Abstract
Background
Vibrio necrotizing fasciitis is a rare and life-threatening soft tissue infection, with fulminant clinical courses and high mortality rates. However, the lack of specific disease characteristics and diagnostic tools during the initial examination may delay diagnosis.
Questions/purposes
We (1) asked whether the clinical indicators could predict laboratory findings during the initial stage of Vibrio necrotizing fasciitis and (2) determined the relationships between the laboratory risk indicator for necrotizing fasciitis (LRINEC) score and the diagnosis of Vibrio infection.
Methods
We retrospectively reviewed 70 patients with 71 episodes of Vibrio necrotizing fasciitis and sepsis. Of the 70 patients, 68 had a history of contact with seawater or raw seafood; 66 had underlying chronic diseases.
Results
Eighteen patients (25.7%) died a mean 18.7 days after admission, and 52 patients survived. A systolic blood pressure of 90 mm Hg or less at the time of admission to the emergency room was associated with mortality. Patients who died had lower leukocyte counts, segmented leukocyte counts, platelet counts, and serum albumin levels compared with the patients who survived and higher counts of band forms of leukocytes. Only eight patients (11%) who survived had a LRINEC score of 6 or greater.
Conclusions
The LRINEC scoring system is not applicable when treating such a highly lethal disease. We propose that severe hypoalbuminemia, severe thrombocytopenia, and increased banded forms of leukocytes are laboratory risk indicators of necrotizing fasciitis that aid in pointing toward initiation of early surgery and predict a higher risk of death.
Level of Evidence
Level III Prognostic study. See the Guidelines for Authors for complete descriptions of levels of evidence.
Introduction
Necrotizing fasciitis and progressive sepsis caused by the Vibrio species is a rare and life-threatening soft tissue infection. It is characterized by rapid invasion and necrosis of the skin, subcutaneous tissue, and fascial planes and is acquired through exposure to warm seawater and raw seafood [7, 8, 11, 19, 25]. The features of this infection include hemorrhagic bullae, subcutaneous bleeding, purpura, necrosis, and gangrene. Necrotizing fasciitis is categorized as Type 1 (mixed infection from aerobic and anaerobic bacteria) [23, 24] or Type 2 (Group A β-hemolytic Streptococcus and Staphylococcus aureus). Vibrio necrotizing fasciitis with sepsis has been categorized as Type 3 necrotizing fasciitis owing to its reported fulminant clinical presentation and high mortality rates (range, 25–100%) [2, 9, 14, 23, 24].
Early diagnosis of Vibrio soft tissue infections and emergency fasciotomy or amputation can reduce the mortality in patients with underlying chronic illnesses [2, 7–9, 14, 23–25, 28]. In 2004, we reported a treatment algorithm for patients with suspected Vibrio necrotizing soft tissue infections that includes emergency fasciotomy or amputation, antibiotic therapy with a third-generation cephalosporin plus tetracycline, and admission to the intensive care unit [28]. However, our mortality rates of patients with Vibrio infections were still as much as 35% to 44% [15, 27, 29]. The lack of specific disease characteristics and diagnostic tools during the initial examination might have delayed the diagnosis and resulted in failure to initiate early aggressive surgery. Wong et al. advocated the laboratory risk indicator for necrotizing fasciitis (LRINEC) score to distinguish necrotizing fasciitis from nonnecrotizing soft tissue infections [32]. The LRINEC score is based on points assigned for six laboratory variables at the time of presentation: C-reactive protein, total leukocyte count, hemoglobin, serum sodium, serum creatinine, and serum glucose. A LRINEC score of 6 or greater reportedly indicates a high risk for the presence of necrotizing fasciitis [32, 33].
Based on previous small series and case reports of 34 patients with Vibrio necrotizing fasciitis, 13 died and 21 survived, resulting in a mortality rate of 38% [15, 16, 26–29]. Hypotensive shock, leukopenia, higher counts of band forms of leukocytes, decreased platelet counts, and severe hypoalbuminemia at the time of presentation were associated with mortality in patients with Vibrio necrotizing fasciitis [15, 27–29]. However, there are no known laboratory parameters that specifically can detect and make an early diagnosis of Vibrio necrotizing fasciitis.
The purposes of this retrospective study were: (1) to determine whether clinical indicators, such as total leukocyte counts, band and segmented forms of leukocytes, platelet counts, and serum albumin level, could predict the presence of an early stage of Vibrio necrotizing fasciitis; (2) to evaluate the mortality of surgery using our laboratory risk indicators; and (3) to quantify the relationships between the LRINEC score and the diagnosis of Vibrio infection.
