Abstract
Toxoplasma gondii is an intracellular parasite that causes severe neurologic and ocular disease in immune-compromised and congenitally infected individuals. There is no vaccine protective against human toxoplasmosis. Herein, immunization of Ld mice with HF10 (HPGSVNEFDF) with palmitic acid moieties or a monophosphoryl lipid A derivative elicited potent IFN-γ production from Ld-restricted CD8+ T cells in vitro and protected mice. CD8+ T cell peptide epitopes from T. gondii dense granule proteins GRA 3, 6, 7, and Sag 1, immunogenic in humans for HLA-A02+, HLA-A03+, and HLA-B07+ cells were identified. Since peptide repertoire presented by MHC class I molecules to CD8+ T cells is shaped by endoplasmic reticulum-associated aminopeptidase (ERAAP), polymorphisms in the human ERAAP gene ERAP1 were studied and associate with susceptibility to human congenital toxoplasmosis (p<0.05). These results have important implications for vaccine development.
Keywords: Toxoplasma gondii, vaccine, HLA Class 1 bound peptides
1. INTRODUCTION
Toxoplasma gondii is an obligate, intracellular, apicomplexan parasite that has a global distribution and a broad host range. The majority of T. gondii strains can be classified into 3 distinct archetypal clonal lineages, type I to III, or atypical, which differ in their patterns of virulence[1]. Infection in humans can cause severe ocular, neurologic, and sometimes systemic disease, especially in immunocompromised and congenitally infected individuals[2]. Proinflammatory cytokines produced by macrophages and lymphocytes, such as tumor necrosis factor-α (TNF-α), interleukin-12 (IL-12), and interferon-γ (IFN-γ) are critical for control of both acute and chronic phases of T. gondii infection [3,4]. In addition, CD8+ T lymphocytes have been shown to be the major cell subset responsible for protective immunity against T. gondii by secreting IFN-γ and developing cytotoxic activity against infected cells [3,5]. Consistent with this, CD8+ cytotoxic T lymphocyte (CTL)-mediated resistance to toxoplasmic encephalitis in H-2d mice has been definitively mapped to the major histocompatibility complex (MHC) class I Ld allele[6]. Moreover, immune Ld-restricted CTLs are strain-specific, killing target cells that are infected with the type II Me49 parasite strain and its derivatives but not those infected with type I strains[7].
At present, there are no chemotherapeutic agents to definitively and completely prevent or cure T. gondii infection in humans[8,9]. A vaccine could solve these problems. The ideal vaccine against toxoplasmosis in humans would include antigens that can elicit a protective T helper cell type 1 (Th1) immune response, and characterized by the generation of long-lived IFN-γ-producing CD8+ T cells. The administration of live attenuated tachyzoites such as the temperature-sensitive ts-4 strain has been found to induce a potent, protective cell-mediated immune response in animals [3,10] (Hutson et al., unpublished data), although live vaccines are generally considered insufficiently safe for use in humans. Studies have identified a number of T. gondii surface antigens (SAG) and secreted proteins such as the dense granule, rhoptry, and microneme proteins as antigens recognized by murine T lymphocytes [11]. Indeed, vaccine formulations based on native, recombinant proteins or DNA vaccine forms of these antigens have resulted in reduced mortality and cyst burden in infected animals [12–14]. Nonetheless, protection was incomplete because sterile immunity was not achieved.
Recently, Blanchard et al. demonstrated that a single decapeptide HF10 (HPGSVNEFDF), derived from the dense granule protein 6 (GRA6), was the protective, immunodominant Ld-restricted epitope in H-2d mice infected with the Prugneaud, a type II, T. gondii strain [15]. Endogenous generation of this immunodominant peptide was critically dependent on the endoplasmic reticulum-associated aminopeptidase (ERAAP), a protease that trims precursor peptides delivered into the endoplasmic reticulum lumen to the requisite 8- to 10-amino acid length for loading onto MHC class I molecules[15]. Ld and human MHC molecules belonging to the HLA-B07 supertype exhibit a similar peptide binding motif as determined empirically from the specificity of their main anchor residues [16,17]. There is functional overlap in Ld and HLA-B07 in peptide binding shown in binding studies and crystal structures of various B7 supertype alleles are similar to Ld [18]. Consequently, it seemed highly likely that HF10 would also be the immunodominant epitope generated during the course of natural infection by type II T. gondii strains in HLA-B07+ individuals. This provided a paradigm for evaluating the protective mechanisms of various HF10-containing vaccine formulations in H-2d and HLA-B07+ transgenic murine models. Interestingly, 3 other distinct Ld-restricted peptides were separately identified as immunogenic epitopes in H-2d mice [19,20], thereby making the model of a single, immunodominant CD8+ T cell epitope in T. gondii infections more complex. Thus, it remains important to define immunogenic epitopes restricted by HLA-B07 and other HLA supertypes for the development of an efficacious vaccine in humans.
The success of a vaccine also depends on other factors, including immuno-stimulatory properties of adjuvants employed and delivery system for both antigen and adjuvant. Conjugation of CD8+ T cell determinants to lipid groups is known to enhance specific cell-mediated responses to target antigens in animals and humans, although mechanisms whereby immunity is achieved remains poorly understood. In particular, palmitoylated lipopeptide constructs have been demonstrated to elicit long-lived, protective cellular responses against a variety of pathogens, including Hepatitis B virus (HBV), influenza virus, and Plasmodium falciparum [21–24]. Lipopeptides hold several advantages over other conventional vaccine formulations; for instance, they are self-adjuvanting and display none of the toxicity-associated side effects of Th1-inducing adjuvant systems such as saponin or QS21 [25]. They are also convenient to store at ambient temperature and can be chemically synthesized, thus avoiding potential contamination problems associated with bacterial or other biosynthetic systems. There have also been a number of studies indicating that lipopeptides and lipid-associated peptide formulations are remarkably effective at inducing mucosal immunity, which represents a major goal for vaccines against T. gondii infection [23,26]. The inclusion of PADRE, a synthetic peptide that binds promiscuously to variants of the human MHC class II molecule DR and is effective in mice, in lipopeptides and DNA vaccine vectors containing CpG motifs, also can augment CD8+ T cell effector functions by inducing CD4+ T helper cells [21,27]. Detoxified derivatives of lipopolysaccharide (LPS), such as monophosphoryl lipid A (MLA), also are potent activators of Th1 responses via the Toll-like receptor 4 (TLR4) signaling pathway in macrophages and dendritic cells [28].
Herein, we designed 3 HF10-containing vaccine formulations comprising 2 lipopeptides that incorporate PADRE, as well as an oil-in-water emulsion that includes a synthetic MLA derivative. The efficacy of these vaccines in eliciting Ld-restricted, CD8+ T cell-mediated IFN-γ production in vitro from H-2d BALB/c mice was examined. To determine if HF10 could be an immunodominant epitope generated for HLA-B07+, we tested HF10 immunized HLA-B07+ transgenic mice and naturally infected HLA-B07+ individuals for their reactivity to HF10 as determined by the levels of IFN-γ secretion. We also utilized bioinformatic algorithms to identify novel, T. gondii-derived, CD8+ T cell epitopes restricted by the HLA-A02, -A03, and -B07 supertypes, which collectively provide broad coverage for over 80 to 90% of the human population worldwide, regardless of ethnicity [29]. Since ERAAP shapes the final repertoire of peptide antigens presented by MHC class I molecules to CD8+ T cells and ERAAP-deficient mice are much more susceptible to T. gondii than control mice [30], we hypothesized that polymorphisms in the human ERAAP gene that is orthologous to mouse ERAAP, ERAP1, might influence the outcome of congenital parasite infection in people. Indeed, our analysis of a cohort of congenitally infected children and their parents using the transmission disequilibrium test (TDT) indicates that congenital toxoplasmosis associates with 2 single nucleotide polymorphisms (SNPs) in ERAP1.
2. METHODS
2.1 Peptides, lipopeptides, and formulated MLA mimetic
All peptides and both HF10-containing lipopeptides were synthesized by Synthetic Biomolecules (San Diego, CA) at >90% purity in lyophilized form. Both lipopeptides have structures similar to previously described lipidated constructs [21,22]. Murine cytomegalovirus (MCMV) pp89-derived peptide YL9 (YPHFMPTNL) and Hepatitis C virus (HCV) core 169 (LF9 (LPGCSFSIF)) were used as irrelevant Ld-restricted and HLA*B0702-restricted peptides respectively. The TLR4 agonist was a MLA mimetic that was synthesized by the Infectious Diseases Research Institute (IDRI, Seattle, WA) as a stable oil-in-water emulsion.
2.2 Mice
BALB/c mice were obtained from Taconic Farms. HLA-B*0702 transgenic mice[31] were produced at Pharmexa-Epimmune (San Diego, CA) and bred at the University of Chicago. All studies were conducted with the approval of the Institutional Animal Care and Use Committee at the University of Chicago.
2.3 Immunizations
Age-matched female BALB/c mice were inoculated subcutaneously (s.c.) at the base of the tail using a 27-gauge needle with 50 μg HF10, 50 μg HF10 and 20 μg EM005, or 50 nmol of each lipopeptide in 100 μl of phosphate-buffered saline (PBS). As controls, mice were injected with PBS or a combination of PBS and 20 μg EM005. Mice that received PBS/EM005, HF10, and HF10/EM005 were boosted twice at 2-week intervals, while mice that received PBS and either of the 2 lipopeptides were boosted once at 21 days after the first immunization. Sex- and age-matched HLA-B*0702 mice were inoculated s.c. at the scruff of the neck with the same peptide immunogens at the same time intervals.
2.4 Peripheral blood mononuclear cells
Cryopreserved peripheral blood mononuclear cells (PBMC) from both T. gondii-seropositive and seronegative individuals were used in the ELISPOT assay. PBMCs were previously isolated from heparinized blood samples by density gradient centrifugation using Histopaque (Sigma-Aldrich), washed twice with PBS, and cryopreserved in AIM-V medium (Gibco) containing 20% FCS and 10% DMSO. All blood samples were obtained with written informed consent from the donors and in accordance with institutional and NIH guidelines.
2.5 T. gondii serotyping and HLA typing of chronically infected individuals
Serotyping of the T. gondii strain in infected individuals was performed by Michael Grigg (National Institutes of Health) as previously described [32]. HLA haplotype analysis of seropositive individuals was determined using serologic typing by Paul Terasaki (UCLA, Tissue Typing Laboratory, Los Angeles, CA) as previously described [33].
2.6 Ex vivo preparation of murine splenocytes
Mice were euthanized 7 to 14 days after the last immunization. Spleens were harvested, pressed through a 70 μm screener to form a single-cell suspension, and depleted of erythrocytes with AKC lysis buffer (160 mM NH4Cl, 10 mM KHCO3, 100 μM EDTA). The remaining splenocytes were washed twice with Hank’s Balanced Salt Solution (HBSS) and resuspended in complete RPMI medium (RPMI-1640 supplemented with 2 mM L-GlutaMax [Invitrogen], 100U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, and 10% FCS) before they were used in subsequent in vitro assays.
2.7 Enzyme-linked immunospot (ELISPOT) assay
For human ELISPOT assays, MSIPS4W10 Multiscreen HTS-IP 96-well plates (Millipore, Bedford, MA) were coated with 50 μl of 15 μg/ml anti-human IFN-γ (1-D1K) monoclonal antibody (mAb) in sterile PBS overnight at 4°C. Wells were washed with sterile PBS and blocked with RPMI-1640 medium containing 10% FCS at room temperature for 2–3 hr. PBMCs were then plated in complete RPMI-1640 medium at 2×105 cells per well. Peptide or peptide pools were added to each well at 10 μg/ml and plates were incubated at 37°C and 5% CO2 for 20–24 hr. Media containing an equivalent concentration of DMSO was used to measure background response levels, and 5 μg/ml of T. gondii lysate antigen (TLA), 2.5 μg/ml of Concavalin A, or 10 μg/ml of a relevant HLA-B07-restricted Epstein-Barr virus (EBV)-derived peptide[31] was used as a positive control. Plates were successively incubated with 100 μl of 1 μg/ml biotinylated anti-human IFN-γ mAb (7B6-1) for 2 hr and streptavidin-conjugated alkaline phosphatase at a 1:1000 dilution for 1 hr at room temperature, before spots were developed using 5-bromo-4-chloro-3-indolyl-phosphate/p-nitro blue tetrazolium chloride (BCIP/NBT). Wells were washed with PBS in between incubation stages. Spot formation was quenched by extensive washing with distilled water. Plates were air-dried overnight at 4°C, and spots counted the next day using an automated ELISPOT reader (CTL ImmunoSpot).
Murine ELISPOT assays were performed similarly, except that anti-mouse IFN-γ mAb (AN18) and the biotinylated anti-mouse IFN-γ mAb (R4-6A2) were used as the cytokine-specific capture antibodies instead. In addition, 5×105 splenocytes were plated per well. All antibodies and reagents used for the ELISPOT assay were obtained from Mabtech (Cincinnati, OH). Cells were plated in at least 3 replicate wells for each condition. Results were expressed as the number of spot forming cells (SFCs) per 106 PBMCs or per 106 murine splenocytes.
2.8 Antibody blocking in ELISPOT
Murine splenocytes were incubated with the relevant blocking antibody for 1.5 to 2 hr at 37°C and 5% CO2 before they were seeded at 2.5 to 5×105 cells per well. Anti-CD4 (RM4-5, BioLegend), Anti-CD8α (53-6.7, BD Biosciences) mAb, and their relevant isotype control (Rat IgG2a, BD Biosciences) were added to each sample at a final concentration of 10 μg/ml. To assay for Ld-restricted IFN-γ production, splenocytes were incubated with 0.5 μg of FcBlock™ (BD Biosciences) per 106 cells for 10–15 min at 4°C and washed before anti-Ld (BioLegend) mAb or its isotype control (mouse IgG2a, BioLegend) was added at a final concentration of 20 μg/ml. Results were expressed as the percentage of SFCs generated in the presence of antibody relative to that in the absence of antibody as follows: percent SFC production = (number of SFCs in presence of antibody)/(number of SFCs in absence of antibody) × 100% ± s.e.m.
2.9 Bioinformatic prediction of CD8+ T cell epitopes
A total of 9 surface and secreted proteins (SAG1, SUSA1, GRA2, GRA3, GRA6, GRA7, ROP2, ROP16, ROP18) from the type II T. gondii strain, Me49, were selected for bioinformatic analysis. Initially, protein sequences were screened for nonameric CD8+ T cell epitopes on the basis of their predicted binding affinity to HLA-A02, -A03, or -B07 supertype molecules using the ARB, SMM, and ANN algorithms, as well as predicted proteasomal cleavage and processing by TAP as previously described [34,35]. A total of 118 unique peptides representing the top 1% of all ranked nonameric peptides were identified (Supplementary Table 1–3). All protein sequences were obtained from ToxoDB 5.1 (http://toxodb.org/toxo/). Subsequently, a new algothythm was utilized (http://www.immuneepitope.org). Peptides from the proteins listed above that were present in both Type I and II parasite with predicted avidities <100 nM were tested in the IFN-γ Elispot assays described below.
2.10 Modeling of peptide/MHC structures
The previously determined crystal structures of Ld (Protein Data Bank accession code 1LDP) and HLA-B*3501 (Protein Data Bank accession code 2CIK), a HLA-B07 supertype molecule, were used as templates to model the respective HF10/MHC complexes. The molecular graphics program PyMol was used to generate the structure figures.
2.11 Parasites
The transgenic T. gondii strain used for in vivo challenges in this work, Pru-FLUC, was derived from the type II Prugneaud (Pru) strain and expresses the firefly luciferase (FLUC) gene constitutively by tachyzoites and bradyzoites. It was created, maintained and utilized as previously described [36].
2.12 Patient cohort and genotyping of ERAP1 and ERAP2
Case-parent trios were from the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) as previously described [37]. Briefly, the NCCCTS consisted of 176 North American children clinically diagnosed with congenital toxoplasmosis and their parents. DNA was successfully extracted from 149 case-parent samples and genotyped at 18 SNPs in ERAP1 and 7 SNPs in ERAP2. Of these 149 infected children, 124 (83%) had clinically confirmed brain calcifications with/without hydrocephalus and/or retinal lesions at birth or time of diagnosis.
2.13 In vivo bioluminescence imaging
Mice infected with Pru-FLUC tachyzoites[36] were imaged at 5 to 7 days post-challenge using the in vivo imaging system (IVIS; Xenogen, Alamedia, CA) as previously described [38]. Briefly, mice were injected i.p. with 200 μl of D-luciferin and immediately anesthetized in an O2-rich induction chamber with 2% isofluorane. After 12 min, mice were imaged in ventral positions and photonic emissions were assessed using Living image® 2.20.1 software (Xenogen). Data were presented as pseudocolor representations of light intensity and mean photons/s/region of interest (ROI).
2.14 MHC-peptide binding assays
Quantitative assays to measure the binding of peptides to HLA class I molecules are based on the inhibition of binding of a radiolabeled standard peptide. MHC molecules were purified by affinity chromatography from EBV transformed or single allele transfected 721.221 cell lines, and assays performed, as described previously [39–41]. Peptides used in this assay included: HLA-A2: VVFVVFMGV (GRA6); FMGVLVNSL (GRA6); FLVPFVVF (GRA3); HLA-A3 KSFKDILPK (SAG1); AMLTAFFLR (GRA6); RSFKDLLKK (GRA7); HLA-B7: LPQFATAAT (GRA7); VPFVVFLVA (GRA3); HPGSVNEFDF (GRA6). Peptides were tested at six different concentrations covering a 100,000-fold dose range in three or more independent assays, and the concentration of peptide yielding 50% inhibition of the binding of the radiolabeled probe peptide (IC50) was calculated. Under the conditions used, where [radiolabeled probe] < [MHC] and IC50 ≥ [MHC], the measured IC50 values are reasonable approximations of the true Kd values [42,43].
2.15 Statistical analyses
Statistical analyses for all in vitro assays were performed using a 2-tailed student’s t test. Peptides were considered immunogenic in mice if they induced IFN-γ spot formation from immunized mice that was significant (p<0.05) relative to: (i) spot formation from control mice, and (ii) spot formation from immunized mice incubated with an irrelevant peptide. Peptides and peptide pools were considered immunogenic in humans if they induced IFN-γ spot formation from PBMCs that was significant (p<0.05) relative to spot formation from the same cells incubated with media containing an equivalent concentration of DMSO. All Elispot experiments were replicated a minimum of twice. The experiment with luciferase expressing parasites used to challenge immunized BALB/c mice was performed with a total of 5–9 mice per treatment group. Different mice were imaged on each of the three days (5–7 days after challenge). For the genetic study, tag SNPs were selected from the HapMap project in ERAP1 and ERAP2 using 10 kb flanking sequence on each side, a MAF cutoff of 5% in CEU and r2 threshold of 0.8. Allelic association analysis was performed for the 124 infected children in the cohort with confirmed clinical findings in the eye and/or brain using a conventional TDT and p values were calculated using Haploview (http://www.broadinstitute.org/haploview). P values of 0.05 or less were considered significant for association with disease.
