Abstract
Background
Technical errors during navigation-assisted bone tumor resection may occur by: (1) incorrect registration of images and corresponding anatomic points of bone sent to the navigation system; and (2) incorrect fusion of two or more images that have been transported to the navigation system.
Questions/purposes
We investigated new methods of navigation surgery to minimize technical errors during the registration and image fusion processes and specifically asked whether a navigated cannula probe would reduce unnecessary soft tissue dissection, and allow percutaneous registration and implantation of a reference base tracker in the margin of bone to be resected.
Methods
We performed direct MRI-guided navigation surgery without image fusion on a patient with osteosarcoma using absorbable pins as temporary implanted bone markers that prevent artifacts on MR images.
Results
Direct MRI-guided navigation surgery was possible using bone markers. A navigated cannula probe allowed percutaneous registration and a navigated blade-shaped probe provided a real-time check on the narrow osteotomy gap. The surgical procedure was facilitated by implantation of a reference base tracker on the margin of bone to be resected.
Conclusions
Our modified technique of MRI-guided navigation surgery for patients with a malignant bone tumor may reduce processing errors by increased accuracy and be helpful for joint preserving surgery.
Introduction
Computer-assisted navigation surgery has been widely used for patients with trauma [3, 8, 18] or those having joint arthroplasty [9, 11] or spine surgery [14]. Navigation also has been used for patients with malignant bone tumors for precise tumor resection and planned reconstruction presuming it will reduce local recurrence rates and enhance functional outcomes by saving adjacent joint and major neurovascular structures [1, 6, 7, 10, 15–17]. Navigation-guided bone surgery requires registration to match the image to the patient’s bone. Investigators have developed two registration methods; one uses temporarily implantable artificial bone markers [1, 5, 14], and the other uses prominent anatomic points of bone [6, 15–17].
To register landmarks, Kirschner wires have been fixed temporarily to the adjacent bone during sacropelvic and femoral bone tumor excisions [1], spine markers have been designed for spine surgery [14], and various bone-marking screws have been developed [5]. Because the aforementioned devices are made of stainless steel or titanium, MR images cannot be used for navigation because the images are degraded and the artifacts prevent accurate interpretation. Therefore, CT often is used for registration and surgery. However, in musculoskeletal tumor surgery, the resection margins usually are determined by MRI rather than CT because MR images present more details of the reactive zone, which is not a bony destruction area but the location potentially affected by tumor. Therefore, MRI is essential for computer-assisted navigation surgery in patients with malignant bone tumors, combined with the CT images using the software provided with the navigation system. Three or four corresponding anatomic bone landmarks of the patients then can be established on the fused CT-MR images [16–18].
Technical errors during navigation-assisted bone tumor resection may have been caused by two principal steps: (1) incorrect registration of images and corresponding points of bone sent to the navigation system; and (2) incorrect fusion of two or more images that have been transported to the navigation system. Although the diaphyses of the long bones such as the femur have no definitive anatomic landmarks, most malignant bone tumors are located in the metaphysis or epiphysis, which have appropriate landmarks for registration. However, registration using anatomic landmarks in the femoral meta-epiphyseal malignant bone tumor causes unnecessary extensive soft tissue dissection and possible tumor contamination.
To minimize technical errors during the registration and image fusion processes for a patient with osteosarcoma of the distal femur, we performed MRI-guided navigation surgery using absorbable pins as temporary implantable bone markers, which prevent artifacts on MR images.
Case Report
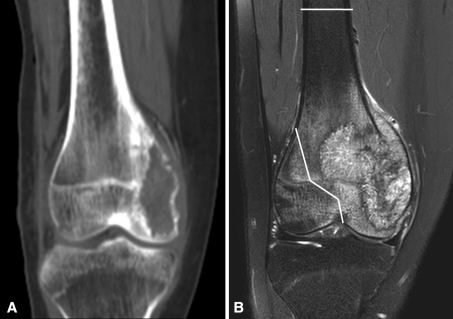
A 15-year-old girl was referred with right knee pain that had persisted for 2 months. She had difficulty bearing full weight and had severe tenderness on her right medial condylar area. There was no history of trauma, and the results of initial blood tests were within the normal range. Plain radiographs showed an osteolytic lesion in the medial femoral condyle with cortical destruction and periosteal reaction. The lesion extended to the juxtaarticular area of the epiphysis and metaphysis of the medial condyle, but the lateral condyle appeared intact on CT images (Fig. 1A). However, on MRI, the lesion extended more widely beyond midline to the lateral condyle (Fig. 1B). No metastases were observed on pulmonary CT and whole-body PET images.
