-C-111-005 Cancer Center of Research Excellence (Taiwan).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
In memoriam, Tiong Loi Ang for his courageous fight against cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.Thompson N, Lyons J. Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol. 2005;5:350–6. doi: 10.1016/j.coph.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Scholl FA, Dumesic PA, Khavari PA. Effects of active MEK1 expression in vivo. Cancer Lett. 2005;230:1–5. doi: 10.1016/j.canlet.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg RA. ras Oncogenes and the molecular mechanisms of carcinogenesis. Blood. 1984;64:1143–5. [PubMed] [Google Scholar]
- 6.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–37. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 7.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 8.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–17. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 11.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 12.Hu MC, Lee DF, Xia W, et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 13.Yang JY, Zong CS, Xia W, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong XY, Chen C, Sun X, et al. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 15.Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–92. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunters A, Fernandez de Mattos S, Stahl M, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 17.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Dan HC, Sun M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 19.Hoeflich KP, O’Brien C, Boyd Z, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res. 2009;15:4649–64. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 20.Meng J, Peng H, Dai B, et al. High level of AKT activity is associated with resistance to MEK inhibitor AZD6244 (ARRY-142886) Cancer Biol Ther. 2009:8. doi: 10.4161/cbt.8.21.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirzoeva OK, Das D, Heiser LM, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–72. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JY, Hung MC. A new fork for clinical application: targeting fork-head transcription factors in cancer. Clin Cancer Res. 2009;15:752–7. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phos-phatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5:195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.