Abstract
Background
To avoid ischemic necrosis, compartment syndrome is a surgical emergency treated with decompression once identified. A potentially lethal, oxidant-driven reperfusion injury occurs after decompression. N-acetylcysteine is an antioxidant with the potential to attenuate the reperfusion injury.
Questions/purposes
We asked whether N-acetylcysteine could preserve striated muscle contractility and modify neutrophil infiltration and activation after simulated compartment syndrome release.
Materials and Methods
Fifty-seven rats were randomized to control, simulated compartment syndrome, and simulated compartment syndrome plus N-acetylcysteine groups. We isolated the rodent cremaster muscle on its neurovascular pedicle and placed it in a pressure chamber. Chamber pressure was elevated above critical closing pressure for 3 hours to simulate compartment syndrome. Experiments were concluded at three times: 1 hour, 24 hours, and 7 days after decompression of compartment syndrome. We assessed twitch and tetanic contractile function and tissue myeloperoxidase activity. Ten additional rats were randomized to control and N-acetylcysteine administration after which neutrophil respiratory burst activity was assessed.
Results
The simulated compartment syndrome decreased muscle contractility and increased muscle tissue myeloperoxidase activity compared with controls. Treatment with N-acetylcysteine preserved twitch and tetanic contractility. N-acetylcysteine did not alter neutrophil infiltration (myeloperoxidase activity) acutely but did reduce infiltration at 24 hours, even when given after decompression. N-acetylcysteine reduced neutrophil respiratory burst activity.
Conclusion
N-acetylcysteine administration before or after simulated compartment syndrome preserved striated muscle contractility, apparently by attenuating neutrophil activation and the resultant oxidant injury.
Clinical Relevance
Our data suggest a potential role for N-acetylcysteine in the attenuation of muscle injury after release of compartment syndrome and possibly in the prophylaxis of compartment syndrome.
Introduction
Acute compartment syndrome (CS) is a condition in which the circulation and function of tissues in an enclosed space are compromised by increased pressure in that space [31]. In clinical practice, fasciotomy remains the only proven treatment of CS [31, 47]. Promptly establishing the diagnosis of CS and timing appropriate surgery remain important clinical challenges [31, 34, 47]; however, fasciotomy is not an entirely benign procedure and is associated with substantial morbidity locally and systemically and with potential mortality [34, 47]. The most important predictor of outcome after CS is time to decompression [40, 48].
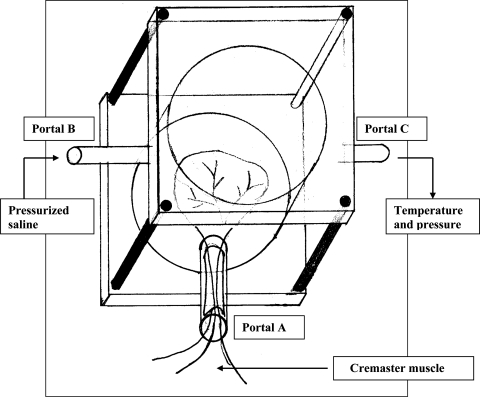
Ischemia-reperfusion injury (IRI) causes local and distant organ injury mediated largely by activated neutrophils [1–3, 5, 10, 12, 58]. Locally, after reperfusion, activated neutrophils cause additional injury to the already ischemic striated muscle through the release of reactive oxygen species (ROS) and proteolytic enzymes [12, 37, 55, 57]. Systemic neutrophil infiltration after IRI manifests as sequestration in distant organs, such as lungs and kidneys [26, 59]. Although there are similarities between IRI and CS-induced striated muscle injury, important differences do exist between the pathophysiologic features of these conditions [20]. In particular, the elevated tissue pressure in a confined space, as occurs in CS, acts synergistically with ischemia to produce a more severe degree of cellular deterioration than ischemia alone [18, 19]. The increased metabolic insult that results from elevated compartment pressure and local microvascular occlusion in CS may render simple tourniquet models, which cause total tissue ischemia attributable to isolated macrovascular compression, less clinically relevant [20]. A clinical feature of early CS, which distinguishes it, for example from acute ischemia, is maintenance of distal pulses indicating patent macrovasculature; that is, in early CS the macrovasculature is intact. A custom-built chamber has been described that is designed to apply external compression to the muscle above the critical closing pressure (∆P) [9, 11], resulting in microvascular occlusion without occluding the macrovascular supply in the neurovascular pedicle (Fig. 1). We believe this replicates more closely the physiologic conditions encountered in early CS than tourniquet models [16, 25, 31].
Fig. 1.
A line drawing illustrates the compartment syndrome simulation chamber. The cremaster muscle pedicle is shown passing into the chamber through Portal A. Warmed saline was introduced through Portal B. Temperature and pressure were monitored at Portal C.
