Abstract
Background
Patient-controlled analgesia is a widely used and effective method of controlling pain after THA. This method is associated with substantial undesirable side effects. Local infiltration has been introduced in an attempt to reduce opioid requirements postoperatively, but its ability to reduce pain without complications is still questioned.
Questions/Purposes
We evaluated patient-controlled analgesia use, pain and satisfaction scores, complication rates, and ropivacaine levels associated with the use of periarticular multimodal drug infiltration in THA.
Patients and Methods
We randomized 64 patients undergoing THA to receive a periarticular intraoperative multimodal drug injection or to receive no injection. All patients received patient-controlled analgesia for 24 hours after surgery. The final assessment was at 6 weeks.
Results
Patients receiving the periarticular injection used less patient-controlled analgesia 6 hours postoperatively. The 24-hour patient-controlled analgesia requirement postsurgery also was less. The visual analog scale score for pain on activity in the postanesthetic care unit was less for patients who received an injection. The visual analog scale satisfaction score was similar in the two groups throughout the followup period. Recorded unbound ropivacaine levels were 2.5 times lower than toxic levels.
Conclusions
Periarticular intraoperative injection with multimodal drugs can reduce postoperative patient-controlled analgesia requirements and pain on activity in patients undergoing THA with no apparent increase in risk.
Level of Evidence
Level I, therapeutic study. See the guidelines online for a complete description of level of evidence.
Introduction
THA is one of the most cost-effective interventions available in modern surgery in terms of increased quality-adjusted life expectancy and the cost per quality-adjusted life year gained [7], but it is associated with considerable postoperative pain [12, 33, 35]. There has been a recent trend toward performing THAs through minimally invasive approaches with proponents claiming benefits including reduced intraoperative blood loss [2], reduced hospital in-patient stay, and reduced postoperative pain [4]. The evolution of these techniques has been accompanied by the introduction of new analgesic regimes, improved patient education, and preoperative preparation. It is unclear how much of the benefit attributed to the minimally invasive approach is attributable to these changes rather than the minimally invasive technique itself. Recent research suggests it may be these rather than the surgical approach that has led to an improvement in outcome [27]. A Cochrane review however concluded preoperative education in isolation had no effect on postoperative pain, function, or length of stay although no account was taken of the surgical technique [21]. Improved perioperative anesthetic regimes reportedly reduce length of stay, reduce postoperative opioid requirements, and improve ambulatory distance in the early rehabilitation period [25].
Continuous epidural and spinal analgesia are commonly used in the context of THA but can be associated with side effects, such as spinal headache, neurogenic bladder, hypotension, respiratory depression, pulmonary hypertension, cardiac compromise, and risk of spinal infection [19, 26]. Opioid analgesia often is associated with side effects, including nausea and vomiting, respiratory depression, drowsiness, pruritus, reduced gut motility, and urinary retention [10, 11]. The use of a lumbar plexus block or femoral and sciatic nerve blocks in THA is becoming more popular but can be associated with technical difficulties [14, 15].
Periarticular infiltration using multimodal analgesia after TKA reportedly reduces postoperative analgesia requirements [5, 34]. However, there is little evidence to support the use of periarticular multimodal injection for postoperative pain relief in the case of THA, although several authors have stated in a review article [28] and an editorial [29] the techniques are used routinely in their departments. These authors state studies are underway, but preliminary data suggest the technique is effective and not associated with an increase in complications [28, 29]. One large nonrandomized case series suggests it is not associated with an increase in side effects or complications [16].
We first hypothesized (1) one periarticular injection using multimodal drugs (an opioid, a NSAID, a long-acting local anesthetic, and epinephrine) would reduce the requirements for postoperative patient-controlled analgesia (PCA). We further hypothesized (2) the injection would reduce the visual analog scale (VAS) scores for pain, (3) improve the VAS scores for patient satisfaction, (4) not increase the operating time, (5) not affect length of hospital stay, and (6) not lead to an increase in complications, and finally (7) the associated increase in unbound ropivacaine levels would not be above reported toxic levels.
