Abstract
Background
Open orthopaedic wounds are ideal sites for infection. Preventing infection in these wounds is critical for reducing patient morbidity and mortality, controlling antimicrobial resistance and lowering the cost of treatment. Localized drug delivery has the potential to overcome the challenges associated with traditional systemic dosing. A degradable, biocompatible polymer sponge (chitosan) that can be loaded with clinician-selected antibiotics at the point of care would provide the patient and clinician with a desirable, adjunctive preventive modality.
Questions/purposes
We asked (1) if an adaptable, porous chitosan matrix could absorb and elute antibiotics for 72 hours for potential use as an adjunctive therapy to débridement and lavage; and (2) if the sponges could elute levels of antibiotic that would inhibit growth of Staphylococcus aureus and Pseudomonas aeruginosa?
Methods
We fabricated a degradable chitosan sponge that can be loaded with antibiotics during a 60-second hydration in drug-containing solution. In vitro evaluation determined amikacin and vancomycin release from chitosan sponges at six time points. Activity tests were used to assess the release of inhibitory levels of amikacin and vancomycin.
Results
Amikacin concentration was 881.5 μg/mL after 1 hour with a gradual decline to 13.9 μg/mL after 72 hours. Vancomycin concentration was 1007.4 μg/mL after 1 hour with a decrease to 48.1 μg/mL after 72 hours. Zone of inhibition tests were used to verify inhibitory levels of drug release from chitosan sponges. A turbidity assay testing activity of released amikacin and vancomycin indicated inhibitory levels of elution from the chitosan sponge.
Clinical Relevance
Chitosan sponges may provide a potential local drug delivery device for preventing musculoskeletal infections.
Introduction
Bacterial infections are serious complications of orthopaedic trauma and surgical procedures. Treatment of infections is difficult and in need of more effective preventive modalities. The avascular nature of some traumatic injury sites is a potential cause for concern when systemically administering antibiotics. The inability to achieve high local levels of antibiotic can lead to systemic overdosing or subtherapeutic levels of antibiotic, leading to systemic toxicity [30, 36] or antimicrobial resistance [15, 30], respectively. Improper peak and trough levels and the inconvenience and cost of monitoring these levels are additional issues associated with systemic antibiotic therapy. Local antibiotic therapy has the potential to overcome these problems by providing high concentrations of antibiotic locally. As McLaren discussed [23], a desirable carrier for therapeutic agent(s) would be degradable. The carrier would also provide predictable elution and degradation rate while allowing flexibility in antibiotics chosen.
Local antibiotic delivery systems have been proposed as alternative therapies to typical prophylactic antibiotic dosing [9, 11, 26, 28, 32, 41]. Several materials are being used as carriers, with polymethylmethacrylate (PMMA) being the most common [23, 25]. PMMA is used in many orthopaedic applications, including arthroplasty and antibiotic-loaded spacers. PMMA provides a consistent, predictable rate of antibiotic elution; however, potential drawbacks to this particular therapy include the need for surgical removal of bead implants and bacterial colonization and subsequent biofilm formation on the implant surface [10, 23, 25]. Like with systemic antibiotics, subtherapeutic release of antibiotic from PMMA can lead to resistance [15, 21]. Another material used in orthopaedic applications as a local delivery system is calcium sulfate [5, 13, 41]. Calcium sulfate provides a bolus release of antibiotic with extended elution from several days to weeks [35, 37]. Calcium sulfate is a biocompatible material that provides osteoconductive properties [33, 38] but has elevated sterile, serous wound drainage [3, 31]. Another degradable material being used in local delivery applications is collagen [7]. Antibiotic-loaded collagen sponges provide high local concentrations of antibiotic in a biocompatible matrix [14, 24]. A possible drawback to these sponges is potentially toxic serum levels of gentamicin in the initial treatment cycle [34]. A limitation with use of calcium sulfate, collagen, and other local delivery systems is a lack of data validating each particular system with multiple antibiotics. Chitosan has also been proposed as a carrier in local drug delivery systems [1, 18, 19]. Chitosan is a biocompatible, biodegradable material that effectively carries antibiotics [1, 27] and is also a Food and Drug Administration-approved hemostatic wound dressing being used by the US military to control hemorrhaging of battlefield injuries [4, 39]. A porous, chitosan matrix capable of being loaded at the point of care could provide clinicians with an adaptable, adjunctive antibiotic delivery system in which tailored dosing in a degradable matrix can be achieved.