Various antioxidant therapies reportedly prevent distant organ and local skeletal muscle injury after IRI [4, 33, 37, 55, 56]. N-acetylcysteine (NAC) is one such antioxidant [28, 44]. In clinical practice, NAC is used primarily to reduce hepatocyte injury after acetaminophen overdose [14, 42]. NAC also has been used as nephroprotective prophylaxis before administration of radiographic intravenous contrast in patients with renal impairment [52]. It protects lung epithelial cells against oxidant injury mediated by activated neutrophils in vitro and against pulmonary oxygen toxicity in vivo [6, 36, 45, 54]. NAC also reduces oxidative burst activity but enhances phagocytosis in rodent and human neutrophils [13, 17]. NAC is particularly suitable for the attenuation of oxidant-mediated injury as it not only has a direct antioxidant effect by means of free-radical scavenging properties but also replenishes depleted cellular glutathione stores restoring intrinsic cellular antioxidant defenses [6, 13, 32, 50]. The widely accepted use of NAC in current clinical practice confers an additional advantage for its potential use in the context of CS, IRI, or trauma, as the toxicity profile of the drug is well established and clinicians already are experienced in its use.
We hypothesized parenteral NAC in the context of CS in a rodent model would (1) preserve striated muscle contractility; (2) decrease neutrophil sequestration (myeloperoxidase activity [MPO]); and (3) reduce neutrophil respiratory burst activity.
Materials and Methods
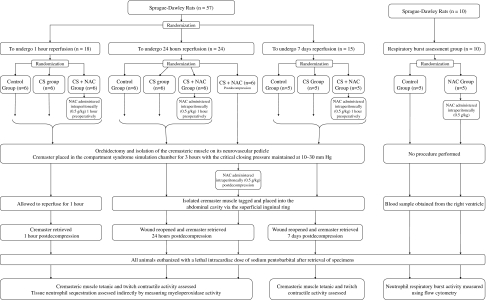
We randomized 57 Sprague–Dawley rats (weight, 300–400 g) into three groups according to the timing of postoperative sample analysis: one group assessed after 1 hour of reperfusion (n = 18), one group assessed 24 hours after reperfusion (n = 24), and one group assessed 7 days after reperfusion (n = 15) (Fig. 2). The animals assigned to each of these three groups then were randomized into subgroups: a control subgroup, a CS subgroup, and an NAC pretreatment subgroup at each time (CS + NAC). The 24-hour group had an additional fourth subgroup given NAC at the time of CS decompression (CS + NACd). Animals in the NAC pretreatment groups received 0.5 g NAC per kg intraperitoneally 1 hour before induction of anesthesia whereas rats in the posttreatment group received the same dose intraperitoneally after muscle decompression; this dose reportedly reduces neutrophil infiltration in a murine model [39]. A power analysis was performed using the effect of NAC on twitch contractile function as the primary research question. Previous investigators showed NAC to reduce neutrophil infiltration and creatine phosphokinase by greater than 50% in a rat hindlimb IRI model [28]. We presumed this would correspond to an improvement in muscle contractile function. From previously published research, the mean contractile force 1 hour after exposure to CS was 47.0 g (SD, 14.45 g) reduced from a peak contractile force of 160.7 g in the control group [27]. We estimated 25% preservation of contractile force would equate to a clinically relevant treatment effect. This corresponds to a 28.4 g difference in contractile force between the CS + NAC and CS groups in our study. With a sample size of six animals per group and alpha value of 0.05, the power of this study to identify this difference was 85.2%. All rats were bred, housed, and fed in an approved facility. The experimental work was performed with the appropriate license.
Fig. 2.
A flowchart shows the study protocol.
All animals were anesthetized with inhalation of halothane, temperature was maintained using a heating lamp, and rats were monitored using a rectal thermometer. All animals underwent orchidectomy with isolation of the cremaster muscle on its neurovascular pedicle using a previously described technique [27]. The cremaster was introduced into the CS simulation chamber (Fig. 1). Arterial blood pressure was measured throughout the experiment by a carotid cannula. In the CS and CS + NAC groups, elevating chamber pressure to 10 mm Hg below diastolic blood pressure for 3 hours simulated CS. The pressure then was released simulating fasciotomy. For control animals, the chamber pressure was not elevated. In the 1-hour groups, the cremaster was reperfused for 1 hour after CS release. Muscle then was divided and samples collected for contractility and tissue MPO activity testing. After removal from the CS simulation chamber in the 24-hour and 7-day groups, the muscle was placed in the abdominal cavity via the superficial inguinal ring. A suture was placed in the muscle to facilitate retrieval and the skin was closed. Animals were resuscitated, given intramuscular analgesia (buprenorphine 0.03 mg), and returned to their cages. They were reanesthetized after 24 hours or 7 days for sample retrieval. In the 24-hour group, samples were collected for assessment of muscle contractility, weight, and tissue MPO activity. In the 7-day group, contractility was the only outcome measure assessed.
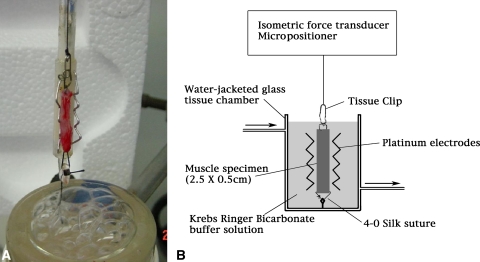
A longitudinal strip of muscle 2.5 cm × 0.5 cm was isolated from a central area of the cremaster muscle parallel to the raphe to ensure consistent fiber alignment. This was used to assess muscle twitch and tetanic contractile force in all groups. The strip was maintained at 37°C in a bicarbonate buffer solution and the pH was corrected to 7.3 to 7.4. The pH and O2 saturation were maintained by constant aeration with a 95% O2, 5% CO2 gas mixture. The muscle strip was mounted, with its long axis in the vertical plane, and suspended between two vertical platinum electrodes approximately 1 cm apart and lowered into a 50-mL water-jacketed glass tissue chamber (Fig. 3). Specimen tension was adjusted to ensure optimum fiber length using a micropositioning device. The optimum tension was determined as that resulting in the maximum contractile force for a given stimulus. The muscle was stimulated using supramaximal pulses (20 V, 2-ms square wave, 40 Hz) obtained from a pulse generator. The isometric contraction of each muscle strip was assessed in response to a timed series of twitch and tetanic electrical stimuli. We measured muscle contractile force using a force transducer and computerized data acquisition program. Twitch and tetanic muscle contractile function were expressed as peak tension achieved in grams of force. After function testing, we weighed the muscle strip to calculate force per gram of tissue.
Fig. 3A–B.
(A) A photograph and (B) schematic diagram illustrate the apparatus for assessing muscle contractile force after twitch and tetanic stimulation. The muscle sample was mounted as shown and lowered into the tissue chamber. Optimal fiber length of the specimen was determined using a micropositioner to identify the length producing maximal contractile force. Measurements were taken using an isometric force transducer.
MPO measurement is a reliable method of quantitatively assessing tissue neutrophil sequestration [29]. We homogenized the muscle in 10 mL of 20 mmol/L potassium phosphate buffer (pH 7.4) containing 0.1 mmol/L EDTA. The homogenate was centrifuged at 20,000 g for 10 minutes at 4°C to pellet the insoluble cellular debris. The samples were freeze-thawed twice. The samples were assayed spectrophotometrically for MPO activity by incubating 10 mL of the homogenate with 290 mL of a solution containing 2.9 mL O-dioniside dihydrochloride in 90 mL distilled water, 10 mL of 50 mmol/L potassium phosphate buffer (pH 6), and hydrogen peroxide. The change in absorbance with time then was measured at 450 nm. We defined one unit of MPO as that degrading 1 mmol of peroxide per minute at 25°C.
We determined the effect of NAC on the neutrophil respiratory burst activity using a separate experiment. Ten animals were randomized to control (n = 5) or to undergo administration of 0.5 mg/kg intraperitoneal NAC (n = 5). No operation was performed on these 10 animals. Blood was drawn 4 hours after NAC administration by cannulation of the right ventricle under halothane anesthesia. Respiratory burst activity was assessed using flow cytometry (BURSTTEST; Orpegen, Heidelberg, Germany) to determine leukocyte oxidative and enzymatic activity using dihydrorhodamine 123 (DHR123) as the fluorogenic substrate [41]. Whole blood (100 μL) was incubated alone (unstimulated neutrophils) or with 10 μL rat serum opsonized Escherichia coli (stimulated neutrophils) for 10 minutes. Ten microliters of DHR123 was added and incubation continued for an additional 10 minutes. The analysis was performed on a cytofluorometer detecting mean channel fluorescence (mcf). A minimum of 5000 cells was collected and analyzed using Lysis™ II software (Becton-Dickinson, San Jose, CA).
We determined differences in contractile force at each time between each of the subgroups. The data for twitch contractile force were normally distributed; however, the results for tetanic contractile force were skewed and we therefore performed log transformation before additional analysis. We determined differences in twitch and tetanic contractile forces between control, CS, CS + NAC, and CS + NACd subgroups at each relevant time using ANOVA for the comparison of multiple means with post hoc Tukey test analysis. The MPO data were skewed and underwent log transformation before analysis. We determined differences in muscle MPO activity between subgroups at 1 hour and 24 hours using one-way ANOVA. The Tukey test was used for post hoc analysis of significant results. For respiratory burst activity, we determined the difference in the effects of NAC on the stimulated and unstimulated neutrophil populations using Student’s unpaired t test. Statistical analyses were performed using MacLab™/2e Chart and Scope (ADInstruments Ltd, Chalgrove, UK).
Results
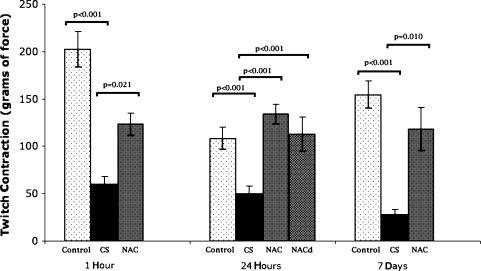
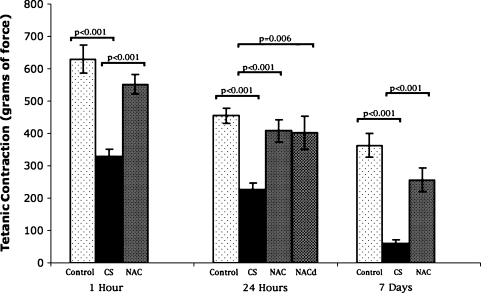
One hour after simulated fasciotomy, CS impaired twitch and tetanic muscle contractile force compared with controls (Table 1; Figs. 4, 5). NAC attenuated this negative effect for twitch and tetanic stimuli. At 24 hours after simulated CS release, twitch and tetanic contractile function were impaired compared with those of controls. NAC attenuated twitch and preserved tetanic contractile force, whether given before or after decompression of CS. Seven days after decompression of CS, muscle twitch and tetanic contractile function were reduced compared with those of controls, but this effect was attenuated in the animals given NAC.