Patients and Methods
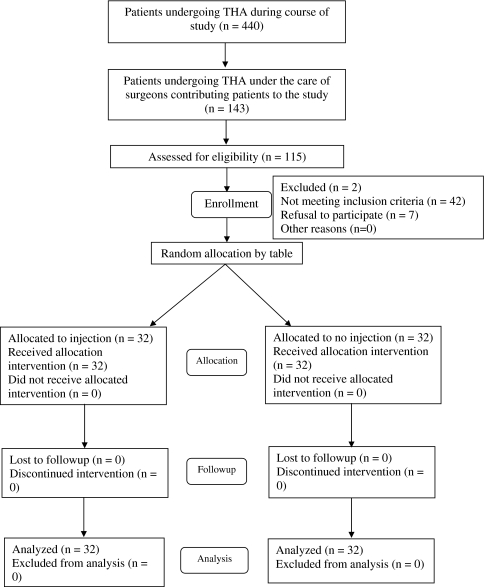
We prospectively enrolled 64 patients (Table 1) undergoing unilateral THA for a diagnosis of osteoarthritis between November 2003 and April 2005. During this time, 452 THAs were performed in our unit in 440 patients for all diagnoses (Fig. 1). Of these 440 patients, 143 were under the care of the surgeons contributing to this study, and of these 143 we assessed 115 for eligibility. Patients were identified at the preoperative assessment stage. We considered patients younger than 80 years weighing from 50 to 120 kg, who were able to provide informed consent for and cooperate with the study with a diagnosis of primary osteoarthritis. We excluded two patients owing to major psychological problems, previous drug dependency, allergies to any of the ingredients of the injection, renal insufficiency, abnormal liver enzymes, a history of stroke or major neurologic deficit, or uncontrolled angina and bifascicular blocks with prolonged QT intervals. Using randomization tables, patients were randomized to one of two groups: 32 patients received an intraoperative periarticular injection and 32 patients did not. Patients and assessors were blinded; owing to the study design, it was not possible to blind the surgeons. A power study, using an estimated 30% decrease in 24-hour PCA consumption as being clinically important, showed statistical significance (alpha = 0.05) was reached using 23 patients in each treatment arm (beta = 0.8); for a significance value of alpha = 0.01, 32 patients were required in each arm. Previous studies that have stated their power calculation have used a 20% decrease in narcotic requirement and other parameters [24] or a 25% reduction in VAS scores [1]. From our previous work, we believed a 30% reduction in PCA requirement would need to be achieved to be significant. No patients were lost to followup at the 6-week end point. All patients consented to inclusion in the study. We had prior approval of our local ethics committee (in accordance with the ethical standards of the responsible committee on human experimentation and with the Declaration of Helsinki of 1975 as revised in 2000).
Table 1.
Demographic data for patients participating in the study
| Demographic variable | Group 1 (injected) | Group 2 (noninjected–control) |
|---|---|---|
| Age (years)* | 61 (34–80) | 65 (39–80) |
| Gender (female: male) | 18:18 | 19:16 |
| Body mass index† | 30.1 (6.9) | 30.6 (7.2) |
| Diagnosis | 32 osteoarthritis | 32 osteoarthritis |
* Values are expressed as mean, with range in parentheses; †values are expressed as mean, with SD in parentheses.
Fig. 1.
A CONSORT flowchart describes patient selection.
The THAs were performed through a direct lateral approach. Operative anesthesia was either general or spinal. The anesthetic regime was standardized. No long-acting analgesics were used and spinal anesthesia was performed using spinal bupivacaine hydrochloride, ranging from 10 to 15 mg. Twenty-three patients in the injected group and 24 in the noninjected group had a general anesthetic. Nine patients in the injected group and eight in the noninjected group had a spinal anesthetic. With these small numbers, there was no difference (chi square test, p = 0.78) between general versus spinal anesthetic, which was selected at the anesthesiologist’s discretion.