Patients and Methods
We retrospectively reviewed the medical records of 76 patients with a history of 77 episodes of Vibrio necrotizing fasciitis and sepsis admitted between June 2002 and December 2008 through an electronic search with a discharge diagnosis of necrotizing fasciitis (ICD-9 code 72886) and our registration database. The cultured specimens, obtained from the wounds or the blood, were confirmed Vibrio species by microbiologic evaluation. The most common symptoms on presentation were pain and swelling of the involved limbs with patchy, edematous, erythematous, and bullous skin lesions (Fig. 1). Broad-spectrum antibiotic therapy with a third-generation cephalosporin plus tetracycline or with penicillin plus gentamicin was administered initially to patients when Vibrio necrotizing fasciitis was suspected owing to a recent history of contact with seawater or raw seafood. Emergency surgery was planned for all patients. However, six patients did not undergo surgery: three refused surgery, two died before anticipated surgery, and one who had no underlying chronic illness survived with intravenous antibiotic therapy. One was a fisherman with hepatitis B who had necrotizing fasciitis of the right forearm, and had an emergency fasciotomy in September 2004 and survived; however, he had a similar episode of the left forearm in May 2005 and died from multiorgan failure. After excluding the six patients without surgery, we had 70 patients with 71 episodes of Vibrio necrotizing fasciitis in the study. There were 54 men and 16 women with a mean age of 53 years (range, 36–68 years) at the time of the episode. Débridement of the necrotic fascia or immediate limb amputation was performed initially in all 70 patients. Thirty-four of these 70 patients were reported previously [26–29]. The minimum followup was 2 days (mean, 6 months; range, 2 days–16 months). No patients were lost to followup.
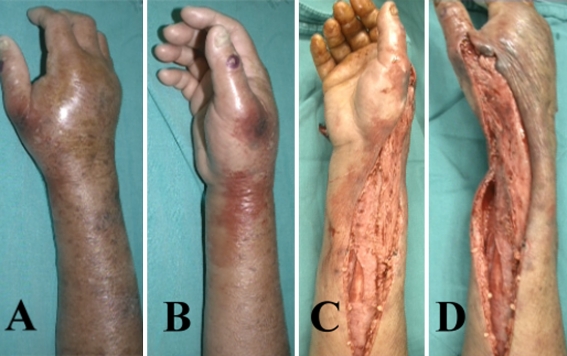
Fig. 1A–D.
A 79-year-old fisherman with liver cirrhosis, hepatitis, and diabetes mellitus had direct contact of his right forearm with a fish. His right forearm had (A) hemorrhagic bullous lesions on the dorsal side and (B) patchy erythema on the medial side before surgery. After emergency fasciotomy, (C) progressive skin necrosis and (D) slightly cyanotic change of the underlying muscles were observed on his forearm. He was treated with skin graft coverage and was discharged 41 days after the first surgery.
In 68 patients with 69 episodes, there was a prior history of having handled raw seafood or prolonged occupational exposure to warm seawater. One patient had a cut on his right arm, and another had an insect bite on his right hand. Emergency fasciotomy or immediate limb amputation initially was performed in all patients. Patients with sepsis and severe clinical presentation were admitted to the intensive care unit after surgery.
From the records we recorded age, gender, comorbidities, presenting signs and symptoms, site, bacteriologic results, predisposing factors, laboratory findings at the time of admission, interval between contact and admission, interval between diagnosis of necrotizing fasciitis and first surgery, length of stay, LRINEC score, and clinical outcomes for each patient.
We categorized the patients into two groups; those who died and those who survived at each episode. Thirteen men and five women with a mean age of 59 years (range, 45–77 years) died. Seventeen had a history of hepatic disease. Seven patients had hepatic dysfunction and diabetes mellitus: three with cirrhosis and diabetes mellitus, and four with cirrhosis, diabetes mellitus, and hepatitis. Ten patients had hepatic dysfunction alone: four had liver cirrhosis, one had hepatitis B and C, two had hepatitis C, one had hepatitis B, one had liver cirrhosis and hepatitis C, and one had alcoholic liver disease. One patient had diabetes mellitus alone. Five of the patients who died had upper limb skin lesions and 13 had lower limb skin lesions. Sixteen patients initially underwent fasciotomy and débridement, and two underwent immediate above-the-knee amputation owing to severe skin lesions and sepsis at the time of presentation in the emergency room. Five patients underwent above-the-knee amputation a few days after fasciotomy owing to progressive uncontrolled sepsis. Three patients received skin grafts, one underwent débridement, and nine had no additional surgery after the fasciotomy. Six of the 18 patients had a body temperature greater than 38.5°C. Seventeen were hypotensive with a systolic blood pressure of 90 mm Hg or less. The mean duration of hospital stay for the patients who died was 19 days (range, 2–78 days).