Fig. 1A–B.
(A) CT and (B) MR coronal images show the osteosarcoma in the right distal femur of our patient. The MR image showed wider involvement of the femoral condyle than the CT image. Therefore, the planned osteotomy sites were marked on the MR image for precise resection without tumor contamination.
A definitive diagnosis of an osteoblastic-type osteosarcoma was established by incisional biopsy. After two cycles of neoadjuvant chemotherapy, MR images showed the reactive zone of the tumor had decreased enough so we could save the lateral femoral condyle (Fig. 2). We planned limb salvage surgery with navigation-assisted unicondylar resection of the distal femur and reconstruction with a shaped osteoarticular allograft. We preoperatively marked the osteotomy sites on the femoral condyle on the MR images for precise resection without tumor contamination and to measure allograft reconstruction. These osteotomy sites included the femoral shaft approximately 11.5 cm from the joint line proximally, the disarticulated medial condyle distally, and a tumor-free lateral condylar area laterally (Fig. 1).
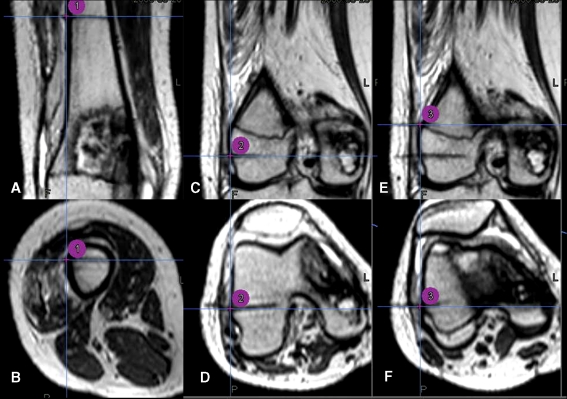
Fig. 2A–F.
MR images were obtained after insertion of three absorbable pins and marked by three-point registration area for condylar-preserving surgery using direct MRI-guided navigation. Intraoperative images as seen on the navigation system show (A) coronal and (B) axial views of the first registration point, (C) coronal and (D) axial views of the second registration point, and (E) coronal and (F) axial views of the third registration point.
Before surgery but on the same day, under local anesthesia, three transcutaneous absorbable pins (OrthoSorb®; DePuy, Raynham, MA) were inserted in the distal femur away from the tumor (Fig. 2), and one transcutaneous 0.8-mm Kirschner wire was inserted to determine the difference in MRI artifacts between the two materials. T2-weighted axial MR images (TR 1500 ms, TE 300 ms, 1-mm slice thickness) (3.0-T unit, Achieva; Philips, Einthoven, The Netherlands) of the corresponding region were obtained. Data sets were imported to the navigation system (Stryker Navigation, Kalamazoo, MI). The patient was taken to the operating room and given general anesthesia. We carefully draped a sterile field so as not to pull out inserted pins protruding through the skin. The conventional pointer probe could not be used in percutaneous registration because there was no central hole for inserting implanted bone markers and because the probe was too thick to fit in the saw kerf of the osteotomy. We therefore calibrated a cannula, blade-shaped probes, and conventional pointer probe (Fig. 3A) for real-time navigation surgery.
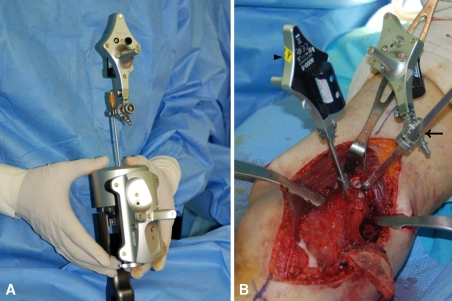
Fig. 3A–B.
(A) Calibration of the cannula probe (arrowhead) for real-time navigation surgery is shown. (B) The intraoperative photograph shows a fixed reference base tracker on the femur approximately 1 cm distal to the proximal osteotomy line (arrowhead) and the navigated blade probe in the proximal osteotomy kerf (arrow).