The injection contained 400 mg ropivacaine (ropivacaine HCl; Astra Zeneca Canada Inc, Ontario, Canada), 30 mg ketorolac (Toradol®; Sabex Inc, Boucherville, Canada), 0.6 mL epinephrine (1:1000; Abbott Laboratories, Abbott Park, IL), and 5 mg preservative-free morphine (Sabex Inc). These were mixed with sterile normal saline and made up to a combined volume of 100 mL in the operating room [5]. During the operation, 20 mL of the mixture was injected into the posterior capsule after femoral component insertion and another 20 mL in the anterior capsule before hip reduction and capsular closure. The remaining 60 mL was placed in the fat and subcuticular tissues. Five patients in each group had blood samples taken at 30 minutes, 1 hour, and 4 hours postoperation to measure venous blood (protein-bound) ropivacaine levels.
All patients received PCA for 24 hours postsurgery (morphine: bolus 1.5 mg, lockout 6 minutes and maximum 15 mg/hour). All patients had a lower limb ultrasound to exclude a deep vein thrombosis at Day 5 postsurgery.
Postoperative PCA consumption was measured for the first 24 hours and the patient’s overall analgesic consumption until discharge was measured and converted to morphine equivalents to allow for comparison of the groups. Other analgesics used by the patients during the postoperative period included Percocet™ (acetaminophen 325–650 mg, oxycodone 5–10 mg; Endo Pharmaceuticals Inc, West Chester, PA), Tylenol® (acetaminophen 325 mg, caffeine 15 mg, codeine 8 mg; McNeil Consumer Healthcare, Fort Washington, PA), and Tylenol® 3 (acetaminophen 300 mg, codeine 30 mg; McNeil Consumer Healthcare). The following conversion ratios were used for calculating morphine equivalents: morphine:oxycodone 1:2 and morphine:codeine 1:20 [13]. The total dose of a drug was calculated for the period of interest and then converted to morphine equivalents for analysis. We measured ropivacaine levels in five patients 4 hours postoperation. The criteria for hospital discharge were the ability to safely walk up and down a flight of stairs and a safe environment for discharge.
Patients were assessed using VAS scores for pain, at rest and during activity, and for patient satisfaction, in the preoperative assessment clinic (2–3 weeks before surgery), on the day of surgery, in the postanesthetic care unit (PACU), at some time during the inpatient stay, and finally at 6 weeks’ followup. The VAS for pain and satisfaction ran from 0 mm (indicating no pain or completely dissatisfied) to 100 mm (indicating extreme pain or completely satisfied) in 10-mm increments. The activity performed relating to the VAS assessment of pain on activity was leg movements while supine within the limits of any regional anesthesia while in the PACU. At other times, the activity was the rehabilitation activity relevant to that stage of the patient’s recovery. Specific note was made of any signs of cardiac (chest pain, shortness of breath, or electrocardiogram changes) or central nervous system toxicity (visual and hearing disturbances, dysarthria, tingling, perioral numbness, dizziness, paresthesia, light-headedness, muscular twitching, or muscular rigidity), or wound complications. We observed wounds daily and noted any wound drainage, erythema, swelling, blisters, desquamation, dehiscence, protuberant suture material, or signs of infection. As part of our routine clinical care during the period of this study, Harris hip scores and WOMAC scores were collected prospectively for all patients (Table 2). These data were available out to 2 years by the time of this study. All patients had a lower limb ultrasound to assess for the presence of deep vein thrombosis before discharge.
Table 2.