Table 1.
Effects of compartment syndrome with or without N-acetylcysteine on muscle contractility and myeloperoxidase activity
| Group | Experiment time after release of CS | ||
|---|---|---|---|
| 1 hour | 24 hours | 7 days | |
| Twitch contraction (g of force) | |||
| Control group | 204 ± 18.6 | 108.5 ± 11.5 | 154.7 ± 14.1 |
| CS group | 59.9 ± 8.3 (p < 0.001 vs control) | 50.4 ± 7.7 (p = 0.026 vs control) | 28.1 ± 5.5 (p < 0.001 vs control) |
| CS + NAC group | 123.5 ± 11.6 (p = 0.021 vs CS) | 134.3 ± 10.4 (p < 0.001 vs CS) | 118.2 ± 22.9 (p = 0.010 vs CS) |
| CS + NACd group | 112.9 ± 18.1 (p < 0.001 vs CS) | ||
| Tetanic contraction (g of force) | |||
| Control group | 629.9 ± 43.4 | 455.3 ± 23.3 | 362 ± 37.2 |
| CS group | 328.5 ± 23.1 (p < 0.001 vs control) | 225.7 ± 21.6 (p < 0.001 vs control) | 59.7 ± 12.1 (p < 0.001 vs control) |
| CS + NAC group | 552 ± 29.4 (p < 0.001 vs CS) | 408.3 ± 34.3 (p < 0.001 vs CS) | 256.3 ± 37 (p < 0.001 vs CS) |
| CS + NACd group | 402.4 ± 52 (p = 0.006 vs CS) | ||
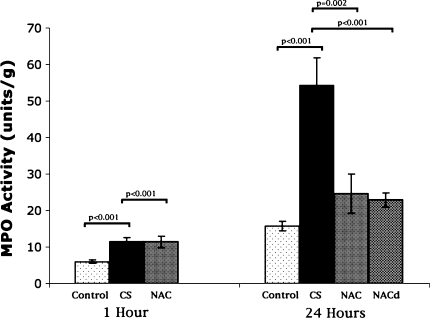
| Myeloperoxidase activity (units/g tissue) | |||
| Control group | 6 ± 0.4 | 15.8 ± 1.3 | |
| CS group | 11.5 ± 1.1 (p < 0.001 vs control) | 54.2 ± 7.7 (p < 0.001 vs control) | |
| CS + NAC group | 11.4 ± 1.5 (p < 0.001 vs control) | 24.6 ± 5.4 (p = 0.002 vs CS) | |
| CS + NACd group | 22.9 ± 1.9 (p < 0.001 vs CS) | ||
Values are expressed as mean ± SD; control = control group; CS = compartment syndrome; CS + NAC = group given N-acetylcysteine before CS; CS + NACd = group given N-acetylcysteine at CS decompression.
Fig. 4.
NAC attenuated the detrimental effects of CS on skeletal muscle twitch contraction at 1 hour and 7 days after release of CS and prevented the effects of CS at 24 hours. NAC prevented CS-induced loss of contractile force at 24 hours even when given after the release of CS. Bar = mean; error bar = SD; control = control group; CS = compartment syndrome group; NAC = group given N-acetylcysteine before CS; NACd = group given N-acetylcysteine at CS decompression.
Fig. 5.
Administration of NAC before onset of CS attenuated the CS-induced reduction in tetanic contraction at all times. This effect was reproduced even when NAC was given after the release of CS. Bar = mean; error bar = SD; control = control group; CS = compartment syndrome group; NAC = group given N-acetylcysteine before CS; NACd = group given N-acetylcysteine at CS decompression.
Muscle MPO activity was increased compared with that of controls 1 hour after simulated release of CS, indicating an increase in muscle neutrophil infiltration (Table 1; Fig. 6). NAC preadministration did not appreciably alter muscle neutrophil infiltration 1 hour after CS. MPO activity increased 24 hours after simulated fasciotomy, indicating pronounced neutrophilic infiltrate compared with that of controls. NAC reduced the MPO activity compared with CS regardless whether given before or after CS decompression.
Fig. 6.
CS increased MPO activity at 1 hour. NAC did not reduce MPO activity compared with CS initially. NAC reduced MPO activity at 24 hours compared with CS alone, whether given before onset of CS or after CS release. Bar = mean; error bar = SD; control = control group; CS = compartment syndrome group; NAC = group given N-acetylcysteine prior to CS; NACd = group given N-acetylcysteine at CS decompression.
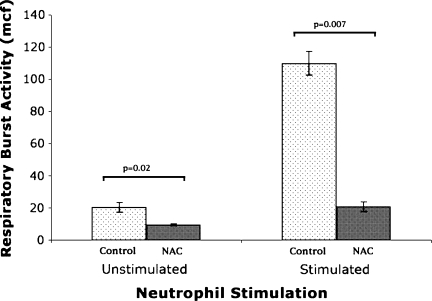
NAC pretreatment reduced neutrophil respiratory burst activity compared with that of controls. This was noted when whole blood was incubated without stimulating the neutrophils and also when incubated with rat serum opsonized E. coli to stimulate the neutrophils (Table 2; Fig. 7).
Table 2.
Neutrophil respiratory burst activity
| Neutrophil population | Control group | NAC group | p Value |
|---|---|---|---|
| Unstimulated (mcf) | 20.5 ± 6.40 (12.5–28.5) | 9.58 ± 1.42 (8.1–11.0) | 0.02 |
| Stimulated (mcf) | 110 ± 38.81 (61.8–158.2) | 20.7 ± 6.76 (12.4–29.1) | 0.007 |
Values are expressed as mean ± SD; ranges in parentheses NAC = N-acetylcysteine; mcf = mean channel fluorescence.
Fig. 7.
NAC inhibited respiratory burst activity as measured by mean channel fluorescence (mcf) in unstimulated and rat opsonized E coli stimulated neutrophil populations. Bar = mean; error bar = SD; control = control group; NAC = group given N-acetylcysteine.
Discussion
Reperfusion after prolonged ischemia results in a metabolic insult to the affected tissue greater than the ischemic injury alone [21, 58]. IRI also results in a systemic inflammatory response with the potential to affect tissues distant from the reperfused area. Acute respiratory distress syndrome, multiorgan failure, and death have been described as consequences of IRI [7, 15]. Neutrophil activation and infiltration have been identified as primary protagonists in the pathophysiology of IRI [10, 21, 22]. CS results in a much greater level of metabolic strain and cellular deterioration than IRI alone, and its treatment is urgent surgical decompression by fasciotomy once identified [18, 19, 37, 40, 49]. Despite the major morbidity associated with CS, there is no widely accepted clinical treatment to attenuate the reperfusion injury that follows fasciotomy. NAC is a potent free-radical scavenger that has intrinsic antioxidant properties and replenishes depleted glutathione stores [36]. It has a well-described, acceptable toxicity profile, proven in a critical-care setting [51]. We hypothesized NAC in a simulated CS injury in a rodent model would preserve striated muscle contractility, decrease neutrophil sequestration, and reduce neutrophil respiratory burst activity.
We recognize certain limitations of our study. First, this is an animal model of simulated compartment syndrome using cremaster muscle, which therefore may not necessarily reflect the human physiologic responses to CS of an extremity and the model does not address neural injury in the involved compartment. The study was designed to determine the potential of NAC to attenuate the reperfusion injury to striated muscle after CS. Although our data suggest improved muscle function in terms of contractility in this model at as much as 7 days recovery, the data do not address nerve injury or long-term recovery of muscle function, therefore functional clinical outcomes cannot be directly inferred. Second, we assessed one dose of NAC (0.5 mg/kg) delivered parenterally but did not investigate the dose–response relationship; additional studies will be required to determine the optimum dose. Despite these limitations, we believe the data provide a valid argument for additional investigation of NAC’s ability to reduce reperfusion injury following CS. Even if clinical muscle function is not maintained owing to nerve impairment, the improved muscle tissue viability as shown by improved contractility, and the reduced oxidant injury as evidenced by reduced myeloperoxidase and respiratory burst activity may correlate to a decrease in the inflammatory activity of the reperfusate and therefore, the systemic consequences of reperfusion.
We found muscle contractility was preserved using NAC given before or after decompression of CS. Liu et al. noted a similar protective effect after IRI as measured by improved arteriolar dilatation and tetanic contraction [30]. NAC also decreases muscle cell death attributable to IRI in mice as measured by malonyldialdehyde levels [8]. Thus, the apparent benefits of NAC seen in IRI models apparently apply in our CS model.
The increased neutrophil infiltration after CS release was not attenuated by NAC at 1 hour, suggesting NAC pretreatment did not alter neutrophil transmigration acutely. At 24 hours, however, there was a definite reduction in MPO activity in the NAC groups, whether given before or after CS. This indicates reduced neutrophil sequestration, implying a delayed protective effect of NAC. Two other studies have shown NAC decreases vascular permeability in rats after free-radical-induced injury [46, 53]. The ability of NAC to maintain endothelial integrity and decrease vascular permeability may explain the reduced neutrophil infiltration.
Activated neutrophils produce superoxide anions, ROS, and proteolytic enzymes, which mediate their injurious effects in reperfusion injury. Neutrophil respiratory burst activity correlates to the level of neutrophil activation and ROS production [38, 58]. The decrease in respiratory burst activity when animals were pretreated with NAC suggests it modulates its effects directly on the neutrophils, reducing their propensity to produce free radicals, possibly by its combined antioxidant and glutathione-replenishing activities.
Making the diagnosis of a CS is a critical clinical episode, particularly in the unconscious patient [34, 35]. Delay to diagnosis and treatment is the most important prognostic factor in CS [40, 48]. Fasciotomy may be associated with substantial surgical morbidity, but even after decompression, focal areas of muscle ischemia and hypoxia may persist [23, 24, 43, 47]. Preadministration with NAC to high-risk patients (such as patients with high-energy tibial fractures) may help reduce muscle injury and edema induced by CS. The efficacy of this agent, even when administered after decompression, has potential clinical applications in the trauma setting and warrants additional assessment.