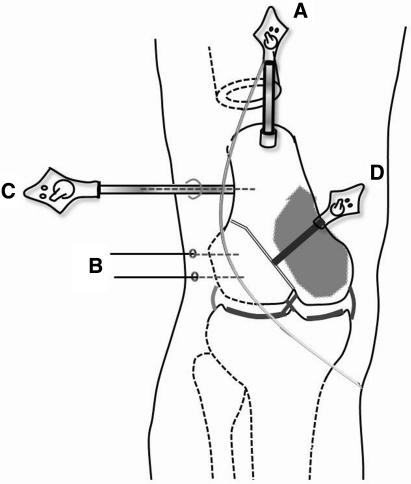
After the soft tissue dissection, we placed a dynamic reference base tracker on the femur approximately 1 cm distal to the proximal femoral osteotomy line (Fig. 3B). We made small skin incisions over the inserted pins and drove the navigated cannula probe percutaneously to the bone surface. Each fiducial point, which was located at the pin center on the cortical surface of the femur, was registered to the corresponding point on MR images of the navigation system (Fig. 2). The system software calculated the registration errors to be less than 1 mm for all points. The time for real-time navigation monitoring from fixation of the reference base tracker to three-point registration was approximately 10 minutes.
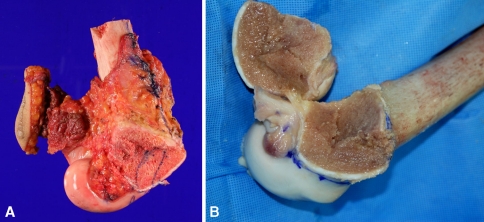
Intraoperatively, the imaginary surgical margin was checked three-dimensionally under the guidance of the navigation system (Fig. 4A–D) by locating the pointer around the tumor (Fig. 4E). The proximal osteotomy was first made just proximal to the dynamic reference base tracker and the distal tumor resection then was performed in a proximal to distal fashion (Fig. 5). When performing osteotomy of the intercondylar area, we intermittently stopped the saw and placed the blade-shaped navigation probe in the cutting plane to obtain three-dimensional confirmation of the location of the tip of the saw on the navigation images to ensure the osteotomy was performed according to the original plan without any surgical errors. The specimen was excised en bloc (Fig. 6A). The femoral attachment of the anterior cruciate ligament was preserved along with the lateral collateral ligament and both menisci. The posterior cruciate and medial collateral ligaments were sacrificed together with the medial condyle of the femur.
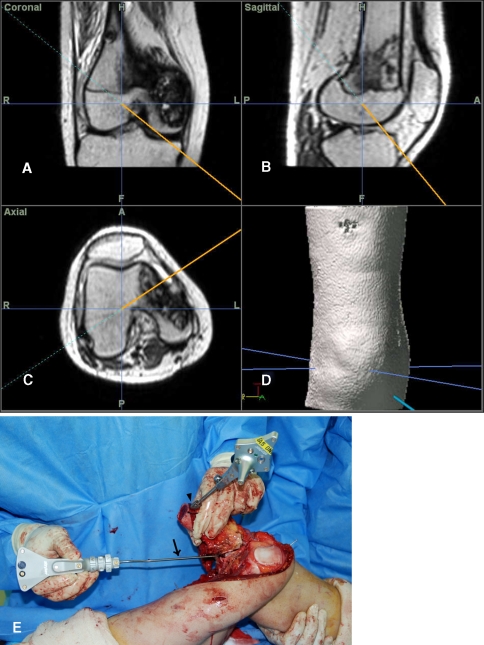
Fig. 4A–E.
Navigation images show intraoperative three-dimensional orientation of (A) coronal, (B) sagittal, (C) axial, and (D) three-dimensional reconstruction views. (E) The reference base tracker fixed just distal to the margin of proximal osteotomy line (arrowhead) facilitated surgical resection by allowing continuous navigational monitoring. The conventional pointer probe (arrow) was located to check the imaginary surgical margin.
Fig. 5.
A schematic view shows a dynamic reference base tracker which is attached to one end of the bone that is to be excised (A); the absorbable pins for registration as bone markers for the MR image (B); a navigated cannula probe (C); and a navigated blade probe (D).
Fig. 6A–B.
The photographs show (A) the resected tumor and (B) the shaped osteoarticular allograft with ligaments for reconstruction.
An osteoarticular allograft was shaped to match the resected specimen as precisely as possible (Fig. 6B) and the skeletal reconstruction performed. The posterior cruciate and medial collateral ligaments were sutured to their corresponding sites of the ligament stumps on the allograft and the medial joint capsule was reconstructed with an allograft-patellar tendon.
The final histologic diagnosis was an osteosarcoma of the osteoblastic type and all resection margins (range, 1–4 cm) were free of tumor cells. Histologic mapping showed a response rate of 90% to neoadjuvant chemotherapy.