Based on the need for more effective methods in preventing infections, we asked the following questions: Can we fabricate an adaptable, chitosan matrix capable of absorbing and eluting antibiotics for 72 hours for potential use as an adjunctive therapy to débridement and lavage? Are the sponges capable of eluting levels of antibiotic that would inhibit growth of Staphylococcus aureus and Pseudomonas aeruginosa and is there a difference in release kinetics between vancomycin and amikacin?
Materials and Methods
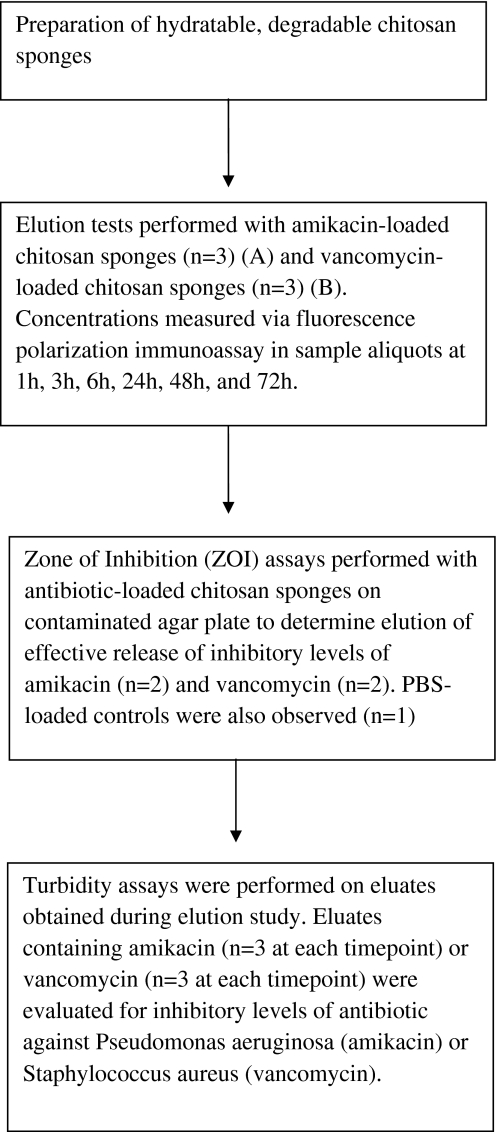
We investigated the ability of a porous chitosan sponge to absorb an antibiotic solution and elute the absorbed drug over 72 hours. According to the East Practice Management Guidelines for prophylactic antibiotic use in open fractures [22], a “second-look” at a wound may be performed at 24-48 hours with subsequent débridements necessary. In vitro elution tests were performed to evaluate release kinetics of amikacin and vancomycin from the chitosan sponge delivery system at six different time points (1 h, 3 h, 6 h, 24 h, 48 h, 72 h) (Fig. 1). Six chitosan sponges were hydrated in antibiotic-containing solution (amikacin or vancomycin) and subjected to elution testing. Radial elution of antibiotic was analyzed using a standard zone of inhibition (ZOI) assay and to assess the capability of the chitosan sponge to elute inhibitory levels of antibiotic before testing for activity using a standard turbidity assay. Eluates taken during the study were subjected to a turbidity assay to assess ability of the delivery system to elute inhibitory levels of amikacin or vancomycin against P. aeruginosa or S. aureus, respectively, within the stated study parameters.
Fig. 1.
Flow chart outlining study design. The elution study and subsequent activity assays were performed with amikacin- or vancomycin-loaded chitosan sponges.
We prepared six chitosan sponges (approximately 40 mm diameter × 6 mm thick) by dissolving 2.0 g of 71% deacetylated chitosan (Primex, Iceland) into 200.0 mL of 1% (v/v) blended lactic/acetic acid solvent (Fisher Scientific, Pittsburgh, PA). The chitosan solution was stirred for 5 hours and then passed over a 180-μm nylon mesh (Gilson, Lewis Center, OH) to remove any nondissolved chitosan particulate. After filtering, 25 mL of chitosan solution was cast into aluminum weighing dishes (Fisher Scientific, 42 mL, fluted) and placed into a −80°C freezer (C90-14A31; Kendro Lab Products, Asheville, NC) for 1 hour. Removal of the frozen samples was followed by placement into a freeze-dryer (FreeZone 2.5; Labconco, Kansas City, MO) for 48 hours. We removed the samples after lyophilization and neutralized them in sodium hydroxide solution (Fisher Scientific). Samples were then rinsed with water until a neutral pH was achieved. The hydrated samples were refrozen for a period of 1 hour at −80°C. The frozen samples were relyophilized for 48 hours. We removed the porous sponges and sterilized them by low-dose gamma irradiation (25–32 kGy).
Elution tests were conducted after hydrating the chitosan sponges for 1 minute in 10 mL of 5 mg/mL antibiotic solution containing either amikacin (Catalog 150342; MP Biomedical, Solon, OH) or vancomycin (Catalog 195540; MP Biomedical). Approximately 7.0 mL of antibiotic solution was absorbed by each sponge. We placed hydrated sponges into 20 mL of 1× phosphate-buffered saline (PBS) (Fisher Scientific) under infinite sink conditions throughout. Samples were gently shaken at 37°C for the duration of the study. One-milliliter aliquots were drawn at 1, 3, 6, 24, 48, and 72 hours. Samples were frozen immediately after acquisition. Complete refreshment of PBS was made at each time point by pipetting. Pipetting the PBS was necessary because of the gelatinous nature of the sponges. We tested samples for antibiotic concentration using a fluorescence polarization immunoassay technique (TDxFLx; Abbott Labs, Abbott Park, IL) using amikacin- and vancomycin-specific reagent kits.
We tested the biologic activity of eluted agents using standard microbiologic techniques. A ZOI assay was used to test the effectiveness of the chitosan sponge delivery system to elute inhibitory levels of active antibiotic. We cut samples into six 40-mg fragments and submerged in 1 mL of 5 mg/mL antibiotic solution (amikacin or vancomycin). The fragments were placed onto a contaminated agar plate containing S. aureus or P. aeruginosa. The plates were incubated for 24 hours and zones of bacterial inhibition were measured and images were taken. The samples were aseptically removed and placed onto another contaminated agar plate for an additional 24 hours. Again, zones of bacterial inhibition were measured and images were taken. The other activity test used was a turbidity assay. Vancomycin samples were tested against a clinical isolate of S. aureus (Cowan I) and amikacin samples were tested against P. aeruginosa (ATCC 27317). Two hundred μL of vancomycin samples was added to 1.75 mL trypticase soy broth (TSB) combined with 20 μL of S. aureus inoculums (104 colony-forming units [CFU]). Amikacin samples (200 μL) were added to 1.75 mL of TSB and 50 μL of P. aeruginosa inoculum (104 CFU). We incubated samples for 24 hours at 37°C and absorbance was read at 530 nm wavelength.
We determined differences in amikacin elution versus vancomycin elution at individual time points using a one-way analysis of variance sorted by time point. All assumptions for normality were met. Statistical analyses were performed using JMP 8.0 (Cary, NC).
Results
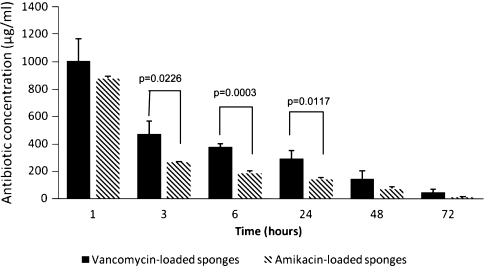
The elution studies showed high concentrations at 1 hour with gradual decreasing concentrations between 3 and 72 hours. Amikacin concentration was 881.5 ± 15.4 μg/mL (Fig. 2) at 1 hour with that value decreasing to 269.2 ± 4.6 μg/mL at 3 hours. After 72 hours, amikacin concentration was still 13.9 ± 3.5 μg/mL. Vancomycin elution was similar with a concentration of 1007.4 ± 162.8 μg/mL measured at 1 hour (Fig. 2). Vancomycin concentration dropped to 472.6 ± 97.6 μg/mL by 3 hours. High concentration of vancomycin was still observed through 72 hours with a measured value of 48.1 ± 23.3 μg/mL. The total recovered amount of drug was between 85% and 98%, respectively. The minimum inhibitory concentration (MIC) of S. aureus (Cowan I) was between 0.5 and 1.0 μg/mL for MP Biomedical vancomycin. The MIC for P. aeruginosa (ATCC 27317) was between 2.0 and 4.0 μg/mL for MP Biomedical amikacin. We observed differences in release of amikacin and vancomycin at 3 hours (p = 0.0226), 6 hours (p = 0.0003), and 24 hours (p = 0.0117).
Fig. 2.
Amikacin and vancomycin release from chitosan sponges. Chitosan sponges were loaded in 5 mg/mL antibiotic solution and antibiotic was eluted in 1× phosphate-buffered saline. Concentrations are given in micrograms per milliliter ± SD (n = 3).
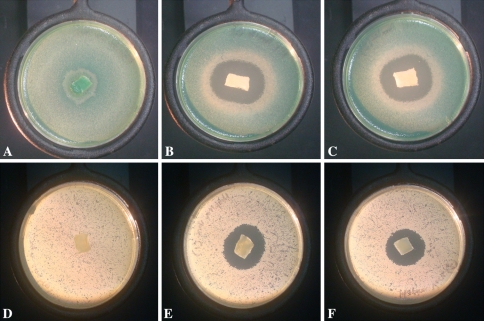
Amikacin samples were active against P. aeruginosa at 1, 3, 6, 24, and 48 hours. Samples fell below the MIC at 72 hours. Bacterial growth was detected in the 72-hour samples (Table 1). Vancomycin samples remained above MIC levels for 72 hours and bacterial growth was not detected in any of those samples (Table 1). Sponges soaked in amikacin and vancomycin had clear zones of inhibition at 24 and 48 hours (Fig. 3). The zones decreased slightly from 24 hours to 48 hours (Table 2).
Table 1.
Results of the turbidity study*
| Time point (hours) | Organism | |
|---|---|---|
| Pseudomonas aeruginosa ATCC 27317 | Staphylococcus aureus Cowan I | |
| Sponge + amikacin | Sponge + vancomycin | |
| 1 | – | – |
| 3 | – | – |
| 6 | – | – |
| 24 | – | – |
| 48 | – | – |
| 72 | + | – |
(−) indicates no bacterial growth detected; (+) bacterial growth detected; *amikacin-containing eluates were tested against P. aeruginosa and vancomycin-containing eluates were tested against S. aureus; time points were 1, 3, 6, 24, 48, and 72 hours with (−) indicating a complete inhibition of bacterial growth and (+) indicating lack of complete inhibition of bacterial growth (n = 3).
Fig. 3A–F.
(A–C) Agar plates with Pseudomonas aeruginosa. (A) Saline-loaded chitosan sponge control and (B–C) chitosan sponges loaded with amikacin. No inhibition of growth was seen with the saline-loaded control. (D–F) Agar plates with Staphylococcus aureus. (D) Saline-loaded chitosan sponge control and (E–F) are chitosan sponges loaded with vancomycin. No inhibition of growth was seen with the saline-loaded control. The images were taken at the 48-hour time point.
Table 2.
Zones of inhibition were measured with amikacin-loaded chitosan sponges (Pseudomonas aeruginosa) and vancomycin-loaded chitosan sponges (Staphylococcus aureus)
| Sample type | 24-hour ZOI (mm) | 48-hour ZOI (mm) |
|---|---|---|
| P. aeruginosa control | 2–3 mm (one corner only) | 0 mm |
| Amikacin (1) | 30–34 mm | 22–24 mm |
| Amikacin (2) | 32–34 mm | 22–24 mm |
| S. aureus control | 1–2 mm (one corner only) | 0 mm |
| Vancomycin (1) | 30–32 mm | 18–20 mm |
| Vancomycin (2) | 30–32 mm | 14–20 mm |
ZOI = zone of inhibition.
Discussion
Traditional systemic antibiotic therapy is not without drawbacks [12, 17, 42]. Improper peak and trough levels and the inconvenience and cost of monitoring these levels are issues that clinicians must face when administering systemic antibiotic therapy. Another concern when administering antibiotics systemically are the avascular zones near an injury site that limit the local influx and availability of given antibiotics. Using a degradable antibiotic delivery system that can be loaded immediately before implantation as an adjunctive therapy could potentially improve patient outcomes after a traumatic injury or surgery. We sought to fabricate an adaptable, porous chitosan matrix capable of absorbing and eluting antibiotics for 72 hours for potential use as an adjunctive therapy to débridement and lavage. Also, we observed active antibiotic release (bactericidal levels) against two commonly pathogenic bacteria, S. aureus and P. aeruginosa, for 48 hours.
Our study is limited by several factors. First, we studied only two antibiotics, one against each specific strain. An expansion of the drug variation would be important to ensure other antibiotics can be similarly delivered. Second, we lacked more time points, particularly those associated with long-term antibiotic release. For the reasons outlined we intended to fabricate a system that would degrade rapidly and elute inhibitory levels of antibiotic over 72 hours. Extended observation of release would provide a more complete insight as to potential use in a more long-term application; however, the rapid release and degradation allows for use as an implantable material for adjunctive use in the prevention of infections. Thirdly, the in vitro results that are gathered are difficult to translate to in vivo efficacy. Release characteristics are different in an in vivo setting and can only translate if a given clinical scenario and wound environment is reproduced [23]. Also, while we have previously published our studies on antibiotics released from chitosan films we had no experience with the current sponge form [27]. The pilot study presented here was needed to assess the potential of the chitosan sponge delivery system as a suitable carrier of antibiotics for prevention of infection.
We designed and fabricated a degradable, porous chitosan sponge capable of rapid absorption of antibiotics in solution. Similar to other degradable drug delivery systems, we observed a burst release of antibiotic during the first few hours of testing [1, 2, 12, 29]. Comparing our results with the well-defined release profile of antibiotic-loaded bone cement (ALBC) shows the antibiotic is released more rapidly from the chitosan sponge. Our delivery system has the advantage of being degradable because chitosan breaks down into simple sugars and is easily handled by the body’s metabolic pathways. ALBC systems offer longer antibiotic release and familiarity in the medical community [8, 16, 20]; however, the possibility of biofilm formation on the surface, potential subtherapeutic release of antibiotics, and the need for retrieval surgeries makes bone cement a less-than-ideal carrier for antibiotics [12]. Our release profiles are similar in form to previous work with antibiotic-loaded chitosan delivery systems [1, 27]. The magnitude of antibiotic release is much higher, however. Sustained release of over 40 μg/mL of vancomycin and over 13 μg/mL of amikacin at 72 hours shows the chitosan sponge delivery system can sustain very high release levels needed for early-stage infection prevention. These in vitro results are not indicative of in vivo kinetics, however. In vitro data is used as a comparative tool that can be used to project in vivo efficacy. The delivery of antibiotics using calcium sulfate is well characterized [6, 11, 40]. Our results are similar to calcium sulfate delivery systems in terms of burst release of antibiotic at the earliest time point. However, published research using calcium sulfate as an antibiotic delivery vehicle observed longer release of antibiotics than detected in the current study [29]. One reason for this difference may be the degradation rate of the carrier. The chitosan sponges we used were designed to degrade within 72 hours. At 72 hours, the chitosan matrix was completely gelatinous in form. Traditional calcium sulfate delivery systems do not degrade this quickly. Differences in elution rates are also related to the porosity of the matrix. The porous nature of the chitosan sponge allowed for rapid hydration but also allowed for rapid diffusion into the surrounding sampling solution. The chitosan sponge system is similar to the collagen sponge antibiotic delivery systems [7] because both are biodegradable, biocompatible materials. Our chitosan sponges differ from the collagen sponges in that the antibiotic was not preloaded into the sponge. We fabricated a sponge capable of being loaded with amikacin or vancomycin at the point of care.
Elution and activity assays were performed on antibiotic-loaded sponges and eluates to characterize the proposed system as a potential antibiotic delivery system. The samples loaded with amikacin were not inhibitory against P. aeruginosa at 72 hours, although the concentration levels indicated they should be sufficient to inhibit growth. The likely reason the samples were noninhibitory is the samples are diluted 1:10 in the assay. Previous work with this strain of P. aeruginosa suggested an MIC between 2 and 4 μg/mL. When factoring in the dilution, the sample fell below the MIC at the 72-hour time point, hence the lack of inhibition observed. When analyzing the results of the activity assays, however, it is valid to say the chitosan sponge releases inhibitory levels of active antibiotic through 48 hours for the stated experimental parameters.
The need to improve local antibiotic delivery to improve clinical outcomes was a major motivation for this study. Our data suggest that a chitosan sponge can deliver concentrations of antibiotics that effectively inhibit the growth of bacteria. We chose chitosan because it is biocompatible and biodegradable, both important properties for drug delivery systems. The data also suggest the possibility of using this sponge loaded at the point of care to deliver antibiotics locally as an adjunctive therapy for treatment and prevention of infections in a broad range of applications, from open traumatic wounds to prophylactic use in surgical sites.
Acknowledgments
We thank Dr. Joseph C. Wenke for study design consultation. We thank Dr. J. Tracy Watson and Dr. Joseph R. Hsu for clinical consultation. Also, we thank wright Medical Technology (Arlington, TN) for assistance in sample processing.
Footnotes
One or more of the authors (WOH) owns stock in Wright Medical Technology and Extremity Innovations. One or more of the authors (SPN, HSC, JDB, WOH) received research funding from OTRP Grant #W81XWH-08-1-0312.
This work was performed at the University of Memphis and the VA Hospital (Memphis, TN).
References
- 1.Aimin C, Chunlin H, Juliang B, Tinyin Z, Zhichao D. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin Orthop Relat Res. 1999;366:239–247. doi: 10.1097/00003086-199909000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Benoit MA, Mousset B, Delloye C, Bouillet R, Gillard J. Antibiotic-loaded plaster of Paris implants coated with poly lactide-co-glycolide as a controlled release delivery system for the treatment of bone infections. Int Orthop. 1997;21:403–408. doi: 10.1007/s002640050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrelli J, Jr, Prickett WD, Ricci WM. Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin Orthop Relat Res. 2003;411:245–254. doi: 10.1097/01.blo.0000069893.31220.6f. [DOI] [PubMed] [Google Scholar]
- 4.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano A, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang W, Colangeli M, Colangeli S, Di Bella C, Gozzi E, Donati D. Adult osteomyelitis: versus débridement plus Osteoset T pellets. Acta Orthop Belg. 2007;73:238–243. [PubMed] [Google Scholar]
- 6.Dahners LE, Funderburk CH. Gentamicin-loaded plaster of Paris as a treatment of experimental osteomyelitis in rabbits. Clin Orthop Relat Res. 1987;219:278–282. [PubMed] [Google Scholar]
- 7.Diefenbeck M, Muckley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37(Suppl 2):S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10, 905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br. 1997;79:590–595. doi: 10.1302/0301-620X.79B4.7420. [DOI] [PubMed] [Google Scholar]
- 9.Frank A, Rath SK, Venkatraman SS. Controlled release from bioerodible polymers: effect of drug type and polymer composition. J Control Release. 2005;102:333–344. doi: 10.1016/j.jconrel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Gallo J, Kolar M, Florschutz AV, Novotny R, Pantucek R, Kesselova M. In vitro testing of gentamicin-vancomycin loaded bone cement to prevent prosthetic joint infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:153–158. doi: 10.5507/bp.2005.019. [DOI] [PubMed] [Google Scholar]
- 11.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong) 2002;10:53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 12.Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;437:91–96. doi: 10.1097/01.blo.0000175713.30506.77. [DOI] [PubMed] [Google Scholar]
- 13.Heijink A, Yaszemski MJ, Patel R, Rouse MS, Lewallen DG, Hanssen AD. Local antibiotic delivery with OsteoSet, DBX, and Collagraft. Clin Orthop Relat Res. 2006;451:29–33. doi: 10.1097/01.blo.0000229319.45416.81. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey JS, Mehta S, Seaber AV, Vail TP. Pharmacokinetics of a degradable drug delivery system in bone. Clin Orthop Relat Res. 1998;349:218–224. doi: 10.1097/00003086-199804000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006;88:2487–2500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 16.Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop. 2003;11:38–47. doi: 10.5435/00124635-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kaloyanides GJ, Pastoriza-Munoz E. Aminoglycoside nephrotoxicity. Kidney Int. 1980;18:571–582. doi: 10.1038/ki.1980.175. [DOI] [PubMed] [Google Scholar]
- 18.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–2349. doi: 10.1016/S0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 19.Kind GM, Bines SD, Staren ED, Templeton AJ, Economou SG. Chitosan: evaluation of a new hemostatic agent. Curr Surg. 1990;47:37–39. [PubMed] [Google Scholar]
- 20.Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res. 1991;264:302–308. [PubMed] [Google Scholar]
- 21.Lewis G. Properties of antibiotic-loaded acrylic bone cements for use in cemented arthroplasties: a state-of-the-art review. J Biomed Mater Res Appl Biomater. 2009;89B:558–574. doi: 10.1002/jbm.b.31220. [DOI] [PubMed] [Google Scholar]
- 22.Luchette FA, Born CT, DeLong WG, Hoff WS, Mullins D, Palumbo F, Pasquale MD. EAST Practice Management Guidelines Work Group: Practice Management Guidelines for Prophylactic Antibiotic Use in Open Fractures 1998. Available at: http://www.east.org/tpg/openfrac.pdf. Accessed August 3, 2009.
- 23.McLaren AC. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res. 2004;427:101–106. doi: 10.1097/01.blo.0000143554.56897.26. [DOI] [PubMed] [Google Scholar]
- 24.Mehta S, Humphrey JS, Schenkman DI, Seaber AV, Vail TP. Gentamicin distribution from a collagen carrier. J Orthop Res. 1996;14:749–754. doi: 10.1002/jor.1100140511. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CL. The current status of material used for depot delivery of drugs. Clin Orthop Relat Res. 2004;427:72–78. doi: 10.1097/01.blo.0000143741.92384.18. [DOI] [PubMed] [Google Scholar]
- 26.Nelson CL, McLaren SG, Skinner RA, Smeltzer MS, Thomas JR, Olsen KM. The treatment of experimental osteomyelitis by surgical débridement and the implantation of calcium sulfate tobramycin pellets. J Orthop Res. 2002;20:643–647. doi: 10.1016/S0736-0266(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 27.Noel SP, Courtney H, Bumgardner JD, Haggard WO. Chitosan films: a potential local drug delivery system for antibiotics. Clin Orthop Relat Res. 2008;466:1377–1382. doi: 10.1007/s11999-008-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry AC, Rouse MS, Khaliq Y, Piper KE, Hanssen AD, Osmon DR, Steckelberg JM, Patel R. Antimicrobial release kinetics from polymethylmethacrylate in a novel continuous flow chamber. Clin Orthop Relat Res. 2002;403:49–53. doi: 10.1097/00003086-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Richelsoph KC, Webb ND, Haggard WO. Elution behavior of daptomycin-loaded calcium sulfate pellets: a preliminary study. Clin Orthop Relat Res. 2007;461:68–73. doi: 10.1097/BLO.0b013e3181123889. [DOI] [PubMed] [Google Scholar]
- 30.Roberts JA, Lipman J. Antibacterial dosing in intensive care: pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin Pharmacokinet. 2006;45:755–773. doi: 10.2165/00003088-200645080-00001. [DOI] [PubMed] [Google Scholar]
- 31.Robinson D, Alk D, Sandbank J, Farber R, Halperin N. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann Transplant. 1999;4:91–97. [PubMed] [Google Scholar]
- 32.Shirtliff ME, Calhoun JH, Mader JT. Experimental osteomyelitis treatment with antibiotic-impregnated hydroxyapatite. Clin Orthop Relat Res. 2002;401:239–247. doi: 10.1097/00003086-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Strocchi R, Orsini G, Iezzi G, Scarano A, Rubini C, Pecora G, Piattelli A. Bone regeneration with calcium sulfate: evidence for increased angiogenesis in rabbits. J Oral Implantol. 2002;28:273–278. doi: 10.1563/1548-1336(2002)028<0273:BRWCSE>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Swieringa AJ, Goosen JH, Jansman FG, Tulp NJ. In vivo pharmacokinetics of a gentamicin-loaded collagen sponge in acute periprosthetic infection: serum values in 19 patients. Acta Orthop. 2008;79:637–642. doi: 10.1080/17453670810016650. [DOI] [PubMed] [Google Scholar]
- 35.Thomas DB, Brooks DE, Bice TG, DeJong ES, Lonergan KT, Wenke JC. Tobramycin-impregnated calcium sulfate prevents infection in contaminated wounds. Clin Orthop Relat Res. 2005;441:366–371. doi: 10.1097/01.blo.0000181144.01306.b0. [DOI] [PubMed] [Google Scholar]
- 36.Tjuljandin SA, Doig RG, Sobol MM, Watson DM, Sheridan WP, Morstyn G, Mihaly G, Green MD. Pharmacokinetics and toxicity of two schedules of high dose epirubicin. Cancer Res. 1990;50:5095–5101. [PubMed] [Google Scholar]
- 37.Turner T, Urban R, Gitelis S, Sumner D, Haggard W, Parr J. Antibiotic delivery from calcium sulfate as a synthetic bone graft substitutes in a rabbit tibial defect model. Trans Orthop Res Soc. 1998;23:597. [Google Scholar]
- 38.Turner TM, Urban RM, Gitelis S, Haggard WO, Richelsoph K. Resorption evaluation of a large bolus of calcium sulfate in a canine medullary defect. Orthopedics. 2003;26(Suppl):s577–s579. doi: 10.3928/0147-7447-20030502-10. [DOI] [PubMed] [Google Scholar]
- 39.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 40.Wenke JC, Owens BD, Svoboda SJ, Brooks DE. Effectiveness of commercially-available antibiotic-impregnated implants. J Bone Joint Surg Br. 2006;88:1102–1104. doi: 10.1302/0301-620X.88B8.17368. [DOI] [PubMed] [Google Scholar]
- 41.Yarboro SR, Baum EJ, Dahners LE. Locally administered antibiotics for prophylaxis against surgical wound infection. An in vivo study. J Bone Joint Surg Am. 2007;89:929–933. doi: 10.2106/JBJS.F.00919. [DOI] [PubMed] [Google Scholar]
- 42.Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130:202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]