Our data suggest antioxidant administration may represent a potential therapeutic strategy for management of striated muscle CS. In our experiments, NAC administration appeared to protect striated muscle against CS-induced injury by attenuating neutrophil activation. This effect was reproduced with NAC administration after the injury, suggesting a potential role for NAC in the attenuation of muscle injury even after release of CS. We suggest NAC may be an appropriate agent for a clinical trial in such a setting.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed in the Royal College of Surgeons in Ireland and Beaumont Hospital.
References
- 1.Anner H, Kaufman RP, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Pulmonary hypertension and leukosequestration after lower torso ischemia. Ann Surg. 1987;206:642–648. doi: 10.1097/00000658-198711000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anner H, Kaufman RP, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Pulmonary leukosequestration induced by hind limb ischemia. Ann Surg. 1987;206:162–167. doi: 10.1097/00000658-198708000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anner H, Kaufman RP, Valeri CR, Shepro D, Hechtman HB. Reperfusion of ischemic lower limbs increases pulmonary microvascular permeability. J Trauma. 1988;28:607–610. doi: 10.1097/00005373-198805000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Barry MC, Kelly CJ, Abdih H, Watson RW, Stapleton P, Sheehan SJ, Redmond HP, Hayes DB. Differential effects of lower limb revascularisation on organ injury and the role of the amino acid taurine. Eur J Vasc Endovasc Surg. 1997;13:193–201. doi: 10.1016/S1078-5884(97)80018-4. [DOI] [PubMed] [Google Scholar]
- 5.Barry MC, Wang JH, Kelly CJ, Sheehan SJ, Redmond HP, Bouchier-Hayes DJ. Plasma factors augment neutrophil and endothelial cell activation during aortic surgery. Eur J Vasc Endovasc Surg. 1997;13:381–387. doi: 10.1016/S1078-5884(97)80080-9. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med. 1991;91:54S–59S. doi: 10.1016/0002-9343(91)90284-5. [DOI] [PubMed] [Google Scholar]
- 7.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–630. doi: 10.1016/S0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 8.Bolcal C, Yildirim V, Doganci S, Sargin M, Aydin A, Eken A, Ozal E, Kuralay E, Demirkilic U, Tatar H. Protective effects of antioxidant medications on limb ischemia reperfusion injury. J Surg Res. 2007;139:274–279. doi: 10.1016/j.jss.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Burton AC. On the physical equilibrium of small blood vessels. Am J Physiol. 1951;164:319–329. doi: 10.1152/ajplegacy.1951.164.2.319. [DOI] [PubMed] [Google Scholar]
- 10.Cavanagh SP, Gough MJ, Homer-Vanniasinkam S. The role of the neutrophil in ischaemia-reperfusion injury: potential therapeutic interventions. Cardiovasc Surg. 1998;6:112–118. doi: 10.1016/S0967-2109(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 11.Clayton JM, Hayes AC, Barnes RW. Tissue pressure and perfusion in the compartment syndrome. J Surg Res. 1977;22:333–339. doi: 10.1016/0022-4804(77)90152-4. [DOI] [PubMed] [Google Scholar]
- 12.Crinnion JN, Homer-Vanniasinkam S, Parkin SM, Gough MJ. Role of neutrophil-endothelial adhesion in skeletal muscle reperfusion injury. Br J Surg. 1996;83:251–254. doi: 10.1002/bjs.1800830234. [DOI] [PubMed] [Google Scholar]
- 13.Davreux CJ, Soric I, Nathens AB, Watson RW, McGilvray ID, Suntres ZE, Shek PN, Rotstein OD. N-acetyl cysteine attenuates acute lung injury in the rat. Shock. 1997;8:432–438. doi: 10.1097/00024382-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan RJ, Meredith TJ. Use of N-acetylcysteine in clinical toxicology. Am J Med. 1991;91:131S–139S. doi: 10.1016/0002-9343(91)90296-A. [DOI] [PubMed] [Google Scholar]
- 15.Haimovici H. Muscular, renal, and metabolic complications of acute arterial occlusions: myonephropathic-metabolic syndrome. Surgery. 1979;85:461–468. [PubMed] [Google Scholar]
- 16.Hartsock LA, O’Farrell D, Seaber AV, Urbaniak JR. Effect of increased compartment pressure on the microcirculation of skeletal muscle. Microsurgery. 1998;18:67–71. doi: 10.1002/(SICI)1098-2752(1998)18:2<67::AID-MICR1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Heller AR, Groth G, Heller SC, Breitkreutz R, Nebe T, Quintel M, Koch T. N-acetylcysteine reduces respiratory burst but augments neutrophil phagocytosis in intensive care unit patients. Crit Care Med. 2001;29:272–276. doi: 10.1097/00003246-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Heppenstall RB, Sapega AA, Izant T, Fallon R, Shenton D, Park YS, Chance B. Compartment syndrome: a quantitative study of high-energy phosphorus compounds using 31P-magnetic resonance spectroscopy. J Trauma. 1989;29:1113–1119. doi: 10.1097/00005373-198908000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Heppenstall RB, Sapega AA, Scott R, Shenton D, Park YS, Maris J, Chance B. The compartment syndrome: an experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;226:138–155. [PubMed] [Google Scholar]
- 20.Heppenstall RB, Scott R, Sapega A, Park YS, Chance B. A comparative study of the tolerance of skeletal muscle to ischemia: tourniquet application compared with acute compartment syndrome. J Bone Joint Surg Am. 1986;68:820–828. [PubMed] [Google Scholar]
- 21.Huda R, Solanki DR, Mathru M. Inflammatory and redox responses to ischaemia/reperfusion in human skeletal muscle. Clin Sci. 2004;107:497–503. doi: 10.1042/CS20040179. [DOI] [PubMed] [Google Scholar]
- 22.Iwahori Y, Ishiguro N, Shimizu T, Kondo S, Yabe Y, Oshima T, Iwata H, Sendo F. Selective neutrophil depletion with monoclonal antibodies attenuates ischemia/reperfusion injury in skeletal muscle. J Reconstr Microsurg. 1998;14:109–116. doi: 10.1055/s-2007-1000152. [DOI] [PubMed] [Google Scholar]
- 23.Jerome SN, Akimitsu T, Korthuis RJ. Leukocyte adhesion, edema, and development of postischemic capillary no-reflow. Am J Physiol. 1994;267:H1329–1336. doi: 10.1152/ajpheart.1994.267.4.H1329. [DOI] [PubMed] [Google Scholar]
- 24.Jerome SN, Kong L, Korthuis RJ. Microvascular dysfunction in postischemic skeletal muscle. J Invest Surg. 1994;7:3–16. doi: 10.3109/08941939409018278. [DOI] [PubMed] [Google Scholar]
- 25.Kearns SR, Daly AF, Sheehan K, Murray P, Kelly C, Bouchier-Hayes D. Oral vitamin C reduces the injury to skeletal muscle caused by compartment syndrome. J Bone Joint Surg Br. 2004;86:906–911. doi: 10.1302/0301-620X.86B6.14177. [DOI] [PubMed] [Google Scholar]
- 26.Kearns SR, Kelly CJ, Barry M, Abdih H, Condron C, Leahy A, Bouchier-Hayes D. Vitamin C reduces ischaemia-reperfusion-induced acute lung injury. Eur J Vasc Endovasc Surg. 1999;17:533–536. doi: 10.1053/ejvs.1999.0833. [DOI] [PubMed] [Google Scholar]
- 27.Kearns SR, Moneley D, Murray P, Kelly C, Daly AF. Oral vitamin C attenuates acute ischaemia-reperfusion injury in skeletal muscle. J Bone Joint Surg Br. 2001;83:1202–1206. doi: 10.1302/0301-620X.83B8.11754. [DOI] [PubMed] [Google Scholar]
- 28.Koksal C. Attenuation of ischemia/reperfusion injury by N-acetylcysteine in a rat hind limb model. J Surg Res. 2003;111:236–239. doi: 10.1016/S0022-4804(03)00094-5. [DOI] [PubMed] [Google Scholar]
- 29.Laight DW, Lad N, Woodward B, Waterfall JF. Assessment of myeloperoxidase activity in renal tissue after ischemia/reperfusion. Eur J Pharmacol. 1994;292:81–88. doi: 10.1016/0926-6917(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 30.Liu K, Chen LE, Seaber AV, Urbaniak JR. S-nitroso-N-acetylcysteine protects skeletal muscle against reperfusion injury. Microsurgery. 1998;18:299–305. doi: 10.1002/(SICI)1098-2752(1998)18:5<299::AID-MICR1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Matsen FA, Winquist RA, Krugmire RB. Diagnosis and management of compartmental syndromes. J Bone Joint Surg Am. 1980;62:286–291. [PubMed] [Google Scholar]
- 32.McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetylcysteine attenuates the decline in muscle Na+, K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin R, Bowler D, Kelly CJ, Kay E, Bouchier-Hayes D. Taurine protects against early and late skeletal muscle dysfunction secondary to ischaemia reperfusion injury. Eur J Surg. 2000;166:375–379. doi: 10.1080/110241500750008916. [DOI] [PubMed] [Google Scholar]
- 34.McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures: the pressure threshold for decompression. J Bone Joint Surg Br. 1996;78:99–104. [PubMed] [Google Scholar]
- 35.McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg Br. 2000;82:200–203. doi: 10.1302/0301-620X.82B2 .9799. [DOI] [PubMed] [Google Scholar]
- 36.Ortolani O, Conti A, Gaudio AR, Moraldi E, Cantini Q, Novelli G. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med. 2000;161:1907–1911. doi: 10.1164/ajrccm.161.6.9903043. [DOI] [PubMed] [Google Scholar]
- 37.Perler BA, Tohmeh AG, Bulkley GB. Inhibition of the compartment syndrome by the ablation of free radical-mediated reperfusion injury. Surgery. 1990;108:40–47. [PubMed] [Google Scholar]
- 38.Robinson JM. Phagocytic leukocytes and reactive oxygen species. Histochem Cell Biol. 2009;131:465–469. doi: 10.1007/s00418-009-0565-5. [DOI] [PubMed] [Google Scholar]
- 39.Rocksén D, Lilliehöök B, Larsson R, Johansson T, Bucht A. Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000;122:249–256. doi: 10.1046/j.1365-2249.2000.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66:93–97. doi: 10.1302/0301-620X.66B1.6693486. [DOI] [PubMed] [Google Scholar]
- 41.Rothe G, Emmendörffer A, Oser A, Roesler J, Valet G. Flow cytometric measurement of the respiratory burst activity of phagocytes using dihydrorhodamine 123. J Immunol Methods. 1991;138:133–135. doi: 10.1016/0022-1759(91)90074-P. [DOI] [PubMed] [Google Scholar]
- 42.Rumack BH. Acetaminophen overdose. Am J Med. 1983;75:104–112. doi: 10.1016/0002-9343(83)90240-1. [DOI] [PubMed] [Google Scholar]
- 43.Sadasivan KK, Carden DL, Moore MB, Korthuis RJ. Neutrophil mediated microvascular injury in acute, experimental compartment syndrome. Clin Orthop Relat Res. 1997;339:206–215. doi: 10.1097/00003086-199706000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Saricaoglu F, Dal D, Salman AE, Atay OA, Doral MN, Salman MA, Kilinç K, Aypar U. Effect of low-dose N-acetyl-cysteine infusion on tourniquet-induced ischaemia-reperfusion injury in arthroscopic knee surgery. Acta Anaesthesiol Scand. 2005;49:847–851. doi: 10.1111/j.1399-6576.2005.00722.x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt H, Schmidt W, Müller T, Böhrer H, Gebhard MM, Martin E. N-acetylcysteine attenuates endotoxin-induced leukocyte-endothelial cell adhesion and macromolecular leakage in vivo. Crit Care Med. 1997;25:858–863. doi: 10.1097/00003246-199705000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt W, Walther A, Gebhard MM, Martin E, Schmidt H. Influence of N-acetylcysteine treatment on endotoxin-induced microcirculatory disturbances. Intensive Care Med. 1998;24:967–972. doi: 10.1007/s001340050697. [DOI] [PubMed] [Google Scholar]
- 47.Shaw CJ, Spencer JD. Late management of compartment syndromes. Injury. 1995;26:633–635. doi: 10.1016/0020-1383(95)00112-M. [DOI] [PubMed] [Google Scholar]
- 48.Sheridan GW, Matsen FA. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58:112–115. [PubMed] [Google Scholar]
- 49.Sirsjö A, Gidlöf A, Nilsson G, Povlsen B. Skeletal muscle blood flow after prolonged tourniquet ischaemia and reperfusion with and without intervening reoxygenation: an experimental study in rats using laser Doppler perfusion imaging. Scand J Plast Reconstr Surg Hand Surg. 1999;33:281–285. doi: 10.1080/02844319950159244. [DOI] [PubMed] [Google Scholar]
- 50.Sjödin K, Nilsson E, Hallberg A, Tunek A. Metabolism of N-acetyl-L-cysteine: some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem Pharmacol. 1989;38:3981–3985. doi: 10.1016/0006-2952(89)90677-1. [DOI] [PubMed] [Google Scholar]
- 51.Spies CD, Reinhart K, Witt I, Meier-Hellmann A, Hannemann L, Bredle DL, Schaffartzik W. Influence of N-acetylcysteine on indirect indicators of tissue oxygenation in septic shock patients: results from a prospective, randomized, double-blind study. Crit Care Med. 1994;22:1738–1746. [PubMed] [Google Scholar]
- 52.Tepel M, Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 53.Laan L, Oyen WJ, Verhofstad AA, Tan EC, ter Laak HJ, Gabreels-Festen A, Hendriks T, Goris RJ. Soft tissue repair capacity after oxygen-derived free radical-induced damage in one hindlimb of the rat. J Surg Res. 1997;72:60–69. doi: 10.1006/jsre.1997.5167. [DOI] [PubMed] [Google Scholar]
- 54.Wagner PD, Mathieu-Costello O, Bebout DE, Gray AT, Natterson PD, Glennow C. Protection against pulmonary O2 toxicity by N-acetylcysteine. Eur Respir J. 1989;2:116–126. [PubMed] [Google Scholar]
- 55.Walker PM, Lindsay TF, Labbe R, Mickle DA, Romaschin AD. Salvage of skeletal muscle with free radical scavengers. J Vasc Surg. 1987;5:68–75. doi: 10.1067/mva.1987.avs0050068. [DOI] [PubMed] [Google Scholar]
- 56.Wang JX, Li Y, Zhang LK, Zhao J, Pang YZ, Tang CS, Zhang J. Taurine inhibits ischemia/reperfusion-induced compartment syndrome in rabbits. Acta Pharmacol Sin. 2005;26:821–827. doi: 10.1111/j.1745-7254.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 57.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 58.Welbourn CR, Goldman G, Paterson IS, Valeri CR, Shepro D, Hechtman HB. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg. 1991;78:651–655. doi: 10.1002/bjs.1800780607. [DOI] [PubMed] [Google Scholar]
- 59.Xiao F, Eppihimer MJ, Young JA, Nguyen K, Carden DL. Lung neutrophil retention and injury after intestinal ischemia/reperfusion. Microcirculation. 1997;4:359–367. doi: 10.3109/10739689709146800. [DOI] [PubMed] [Google Scholar]