Among the 52 patients with 53 episodes (including the patient who survived the first episode but died after the second episode) and who were alive at 6 months followup, there were 41 men and 11 women with a mean age of 64 years (range, 36–86 years) at the time of the episode. Seven of these 52 patients had hepatic dysfunction and diabetes mellitus, 17 had hepatic dysfunction alone, and six had diabetes mellitus alone. Four patients had diabetes mellitus and gout or a recurrent history of steroid intake; four were receiving steroid therapy; and three had gout with steroid intake. Eight patients had other chronic underlying diseases; two had chronic renal insufficiency; two had valvular heart disease; and one patient each had chronic obstructive pulmonary disease, rheumatoid arthritis, a history of drug abuse, and renal cell carcinoma with metastasis. Four patients denied any chronic illness. Twenty-three of the 52 surviving patients had upper limb involvement (one patient had two episodes of upper limb involvement, therefore there were 24 episodes in which patients survived) and 29 had lower limb involvement. Owing to progressive skin involvement after the fasciotomy, three patients underwent an above-knee amputation, two had above-the-elbow amputations, one had a below-the-knee amputation, and one had a finger amputation. Twenty-nine patients had skin grafts, five had flap reconstruction, six had débridement with repair, and six had only wound care after the initial fasciotomy. Seventeen of the 52 patients were febrile (body temperature greater than 38.5°C) and 24 had a systolic blood pressure of 90 mm Hg or less at presentation in the emergency room. The mean duration of hospital stay for the patients in the survival group was 33 days (range, 6–80 days).
We compared differences in patient characteristics, clinical presentations, underlying chronic diseases, locations of infection, first operative procedure, laboratory data, and hospital courses between these two groups; we used the Wilcoxon rank-sum test for continuous variables (age, interval, hospital stay, leukocyte count, band forms, segmented forms, platelet count, albumin) and Fisher’s Exact test for categorical variables (gender, underlying chronic disease, wound location, first operation, mortality rate of period, LRINEC scores, systolic blood pressure ≤ 90 mm Hg, leukocyte count ≤ 7000, band forms > 7%, segmented forms ≤ 70%, platelet count ≤ 80,000, albumin ≤ 2). To identify risk factors for initiation of surgery we used multivariate logistic regression analysis to examine parameters that showed a difference (p < 0.5) in a univariate analysis. Statistical analyses were performed using SPSS Version 12.0 statistic software (SPSS, Chicago, IL).
Results
Age, gender, interval between contact and admission, interval between diagnosis of necrotizing fasciitis and first surgery, wound location, nature of first surgery, fever, and numbers of lymphocyte forms of leukocytes were similar between patients who died and those who survived (Table 1). Patients with hepatic dysfunction and diabetes mellitus had a higher mortality rate than those with hepatic disease or diabetes mellitus alone. A systolic blood pressure of 90 mm Hg or less at the time of presentation to the emergency room also was associated (p < 0.001) with mortality. By univariate analysis, patients who died had lower total and segmented leukocyte counts, platelet counts, and serum albumin levels compared with patients who survived (Table 2). Patients who died had higher counts of band forms of leukocytes than those who survived. The multivariate analysis identified six variables predicting mortality (Table 3): band forms, segmented forms, albumin, systolic blood pressure ≤ 90 mm Hg, platelet count ≤ 80,000, and albumin ≤ 2.
Table 1.