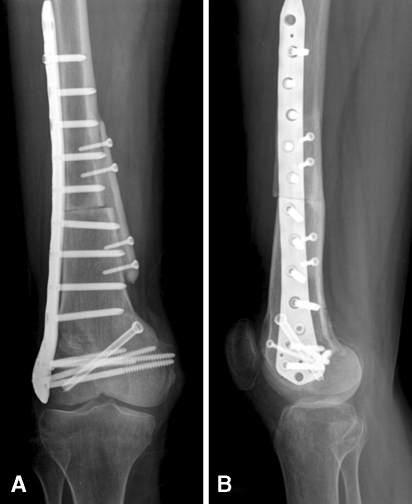
Postoperatively, the patient began ROM exercises 1 week after surgery. Four weeks after surgery, 105° flexion and full extension were achieved and the patient began partial weightbearing walking with crutches. The third month after surgery, 120° flexion and full weightbearing were possible. The patient also received adjuvant chemotherapy. There were no postoperative complications, local recurrence, or distant metastasis during the 28-month followup. The patient walked well without walking assists. The followup plain radiographs showed a well-maintained medial femoral osteoarticular allograft (Fig. 7). The mean Musculoskeletal Tumor Society [4] and Toronto Extremity Salvage Score [2] functional scores at last followup were 27 and 87%, respectively.
Fig. 7A–B.
(A) Anteroposterior and (B) lateral radiographs obtained at the 24-month followup show a well-maintained femoral osteoarticular allograft.
Discussion
Image-guided navigation surgery has been widely used for craniomaxillofacial surgery without artificial bone markers owing to accurate and direct image-to-patient registration. In orthopaedic oncology, anatomic bony landmarks typically have been registered using the paired-points method [10, 15–17]. Most primary bone tumors occur in the epiphysis or metaphysis where there are prominent landmarks, but using them for registration can result in additional exposure and potential tumor contamination. Furthermore, in older patients or those with pathologically altered bone structure, identification of anatomic landmarks and registration are relatively imprecise [14]. Therefore, implantable bone markers would be useful for precise registration during the computer-assisted navigation surgery, especially in the epiphysis or metaphysis involving malignant bone tumors. CT reveals the bone structure in more detail than MRI and allows more precise identification of anatomic bony landmarks. However, because the boundary of the tumor without definitive bony destruction is difficult to assess using CT images, MR images are necessary to more accurately assess the bone tumor margin. Therefore, fusion of CT-MR images is required for surgery, although there sometimes are errors in the fusion process that can be as large as 6 mm or greater [13, 15].
In our patient, we fixed one absorbable transcutaneous pin to the diaphysis of the distal femur and two pins in the lateral condyle to avoid the muscular area so as not to cause tumor contamination. Consequently, we believe it is important to develop longer MRI bone marker pins. We could easily perform the registration process by inserting our navigated cannula probe percutaneously along the absorbable pin (Fig. 7C), and we also could perform reregistration at any time during the operation if we did not remove the inserted markers. It is advantageous to use a bone marker rather than paired-point registration. A bone marker minimizes registration errors and facilitates the registration process without needing to expose the bony landmarks and without the risk of tumor contamination. Because the conventional pointer probes cannot be inserted in the narrow kerf from the bone saw, one could register the bone surface. A navigation diathermy electrode needle has been used as an alternative approach [15, 16]. We developed a blade-shaped probe equipped with a navigation tracker for the saw-cut space (Fig. 7D).
Stiehl used a reference base tracker placed in retained bone to perform navigated TKA [12]. For bone tumor surgery, we believe it is better to place the reference base tracker in the resected bone during navigational monitoring while maintaining the initial registration with the tracker. If the reference tracker is placed in retained bone, navigational monitoring would not be possible after the proximal (ie, farthest from the joint) osteotomy, and the farthest osteotomy (ie, closest to the joint) should be performed first. However, this approach makes the neurovascular dissection more difficult. Alternatively with our technique with the proximal osteotomy performed first, the bone to be resected could be mobilized in any direction without disturbing the navigation system by fixing a dynamic reference base tracker to the resected bone (Fig. 4E). The disadvantage of our technique is that it might increase the risk of tumor contamination, so we precisely planned the proximal osteotomy and then fixed the reference base tracker at the edge of the bone to be excised near the proximal osteotomy line (Fig. 7A).
One unsolved problem is the difficulty of trimming the allograft to match the excised bone, and an accurate method is needed to facilitate allograft shaping according to the planned excision.
Our modified technique of MRI-guided navigation surgery for malignant bone tumors reduces processing errors and potentially allows more accurate resection based on the MRI findings while preserving a maximum amount of the normal joint and ensuring oncologic principles. To determine whether the approach actually reduces recurrences, however, one would have to have a large number of patients with adequate followup.
Acknowledgments
We thank Dr Yoon Whan Roh and physician assistant Byuk Kim for help with preparation and assistance of this surgery.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Orthopaedic Oncology Clinic, National Cancer Center, Gyeonggi-do, Korea.
References
- 1.Cho HS, Kang HG, Kim HS, Han I. Computer-assisted sacral tumor resection: a case report. J Bone Joint Surg Am. 2008;90:1561–1566. doi: 10.2106/JBJS.G.00928. [DOI] [PubMed] [Google Scholar]
- 2.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 3.Easley M, Chuckpaiwong B, Cooperman N, Schuh R, Ogut T, Le IL, Reach J. Computer-assisted surgery for subtalar arthrodesis: a study in cadavers. J Bone Joint Surg Am. 2008;90:1628–1636. doi: 10.2106/JBJS.G.00513. [DOI] [PubMed] [Google Scholar]
- 4.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 5.Grayeli AB, Esquia-Medina G, Nguyen Y, Mazalaigue S, Vellin JF, Lombard B, Kalamarides M, Ferrary E, Sterkers O. Use of anatomic or invasive markers in association with skin surface registration in image-guided surgery of the temporal bone. Acta Otolaryngol. 2009;129:405–410. doi: 10.1080/00016480802579025. [DOI] [PubMed] [Google Scholar]
- 6.Handels H, Ehrhardt J, Plötz W, Pöppl SJ. Three-dimensional planning and simulation of hip operations and computer-assisted construction of endoprostheses in bone tumor surgery. Comput Aided Surg. 2001;6:65–76. doi: 10.3109/10929080109145993. [DOI] [PubMed] [Google Scholar]
- 7.Hüfner T, Kfuri M, Jr, Galanski M, Bastian L, Loss M, Pohlemann T, Krettek C. New indications for computer-assisted surgery: tumor resection in the pelvis. Clin Orthop Relat Res. 2004;426:219–225. doi: 10.1097/01.blo.0000138958.11939.94. [DOI] [PubMed] [Google Scholar]
- 8.Kahler DM. Navigated long-bone fracture reduction. J Bone Joint Surg Am. 2009;91(suppl 1):102–107. doi: 10.2106/JBJS.H.01286. [DOI] [PubMed] [Google Scholar]
- 9.Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18:515–520. doi: 10.1016/j.jse.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Reijnders K, Coppes MH, Hulzen AL, Gravendeel JP, Ginkel RJ, Hoekstra HJ. Image guided surgery: new technology for surgery of soft tissue and bone sarcomas. Eur J Surg Oncol. 2007;33:390–398. doi: 10.1016/j.ejso.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Rubash HE, Pagnano MW. Navigation in total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 5):17. doi: 10.2106/JBJS.I.00346. [DOI] [PubMed] [Google Scholar]
- 12.Stiehl JB. Transepicondylar distal femoral pin placement in computer assisted surgical navigation. Comput Aided Surg. 2007;12:242–246. doi: 10.1080/10929080701517517. [DOI] [PubMed] [Google Scholar]
- 13.West J, Fitzpatrick JM, Wang MY, Dawant BM, Maurer CR, Jr, Kessler RM, Maciunas RJ, Barillot C, Lemoine D, Collignon A, Maes F, Suetens P, Vandermeulen D, Elsen PA, Napel S, Sumanaweera TS, Harkness B, Hemler PF, Hill DL, Hawkes DJ, Studholme C, Maintz JB, Viergever MA, Malandain G, Woods RP. Comparison and evaluation of retrospective intermodality brain image registration techniques. J Comput Assist Tomogr. 1997;21:554–556. doi: 10.1097/00004728-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Winkler D, Vitzthum HE, Seifert V. Spinal markers: a new method for increasing accuracy in spinal navigation. Comput Aided Surg. 1999;4:101–104. doi: 10.3109/10929089909148165. [DOI] [PubMed] [Google Scholar]
- 15.Wong KC, Kumta SM, Antonio GE, Tse LF. Image fusion for computer-assisted bone tumor surgery. Clin Orthop Relat Res. 2008;466:2533–2541. doi: 10.1007/s11999-008-0374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br. 2007;89:943–947. doi: 10.1302/0301-620X.89B7.19067. [DOI] [PubMed] [Google Scholar]
- 17.Wong KC, Kumta SM, Chiu KH, Cheung KW, Leung KS, Unwin P, Wong MC. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput