Abstract
Objective
Increased fat infiltration in skeletal muscle has been associated with diabetes. Quantitative computed tomography (QCT) can be used to measure muscle density, which reflects the lipid content of skeletal muscle such that greater fat infiltration in skeletal muscle is associated with lower muscle density. The relative contribution of genetic and environmental factors to fat infiltration in skeletal muscle has not been assessed. Therefore, our aim is to determine genetic and environmental contributions to measures of skeletal muscle composition, and describe their associations with type 2 diabetes in multigenerational families of African ancestry.
Methods and Procedures
Peripheral QCT (pQCT) measures of skeletal muscle density were obtained for the calf in 471 individuals (60% women; mean 43 years) belonging to eight large, multigenerational Afro-Caribbean families (mean family size 51 individuals; 3,535 relative pairs).
Results
The proportion of variance in muscle density due to additive genetic effects (residual heritability) was 35.0% (P < 0.001) and significant covariates (age, gender, BMI, and parity) explained 55.0% of the total phenotypic variation in muscle density. Muscle density was lower (P < 0.001) in 62 diabetics (69.5 mg/cm3) than in 339 nondiabetics (74.3 mg/cm3) and remained significant after adjusting for age, gender, and BMI (P = 0.005) or age, gender, and waist circumference (P = 0.01).
Discussion
Our results provide new evidence that ectopic lipid deposition in skeletal muscle is a heritable trait and is associated with diabetes, independent of overall and central obesity in families of African heritage. Genome-wide screens and candidate gene studies are warranted to identify the genetic factors contributing to ectopic deposition of skeletal muscle fat.
INTRODUCTION
Skeletal muscle accounts for ~80% of glucose disposal in the human body, and therefore, is crucial for maintaining glucose homeostasis (1). Emerging evidence suggests that ectopic fat accumulation in skeletal muscle may be a major cause of insulin resistance and diabetes (2–4).
Obesity and diabetes disproportionately affect populations of African origin (5,6). A number of studies reported higher fasting insulin and insulin resistance in African Americans than in other ethnicities across all ages, as well as among diabetic and nondiabetic, or lean and obese individuals of African origin (7–11). The underlying basis for these ethnic differences is still unclear. The increased prevalence of diabetes in populations of African origin cannot be explained entirely by differences in overweight and obesity measured by BMI (11). Even differences in abdominal visceral and abdominal subcutaneous adipose tissue (SAT) fail to completely explain ethnic differences in insulin sensitivity (12,13). Some investigators postulate that adipose tissue infiltration in skeletal muscle may at least partially account for ethnic differences in insulin sensitivity and diabetes risk (12–14). Indeed, we and others have demonstrated greater adipose infiltration in skeletal muscle despite comparable total body fat among individuals of African heritage compared with whites (13,15–17).
Body fat and fat distribution are highly heritable traits (18–21). However, to our knowledge, the relative contribution of genetic and environmental factors to adipose tissue infiltration in skeletal muscle has not been assessed. Therefore, the primary aim of this study was to determine the magnitude of genetic and environmental influences on peripheral quantitative computed tomography (pQCT) measures of skeletal muscle composition in large, multigenerational families of African origin, and to describe the association with type 2 diabetes.
METHODS AND PROCEDURES
Study sample
In 2003, we began The Tobago Family Health Study to understand better the role of inheritance, lifestyle, and body weight and composition in the etiology of several common chronic diseases. To date, we have recruited 471 individuals aged 18–103 years (mean age 43 years) belonging to eight multigenerational families (mean family size 51 individuals) of African origin on the Caribbean island of Tobago. These eight multigenerational families are of the following sizes: 102, 26, 49, 28, 113, 21, 38, and 94. Among these 471 individuals, we have the following relationships: 361 parent-offspring, 495 full siblings, 101 grandparent–grandchildren, 1,137 avuncular, 61 half-sibs, and 1,380 cousins (3,535 relative pairs). To be eligible, a proband had to be Afro Caribbean, have had a spouse who was willing to participate in the study, and have at least six living offspring and/or siblings aged ≥18 years who were residing in Tobago. Because we were interested in establishing a community-based sample of families, probands and their family members were recruited without regard to their health status. Written informed consent was obtained from every participant, using forms and procedures approved by the Tobago Ministry of Health and Social Services and University of Pittsburgh Institutional Review Boards.
Data collection
Information on a wide range of lifestyle habits (current smoking (yes/no), current alcohol intake (more than one drink per week (yes/no)), walking (minutes per week), TV viewing (hours/week)), medical conditions, medication use, and reproductive characteristics in women (age at menarche, menopause, parity, oral contraceptive use, etc) were assessed using standardized interviewer-administered questionnaires that were reviewed with participants in the study clinic. We recorded information on walking, because walking is the predominant form of physical activity on the island. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Weight was recorded to the nearest 0.1 kg without shoes on a balance-beam scale.
pQCT measures
A lower-leg pQCT scan was performed using the Stratec XCT-2000 device (Orthometrix, White Plains, NY) in order to evaluate the total, muscle, and fat cross-sectional areas of the calf. Scans were obtained at 66% of the tibial length, proximal to the terminal end of the tibia. This site was chosen as it is the region of the lower leg with the largest circumference of the calf with very little variability across individuals (22). Images of cross-sectional area of the muscle, fat tissue, and density of skeletal muscle were analyzed by using the Stratec analysis software (Version 5.5 D; Orthometrix, White Plains, NY). To maintain consistency, all images were analyzed by a single investigator. Using various edge detection and thresholding steps, the pQCT image can be segmented into various bone and soft tissue measures. For this project, we selected measures of the calf cross-sectional area total adipose tissue (TAT), cross-sectional area SAT, and the average calf muscle density. TAT was expressed in mm2. SAT percentage (%) was determined as: (SAT cross-sectional area/TAT cross-sectional area) × 100. Muscle density is expressed in mg/cm3 and is a valid measure of adipose tissue infiltration in skeletal muscle (23). The coefficients of variation for pQCT measures were determined by repeat pQCT scanning in 15 individuals. The coefficient of variations for TAT, SAT, and muscle density were 10.8, 3.8, and 4.1%, respectively.
Blood sample collection, glucose measurement, and classification of diabetes
Blood samples were obtained in the morning by venipuncture after a 12-h fast. Whole blood was drawn into sterile red top (serum) tubes, stood at room temperature for a minimum of 20 min to clot before centrifugation, and the serum aliquoted into 1.0 ml cryovials and frozen at −20 °C, and transferred to −80 °C within a month. Serum glucose was available for 401 participants and was measured using an enzymatic procedure (24). The coefficient of variation between runs is 1.8%. Diabetes was defined as fasting serum glucose ≥126 mg/dl (25) or currently taking antidiabetic medication.
Statistical analyses
First, the distributions of all the traits were assessed for non-normality and data were transformed before statistical analysis to reduce non-normality. Subsequently, all outliers (±3.5 s.d.) were removed for each trait, and no more than four values were removed for a single variable. We first performed a combined forward and backward stepwise linear regression analysis, ignoring the nonindependence of the subjects, using the R statistical package (R, Version 2.2.1; R Foundation for Statistical Computing, Vienna, Austria) (26). At this initial screening stage, we used a liberal significance level (P ≤ 0.10) to keep the covariate in the model. We subsequently evaluated each of the potentially significant covariates, using a variance components framework that takes into account the correlations among family members. Because of their presumed relationships with the obesity-related traits, age, gender, and BMI were also evaluated as potential covariates in every variance components model, even if they were not significant at the initial screening stage. These analyses were performed using the program SOLAR (Solar, Version 2.1.4; Southwest Foundation for Biomedical Research, San Antonio, TX) (27). On the basis of the pedigree information, the phenotype information, and the potentially significant covariates, quantitative genetic methods were used to model the total variation in all phenotypic parameters as a function of the mean trait value (additive genetic effects, heritability residual, h2r), effects attributed by the measured covariates, and the uncertain variation due to residual genetic and unmeasured environmental impact plus random errors. To compare the effects of measured covariates (age, gender, BMI, current smoking, current alcohol intake, minutes walking per week, postmenopausal status, parity, age at menarche, and oral contraceptive use) across all traits (in original or its transformed form), we report the strength of association between covariates and pQCT traits as a percent difference in the pQCT trait per unit of the covariate, instead of the nonstandardized β-coefficients. For continuous covariates, the unit range was every 10 years for age and every 1 kg/m2 for BMI. The unit range for dichotomous covariates equaled 1 (28). The percentage difference between youngest and oldest age groups was calculated as: ((trait mean ≥60 age group - trait mean 18–29 years age group)/(trait mean 18–29 years age group)) × 100% for each gender. Finally, All P values that tested for gender differences, differences between age groups, or differences between individuals with and without diabetes were computed using SOLAR univariate regression analysis, which accounts for the nonindependence of the family data.
RESULTS
General characteristics of the study populations are presented in Table 1. Mean age of 187 men and 284 women was ~43 years and ranged from 18 to 103 years. Participants were predominantly women (60.3%) and moderately overweight (BMI 28.30 ± 6.40 kg/m2). More men than women smoked (11% vs. 1%) and drank alcohol (29% vs. 9%) on a regular basis. Approximately 1/3 of the women were postmenopausal and 1/3 used oral contraceptives. Men were significantly taller, weight was similar in both genders, and BMI was significantly greater in women.
Table 1.
General characteristics of the tobago Family health study participants: total and by gender (unadjusted means ± s.d.)
| Total (N = 471) | Men (N = 187) | Women (N = 284) | |
|---|---|---|---|
| Age (years) | 42.68 ± 16.81 | 42.80 ± 16.90 | 42.60 ± 16.77 |
| Anthropometric | |||
| BMI (kg/m2)a | 28.30 ± 6.40 | 26.68 ± 4.9 | 29.38 ± 7.0 |
| Waist circumference (cm) | 89.87 ± 15.4 | 90.06 ± 12.4 | 89.74 ± 17.2 |
| Weight (kg) | 82.25 ± 18.6 | 83.67 ± 17.3 | 81.30 ± 19.3 |
| Height (cm)a | 170.68 ± 8.6 | 177.02 ± 7.4 | 166.48 ± 6.4 |
| Lifestyle | |||
| Alcohol use (>1 drink per week) (%)a | 13.2 | 28.9 | 2.8 |
| Current smoking (%)a | 4.9 | 11.4 | 0.7 |
| TV viewing (hours/week) | 15.92 ± 8.0 | 16.60 ± 8.5 | 15.46 ± 7.7 |
| Time spent walking per week (minutes/week) | 47.90 ± 96.7 | 52.57 ± 72.7 | 44.80 ± 109.8 |
| Reproductive | |||
| Use of oral contraceptives (%) | N/A | N/A | 33.1 |
| Ever pregnant (%) | N/A | N/A | 76.7 |
| Number of pregnancies | N/A | N/A | 3.54 ± 2.6 |
| Postmenopausal status (%) | N/A | N/A | 31.6 |
| Medical conditions | |||
| Diabetes (%)b | 15.4 | 12.4 | 17.2 |
P value for gender difference <0.001.
Diabetes prevalence was estimated based on only 401 individuals who had available glucose data.
We tested gender differences in lower extremity pQCT body composition traits after adjusting for age and height. Women had more cross-sectional TAT in the calf than men (3,172 mm2 vs. 1,672.5 mm2, P < 0.001). SAT represented a higher percentage of the TAT cross-sectional area in women than men (88.7% vs. 78.4%, P < 0.001), whereas skeletal muscle density was lower in women than in men (72.4 mg/cm3 vs. 75.2 mg/cm3, P < 0.001), indicating more adipose tissue infiltration in the skeletal muscle of female participants.
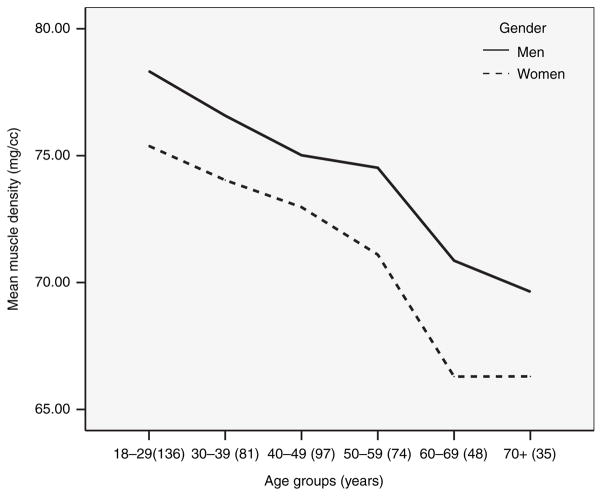
We next examined muscle density by gender and 10-year age group after adjusting for height (Figure 1). Women had lower muscle density than men in every age group (all P values < 0.05), although, among the eldest individuals the magnitude of the difference in muscle density did not reach statistical significance possibly due to the small sample size in the oldest age group. Muscle density decreased by 10% in men and by 12% in women between the participants in the youngest group (18–29 years) and participants ≥60 years (Figure 1).
Figure 1.
Lower extremity skeletal muscle adipose tissue infiltration across age and gender. All P values for gender differences between age groups after adjustment for height <0.05, except for oldest groups (≥70 years); sample size shown in the parentheses.
Table 2 shows the associations of anthropometric and lifestyle factors with pQCT body composition traits. Percent SAT and muscle density decreased 1–5% every 10 years. Men had up to 10% less TAT and percent SAT, but had 10% denser muscle than women. A one-unit increase in BMI was associated with 1–5% increase in TAT, up to 1% increase in SAT, and 1–5% decrease in muscle density. Smoking had a negative association with TAT, which was 1–5% lower in smokers than in nonsmokers. Regular consumption of alcohol, physical activity (measured as minutes of walking per week), and post-menopausal status had no impact on any of the pQCT measures. Women who were taking oral contraceptives had 1–5% more TAT, whereas women who were pregnant at least once in their lifetime had 5–10% less TAT and 1–5% denser muscle.
Table 2.
| Age (years) | Gender (M/F) F = 0 | BMI (kg/m2) | Current smoking (Y/N) | Ever pregnant (Y/N) | OC intake (Y/N) | h2r ± s.e. | % of variance explained by age and gender | % of variance explained by all significant covariates | |
|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | +** | N/A | −−*** | ++*** | 0.44 + 0.11*** | 0.07 | 0.13 | ||
| Total adipose tissue (mm2) | −−−−*** | ++*** | −−−* | −−−** | ++* | 0.59 + 0.10*** | 0.36 | 0.63 | |
| Subcutaneous adipose tissue (%) | −−*** | −−−−*** | +*** | 0.29 + 0.09*** | 0.37 | 0.40 | |||
| Muscle density (mg/cm3) | −−−*** | ++++*** | −−*** | +++* | 0.35 + 0.09*** | 0.42 | 0.55 |
Symbols are used to indicate positive (+) and negative (−) associations with body composition phenotypes.
The strength of the correlation is indicated by the number of symbols: four symbols indicate 10% or greater difference; three symbols, a 5–10% difference; two symbols, a 1–5% difference and one symbol, <1% difference.
Only variables which showed a significant association with peripheral quantitative computed tomography (pQCT) traits are shown. We tested for age, gender, BMI, current smoking, current alcohol intake, minutes walking per week, postmenopausal status, pregnancy history, age at menarche, oral contraceptive (OC) use.
P < 0.05;
P < 0.01;
P < 0.001.
Table 2 also shows residual heritability estimates for all pQCT body composition traits. After removing the variation attributable to all significant covariates (Table 2), which explained 40–63% of the total phenotypic variation in pQCT measured phenotypes, and 13% of the total phenotypic variation in BMI, all tested traits were significantly heritable, with the proportion of variance in pQCT fat traits due to additive genetic effects (residual heritability) ranging from 29 to 59%. Residual heritability of BMI was 44%. Interestingly, age and gender accounted for most of the variation in percent SAT due to measured covariates, and were also important correlates of muscle density (Table 2). These results indicate that genetic factors contribute significantly to the observed variation in pQCT adipose tissue traits and BMI.
In addition, considering existing gender differences in pQCT traits, and reported gender-specific genetic effects for fat and fat-free mass (29), we further estimated gender-specific heritability. Residual gender-specific heritability for all measured traits was significant (all P values < 0.05) and there were no significant differences in genetic effects between the genders (h2rmen and h2rwomen for BMI: 39 and 49%, respectively; h2rmen and h2rwomen for TAT: 62 and 69%, respectively; h2rmen and h2rwomen for percent SAT: 26 and 39%, respectively; and h2rmen and h2rwomen for muscle density: 32% in both genders).
After adjusting for age and gender, no difference in BMI between individuals with and without diabetes was observed, but TAT cross-sectional area (P = 0.003) was higher in diabetics compared with nondiabetics. Furthermore, percent SAT was lower (P = 0.01) and muscle was less dense (P = 0.005) in diabetics than in nondiabetics, independent of age, gender, and total obesity (BMI) (Table 3). The difference in percent SAT and muscle density between diabetic and nondiabetic individuals remained significant even after including age, gender, and central obesity (waist circumference), as covariates (both P values < 0.05, data not shown).
Table 3.
BMI and lower extremity pQct measured body composition: associations with diabetes (unadjusted means ± s.d.)
| Characteristics | Diabetic (n = 62) | Nondiabetic (n = 339) | P valuea |
|---|---|---|---|
| BMI (kg/m2)a | 31.2 ± 6.6 | 27.8 ± 6.3 | 0.41 |
| Total adipose tissue (mm2)a | 2,701.5 ± 1,498.3 | 2,497.7 ± 1,226.4 | 0.002 |
| Subcutaneous adipose tissue (%)b | 78.6 ± 17.7 | 85.6 ± 11.4 | 0.01 |
| Muscle density (mg/cm3)b | 69.5 ± 6.5 | 74.3 ± 4.3 | 0.005 |
pQCT, peripheral quantitative computed tomography.
P value after adjusting for age and gender.
Adjusted for age, gender, and BMI.
DISCUSSION
To our knowledge, this report is the first to investigate the heritability of QCT measures of adipose tissue infiltration in skeletal muscle. Our results suggest that a substantial degree of variability in fat infiltration in muscle is explained by genetic factors, with 35% of the variance attributable to polygenic influences, after accounting for environmental factors. These findings support the hypothesis that genetic factors may contribute to ectopic lipid infiltration in skeletal muscle among men and women of African origin, and suggest that further studies are warranted to identify the genes and allelic variants contributing to adipose tissue infiltration in skeletal muscle.
Our results also show that compared to men, women have greater adipose tissue infiltration in the skeletal muscle, independent of gender differences in age and overall obesity. Further, in both genders muscle density decreased across age groups. Previous studies in elderly white and African Americans have also shown that women have substantially lower skeletal muscle density (30). Moreover, a biopsy study has shown that women aged ≥60 have greater fat infiltration in skeletal muscle (31). Cree et al. compared intramyocellular lipids measured by magnetic resonance spectroscopy in young (aged 20–32 years) and elderly (aged 65–74 years) and reported increased fat infiltration in the muscle of elderly compared to younger individuals (32). Our findings across age and between genders among Afro-Caribbean families are consistent with these reports.
In addition to age and gender, we examined a wide range of lifestyle and environmental factors that might contribute to lower extremity body composition and fat infiltration. With increased level of BMI, TAT, and SAT also increased, whereas the density of the muscle decreased, indicating more fat infiltration in the muscle of individuals with higher BMI. Previous studies of QCT which obtained midthigh muscle composition reported similar associations with measures of total adiposity (3,33,34). Participants of our study who smoked cigarettes had less TAT and lower BMI than nonsmokers, which is consistent with previous reports where cigarette smoking has been associated with a lower BMI (35) and lower total body fat (35). Parity had a strong negative association with both TAT and adipose tissue infiltration in the muscle. This is not surprising, considering that parity has been associated with changes in body fat distribution (36,37), possibly due to insulin resistance associated with pregnancy (38), hormonal changes due to fewer ovulatory cycles (37), and specific accumulation of fat mass in the femoral area during pregnancy (39).
The physiological and molecular mechanisms responsible for lipid accumulation in skeletal muscle are still unclear. Some have proposed that lipid accumulation in skeletal muscle may be due to a reduction in the utilization of fatty acids (i.e., reduced lipolysis and lipid oxidation) (40,41). Others have shown in animal models that increased activity of acyl-CoA synthetase enzyme in skeletal muscle may contribute to the increased efficiency of fatty-acid uptake and lipid accumulation in skeletal muscle of high fat–fed rats compared with lean controls (42). Finally, other investigators have suggested that impaired skeletal muscle mitochondrial function may predispose individuals to increase fatty infiltration in skeletal muscle (43). Still, the precise causes that underlie this link remain to be further investigated. This study suggests that there is a familial predisposition to fat infiltration in skeletal muscle. The identification of the specific genetic factors involved may provide fundamental insight on the biological pathways contributing to ectopic lipid accumulation in muscle.
It has been previously reported that diabetic individuals of European origin have lower total and subcutaneous thigh fat than controls (44). Other studies in African Americans and individuals of European origin reported an association between QCT obtained intermuscular fat and insulin resistance (45,46), and additionally, that individuals with diabetes have lower skeletal muscle density than those with normal glucose tolerance after adjusting for total fat mass (47). In the Health Aging and Body Composition Study, older men and women with metabolic syndrome had lower muscle density values (48). Similarly, individuals with diabetes from our study had greater adipose tissue infiltration in skeletal muscle, independent of total and central adiposity. Although the molecular mechanisms linking increased skeletal muscle adipose tissue accumulation and the development of insulin resistance are unclear, several human and animal studies have shown that fatty acid excess disrupts various aspects of the insulin signaling cascade (49). Increased fat deposition in skeletal muscle may interfere with glucose utilization by reducing tyrosine phosphorylation of the insulin receptor substrate, and it may also impair the insulin receptor substrate/phosphatidylinositol 3-kinase (PI3K) pathway and growth-factor-regulated protein kinase B (Akt/PKB) pathway of insulin signaling, insulin-stimulated skeletal muscle glycogen synthesis and glucose transport (43,49–51). This could cause insulin resistance in skeletal muscle and contribute, therefore, to the development of type 2 diabetes. Also, skeletal muscle adipose tissue accumulation can contribute to the production of reactive oxygen species, which activates the glycosylation (52). However, our understanding of the mechanisms linking fat accumulation in skeletal muscle and diabetes, and the reasons why some individuals store more fat in insulin-sensitive tissues than others are still limited. A better understating of these issues may lead to the development of new insulin-sensitizing medications.
Previous studies reported that African Americans (both women and men) have more intermuscular fat compared to whites and Asians, even after adjustment for differences in total adiposity and other potential covariates (16,46). Other studies have shown that middle-aged and older African-American women have lower muscle density than white women (13,53). The underlying mechanisms for black–white differences in ectopic fat accumulation are still unknown, but some studies have found decreased fat oxidation (54) and decreased lipolysis (55) in African-American women compared to matched white women. Other studies suggest that ectopic deposition of lipids may be higher in obese African-American women due to a higher rate of fatty-acid uptake and a higher expression of fatty acid transporting proteins (56). Importantly, no studies were conducted in populations of African origin outside of the United States. Also, to our knowledge, no studies have examined the possible genetic mechanisms for individual variability in ectopic adipose tissue accumulation in skeletal muscle. Future genetic studies in this Afro-Caribbean population may help explain why individuals of African origin are at increased risk for the development of obesity, insulin resistance, and type 2 diabetes, and shed some light on the potential causes of the increased ectopic fat accumulation in individuals of African origin.
There are several potential limitations of our study. First, QCT cannot directly measure the lipid content or detect the location of fat storage within or surrounding myocytes. Most previous studies have utilized a single CT slice of the midthigh to assess fat infiltration in muscle (23,34,48). By obtaining a single slice in the calf muscle, we were able to only measure a relatively small depot of skeletal muscle adipose tissue compared to CT measures of the midthigh. However, a recent study has shown that CT muscle density of the midthigh is significantly correlated with muscle density of the calf (r = 0.62, P < 0.01), and additionally, that muscle density measured by CT of the soleus muscle is associated with intramyocellular lipid measured by magnetic resonance spectroscopy (r = −0.64, P < 0.01) (57). The authors therefore concluded that both CT and magnetic resonance spectroscopy are reliable methods for assessing skeletal muscle lipid content. Further, due to the relatively small sample size and higher number of female participants, our findings of no significant differences in genetic effects between the genders should be interpreted with caution and should be tested further in future larger studies. Also, our relatively small total sample size and total number of pedigrees may have influenced our heritability estimates. However, Wijsman and Amos (58) and Slate et al. (59) showed that extended pedigrees, such as ours, may be more powerful than nuclear pedigrees or sib-pairs in detecting and locating simulated disease loci (or quantitative strait loci), and with fewer false positives. Finally, heritability estimates do not provide insight on the number of loci that contribute to the variation in muscle composition related traits and therefore, future studies of the genetic mechanisms contributing to skeletal muscle composition are needed.
In conclusion, our study provides the first comprehensive epidemiologic and genetic analysis of adipose tissue infiltration in skeletal muscle. Our results suggest that ectopic fat deposition in skeletal muscle is a heritable trait and is associated with diabetes, independent of overall and central obesity. Additional studies are warranted to identify the specific genes that influence ectopic adipose tissue infiltration in skeletal muscle. Such studies may provide new insight into the pathophysiology and genetic susceptibility to diabetes and suggest new therapeutic targets for the treatment and prevention of insulin resistance.
Acknowledgments
I.M.-G. is supported by the Individual National Research Service Award for postdoctoral training from the National Heart, Lung, and Blood Institute (grant F32 HL083641). This study was supported, in part, by funding or in-kind services from the Division of Health and Social Services, Tobago House of Assembly, the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK046204), and the National Institute of Arthritis and Musculoskeletal Diseases (grant R03-AR050107). We thank the participants of the Tobago Family Health Study and all supporting staff.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Shulman GI, Rothman DL, Jue T, et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 2.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 4.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 7.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 8.Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM, Howard G, Mayer E, et al. Insulin sensitivity and acute insulin response in African-Americans, non-Hispanic whites, and Hispanics with NIDDM: the Insulin Resistance Atherosclerosis Study. Diabetes. 1997;46:63–69. doi: 10.2337/diab.46.1.63. [DOI] [PubMed] [Google Scholar]
- 10.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 11.Palaniappan LP, Carnethon MR, Fortmann SP. Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care. 2002;25:1351–1357. doi: 10.2337/diacare.25.8.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus MA, Murphy L, Pi-Sunyer FX, Albu JB. Insulin sensitivity and serum triglyceride level in obese white and black women: relationship to visceral and truncal subcutaneous fat. Metabolism. 1999;48:194–199. doi: 10.1016/s0026-0495(99)90033-1. [DOI] [PubMed] [Google Scholar]
- 13.Ryan AS, Nicklas BJ, Berman DM. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res. 2002;10:336–344. doi: 10.1038/oby.2002.47. [DOI] [PubMed] [Google Scholar]
- 14.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: the Healthy Transitions Study. Obes Res. 2001;9:10–16. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- 15.Munoz J, Gower BA. Relationship between serum leptin concentration and low-density muscle in postmenopausal women. J Clin Endocrinol Metab. 2003;88:1157–1161. doi: 10.1210/jc.2002-020959. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miljkovic-Gacic I, Sheu YT, Cauley JA, et al. Increased adipose tissue infiltration in skeletal muscle despite lower total body fat among older men of African origin. Obesity. 2006;14(Suppl):A194. [Google Scholar]
- 18.Samaras K, Spector TD, Nguyen TV, et al. Independent genetic factors determine the amount and distribution of fat in women after the menopause. J Clin Endocrinol Metab. 1997;82:781–785. doi: 10.1210/jcem.82.3.3803. [DOI] [PubMed] [Google Scholar]
- 19.Hsu FC, Lenchik L, Nicklas BJ, et al. Heritability of body composition measured by DXA in the diabetes heart study. Obes Res. 2005;13:312–319. doi: 10.1038/oby.2005.42. [DOI] [PubMed] [Google Scholar]
- 20.Luke A, Guo X, Adeyemo AA, et al. Heritability of obesity-related traits among Nigerians, Jamaicans and US black people. Int J Obes Relat Metab Disord. 2001;25:1034–1041. doi: 10.1038/sj.ijo.0801650. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Cooper RS, Luke A, et al. A genome-wide scan for obesity in African-Americans. Diabetes. 2002;51:541–544. doi: 10.2337/diabetes.51.2.541. [DOI] [PubMed] [Google Scholar]
- 22.Simonsick EM, Maffeo CE, Rogers SK, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M264–M274. doi: 10.1093/gerona/52a.5.m264. [DOI] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 24.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20:586–590. [PubMed] [Google Scholar]
- 25.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 26.Becker RA, Chambers JM, Wilks AR. The New S Language. Chapman & Hall; London: 1988. [Google Scholar]
- 27.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cauley JA, Fullman RL, Stone KL, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16:1525–1537. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 29.Lecomte E, Herbeth B, Nicaud V, et al. Segregation analysis of fat mass and fat-free mass with age- and sex-dependent effects: the Stanislas Family Study. Genet Epidemiol. 1997;14:51–62. doi: 10.1002/(SICI)1098-2272(1997)14:1<51::AID-GEPI4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 31.Forsberg AM, Nilsson E, Werneman J, Bergstrom J, Hultman E. Muscle composition in relation to age and sex. Clin Sci (Lond) 1991;81:249–256. doi: 10.1042/cs0810249. [DOI] [PubMed] [Google Scholar]
- 32.Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 33.Ding J, Visser M, Kritchevsky SB, et al. The association of regional fat depots with hypertension in older persons of white and African American ethnicity. Am J Hypertens. 2004;17:971–976. doi: 10.1016/j.amjhyper.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23:126–132. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 35.Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. Int J Obes (Lond) 2005;29:236–243. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- 36.Bjorkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C. Reproductive history in relation to relative weight and fat distribution. Int J Obes Relat Metab Disord. 1996;20:213–219. [PubMed] [Google Scholar]
- 37.Rossner S. Pregnancy, weight cycling and weight gain in obesity. Int J Obes Relat Metab Disord. 1992;16:145–147. [PubMed] [Google Scholar]
- 38.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl J Med. 1989;321:1214–1219. doi: 10.1056/NEJM198911023211802. [DOI] [PubMed] [Google Scholar]
- 39.den Tonkelaar I, Seidell JC, van Noord PA, Baanders-van Halewijn EA, Ouwehand IJ. Fat distribution in relation to age, degree of obesity, smoking habits, parity and estrogen use: a cross-sectional study in 11,825 Dutch women participating in the DOM-project. Int J Obes. 1990;14:753–761. [PubMed] [Google Scholar]
- 40.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 42.Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes. 2002;51:1477–1484. doi: 10.2337/diabetes.51.5.1477. [DOI] [PubMed] [Google Scholar]
- 43.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dube MC, Joanisse DR, Prud’homme D, et al. Muscle adiposity and body fat distribution in type 1 and type 2 diabetes: varying relationships according to diabetes type. Int J Obes (Lond) 2006;30:1721–1728. doi: 10.1038/sj.ijo.0803337. [DOI] [PubMed] [Google Scholar]
- 45.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 46.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 48.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 49.Hulver MW, Dohm GL. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc Nutr Soc. 2004;63:375–380. doi: 10.1079/pns2004351. [DOI] [PubMed] [Google Scholar]
- 50.Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 2004;19:183–190. doi: 10.1152/physiol.00007.2004. [DOI] [PubMed] [Google Scholar]
- 51.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119(5 Suppl 1):S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care. 2002;5:545–549. doi: 10.1097/00075197-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 54.Hickner RC, Privette J, McIver K, Barakat H. Fatty acid oxidation in African-American and Caucasian women during physical activity. J Appl Physiol. 2001;90:2319–2324. doi: 10.1152/jappl.2001.90.6.2319. [DOI] [PubMed] [Google Scholar]
- 55.Barakat H, Hickner RC, Privette J, et al. Differences in the lipolytic function of adipose tissue preparations from Black American and Caucasian women. Metabolism. 2002;51:1514–1518. doi: 10.1053/meta.2002.35589. [DOI] [PubMed] [Google Scholar]
- 56.Bower JF, Davis JM, Hao E, Barakat HA. Differences in transport of fatty acids and expression of fatty acid transporting proteins in adipose tissue of obese black and white women. Am J Physiol Endocrinol Metab. 2006;290:E87–E91. doi: 10.1152/ajpendo.00194.2005. [DOI] [PubMed] [Google Scholar]
- 57.Larson-Meyer DE, Smith SR, Heilbronn LK, et al. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14:73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wijsman EM, Amos CI. Genetic analysis of simulated oligogenic traits in nuclear and extended pedigrees: summary of GAW10 contributions. Genet Epidemiol. 1997;14:719–735. doi: 10.1002/(SICI)1098-2272(1997)14:6<719::AID-GEPI28>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 59.Slate J, Pemberton JM, Visscher PM. Power to detect QTL in a free-living polygynous population. Heredity. 1999;83:327–336. doi: 10.1038/sj.hdy.6885830. [DOI] [PubMed] [Google Scholar]