Preoperative and postoperative hip scores
| Scoring system | Group 1 (injected) | Group 2 (noninjected–control) | p Value for Group 1 vs Group 2 | All patients | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Harris hip score | |||||||
| Preoperative | 45.9 | 10.0 | 43.6 | 15.6 | 0.49 | 44.8 | 11.4 |
| 3 months | 88.2 | 9.1 | 81.6 | 25.1 | 0.17 | 84.8 | 9.5 |
| 1 year | 93.1 | 8.2 | 90.6 | 21.4 | 0.54 | 91.8 | 8.3 |
| 2 years | 92.5 | 10.7 | 90.9 | 22.8 | 0.72 | 91.5 | 9.0 |
| WOMAC | |||||||
| Preoperative | 45.0 | 20.6 | 39.2 | 21.3 | 0.27 | 42.3 | 20.2 |
| 3 months | 80.0 | 13.5 | 78.2 | 23.1 | 0.70 | 79.0 | 15.1 |
| 1 year | 85.1 | 15.6 | 78.0 | 25.3 | 0.18 | 81.5 | 17.7 |
| 2 years | 69.3 | 23.5 | 76.6 | 25.9 | 0.24 | 73.2 | 21.6 |
We determined differences in postoperative PCA requirements, postoperative VAS scores for pain and satisfaction, the operating time, and the length of hospital stay between the injected and noninjected cohorts using a two-tailed unpaired t test. In all five of these analyses, the data passed the Kolmogorov-Smirnov test for normality (p < 0.05). We determined the difference in the incidence of complications between the injected and noninjected cohorts using Fisher’s exact test (nonparametric). SPSS® (Version 11.5; SPSS Inc, Chicago, IL) was used for the analyses.
Results
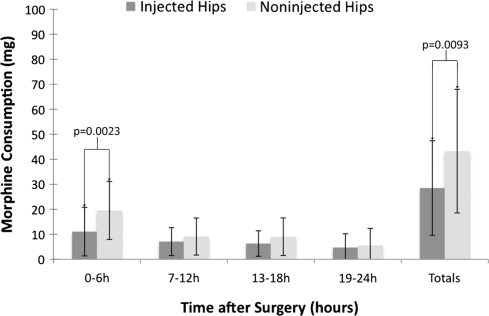
Patients undergoing THA who had received the multimodal drug infiltration used less (p = 0.0023) PCA at 6 hours postoperation (11.1 mg morphine [SD, 9.7 mg] versus 19.6 mg morphine [SD, 11.6 mg]). The overall PCA requirement was less (p = 0.0093) during the first 24 hours after surgery for the injected patients (28.5 mg morphine [SD, 18.9 mg] versus 43.3 mg morphine [SD, 24.7 mg]) (Fig. 2).
Fig. 2.
A graph showing 24-hour PCA morphine consumption in patients after THA. Patients undergoing THA receiving the multimodal drug infiltration used less (p = 0.0023) PCA at 6 hours postoperation and less (p = 0.0093) overall PCA during the first 24 hours after surgery. Data are presented as means (bars) with SD (error bars).
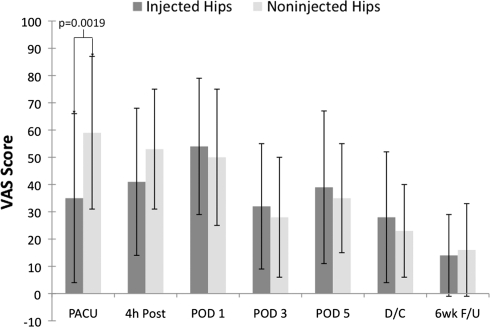
The VAS score for pain on activity was lower (p = 0.0019) in the injected group in the PACU (0.35 [SD, 0.31] versus 0.59 [SD, 0.28]) (Fig. 3). There was no difference between the VAS scores for pain at rest.
Fig. 3.
VAS scores for pain in patients after THA are shown. The VAS score for pain on activity was lower (p = 0.0019) in the injected group in the PACU. PACU = postanesthetic care unit; 4 h Post = 4 hours postoperative; POD1 = Postoperative Day 1; POD3 = Postoperative Day 3; POD5 = Postoperative Day 5; D/C = discharge; 6wk F/U = 6 weeks’ followup. Data are presented as means (bars) with SD (error bars).