3. RESULTS
3.1 Lipopeptides and HF-10 with MLA elicit potent IFN-γ-production from Ld-restricted CD8+ T cells in vitro and partially protect mice
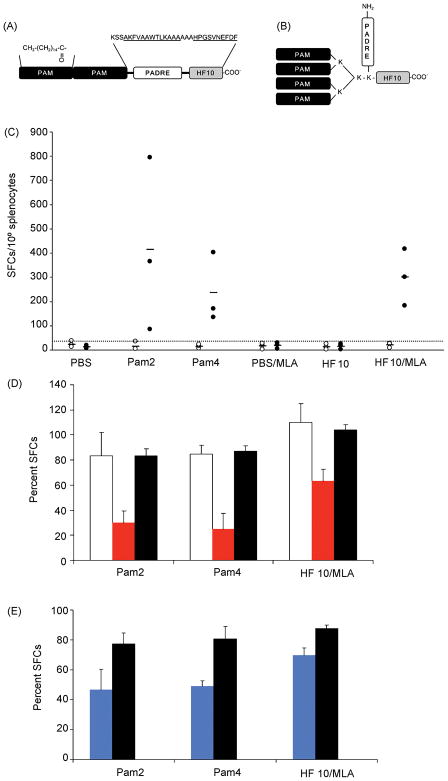
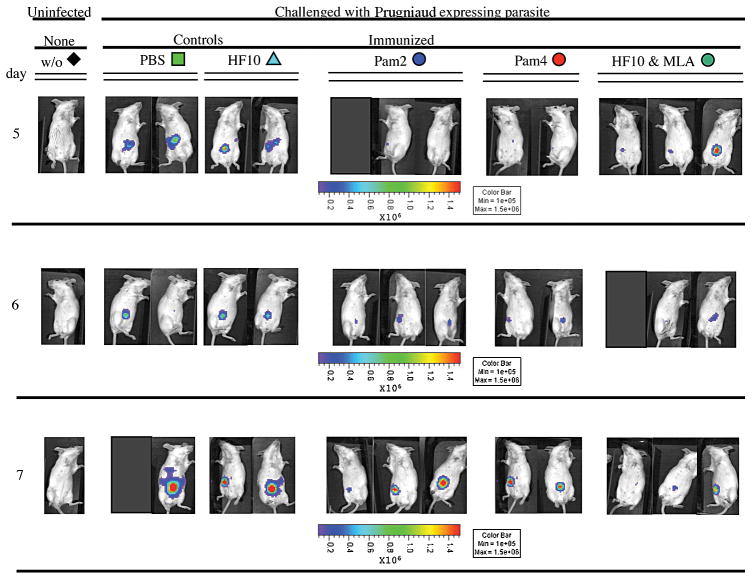
IFN-γ was defined as a key mediator of protective immunity during both acute and chronic phases of disease in mice [4]. To evaluate adjuvancy of lipid groups and MLA in vaccines against T. gondii infection, we designed and studied ability of 2 lipopeptides (Figure 1A,B) and a MLA/HF10 mixture to induce antigen-specific IFN-γ production in H-2d mice. BALB/c (H-2d) mice were immunized with the HF10-adjuvant formulations and spleens harvested 7 to 14 days after the last inoculation. Frequency of IFN-γ-secreting splenic cells generated in immunized mice and control mice was measured and compared using an ex vivo ELISPOT assay. Both lipopeptides and MLA/HF10 elicited significantly higher HF10-specific IFN-γ production relative to control mice (Figure 1C, p<0.01). In addition, there was no significant difference in IFN-γ spot formation among mice that received the 3 different vaccine formulations (p>0.05). To assess the relative contribution of CD8+ T cells to overall IFN-γ response, we blocked activity of splenic CD4+ and CD8+ T cells using antibodies (Ab) specific to those 2 surface co-receptors. IFN-γ spot formation was significantly reduced in the presence of anti-CD8 Ab (p<0.01) but not anti-CD4 Ab when compared to spot formation induced by isotype control Ab (Figure 1D). Ld restriction was also demonstrated by abrogation of IFN-γ responses with Ab to Ld but not with its isotype control Ab (Figure 1E, p<0.05). Thus, all 3 adjuvant systems elicited significant frequencies of IFN-γ-producing Ld-restricted CD8+ T cells in immunized mice. Mice were partially protected, i.e. reduced luciferase expessing parasites, by these immunizations (Figure 2). Protection was present for each of the immunized groups of mice, each of which had spleen cells that produced IFN-γ in ELISPOT assays when cultured with HF10 in parallel experiments. All adjuvanted immunizations protected mice on each of the 3 days tested. Different mice were imaged on each of the 3 days, 5–7 days after challenge. There were a total of 5–9 mice in each control or immunized group.
Figure 1. Schematic representation of lipopeptide constructs used and data demonstrating that immunization of H-2d mice with lipopeptides and HF10/MLA elicits significant Ld-restricted, CD8+ T cell-mediated IFN-γ production.
(A) Pam2 lipopeptide contains 2 covalently linked palmitoyl moieties at the N terminus and 2 spacers comprising 3 amino acids each. (B) Pam4 lipopeptide consists of PADRE and HF10 separated by a fan-like structure that contains 4 lysines and 4 branching palmitoyl groups. Amino acid sequence of PADRE, HF10, and intervening spacers are indicated in single-letter code above the peptides, with those for PADRE and HF10 underlined. The N and C termini of each lipopeptide and the α- and ε-amino groups of certain lysines in Pam4 are also indicated for clarity. Pam: palmitoyl moiety. (C) Splenocytes from immunized (Pam2 and Pam4 lipopeptides and HF10/MLA) and control H-2d mice (PBS, PBS/MLA, or HF10 alone) were stimulated with HF10 (filled circles) or irrelevant peptide YL9 (unfilled circles) and frequency of IFN-γ-producing cells in each case was determined using an ex vivo ELISPOT assay. Each symbol represents an individual mouse; small horizontal bars indicate the mean of each group. The dotted line represents the cutoff value determined as the maximal mean of the control mice + 3 S.D. (D) Splenocytes were incubated with antibodies to CD4 (unfilled bars), CD8α (red bars), and their common isotype control (filled bars) for 60–90 min prior to stimulation with HF10. Data are normalized to the number of spots generated in the absence of antibody and presented as the percent production of SFCs. (E) As in (D), except that anti-Ld (blue bars) Ab and its isotype control (filled bars) were added instead. *, p<0.05; **, p<0.01.
Figure 2. Dissemination and replication of T. gondii luciferase expressing parasites were partially reduced in H-2d mice immunized with lipopeptides and MLA/HF10 when assayed from 5–7 days after challenge.
Different mice were imaged on each day. Mice from each of the groups of immunized mice that have T cells that produced IFN-γ in response to HF10 were protected compared with unimmunized control mice that did not produce IFN-γ. There were a total of 5–9 mice tested in each control or immunization group.
3.2 Absence of Immunogenicity of HF10 in HLA-B*0702 transgenic mice
Studies have shown that many polymorphic MHC class I molecules bind overlapping peptide repertoires, which are to a large extent determined by anchor residues at the second and C-terminal positions of presented peptides. We noted that Ld shares the same peptide binding motif as the HLA-B07 supertype of human MHC molecules; both present peptides containing proline at the second position and aliphatic or hydrophobic residues at the C-terminal end [16,17]. Consequently, we hypothesized that immunogenic T. gondii-derived epitopes that are bound by Ld in mice (such as HF10) might be similarly presented by HLA-B07 supertype molecules in humans. Bioinformatic analysis also predicted a relatively high binding affinity of HLA-B07 supertype molecules to HF10. We therefore immunized HLA-B*0702 mice with the same HF10-containing vaccine formulations that were previously administered to BALB/c mice and demonstrated to elicit significant IFN-γ-producing CD8+ T cells in vitro. Splenocytes were similarly harvested from immunized HLA-B*0702 mice and stimulated with HF10 or an irrelevant HLA-B07-restricted peptide, LF9 in an ELISPOT assay. Contrary to our prediction, neither lipopeptide nor the MLA/HF10 combination generated significant numbers of IFN-γ spots relative to the control mice, although very modest but not significant levels of IFN-γ were produced to HF10 compared to those for LF9 in the immunized mice (Figure 3A). The functionality of our experimental system was confirmed using a positive control group of mice that were inoculated with an immunogenic, HLA-B07-restricted, EBV-derived peptide and which produced significant frequencies of IFN-γ spots in response to the relevant EBV antigen (data not shown). Thus, HF10 was not immunogenic in HLA-B*0702 mice when administered as lipopeptide or with MLA.
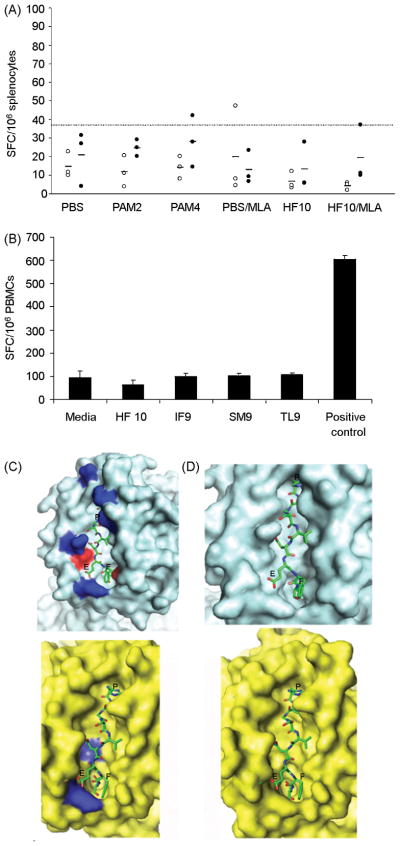
Figure 3. HF10 was not immunogenic in HLA-B*0702 mice and Ld-restricted peptides HF10, IF9, SM9, and TL9 were not recognized by T. gondii-infected HLA-B07+ humans, likely due to differences between Ld and HLA-B07 surface and electrostatic complementarity for HF10 and size of peptide-binding pockets.
(A) Splenocytes from immunized (Pam2 and Pam4 lipopeptides and HF10/MLA) and control HLA-B*0702 mice (PBS, PBS/MLA, or HF10 alone) were stimulated with HF10 (filled circles) or irrelevant peptide LF9 (unfilled circles) and frequency of IFN-γ-producing cells in each case was determined using an ELISPOT assay. Each symbol represents an individual mouse; small horizontal bars indicate mean of each group. Dotted line represents cutoff value determined as maximal mean of control mice ± 3 S.D. (B) PBMCs from T. gondii-seropositive HLA-B07+ humans were stimulated with 10 μg/ml of 4 Ld-restricted peptides (HF10, IF9, SM9, TL9) previously shown to be immunogenic in mice. Frequency of SFCs was measured using an ELISPOT assay. Data (mean ± s.e.m.) are from 1 seropositive individual and representative of 2 to 4 individuals per peptide tested. Media: negative control containing equivalent concentration of DMSO relative to peptide-stimulated wells; Positive control: 10 μg/ml of an immunogenic HLA-B07-restricted peptide derived from Epstein-Barr virus (EBV). (C,D) Predicted structures of murine Ld and human HLA-B07 supertype pMHC complexes were modeled by superimposing HF10 peptide (presented as a ‘ball-and-stick’) into previously determined peptide-binding pocket of Ld (cyan) and HLA-B*3501 (yellow), a HLA-B07 supertype member. (C) Surface electrostatics of peptide-binding pocket of both MHC molecules are indicated in blue (basic patches) and red (acidic patches). Remaining regions represent nonpolar, hydrophobic patches. (D) A close-up view of pMHC region shown in (C). Relative positions of Pro, Glu, and C-terminal Phe of HF10 (HPGSVNEFDF) are indicated in single-letter code above corresponding residues of peptide.
3.3 Absense of Immunogenicity of HF10 and 3 other Ld-restricted T. gondii epitopes in HLA-B07+ humans
Although relevance of HLA-B*0702 mice as a model system for identifying human HLA-B07-restricted epitopes has been previously described [31], it was still possible that H-2b molecules which are also expressed in the transgenic mice to interfere with presentation of HF10 by HLA-B*0702. To directly determine immunogenicity of HF10 in humans, we tested PBMCs from T. gondii-infected donors for their reactivity to HF10 using an ELISPOT assay. All donors were seropositive for the type II strain of T. gondii (data not shown) and expressed HLA-B07 supertype alleles (referred to as HLA-B07+). None of the 4 seropositive individuals tested at times from acquisition of their infection ranging from months to 35 years exhibited significant IFN-γ responses to HF10 (Figure 3B), even when peptide concentration was titrated upwards to 50 μg/ml and stimulation period was extended to 48 hrs (data not shown). We therefore concluded that HF10 was not the type II T. gondii epitope that was naturally processed and presented by HLA-B07 supertype molecules to CD8+ T cells in humans.
It was notable that HF10 did not represent the only Ld-restricted epitope that has been identified to be immunogenic in T. gondii-infected mice. Recent work by Frickel et al. suggest that 2 unique nonameric epitopes (ROP7-derived IF9 [IPAAAGRFF] and GRA4-derived SM9 [SPMNGGYYM]) were generated in a stage-specific manner during natural infections in BALB/c mice [20]. In addition, a SAG1-derived peptide TL9 (TPTENHFTL) was found to be immunogenic and partially protective in H-2d mice [19]. We hypothesized that 1 or more of these Ld-bound epitopes might be recognized in HLA-B07+ individuals instead. However, none of the peptides was found to elicit IFN-γ production by HLA-B07+ human cells (Figure 3B).
Thus, there appeared to be a disparity between peptide repertoires naturally presented by Ld and HLA-B07 supertype molecules to CD8+ T cells in mice and humans despite their similar binding motifs. To test this hypothesis, we modeled Ld/HF10 and HLA-B07/HF10 peptide/MHC (pMHC) structures based on previously determined crystal structures of pMHC complexes involving Ld and HLA-B*3501 [44,45], a HLA-B07 supertype molecule [17]. The peptide-binding pocket of both murine and human MHC molecules displayed good surface complementarity to bound peptide, with no obvious predicted steric occlusion between peptide and MHC molecule (Figure 3C,D). A moderate electrostatic complementarity was also observed, with basic patches of peptide-binding pocket proximal to acidic residues (Glu and Asp) in HF10 and hydrophobic regions of peptide-binding pocket surrounding remaining nonpolar residues of HF10 (Figure 3C,D). Hence there did not appear to be significant differences in modeled structures of both pMHC complexes that might indicate a bias for binding of HF10 to Ld instead of HLA-B*3501, or that Ld and HLA-B07 supertype molecules would bind significantly different peptide repertoires. Our preliminary structural analysis (Figure 3C,D) thus was consistent with bioinformatic predictions of overlapping binding motifs of Ld and HLA-B07 supertype molecules. Although there were no differences in configuration of Ld or individual HLA-B07 supertype members there were differences both in size (Ld larger) and charge that we thus hypothesized must be responsible for the lack of immunogenicity of HF10 and other Ld binding peptides for HLAB7 humans and transgenic mice.
3.4 Identification of candidate CD8+ T cell epitopes restricted by HLA-A02, -A03, and -B07 supertypes
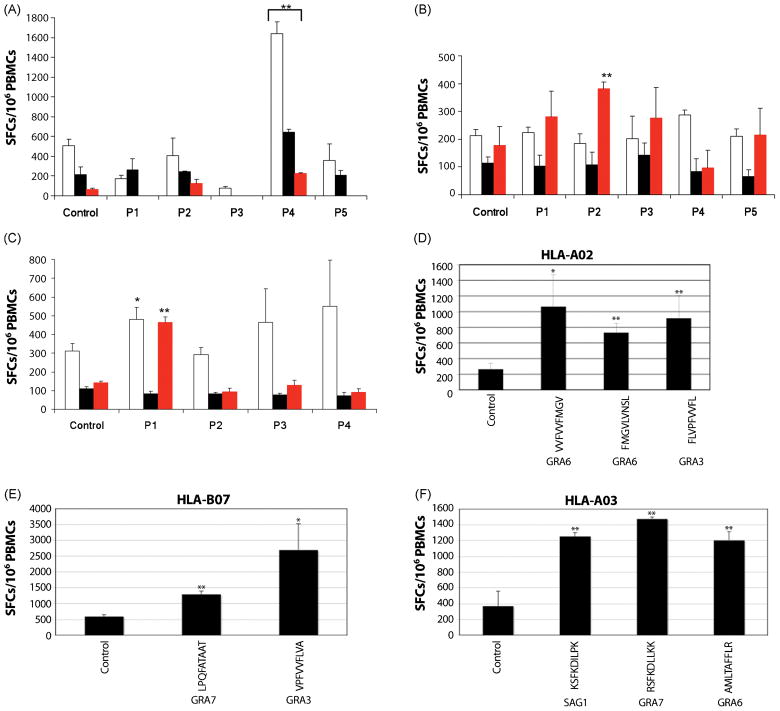
Since the GRA6 peptide HF10, and the ROP7, GRA4, and SAG1 peptides that bound Ld did not elicit a CD8+ T cell response in the HLA B7 persons tested or the HLA B7 mice, to attempt to directly identify CD8+ T cell epitopes in humans, we utilized several bioinformatic algorithms to screen a panel of 9 candidate proteins for potential epitopes that might be endogenously processed and presented by the HLA-A02, -A03, and -B07 supertypes. Candidate proteins include 2 surface antigens (SAG1, and SUSA1, a surface marker specific to the slow-growing, bradyzoite form of T. gondii) and 7 secreted proteins (GRA2, GRA3, GRA6, GRA7, ROP2, ROP16, ROP18) that have previously been reported to be highly immunogenic in mice and humans or to reach host cell cytoplasm, as is necessary for entry into a MHC class I processing pathway [46]. A total of 118 unique nonameric peptides were identified (42 for HLA-A02, 40 for HLA-A03, and 36 for HLA-B07) and screened in pools (Tables A1–A3) for reactivity in MHC-matched individuals who were seropositive for the type II strain of T. gondii. We observed that 1 HLA-A02-restricted pool, A2.P4, induced significant IFN-γ production from all 3 HLA-A02 tested individuals (p<0.01, Table 1 and Figure 4A). Intriguingly, only 1 out of 3 donors responded significantly to the HLA-A03-restricted pool, A3.P2, or the HLA-B07-restricted pool, B7.P1 (Table 1 and Figure 4B,C). Moreover, the B7.P1 pool also elicited modest IFN-γ spot formation from a second HLA-B07+ individual (Figure 4C, p=0.087). This pool might therefore contain peptides that are only moderately immunogenic in HLA-B07+ humans, possibly due to an intermediate peptide binding affinity for HLA-B07 supertype molecules. Given the considerable genetic and environmental variability across the human population, it is not entirely surprising that individuals might respond differently to the same peptides. Nevertheless, the 3 aforesaid peptide pools, A2.P4, A3.P2, and B7.P1, represented interesting targets for further deconvolution studies so that individual immunogenic epitopes might be defined.
Table 1.
Immunogenicity of predicted Me49-derived epitopes in seropositive individuals
| Patient number | HLA haplotypea |
Peptide pool testedd | Immunogenicitye |
|||||
|---|---|---|---|---|---|---|---|---|
| HLA-Ab | HLA-Bc | Pool 1 | Pool 2 | Pool 3 | Pool 4 | Pool 5 | ||
| 1 | A*02 | B*07 | A2 | − | − | − | + | − |
| 2 | A*01, A*02 | B*07, B*08 | A2 | − | − | ND | + | − |
| 3 | A*02 | B*27, B*62 | A2 | ND | − | ND | + | ND |
| 4 | A*02, A*11 | B*07, B*51 | A3 | − | + | − | − | − |
| 5 | A*01, A*03 | B*07, B*08 | A3 | − | − | − | − | − |
| B7 | − | − | − | − | − | |||
| 6 | A*03, A*24 | B*07, B*61 | A3 | − | − | − | − | − |
| B7 | + | − | − | − | − | |||
| 7 | A*03, A*31 | B*07, B*51 | B7 | − | − | − | − | − |
HLA-A and HLA-B alleles of the seropositive individual.
HLA-A*02 alleles are classified under the HLA-A02 supertype; HLA-A*03, -A*11, and A*31 alleles are classified under the HLA-A03 supertype.
HLA-B*07 and -B*51 alleles are classified under the HLA-B07 supertype.
The HLA supertype-restriction of the peptide pool tested in each individual. Peptide pools were screened only in HLA supertype-matched people. A complete list of peptide pools and their constitutent peptides can be found in Tables A1 to A3.
Each peptide pool (pool 1–5) was tested for its ability to elicit IFN-γ spot formation from PBMCs of a HLA supertype-matched individual using an ELISPOT assay.
+, statistically significant response (p < 0.01);
−, response not statistically significant (p > 0.05); ND, not done.
Figure 4. Peptide screening in MHC-matched T. gondii-infected individuals identified 2 immunogenic peptide pools restricted by the HLA-A02 and -A03 supertypes and peptides eliciting IFN-γ in persons with HLA-A02, -A03, and B07.
(A) 42 HLA-A02-restricted peptides were divided in 5 pools with 8 to 9 peptides per pool and tested for reactivity in PBMCs isolated from HLA-matched T. gondii-seropositive individuals in an ex vivo ELISPOT assay. (B) As in (A), except that 40 HLA-A03-restricted peptides were used instead. (C) As in (B), except that 36 HLA-B07-restricted peptides were used instead. Data (mean ± s.e.m.) are from 3 T. gondii-seropositive individuals per HLA supertype; bars of the same color represent the same individual in each HLA supertype group. Control: media containing an equivalent concentration of DMSO relative to the peptide-stimulated wells; P1 to P5: peptide pools as described in Tables A1–A3. *, p=0.087; **, p<0.01. (D,E,F) In D, E and F, representative data for 1 person of each haplotype are shown. Three A02, 2 B07, and 4 A3-11 seronegative control persons did not respond to these peptides. Thus, individual peptides for each HLA supertype that can elicit high IFN-γ production were identified. Controls are the same as in A, B, and C. Peptide sequences and proteins from which they are derived are shown. *, p=0.06; **, p<0.05.
Subsequent studies were performed following the studies with deconvolution of peptide pools found with the initial algorhytms decribed above. Selection of additional predicted best binding peptides was based on peptides identified using a new algorithm at http://www.immuneepitope.org. We selected peptides with predicted binding avidities <100 nM that were present in both Type I and II parasites. Those peptides that elicited IFN-γ from T cells from all of the seropositive persons of the specific haplotype are: HLA-A02 (GRA6[VVFVVFMGV], GRA6[FMGVLVNSL], GRA3[FLVPFVVFL]), HLA-B07 (GRA7[LPQFATAAT], GRA3[VPFVVFLVA]), and HLA-A03 (SAG1[KSFKDILPK], GRA7[RSFKDLLKK], GRA6[AMLTAFFLR]). Figures 4D,E,F show data from one seropositive person of each haplotype. The data in Figures 4D, E and F show responses that are representative of data from each of the seropositive persons. Specifically, four of 4 seropositive persons of each haplotype (4 with A2, 4 with A3-11, and 4 with B7) responded to each of the peptides identified and listed above, for their haplotype. Three of these seropositive persons were then tested with these 8 peptides pooled and all three also responded to the pooled peptides. For seronegative persons, 0 of 3 A2 persons, 0 of 4 A3-11 persons and 0 of 2 B7 persons responded to the peptides pooled. These data for seronegative persons are not shown, as there was no response to these peptides
These are the first T. gondii octamer/nonamer peptides predicted to bind to HLA Class I molecules that have been identified. These are the first T. gondii octamer/nonamer peptides that have been found to elicit IFN-γ production by human T cells from seropositive and not seronegative persons.
3.5 Binding Assays
Binding assays identified physiologically relevant differences in binding avidity for the peptides noted to stimulate T cell production of IFN-γ in each of the seropositive persons of each supermotif haplotype and none of the seronegative controls of each supermotif hypothesized. (Table 2). HF10 (HPGSVNEFDF) also was tested. With the exception of HPGSVNEFDF all of the peptides identified were good binders, binding 3 or more alleles within the respective supertype with an affinity of 500 nM, or better. The HF10 peptide, HPGSVNEFDF bound several HLA B7 alleles with only weak affinity (500–5000 nM), but none with high affinity This finding explains the lack of response to HF10 in people and mice HLA-B07.
Table 2.
Toxoplasma gondii peptide binding assays
| A2-supertype peptides | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peptide ID | Sequence | Len | Protein | HLA A02-supertype binding capacity (IC50 nM) |
Alleles bound | ||||
| A*0201 | A*0202 | A*0203 | A*0206 | A*6802 | |||||
| 7103.0001 | VVFVVFMGV | 9 | GRA6 | 135 | 3451 | 19 | 0.92 | 39 | 4 |
| 7103.0002 | FMGVLVNSL | 9 | GRA6 | 239 | 5.3 | 2.8 | 553 | 4335 | 3 |
| 7103.0003 | FLVPFVVFL | 9 | GRA3 | <0.1 | <0.1 | 0.11 | 3.5 | 1.5 | 5 |
| A3-supertype peptides | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peptide ID | Sequence | Len | Protein | HLA A03-supertype binding capacity (IC50 nM) |
Alleles bound | |||||
| A*0301 | A*1101 | A*3101 | A*3301 | A*6801 | A*3001 | |||||
| 7103.0004 | KSFKDILPK | 9 | SAG1 | 20 | 54 | 129 | - | 11870 | 200 | 4 |
| 7103.0005 | AMLTAFFLR | 9 | GRA6 | 36 | 3.6 | 0.31 | 26 | 263 | 12056 | 5 |
| 7103.0006 | RSFKDLLKK | 9 | GRA7 | 14 | 14 | 303 | - | 10553 | 145 | 4 |
| B7-supertype peptides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide ID | Sequence | Len | Protein | HLA B07-supertype binding capacity (IC50 nM) |
Alleles bound | ||||||
| B*0702 | B*3501 | B*3503 | B*4201 | B*5101 | B*5301 | B*5401 | |||||
| 7103.0007 | LPQFATAAT | 9 | GRA7 | 14 | 4045 | 6630 | 17 | 15388 | - | 106 | 3 |
| 7103.0008 | VPFVVFLVA | 9 | GRA3 | 18 | 61936 | 20589 | 36 | 3016 | 30896 | 0.87 | 3 |
| 7103.0009 | HPGSVNEFDF | 10 | GRA6 | 3621 | 2093 | 11457 | 877 | 14738 | 5521 | 854 | 0 |
3.6 Congenital toxoplasmosis associates with ERAP1 in humans
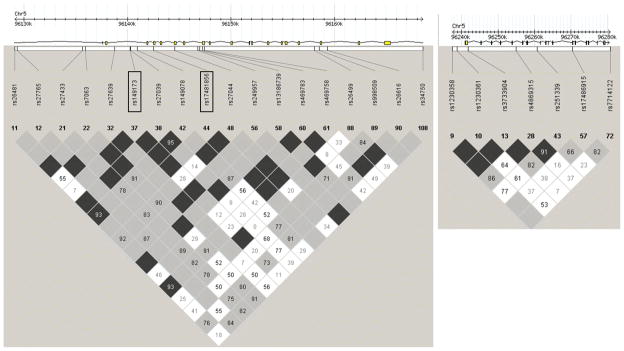
Previous studies have identified ERAAP as a ‘susceptibility gene’ for T. gondii infection in mice because ERAAP is required for proteolytic generation of the immunodominant HF10 epitope. We hypothesized that ERAAP might also play a critical immunomodulatory role in humans by shaping the peptide repertoire presented by MHC class I molecules to protective CD8+ T cells. To test this hypothesis, we genotyped a total of 32 tag SNPs that were selected in the human ERAAP genes, ERAP1 (also ARTS-1) which is the homologue of the mouse ERAAP gene and ERAP2 (also L-RAP) in a North American cohort of case-parent trios comprising 124 congenitally infected children, many with ocular and/or brain disease, using a r2 threshold of 0.8. Overall, 25 SNPs passed all the genotyping QCs. We found that 2 SNPs in ERAP1, rs149173 and rs17481856, associated with congenital toxoplasmosis (Table 3, p<0.05), while none of the 7 SNPs in ERAP2 was observed to associate with disease (Table 3, p>0.05). The 2 SNPs that showed significant association with congenital disease, rs149173 and rs17481856, were in high LD with each other (r2 = 0.4) and are therefore likely part of the same associated haplotype (Figure 5). This suggests that one of those SNPs or an etiological variant in strong LD with these 2 markers accounts for the observed association with susceptibility to this congenital disease. Although neither rs149173 nor rs17481856 result in nonsynonymous amino acid changes in ERAP1 other variants in strong LD with these likely are responsible for the observed association through a change in the protein sequence or through regulation of ERAP1 expression in vivo (for example rs27044 causes Glu to Gln change and has r2 of 0.4 as does rs17481856, although slightly less associated with the trait). This work provides the first evidence for ERAP1 as a ‘susceptibility gene’ in T. gondii infection in humans and confirms the importance of MHC Class I antigen processing in humans in response to this infection.
Table 3.
Detailed information on the 25 genotyped SNPs in ERAP1 and ERAP2 and their associated TDT p values.
| SNP (dbSNP) | Bp Position | Position in Gene | AA changea | NCCCTS patient cohort | P valued | |
|---|---|---|---|---|---|---|
| Overtransmitted alleleb | T/U frequencyc | |||||
| ERAP1 (reverse strand Chr 5q15) | ||||||
| rs26481 | 96129108 | Intron 19 | – | T/C | 1.18333 | 0.5791 |
| rs27765 | 96129345 | Intron 19 | – | G/A | 0.29444 | 0.3657 |
| rs27433 | 96135691 | Intron 19 | – | T/G | 0.75903 | 0.3692 |
| rs7063 | 96135967 | Intron 19 | – | T/A | 0.93056 | 0.7576 |
| rs27639 | 96138798 | Intron 18 | – | T/G | 0.67292 | 0.1615 |
| rs149173 | 96140949 | Intron 18 | – | T/C | 1.09028 | 0.0077 |
| rs27039 | 96140306 | Intron 18 | – | C/T | 1.26319 | 0.1161 |
| rs149078 | 96140949 | Intron 18 | – | T/C | 0.96667 | 0.063 |
| rs17481856 | 96142564 | Exon 17 | – | C/T | 0.62847 | 0.0253 |
| rs27044 | 96144608 | Exon 15 | Gln-730-Glu | C/G | 1.05139 | 0.0782 |
| rs249957 | 96146798 | Intron 13 | – | A/G | 0.29653 | 1 |
| rs13186739 | 96146999 | Intron 13 | – | T/C | 0.80347 | 0.7389 |
| rs469783 | 96147280 | Exon 13 | – | A | 1.26319 | 0.1161 |
| rs469758 | 96147471 | Intron 12 | – | T/C | 1.26319 | 0.1161 |
| rs26499 | 96158099 | Intron 4 | – | C/T | 1.14375 | 1 |
| rs998509 | 96158551 | Intron 4 | – | G/A | 0.46458 | 0.6547 |
| rs26616 | 96159348 | Intron 3 | – | C/G | 0.88333 | 0.1172 |
| rs34750 | 96168562 | Intron 1 | – | C/G | 0.92986 | 0.6394 |
| ERAP2 (forward strand Chr 5q15) | ||||||
| rs1230358 | 96237497 | 5′ UTR | – | A/T | 0.97014 | 0.3428 |
| rs1230361 | 96238927 | 5′ UTR | – | C/A | 0.88542 | 0.3173 |
| rs3733904 | 96241929 | Intron 1 | – | A/G | 0.84167 | 0.1573 |
| rs4869315 | 96255028 | Intron 5 | – | C/G | 1.34861 | 0.1736 |
| rs251339 | 96260794 | Intron 8 | – | T/C | 1.34653 | 0.0687 |
| rs17486915 | 96270475 | Exon 13 | – | C/T | 0.25347 | 0.763 |
| rs7714122 | 96280773 | 3′ UTR | – | C | 0.37986 | 0.6171 |
Where applicable.
Allele that was overtransmitted from heterozygous parents to affected offspring presented as overtransmitted allele/under-transmitted allele.
The ratio of transmitted to under-transmitted alleles from heterozygous parents to affected offspring.
p value for the degree of genetic association between the indicated SNP and ocular and/or brain disease in congenital toxoplasmosis was determined using TDT analysis in Haploview. Statistically significant associations (p < 0.05) were bolded
Figure 5. Gene structure and LD plots for human ERAP1 and ERAP2.
Upper diagrams show the positions of genotyped SNPs relative to the intron/exon structure of each gene. Lower diagrams show the LD plots generated in Haploview using data for each gene from our North American patient cohort. LD values (D′ × 100) between markers are indicated at the intercept of the 2 markers on the matrix. Where there is no value, D′=1 (i.e. 100). The black (high) through grey to white (low) shading indicates the degree of confidence in the estimate of LD between the markers. The 2 SNPs in ERAP1 that were found to associate with congenital disease (rs149173 and rs17481856) are outlined in black rectangles. Left panel, ERAP1; right panel, ERAP2.
4. DISCUSSION
In this study, we showed that palmitoylated lipopeptides elicit potent IFN-γ production in vitro, comparable to that induced by the synthetic TLR4 agonist, MLA. Furthermore, IFN-γ responses were primarily dependent on antigen-specific, Ld-restricted CD8+ T cells, which are critical mediators of protective immunity in T. gondii-infected mice. However, HF1O was not immunogenic in HLA-B*0702 mice, and neither HF10 nor 3 other Ld-restricted T. gonii candidate peptides were found to be immunogenic in infected HLA-B07+ humans, even though bioinformatic and structural analyses suggest that both Ld and HLA-B07 supertype molecules bind extensively overlapping peptide repertoires. We then identified additional epitopes from T. gondii dense granule protein GRA6, GRA7, GRA3, and surface antigen 1, SAG1, that are immunogenic in people expressing HLA-A02, -A03, and -B07 supertype alleles. In addition, we showed for the first time that 2 SNPs in ERAP1 that were in strong LD with each other associated with susceptibility to congenital toxoplasmosis in humans.
Administration of HF10 with adjuvants protects mice. In Figure 1C we show that HF10 alone does not induce IFN-γ but HF10 included in lipopeptide constructs does. Immunization with lipopeptides protected mice against challenge with T. gondii. We selected lipopeptides to test because this formulation has successfully induced other CD8+ T cell reponses in humans as well as mice [21–24]. The lipopeptides we used were selected because of prior demonstrations by others that they were immunogenic and stable. Specifically, Livingston et al (21) used a 2 PAM Cys lipopeptide formulation in studies in humans, finding this to be effective in eliciting an immune response against hepatitis B. Deliyannis et al (22) used a 4 PAM Cys lipopeptide formulation in studies in mice, finding this to be effective in producing prolonged memory CD8 + T cells directed against influenza virus. Thus, these constructs were selected for our proof of principal studies to attempt to induce a protective immune response against T. gondii. In our laboratory for these studies, lipopeptides have been active in eliciting IFN-γ, in experiments performed during a period of 8 months after they were synthesized. They were stored at −20 degrees C during this time prior to care.
The new MLA-like adjuvant was provided by The Infectious Disease Research Institute Seattle (IDRI) and it has been very effective as an adjuvant providing CD4+ T cell help for immunizations against other protozoan infections [25] [S. Reed, IDRI, Seattle, personal communication to RMc, 2009]. Our studies provide initial evidence in vitro and in vivo for efficacy of lipopeptides as inducing protective immune responses against T. gondii. MLA also was an effective adjuvant. While MLA promotes a Th1 response by activating TLR4, mechanisms by which lipopeptides enhance cell-mediated immune responses remain to be defined, with speculation that they may be TLR2 ligands. Earlier studies have suggested that lipopeptides upregulate antigen presentation by dendritic cells and macrophages to T cells, possibly by associating with hydrophobic cell membranes, or otherwise inducing maturation of professional antigen-presenting cells and consequent release of pro-inflammatory cytokines [23,47,48]. Lipopeptides represent attractive candidates as vaccines, because they are safe, easy to manufacture and store, and do not require additional adjuvants [21–24]. Future studies will investigate relative immunogenicity of other lipid-based vaccines such as NISV or bilosome enclosed peptides or DNA vaccines, as well as efficacy of different routes of vaccine delivery and use of MLA with lipopeptides. Both lipopeptides and liposomes have also been shown to induce mucosal immune responses, which are crucial for limiting toxoplasmic pathology and transmission since initial infection in humans occurs via the gastrointestinal tract through ingestion of oocysts or encysted bradyzoites in undercooked meat [11], except in cases of congenital infection. We will also determine whether sterile, long-term immunity can be attained in immunized mice when challenged with a type II strain of T. gondii and whether co-delivery of cytokines such as interleukin-15 (IL-15), IL-12, or inhibition of TOR with rapamycin enhance antigen-specific recall responses.
Initially, quite, surprisingly, our results in HLA-B*0702 mice and HLA-B07+ individuals suggested that HF10 was not the immunogenic epitope presented by HLA-B07 supertype molecules to CD8+ T cells in mice and humans. We confirmed that the assay was satisfactory and robust with both EBV and later with specific T. gondii peptides. This lack of immunogeneticity of HF10 was confirmed in our studies with HLA-B07 transgenic mice, where other peptides were immunogenic. This finding of lack of immunogeneticity of T. gondii’s HF10, GRA4, ROP7, and SAG1 peptides was unexpected since both HLA-B07 supertype molecules and murine Ld exhibit the ‘X-P’ peptide binding motif. We predicted that one of several factors might contribute to this observation. HLA-B07 supertype molecules might bind to HF10 with a lower functional avidity relative to Ld due to differences in size and charge in structures of their peptide-binding pockets. Length of the HF10 decapeptide might fit better in the larger Ld binding pocket or prevent productive interactions between the HLA-B07/HF10 complex and the human CD8+ T cell receptor. In this scenario, presentation of HF10 by Ld might be the exception rather than the rule. A lack of appropriate precursors in human T cell repertoires that recognize the HLA-B07/HF10 complex would also prevent induction of a significant, detectable human cytokine response. It is also possible that GRA6 is not endogenously processed to yield HF10 in humans the same way it is in mice due to differences in MHC class I antigen processing pathways in humans and mice. Binding studies provided the empirical evidence to resolve these questions. These data confirmed that the peptides we identified, using our bioinformatics approaches followed by testing responses of T cells from humans, avidly bind HLA A2, A3-11 or B7, and are consistent with those that elicit IFNγ producing T cells. However, HF10 was not an avidly binding peptide for HLA B07 explaining our empirical results in which HF10 did not stimulate human HLA B7 lymphocytes from infected persons to produce IFNγ nor was HF10 immunogeneic in HLA B7 mice.
Specifically, this work identifies, for the first time, the importance of nine specific T. gondii peptides present in both Type I and II parasites in eliciting production of interferon γ by human CD8+T cells for persons with the three major HLA supermotifs. These supermotifs are present in ~90% of humans in the world. It also identifies the parasite proteins from which these peptides are derived. Thereby, we demonstrate that these proteins have peptides that traffic into an MHC Class 1 pathway during this infection in humans. These are proteins which the parasite secretes or that are on the parasite surface. In addition this work defines the critical role of ERAP1, an aminopeptidase in the MHC Class I antigen processing pathway, in susceptibility and resistance to the parasite in human congenital toxoplasmosis. This emphasizes the importance of MHC Class I antigen processing in defense of humans against this obligate intracellular parasite. This is of special interest because this parasite is sequestered in a vacuole in the host cell away from host cell cytoplasm. This work proves that in human cells these peptides must be trafficked from secreted or surface proteins of the parasite into an MHC Class I pathway. Thus, this work provides the foundation and the molecular tools for studying mechanisms of cell-mediated immune responses to T. gondii in humans or transgenic mice with human MHC Class I molecules. It also provides a paradigm for defining peptides important in MHC Class 1 responses for obligate intracellular organisms in humans. This approach will be useful for defining protective immune responses against other pathogens, and in fact potentially for a variety of other types of pathologic processes and diseases as well.
Although this supermotif binding algorhythm approach ultimately proved to be exceptionally useful to predict the T. gondii peptides that human T cells do recognize, it first provided new insights into potential confounding aspects of extrapolating information from Class I MHC antigen processing and presentation in murine models to peptides recognized by CD8+T cells following human MHC Class I antigen presentation. The importance of other features for peptide binding to murine and human MHC molecules was demonstrated. Specifically, initial algorhythms and work predicted that the shared amino acids at the second and 8–9th position of the peptides that binds in the antigen presenting molecule groove of HLA B7 and a critical murine MHC molecule Ld are the same. This indicated that HLA B7 and Ld should bind the same peptides and present them to T cells. However, the experiments performed to test these predictions did not result in presentation of the same peptides with the same efficiency for Ld mice as for B7 mice or B7 humans. In fact, we first predicted those peptides defined recently to bind Ld would be the needed clue for the work with human HLA B7 cells and HLA B7 transgenic mice. However, HLA B7 human’s T cells, from infected persons, that recognize other peptides with this shared binding motif did not recognize those peptides defined recently as critical for presentation by murine Ld.
Blanchard et al utilized ERAAP knock out mice to prove that MHC Class I processing was essential for Ld presentation of HF10 and resistance of mice to T. gondii. It was therefore of interest to determine whether ERAAP would influences outcomes of congenital toxoplasmosis. If it did so, it would provide further evidence that MHC Class I presentation and antigen processing are important for protection/resistance in humans with congenital toxoplasmosis. Intriguingly, humans express 2 ERAAP genes, ERAP1 and ERAP2, while mice have only 1 known ERAAP gene. ERAP1 closely resembles murine ERAAP in its substrate specificity and function, but ERAP2, which has 49% homology with ERAP1, exhibits a significantly different substrate specificity from ERAP1 [49]. Activity and contribution of ERAP1 and ERAP2 towards shaping of final peptide-MHC repertoire in humans remain poorly understood, although both proteases might act in tandem or as heterodimers to trim precursor antigens [49,50]. Blanchard et al. demonstrated, ERAAP-deficient H-2d mice generate significantly lower frequencies of HF10-specific CD8+ T cells and are consequently not protected from T. gondii infection. Herein, we provide evidence that polymorphisms in the human ERAAP gene, ERAP1, also confer increased susceptibility to congenital toxoplasmosis in humans.
We propose that an effective approach for development of a vaccine against T. gondii infection is an immunosense vaccine; one that considers not only adjuvants and CD8+ T cell epitopes but also MHC-restriction of putative epitopes, antigen specificity of different archetypal clonal and nonarchetypal strains and of different life-cycle stages of the parasite, as well as other genetic factors that influence in vivo generation of immunogenic epitopes and quality of immune responses. It will be useful to identify a panel of immunogenic epitopes derived from all 3 strains of T. gondii that are presented by the broad-coverage HLA supertypes, such as HLA-A02, HLA-A03, HLA-B07, HLA-A-24, and HLA-B44. The putative secretome of T. gondii, which includes the rhoptry and dense granule proteins, and surface antigens, represents a central source of antigens that can access the MHC class I processing pathway. Consistent with this, our peptides that have been identified as immunogenic appear to be derived from secreted proteins and a surface antigen, SAG1. In addition, some CD4+ T cell epitopes bound by the major HLA Class II alleles, have been identified [31,51] and are more promiscuous so they could be included to induce specific T. gondii CD4+ T cells, and epitopes that induce humoral immunity are known as well. We propose that collectively, these epitopes, properly adjuvanted, will elicit an immune response that is sufficiently potent and comprehensive to protect against toxoplasmosis in humans. Support for this approach comes from several lines of evidence: It appears that sterile infections have occurred naturally when an initial infection of pigs completely protected some immunized animals against subsequent infection [52]. A robust experience with comprehensive maternal serologic screening to detect seroconversion and congenital T. gondii infection in France for immunologically normal pregnant women demonstrated that, with very rare exception, only those who acquire the infection for the first time while pregnant transmit the infection to their fetus (Desmonts G, personal communication to McLeod R, 1990). The present work begins to provide a foundation for determining whether an immunosense vaccine will be protective.
Supplementary Material
Acknowledgments
We thank C. Oseroff for helpful suggestions, P. Terasaki for HLA-typing, J. Boothroyd and S. Kim for the luciferase expressing parasite, N. Blanchard and N. Shastri for sharing pre-publication data and other suggestions, S. Reed for adjuvants, T. Gajewski and M. Alegre for their helpful comments, and families and collaborating physicians/scientists in the NCCCTS who made this work possible. We gratefully acknowledge support of this work by gifts from the Fin Charity Trust, R Blackfoot, R Thewind, A Akfortseven, S Gemma, S Jackson, AK Bump, the Rooney Aldens, the Dominique Cornwell and Peter Mann Family Foundation, the Morel, Rosenstein, Kapnick, Taub, Latsko, and Kiewit families, Intervet/Schering Plough, and Toxoplasmosis Research Institute. This work also was supported by DMID-NIAID U01 AI77887 (RM) and the Intramural Research Program (NIH, NIAID [MG]) and The Research to Prevent Blindness Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ajzenberg D, Banuls AL, Su C, Dumetre A, Demar M, Carme B, et al. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int J Parasitol. 2004;34:1185–96. doi: 10.1016/j.ijpara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Ryning FW, McLeod R, Maddox JC, Hunt S, Remington JS. Probable transmission of Toxoplasma gondii by organ transplantation. Ann Intern Med. 1979;90:47–9. doi: 10.7326/0003-4819-90-1-47. [DOI] [PubMed] [Google Scholar]
- 3.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–92. [PubMed] [Google Scholar]
- 4.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 5.Brown CR, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–41. [PubMed] [Google Scholar]
- 6.Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, et al. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–28. [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JJ, Roberts CW, Pope C, Roberts F, Kirisits MJ, Estes R, et al. In vitro correlates of Ld-restricted resistance to toxoplasmic encephalitis and their critical dependence on parasite strain. J Immunol. 2002;169:966–73. doi: 10.4049/jimmunol.169.2.966. [DOI] [PubMed] [Google Scholar]
- 8.McLeod R, Boyer K, Karrison T, Kasza K, Swisher C, Roizen N, et al. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis. 2006;42:1383–94. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 9.McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:320–44. doi: 10.1590/s0074-02762009000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLeod R, Frenkel JK, Estes RG, Mack DG, Eisenhauer PB, Gibori G. Subcutaneous and intestinal vaccination with tachyzoites of Toxoplasma gondii and acquisition of immunity to peroral and congenital toxoplasma challenge. J Immunol. 1988;140:1632–7. [PubMed] [Google Scholar]
- 11.Golkar M, Shokrgozar MA, Rafati S, Musset K, Assmar M, Sadaie R, et al. Evaluation of protective effect of recombinant dense granule antigens GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant against Toxoplasma chronic infection in mice. Vaccine. 2007;25:4301–11. doi: 10.1016/j.vaccine.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 12.Leyva R, Herion P, Saavedra R. Genetic immunization with plasmid DNA coding for the ROP2 protein of Toxoplasma gondii. Parasitol Res. 2001;87:70–9. doi: 10.1007/s004360000296. [DOI] [PubMed] [Google Scholar]
- 13.Lourenco EV, Bernardes ES, Silva NM, Mineo JR, Panunto-Castelo A, Roque-Barreira MC. Immunization with MIC1 and MIC4 induces protective immunity against Toxoplasma gondii. Microbes Infect. 2006;8:1244–51. doi: 10.1016/j.micinf.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen HV, Lauemoller SL, Christiansen L, Buus S, Fomsgaard A, Petersen E. Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect Immun. 1999;67:6358–63. doi: 10.1128/iai.67.12.6358-6363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, et al. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol. 2008;9:937–44. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corr M, Boyd LF, Frankel SR, Kozlowski S, Padlan EA, Margulies DH. Endogenous peptides of a soluble major histocompatibility complex class I molecule, H-2Lds: sequence motif, quantitative binding, and molecular modeling of the complex. J Exp Med. 1992;176:1681–92. doi: 10.1084/jem.176.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 2008;9:1. doi: 10.1186/1471-2172-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sette A, Sidney J, Livingston BD, Dzuris JL, Crimi C, Walker CM, et al. Class I molecules with similar peptide-binding specificities are the result of both common ancestry and convergent evolution. Immunogenetics. 2003;54:830–41. doi: 10.1007/s00251-002-0530-0. [DOI] [PubMed] [Google Scholar]
- 19.Caetano BC, Bruna-Romero O, Fux B, Mendes EA, Penido ML, Gazzinelli RT. Vaccination with replication-deficient recombinant adenoviruses encoding the main surface antigens of toxoplasma gondii induces immune response and protection against infection in mice. Hum Gene Ther. 2006;17:415–26. doi: 10.1089/hum.2006.17.415. [DOI] [PubMed] [Google Scholar]
- 20.Frickel EM, Sahoo N, Hopp J, Gubbels MJ, Craver MP, Knoll LJ, et al. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J Infect Dis. 2008;198:1625–33. doi: 10.1086/593019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livingston BD, Alexander J, Crimi C, Oseroff C, Celis E, Daly K, et al. Altered helper T lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in humans. J Immunol. 1999;162:3088–95. [PubMed] [Google Scholar]
- 22.Deliyannis G, Jackson DC, Ede NJ, Zeng W, Hourdakis I, Sakabetis E, et al. Induction of long-term memory CD8(+) T cells for recall of viral clearing responses against influenza virus. J Virol. 2002;76:4212–21. doi: 10.1128/JVI.76.9.4212-4221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology. 2002;106:113–21. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines--yesterday, today, and tomorrow. Lancet Infect Dis. 2002;2:425–31. doi: 10.1016/s1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 25.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol. 2002;169:4905–12. doi: 10.4049/jimmunol.169.9.4905. [DOI] [PubMed] [Google Scholar]
- 27.Ishioka GY, Fikes J, Hermanson G, Livingston B, Crimi C, Qin M, et al. Utilization of MHC class I transgenic mice for development of minigene DNA vaccines encoding multiple HLA-restricted CTL epitopes. J Immunol. 1999;162:3915–25. [PubMed] [Google Scholar]
- 28.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, et al. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002;10:S32–S7. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 29.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–12. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 30.Hammer GE, Kanaseki T, Shastri N. The final touches make perfect the peptide-MHC class I repertoire. Immunity. 2007;26:397–406. doi: 10.1016/j.immuni.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Alexander J, Oseroff C, Sidney J, Sette A. Derivation of HLA-B*0702 transgenic mice: functional CTL repertoire and recognition of human B*0702-restricted CTL epitopes. Hum Immunol. 2003;64:211–23. doi: 10.1016/s0198-8859(02)00786-3. [DOI] [PubMed] [Google Scholar]
- 32.Kong JT, Grigg ME, Uyetake L, Parmley S, Boothroyd JC. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis. 2003;187:1484–95. doi: 10.1086/374647. [DOI] [PubMed] [Google Scholar]
- 33.Mack DG, Johnson JJ, Roberts F, Roberts CW, Estes RG, David C, et al. HLA-class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol. 1999;29:1351–8. doi: 10.1016/s0020-7519(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 34.Botten J, Alexander J, Pasquetto V, Sidney J, Barrowman P, Ting J, et al. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J Virol. 2006;80:8351–61. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen M, Lundegaard C, Lund O, Kesmir C. The role of the proteasome in generating cytotoxic T-cell epitopes: insights obtained from improved predictions of proteasomal cleavage. Immunogenetics. 2005;57:33–41. doi: 10.1007/s00251-005-0781-7. [DOI] [PubMed] [Google Scholar]
- 36.Kim SK, Karasov A, Boothroyd JC. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect Immun. 2007;75:1626–34. doi: 10.1128/IAI.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamieson SE, de Roubaix LA, Cortina-Borja M, Tan HK, Mui EJ, Cordell HJ, et al. Genetic and epigenetic factors at COL2A1 and ABCA4 influence clinical outcome in congenital toxoplasmosis. PLoS One. 2008;3:e2285. doi: 10.1371/journal.pone.0002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeij JP, Arrizabalaga G, Boothroyd JC. A cluster of four surface antigen genes specifically expressed in bradyzoites, SAG2CDXY, plays an important role in Toxoplasma gondii persistence. Infect Immun. 2008;76:2402–10. doi: 10.1128/IAI.01494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidney J, Assarsson E, Moore C, Ngo S, Pinilla C, Sette A, et al. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008;4:2. doi: 10.1186/1745-7580-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidney J, Southwood S, Mann DL, Fernandez-Vina MA, Newman MJ, Sette A. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum Immunol. 2001;62:1200–16. doi: 10.1016/s0198-8859(01)00319-6. [DOI] [PubMed] [Google Scholar]
- 41.Sidney J, Southwood S, Oseroff C, del Guercio MF, Sette A, Grey HM. Measurement of MHC/peptide interactions by gel filtration. Curr Protoc Immunol. 2001;Chapter 18(Unit 18):3. doi: 10.1002/0471142735.im1803s31. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 43.Gulukota K, Sidney J, Sette A, DeLisi C. Two complementary methods for predicting peptides binding major histocompatibility complex molecules. J Mol Biol. 1997;267:1258–67. doi: 10.1006/jmbi.1997.0937. [DOI] [PubMed] [Google Scholar]
- 44.Hourigan CS, Harkiolaki M, Peterson NA, Bell JI, Jones EY, O’Callaghan CA. The structure of the human allo-ligand HLA-B*3501 in complex with a cytochrome p450 peptide: steric hindrance influences TCR allo-recognition. Eur J Immunol. 2006;36:3288–93. doi: 10.1002/eji.200636234. [DOI] [PubMed] [Google Scholar]
- 45.Speir JA, Garcia KC, Brunmark A, Degano M, Peterson PA, Teyton L, et al. Structural basis of 2C TCR allorecognition of H-2Ld peptide complexes. Immunity. 1998;8:553–62. doi: 10.1016/s1074-7613(00)80560-9. [DOI] [PubMed] [Google Scholar]
- 46.Cesbron-Delauw MF, Capron A. Excreted/secreted antigens of Toxoplasma gondii--their origin and role in the host-parasite interaction. Res Immunol. 1993;144:41–4. doi: 10.1016/s0923-2494(05)80096-3. [DOI] [PubMed] [Google Scholar]
- 47.Schild H, Deres K, Wiesmuller KH, Jung G, Rammensee HG. Efficiency of peptides and lipopeptides for in vivo priming of virus-specific cytotoxic T cells. Eur J Immunol. 1991;21:2649–54. doi: 10.1002/eji.1830211102. [DOI] [PubMed] [Google Scholar]
- 48.Tsunoda I, Sette A, Fujinami RS, Oseroff C, Ruppert J, Dahlberg C, et al. Lipopeptide particles as the immunologically active component of CTL inducing vaccines. Vaccine. 1999;17:675–85. doi: 10.1016/s0264-410x(98)00250-3. [DOI] [PubMed] [Google Scholar]
- 49.Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol. 2005;6:689–97. doi: 10.1038/ni1208. [DOI] [PubMed] [Google Scholar]
- 50.Hammer GE, Gonzalez F, Champsaur M, Cado D, Shastri N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat Immunol. 2006;7:103–12. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- 51.Beghetto E, Nielsen HV, Del Porto P, Buffolano W, Guglietta S, Felici F, et al. A combination of antigenic regions of Toxoplasma gondii microneme proteins induces protective immunity against oral infection with parasite cysts. J Infect Dis. 2005;191:637–45. doi: 10.1086/427660. [DOI] [PubMed] [Google Scholar]
- 52.Dubey JP, Speer CA, Shen SK, Kwok OC, Blixt JA. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J Parasitol. 1997;83:870–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.