Comparison of patients who died and who survived at each episode
| Variable | Number of patients (70)† Number of episodes (n = 71) | p Value | ||
|---|---|---|---|---|
| Deaths (n = 18) | Survivals (n = 53) | |||
| Age (years) | Mean | 59.5 | 64.3 | 0.09 |
| Gender | ||||
| Male | 13 | 42 | 0.1 | |
| Female | 5 | 11 | ||
| Timing from seawater contact | Mean | 2.83 | 2.33 | 0.161 |
| to presentation in ER (days) | ||||
| Timing from treatment in ER to first operation (hours) | Mean | 11.67 | 15.58 | 0.34 |
| ≤ 12 | 12 | 42 | 0.22 | |
| > 12 | 6 | 11 | ||
| Underlying chronic disease | ||||
| Hepatic dysfunction and DM | 7 | 7 | 0.02* | |
| Hepatic dysfunction alone | 10 | 17 | 0.069 | |
| Diabetes mellitus alone | 1 | 6 | 0.311 | |
| Diabetes mellitus with others | 0 | 4 | ||
| Steroid intake | 0 | 4 | ||
| Gout with steroid intake | 0 | 3 | ||
| Others | 0 | 8 | ||
| None | 0 | 4 | ||
| Wound location | 0.098 | |||
| Upper extremity | 5 | 19 | ||
| Lower extremity | 13 | 29 | ||
| Finger | 0 | 5 | ||
| First operation | ||||
| Fasciotomy | 16 | 52 | 0.142 | |
| Amputation | 2 | 1 | ||
| Final operation | ||||
| Amputation | 5 | 7 | ||
| Split-thickness skin graft | 3 | 29 | ||
| Flap | 0 | 5 | ||
| Debridement | 1 | 6 | ||
| Without secondary operation | 9 | 6 | ||
| Systolic blood pressure (mm Hg) | 0.000* | |||
| ≤ 90 | 17 | 24 | ||
| > 90 | 1 | 29 | ||
| Body temperature (°C) | 0.226 | |||
| > 38.5 | 6 | 17 | ||
| < 38.5 | 12 | 36 | ||
| Mortality rate | 0.095 | |||
| 2002 to 2005 | 32.30% | 11 | 23 | |
| 2006 to 2008 | 18.90% | 7 | 30 | |
| Hospital days | Mean | 18.78 | 32.92 | 0.004* |
*Mean p < 0.05 and the difference was significant; †one male patient survived the first episode but died after the second episode, therefore his data are included in the death and survival categories here.
Table 2.
Comparison of bacteriologic and laboratory data at each episode
| Variable | Number of patients (70)† All episodes (n = 71) | p Value | ||
|---|---|---|---|---|
| Deaths (n = 18) | Survivals (n = 53) | |||
| Pathogen | ||||
| Vibrio vulnificus | 11 | 48 | ||
| Vibrio cholerae non-O1 | 5 | 3 | ||
| Vibrio parahaemolytics | 1 | 1 | ||
| Vibrio fluvialis | 1 | 1 | ||
| Positive culture | ||||
| Wound | 9 | 24 | ||
| Blood | 1 | 7 | ||
| Wound and blood | 8 | 22 | ||
| Leukocyte count (cells/mm3) | Mean | 7266.7 ± 3467 | 13,114.7 ± 4963 | 0.0002* |
| < 7000 | 10 | 10 | 0.004* | |
| > 7000 | 8 | 43 | ||
| Band forms (%) | Mean | 16.36 ± 3.47 | 6.63 ± 0.84 | 0.014* |
| > 7 | 13 | 20 | 0.012* | |
| < 7 | 5 | 33 | ||
| Segmented forms (%) | Mean | 66.14 ± 5.13 | 80.31 ± 1.23 | 0.015* |
| ≤ 70 | 8 | 7 | 0.009* | |
| > 70 | 10 | 47 | ||
| Lymphocyte forms (%) | Mean | 8.61 ± 1.39 | 7.14 ± 0.67 | 0.351 |
| Platelet count (per mm3) | Mean | 78,444.4 ± 10,833.6 | 135,264 ± 10,904.6 | 0.005* |
| ≤ 80,000 | 12 | 10 | 0.000* | |
| > 80,000 | 6 | 43 | ||
| Albumin (g/dL) | Mean | 1.91 ± 0.44 | 2.44 ± 0.57 | 0.001* |
| ≤ 2 | 12 | 13 | 0.002* | |
| > 2 | 6 | 40 | ||
*Mean p < 0.05 and the difference was significant; †one male patient survived the first episode but died after the second episode, therefore his data are included in the death and survival categories here.
Table 3.
Risk factors for mortality of Vibrio necrotizing fasciitis identified by multivariate analysis
| Variable | Odds ratio | p Value |
|---|---|---|
| (95% confidence interval) | ||
| Continuous variables | ||
| Leukocyte count | 1 (1–1) | 0.086 |
| Band forms | 0.866 (0.788–0.951) | 0.003* |
| Segmented forms | 0.953 (0.909–1) | 0.049* |
| Platelet count | 0.237 (1–1) | 0.237 |
| Albumin | 6.213 (1.134–34.03) | 0.035* |
| Categorical variables | ||
| Systolic blood pressure ≤ 90 mm Hg | 40.39 | 0.011* |
| Leukocyte count ≤ 7000 | 2.571 | 0.308 |
| Band forms > 7% | 1.253 | 0.815 |
| Segmented forms ≤ 70% | 1.373 | 0.748 |
| Platelet count ≤ 80,000 | 43.849 | 0.009* |
| Albumin ≤ 2 | 16.564 | 0.021* |
*Mean p < 0.05 and the difference was significant.
Eighteen of the 70 patients died, resulting in a mortality rate of 25.7%. The mortality rate from 2002 to 2005 was 32% (11/34), and from 2006 to 2008 was 19% (seven of 37) (Table 1). Cultures confirmed Vibrio vulnificus in 58 patients with 11 deaths, Vibrio cholerae non-O1 in eight with five deaths, Vibrio parahaemolytics in one, and Vibrio fluvialis in two with one death. The patient with two episodes had Vibrio vulnificus infection during the first episode and died after the second episode of Vibrio parahaemolytics infection (Table 2).
The mean LRINEC scores were similar in patients who died and those who survived: 2.44 (range, 0–5) and 3.3 (range, 0–9), respectively (Table 4). Only eight patients (11%) had a LRINEC score of 6 or greater.
Table 4.
Laboratory risk indicator for necrotizing fasciitis (LRINEC) score at each episode
| Variable | Score | Deaths (n = 18)† | Survivals (n = 53)† | p Value |
|---|---|---|---|---|
| C-reactive protein, mg/L | 61.28 | 92.26 | ||
| < 150 | 0 | 18 | 43 | 0.04* |
| ≥ 150 | 4 | 0 | 10 | |
| Total leukocyte count, cells/mm3 | 7.266 | 13.114 | ||
| < 15 | 0 | 16 | 32 | |
| 15–25 | 1 | 2 | 21 | 0.02* |
| > 25 | 2 | 0 | 0 | |
| Hemoglobin, g/dL | 12.74 | 12.68 | ||
| > 13.5 | 0 | 8 | 20 | |
| 11–13.5 | 1 | 8 | 22 | 0.3 |
| < 11 | 2 | 2 | 11 | |
| Sodium, mmol/L | 137.5 | 137.1 | ||
| > 135 | 0 | 14 | 42 | 0.5 |
| < 135 | 2 | 4 | 11 | |
| Creatinine, umol/L | 188.33 | 144.62 | ||
| ≤ 141 | 0 | 10 | 34 | 0.35 |
| > 141 | 2 | 8 | 19 | |
| Glucose, mmol/L | 8.29 | 8.47 | ||
| ≤ 10 | 0 | 13 | 38 | 0.61 |
| > 10 | 1 | 5 | 15 | |
| Total score (mean) | 2.44 | 3.3 | ||
| > 6 | 0 | 8 | 0.08 | |
| < 6 | 18 | 45 |
*Mean p < 0.05 and the difference was significant; †one male patient survived the first episode but died after the second episode, therefore his data are include in the death and survival categories here.
Discussion
Vibrio necrotizing fasciitis is a rare and life-threatening soft tissue infection, with fulminant clinical courses and high mortality rates. However, the absence of specific disease characteristics and diagnostic tools during the initial examination may delay diagnosis and result in failure to initiate aggressive operative intervention. We therefore asked whether clinical indicators could predict laboratory findings during the initial stage of Vibrio necrotizing fasciitis, and attempted to determine the relationships between the laboratory risk indicator for necrotizing fasciitis (LRINEC) score and the diagnosis of Vibrio infection.
Our study has several limitations. First, it is retrospective. We previously used an electronic search with a discharge diagnosis of necrotizing fasciitis (ICD-9 code 72886), but found some patients with Vibrio necrotizing fasciitis were not included in the system and we had inaccurate descriptions in the medical records and some variables. To resolve this problem, in 2006 we designed a registration form and created a database to record data for the patients with necrotizing fasciitis confirmed by surgery. Second, it took more than 3 days to obtain the results from the microbiology laboratory. Although we performed emergency fasciotomy and prescribed broad-spectrum antibiotic therapy with a third-generation cephalosporin plus tetracycline for patients who had a contact history of seawater or raw seafood and suspected Vibrio necrotizing fasciitis, we identified no organism in 23 patients. The delay or low sensitivity of the microbiologic result might alter the appropriate use of antibiotic therapy. In the future we plan to use a PCR method [35] for early detection and confirmation of the pathogens. Earlier detection and treatment might change the findings.
Diagnosis is difficult during the early stages of Vibrio necrotizing fasciitis because the skin may not show typical signs, and this can be suspected only in patients with a history of contact with seawater or raw seafood. Skin presentations of Vibrio necrotizing fasciitis are characterized by swelling, hemorrhagic bullae, subcutaneous bleeding, skin necrosis, expanding purpura, and gangrene at the time of presentation, which is considered an ominous sign for early surgical intervention at our institution [12, 13, 27, 29]. All imaging modalities reportedly have a low sensitivity for detecting necrotic soft tissue infections and a low specificity [1, 5, 9, 23, 24]. Wall et al. reported that, on admission, a leukocyte count greater than 15,400 cells/mm3 and/or a serum sodium level less than 135 mmol/L were associated with necrotizing soft tissue infections [31]. However, 48 patients in our study had a leukocyte count less than 15,000 cells/mm3 and 56 patients had a serum sodium level greater than 135 mmol/L. We also found patients with Vibrio necrotizing fasciitis with hypotensive shock, decreased platelet count, leukopenia, low segmented leukocyte counts, high counts of band forms of leukocytes, severe hypoalbuminemia, and a combination of hepatic dysfunction and diabetes mellitus at presentation to the emergency room had a greater mortality rate. Therefore, prompt identification of the laboratory risk factors for mortality is essential to make an early diagnosis and initiate early surgery. By multivariate logistic regression analysis the following factors predicted mortality: segmented leukocyte counts, band form counts of leukocytes, albumin levels, a systolic blood pressure of 90 mm Hg or less, platelet count of 80,000 cells/mm3 or less, and serum albumin level of 2 g/dL or less. However, for the normal ranges of the segmented leukocyte count, 42% to 74%, we consider severe hypoalbuminemia, severe thrombocytopenia, and increased banded forms of leukocytes can be risk indicators of necrotizing fasciitis that enable determination of timing of the surgical intervention and prediction of the possibility of mortality.
With early surgery based on these laboratory findings, the mortality rate of 32% during the 4-year period from 2002 to 2005 was reduced to a mortality rate of 19% during a 3-year period from 2006 to 2008.
Wong et al. created the LRINEC score to distinguish necrotizing fasciitis from nonnecrotizing soft tissue infections, in which a LRINEC score of 6 or greater would indicate a high risk for the presence of necrotizing fasciitis [32, 33]. However, the mean LRINEC score for patients with Vibrio infection was less than 6 and only eight patients in the survival group had a LRINEC score of 6 or greater. Our data suggest the LRINEC scoring system is inappropriate for determining early management of patients with suspected Vibrio necrotizing fasciitis on admission to emergency care.
All Vibrio species can release toxic factors into the circulation, thereby giving rise to similar clinical characteristics and fulminant sepsis [3, 6, 18, 22, 36]. Vibrio vulnificus can produce many extracellular toxins, such as hemolysin, protease, lipase, cytolysin, hyaluronidase, mucinase, DNase, and sulfatase [4, 6, 36]. Vibrio cholerae non-O1 produces a heat-stable enterotoxin (NAG-ST), cholera-like toxin, El Tor (HlyA) hemolysin, Shiga-like toxin, and a cell-associated hemagglutinin/protease [3, 10, 18, 20, 22, 36]. Vibrio fluvialis produces various extracellular toxic factors, such as lipase, protease, and hemolysin [17, 21, 34]. Vibrio parahaemolyticus is associated with a thermostable direct hemolysin (TDH) and/or a TDH-related hemolysin (TRH) encoded by the TDH and TRH genes, respectively [30, 36]. We found Vibrio vulnificus the most common species and the cause of death in 11 of 59 patients (mortality rate 19%). Although Vibrio cholerae non-O1 often causes endemic diarrhea, it was the cause of death for five of eight of our patients, and may cause primary or secondary bacteremia more often than the other Vibrio species [29].
Necrotizing fasciitis and sepsis caused by Vibrio species should be highly suspected when patients present with more than just a history of chronic illness and recent history of exposure to seawater or raw seafood. The LRINEC scoring system cannot be used for treating such a highly lethal disease. We suggest severe hypoalbuminemia, severe thrombocytopenia, and increased banded forms of leukocytes are laboratory risk indicators of necrotizing fasciitis that could be used to initiate early surgery and predict mortality. Our data suggest early surgery based on these findings reduced the mortality rate from 32% to 19%.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these case reports that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Chang Gung Memorial Hospital at Chia-Yi.
References
- 1.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705–710. doi: 10.1086/511638. [DOI] [PubMed] [Google Scholar]
- 2.Angoules AG, Kontakis G, Drakoulakis E, Vrentzos G, Granick MS, Giannoudis PV. Necrotizing fasciitis of upper and lower limb: a systematic review. Injury. 2007;38(suppl):18–25. doi: 10.1016/j.injury.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Begum K, Ahsan CR, Ansaruzzaman M, Dutta DK, Ahmad QS, Talukder KA. Toxin(s), other than cholera toxin, produces by environmental non O1 non O139 Vibrio cholerae. Cell Mol Immunol. 2006;3:115–121. [PubMed] [Google Scholar]
- 4.Borenstein M, Francisco K. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245–248. doi: 10.1016/S0733-8635(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 5.Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13:433–439. doi: 10.1097/MCC.0b013e32825a6a1b. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty S, Nair GB, Shinoda S. Pathogenic vibrios in the nature aquatic environment. Rev Environ Health. 1997;12:63–80. doi: 10.1515/reveh.1997.12.2.63. [DOI] [PubMed] [Google Scholar]
- 7.Chiang SR, Chuang YC. Vibrio vulnificus infection: clinical manifestations, pathogenesis, and antimicrobial therapy. J Microbiol Immunol Infect. 2003;36:81–88. [PubMed] [Google Scholar]
- 8.Chuang YC, Yuan CY, Liu CY, Lan CK, Huang AH. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin Infect Dis. 1992;15:271–276. doi: 10.1093/clinids/15.2.271. [DOI] [PubMed] [Google Scholar]
- 9.Fontes RA, Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8:151–158. doi: 10.5435/00124635-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Honda T, Booth BA, Boesman-Finkelsten M, Finkelstein RA. Comparative study of Vibrio cholerae non-O1 protease and soluble hemagglutinin with those of Vibrio cholerae O1. Infect Immun. 1987;55:451–454. doi: 10.1128/iai.55.2.451-454.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard RJ, Bennett NT. Infections caused by halophilic marine Vibrio bacteria. Ann Surg. 1993;217:525–531. doi: 10.1097/00000658-199305010-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiao CT, Lin LJ, Shiao CJ, Hsiao KY, Chen IC. Hemorrhagic bullae are not only skin deep. Am J Emerg Med. 2008;26:316–319. doi: 10.1016/j.ajem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med. 2008;26:170–175. doi: 10.1016/j.ajem.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Hsueh PR, Lin CY, Tang HJ, Lee HC, Liu JW, Liu YC, Chuang YC. Vibrio vulnificus in Taiwan. Emerg Infect Dis. 2004;10:1363–1368. doi: 10.3201/eid1008.040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang KC, Hsieh PH, Huang KC, Tsai YH. Vibrio necrotizing soft-tissue infection of the upper extremity: factors predictive of amputation and death. J Infect. 2008;57:290–297. doi: 10.1016/j.jinf.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Huang KC, Hsu RWW. Vibrio fluvialis hemorrhagic cellulitis and cerebritis. Clin Infect Dis. 2005;40:e75–e77. doi: 10.1086/429328. [DOI] [PubMed] [Google Scholar]
- 17.Kothary MH, Lowman H, Mccardell BA, Tall BD. Purification and characterization of enterotoxigenic El Tor-like hemolysin produced by Vibrio fluvialis. Infect Immun. 2003;71:3213–3220. doi: 10.1128/IAI.71.6.3213-3220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CJ, Chiu CT, Lin DY, Sheen IS, Lien JM. Non-O1 Vibrio cholerae bacteremia in patients with cirrhosis: 5-year experience from a single medical center. Am J Gastroenterol. 1996;91:336–340. [PubMed] [Google Scholar]
- 19.Morris JG., Jr Cholera and other types of vibriosis: a story of human pandemics and oysters of half shell. Clin Infect Dis. 2003;37:272–280. doi: 10.1086/375600. [DOI] [PubMed] [Google Scholar]
- 20.Ou TY, Liu JW, Leu HS. Independent prognostic factors for fatality in patients with invasive Vibrio cholerae non-O1 infections. J Microbiol Immunol Infect. 2003;36:117–122. [PubMed] [Google Scholar]
- 21.Rahim Z. KMS Aziz. Facors affecting production of haemolysin by strains of Vibrio fluvialis. J Diarrhoeal Dis Res. 1996;14:113–116. [PubMed] [Google Scholar]
- 22.Ramamurthy T, Bag PK, Pal A, Bhattacharya SK, Bhattacharya MK, Shimada T, Takeda T, Karasawa T, Kurazono H, Takeda Y, Balakrish Nair G. Virulence patterns of Vibrio cholerae non-O1 strains isolated from hospitalized patients with acute diarrhea in Calcutta, India. J Med Microbiol. 1993;39:310–317. doi: 10.1099/00222615-39-4-310. [DOI] [PubMed] [Google Scholar]
- 23.Salcido RS. Necrotizing fasciitis: reviewing the causes and treatment strategies. Adv Skin Wound Care. 2007;20:288–293. doi: 10.1097/01.ASW.0000269317.76380.3b. [DOI] [PubMed] [Google Scholar]
- 24.Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279–288. doi: 10.1016/j.jamcollsurg.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Seal DV. Necrotizing fasciitis. Current Opinion in Infect Dis. 2001;14:127–132. doi: 10.1097/00001432-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Tsai YH, Cheng CC, Huang TJ, Hsu RWW. Necrotizing fasciitis and primary sepsis caused by Vibrio fluvialis: a case report. Injury Extra. 2005;36:546–549. doi: 10.1016/j.injury.2005.05.016. [DOI] [Google Scholar]
- 27.Tsai YH, Hsu RWW, Huang TJ, Hsu WH, Huang KC, Li YY, Peng KT. Necrotizing soft tissue infections and sepsis caused by Vibrio vulnificus compared with those caused by Aeromonas species. J Bone Joint Surg Am. 2007;89:631–636. doi: 10.2106/JBJS.F.00580. [DOI] [PubMed] [Google Scholar]
- 28.Tsai YH, Hsu RWW, Hung KC, Chen CH, Cheng CC, Peng KT, Huang TJ. Systemic vibrio infection presenting as necrotizing fasciitis and sepsis: a series of thirteen cases. J Bone Joint Surg Am. 2004;86:2497–2502. doi: 10.2106/00004623-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Tsai YH, Huang TJ, Hsu RWW, Weng YJ, Hsu WH, Huang KC, Peng KT. Necrotizing soft-tissue infections and primary sepsis caused by Vibrio vulnificus and Vibrio cholerae non-O1. J Trauma. 2009;467:846–849. doi: 10.1097/TA.0b013e31816a9ed3. [DOI] [PubMed] [Google Scholar]
- 30.Vongxay K, Wang S, Zhang X, Wu B, Hu H, Pan Z, Chen S, Fang W. Pathogenetic characterization of Vibrio parahaemolyticus isolates from clinical and seafood sources. Int J Food Microbiol. 2008;126:71–75. doi: 10.1016/j.ijfoodmicro.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Wall DB, Klein SR, Black S, Virgilio C. A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg. 2000;191:227–231. doi: 10.1016/S1072-7515(00)00318-5. [DOI] [PubMed] [Google Scholar]
- 32.Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (laboratory risk indicator for necrotizing fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32:1535–1541. doi: 10.1097/01.CCM.0000129486.35458.7D. [DOI] [PubMed] [Google Scholar]
- 33.Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis. 2005;18:101–106. doi: 10.1097/01.qco.0000160896.74492.ea. [DOI] [PubMed] [Google Scholar]
- 34.Wong HC, Ting SH, Shieh WR. Incidence of toxigenic vibrios in foods available in Taiwan. J Appl Bacteriol. 1992;73:197–202. doi: 10.1111/j.1365-2672.1992.tb02978.x. [DOI] [PubMed] [Google Scholar]
- 35.Wu ZH, Lou YL, Lu YY, Yan J. Development of quantitative real-time polymerase chain reaction for the detection of Vibrio vulnificus based on hemolysin coding system. Biomed Environ Sci. 2008;21:296–301. doi: 10.1016/S0895-3988(08)60045-8. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XH, Austin B. Haemolysins in Vibrio species. J Appl Microbiol. 2005;98:1011–1019. doi: 10.1111/j.1365-2672.2005.02583.x. [DOI] [PubMed] [Google Scholar]