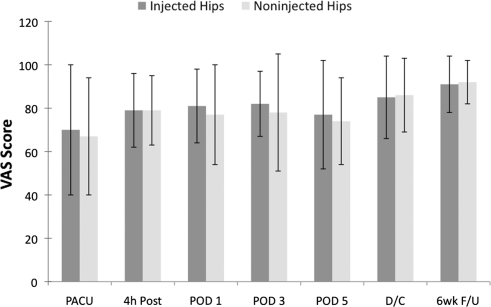
The VAS scores for patient satisfaction were similar between the groups (Fig. 4).
Fig. 4.
VAS scores for satisfaction in patients after THA are shown. The VAS scores for patient satisfaction were similar between the groups. PACU = postanesthetic care unit; 4 h Post = 4 hours postoperative; POD1 = Postoperative Day 1; POD3 = Postoperative Day 3; POD5 = Postoperative Day 5; D/C = discharge; 6wk F/U = 6 weeks’ followup. Data are presented as means (bars) with SD (error bars).
The average operating times (skin incision until dressing application) were similar (p = 0.86) for the two groups: 85.5 minutes (SD, 19.7 minutes) for the injected group and 84.7 minutes (SD, 14.9 minutes) for the noninjected group.
There was no difference (p = 0.24) in average hospital stay (125.8 hours [SD, 30.6] for injected group versus 139.0 hours [SD, 54.7] for the noninjected group).
The rate of wound complications was similar (p = 0.35) in the two groups. Three patients had a minor wound problem in the injected group and one in the noninjected group. These included three patients with blisters related to their dressings and one prominent suture requiring trimming. One patient in the injected group had a deep vein thrombosis postoperatively.
The maximum dose of unbound ropivacaine observed among the five patients was 60 ng/mL, which is 2.5 times below the toxic levels reported by Knudsen et al. [18] (150 ng/mL). No cardiac or central nervous system toxicity was observed.
Discussion
Postoperative pain after THA is common [12, 33, 35]. The cause of pain may be a result of trauma to the bone or soft tissues. The optimal form of pain relief is one that is applied preoperatively, perioperatively, and postoperatively to try to avoid the establishment of pain hypersensitivity [3]. Good pain relief allows for effective postoperative rehabilitation [25]. We performed a study to ascertain whether one periarticular injection using multimodal drugs would achieve a reduction in postoperative PCA requirements. We also aimed to establish whether the injection would lead to a reduction in the VAS scores for pain, lead to an improvement in the VAS scores for patient satisfaction, not increase the operating time, not affect length of hospital stay, and not lead to an increase in complications, and that the associated increase in unbound ropivacaine levels would not be greater than reported toxic levels.
We acknowledge several limitations. The first relates to its size. The study was underpowered to establish whether the observed reduction in postoperative PCA requirements would translate into a lower incidence of the side effects associated with postoperative opioid use. Second, we explored only the use of this analgesic modality with one surgical approach to the hip and the findings might not apply to other joints (eg, the knee). Third, we focused on the early postoperative period; although we would not expect a difference in the groups beyond 6 weeks, additional study would evaluate whether this is the case. This would be of added value in terms of determining whether the reduced PCA requirements translate into a lower incidence of chronic pain developing in these patients. A postal questionnaire followup of patients in Denmark who had THA indicated the incidence of substantial pain after THA may be as much as 12.1% and the state of this pain was related to the recalled early postoperative pain experience [23].
We observed a decrease in the PCA requirements of patients receiving an intraoperative injection, which targets numerous sites of action. Inflamed tissues have abundant opioid receptors [20, 32]. These receptors are expressed shortly after trauma and are an effective site for sensory blockade by opioids [31]. NSAIDs exert their analgesic effect by numerous pathways in the periphery including inhibition of prostaglandin synthesis, inhibition of cyclooxygenase 2 activity, and in some NSAIDs, inhibition of the lipoxygenase pathway and interference with G-protein-mediated signal transduction [6]. The reduction in PCA use was substantial in the PACU with no difference between the groups at 7 to 24 hours. This early substantial reduction was reflected in a substantially lower total during the 24-hour period. Peters et al. [25] observed reduced postoperative narcotic requirements with one intraoperative periarticular injection. Andersen et al. [1] observed reduced postoperative narcotic requirements with an intraoperative periarticular injection and top-up via catheter after 8 hours. Kerr and Kohan [16] reported a series of 325 patients undergoing THAs, TKAs, and hip resurfacings who received a perioperative injection and additional infiltration via a catheter at 15 to 20 hours postoperation. They reported satisfactory pain control in all patients with no morphine required in two-thirds of their patients.
The VAS scores for pain for our patients showed reduced pain on activity in the PACU only. There was no difference in the VAS scores for pain at rest. Peters et al. [25] observed reduced pain on activity on Postoperative Day 2 but at no other time. They also reported reduced pain at rest on Postoperative Days 1 and 2; interestingly, there was increased pain at rest in the injected group on the day of surgery. Kerr and Kohan [16] did not compare their data with a noninjected cohort. Hebl et al. [14] reported on two groups of 20 patients who had minimally invasive THA and TKA with an anesthetic protocol using a peripheral nerve blockade. Good pain relief was achieved but with side effects and a 3% rate of failed blocks.
We observed similar VAS satisfaction scores in the two groups. This result was surprising given there was a decrease in pain on activity scores in the PACU. An increase in pain reportedly correlates with a decrease in patient satisfaction [22]. Parvataneni et al. [24] reported an increase in patient satisfaction in their series of patients undergoing THA who received a perioperative injection. Identifying changes in patient satisfaction in the early postoperative period is a recognized problem [17]. It is possible the VAS scale was not a sensitive enough test to detect the changes in satisfaction or a greater reduction in the VAS pain score would be required in our population to be reflected in an improved VAS satisfaction score.
We observed no difference in the operative time for our patients. Failed peripheral nerve blocks [14] can increase the overall theater time for a patient undergoing TKA [34]. The technique used in our study did not affect the operative time and patients in both groups underwent the same perioperative preparation, so although not formally measured, it is reasonable to assume the overall theater time is not increased by this technique. This may represent an increased efficiency over nerve blocks.
Our injected group was not discharged in a shorter time than the noninjected group. Others investigating periarticular hip injections alone have reported more rapid discharge of their patients who received injections [25]. A more rapid discharge also has been reported with the use of a catheter for additional instillation after perioperative injection [1, 16]. Our criteria for discharge did not differ between the groups, nor did it in these other studies. It is possible the expectations of staff were influencing the actual discharge time. A more thorough investigation of the factors involved in discharge would be required to ascertain whether this was the case.
The complication rates were similar for our two groups. Another series investigating the technique also reported no difference in complication rates [25]. In the trial of an intraoperative injection followed by top-up via a catheter [1], there was an episode of deep infection in the injected group although overall no difference in complication rate. We believe one intraoperative injection is preferable to performing a top-up via catheter postoperatively. Although aseptic technique can be used to achieve this, it is a potential port for the introduction of bacteria. Continuous infusion of opioid and bupivacaine postoperatively in patients who have a TKA is associated with prolonged wound drainage [9].
Ropivacaine is a local anesthetic pharmacokinetically similar to bupivacaine but has a lower toxicity profile and longer duration of action [8, 18]. We measured circulating blood levels of ropivacaine in patients receiving injections. All measurements were well within the reported limits for toxicity [28]. The addition of epinephrine helps to reduce the toxicity of the local anesthetic by keeping it localized to the area of injection [30].
Our data suggest periarticular intraoperative injection with multimodal drugs around THA can reduce postoperative PCA requirements with no apparent increase in risk.
Acknowledgments
We acknowledge Dr. L. Kohan and Dr. D. Kerr of Sydney, Australia, for their work in developing the multimodal drug combination used in this study. We also thank Prof C. H. Rorabeck and Dr. R. Bhandari for contributions to work with this research and the previous investigation of multimodal drug infiltration in TKAs.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at London Health Sciences Centre.
References
- 1.Andersen KV, Pfeiffer-Jensen M, Haraldsted V, Søballe K. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty: a randomized clinical trial of an intraarticular technique versus epidural infusion in 80 patients. Acta Orthop. 2007;78:180–186. doi: 10.1080/17453670710013654. [DOI] [PubMed] [Google Scholar]
- 2.Asayama I, Kinsey TL, Mahoney OM. Two-year experience using a limited-incision direct lateral approach in total hip arthroplasty. J Arthroplasty. 2006;21:1083–1091. doi: 10.1016/j.arth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Badner NH, Bourne RB, Rorabeck CH, MacDonald SJ, Doyle JA. Intra-articular injection of bupivacaine in knee-replacement operations: results of use for analgesia and for preemptive blockade. J Bone Joint Surg Am. 1996;78:734–738. doi: 10.2106/00004623-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Berger RA, Jacobs JJ, Meneghini RM, Della Valle C, Paprosky W, Rosenberg AG. Rapid rehabilitation and recovery with minimally invasive total hip arthroplasty. Clin Orthop Relat Res. 2004;429:239–247. doi: 10.1097/01.blo.0000150127.80647.80. [DOI] [PubMed] [Google Scholar]
- 5.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW. Efficacy of periarticular multimodal drug injection in total knee arthroplasty: a randomized trial. J Bone Joint Surg Am. 2006;88:959–963. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 6.Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52(suppl 5):13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 7.Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA. 1996;275:858–865. doi: 10.1001/jama.275.11.858. [DOI] [PubMed] [Google Scholar]
- 8.Denson DD, Myers JA, Hartrick CT, Pither CP, Coyle DE, Raj PP. The relationship between free bupivacaine concentration and central nervous system toxicity. Anesthesiology. 1984;61:A211. doi: 10.1097/00000542-198409001-00211. [DOI] [Google Scholar]
- 9.DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392:226–231. doi: 10.1097/00003086-200111000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Frater RA, Moores MA, Parry P, Hanning CD. Analgesia-induced respiratory depression: comparison of meptazinol and morphine in the postoperative period. Br J Anaesth. 1989;63:260–265. doi: 10.1093/bja/63.3.260. [DOI] [PubMed] [Google Scholar]
- 11.Gedney JA, Liu EH. Side-effects of epidural infusions of opioid bupivacaine mixtures. Anaesthesia. 1998;53:1148–1155. doi: 10.1046/j.1365-2044.1998.00636.x. [DOI] [PubMed] [Google Scholar]
- 12.Giuffre M, Asci J, Arnstein P, Wilkinson C. Postoperative joint replacement pain: description and opioid requirement. J Post Anesth Nurs. 1991;6:239–245. [PubMed] [Google Scholar]
- 13.Hallenbeck JL. Palliative Care Perspectives: Chapter 4: Pain Management: Conversion among Different Opioids. Copyright 2003 by Oxford University Press, New York, NY. Available at: http://www.mywhatever.com/cifwriter/library/70/4932.html. Accessed October 13, 2009.
- 14.Hebl JR, Kopp SL, Ali MH, Horlocker TT, Dilger JA, Lennon RL, Williams BA, Hanssen AD, Pagnano MW. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total hip arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):63–70. doi: 10.2106/JBJS.E.00491. [DOI] [PubMed] [Google Scholar]
- 15.Indelli PF, Grant SA, Nielsen K, Vail TP. Regional anesthesia in hip surgery. Clin Orthop Relat Res. 2005;441:250–255. doi: 10.1097/01.blo.0000192355.71966.8e. [DOI] [PubMed] [Google Scholar]
- 16.Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79:174–183. doi: 10.1080/17453670710014950. [DOI] [PubMed] [Google Scholar]
- 17.Kiebzak GM, Vain PA, Gregory AM, Mokris JG, Mauerhan DR. SF-36 general health status survey to determine patient satisfaction at short-term follow-up after total hip and knee arthroplasty. J South Orthop Assoc. 1997;6:169–172. [PubMed] [Google Scholar]
- 18.Knudsen K, Beckman Suurkula M, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78:507–514. doi: 10.1093/bja/78.5.507. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney OM, Noble PC, Davidson J, Tullos HS. The effect of continuous epidural analgesia on postoperative pain, rehabilitation, and duration of hospitalization in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:30–37. [PubMed] [Google Scholar]
- 20.Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty. 1997;12:546–552. doi: 10.1016/S0883-5403(97)90178-9. [DOI] [PubMed] [Google Scholar]
- 21.McDonald S, Hetrick S, Green S. Pre-operative education for hip or knee replacement. Cochrane Database Syst Rev. 2004;1:CD003526. doi: 10.1002/14651858.CD003526.pub2. [DOI] [PubMed] [Google Scholar]
- 22.McNeill JA, Sherwood GD, Starck PL, Thompson CJ. Assessing clinical outcomes: patient satisfaction with pain management. J Pain Symptom Manage. 1998;16:29–40. doi: 10.1016/S0885-3924(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 23.Nikolajsen L, Brandsborg B, Lucht U, Jensen TS, Kehlet H. Chronic pain following total hip arthroplasty: a nationwide questionnaire study. Acta Anaesthesiol Scand. 2006;50:495–500. doi: 10.1111/j.1399-6576.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 24.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6 suppl 2):33–38. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Peters CL, Shirley B, Erickson J. The effect of a new multimodal perioperative anesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after total joint arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):132–138. doi: 10.1016/j.arth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Pettine KA, Wedel DJ, Cabanela ME, Weeks JL. The use of epidural bupivacaine following total knee arthroplasty. Orthop Rev. 1989;18:894–901. [PubMed] [Google Scholar]
- 27.Pour AE, Parvizi J, Sharkey PF, Hozack WJ, Rothman RH. Minimally invasive hip arthroplasty: what role does patient preconditioning play? J Bone Joint Surg Am. 2007;89:1920–1927. doi: 10.2106/JBJS.F.01153. [DOI] [PubMed] [Google Scholar]
- 28.Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12–15. doi: 10.1016/j.arth.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Röstlund T, Kehlet H. High-dose local infiltration analgesia after hip and knee replacement: what is it, why does it work, and what are the future challenges? Acta Orthop. 2007;78:159–161. doi: 10.1080/17453670710013627. [DOI] [PubMed] [Google Scholar]
- 30.Solanki DR, Enneking FK, Ivey FM, Scarborough M, Johnston RV. Serum bupivacaine concentrations after intraarticular injection for pain relief after knee arthroscopy. Arthroscopy. 1992;8:44–47. doi: 10.1016/0749-8063(92)90134-W. [DOI] [PubMed] [Google Scholar]
- 31.Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993;76:182–191. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- 32.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 33.Thomas T, Robinson C, Champion D, McKell M, Pell M. Prediction and assessment of the severity of post-operative pain and of satisfaction with management. Pain. 1998;75:177–185. doi: 10.1016/S0304-3959(97)00218-2. [DOI] [PubMed] [Google Scholar]
- 34.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–289. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]
- 35.Weller R, Rosenblum M, Conard P, Gross JB. Comparison of epidural and patient-controlled intravenous morphine following joint replacement surgery. Can J Anaesth. 1991;38:582–586. doi: 10.1007/BF03008188. [DOI] [PubMed] [Google Scholar]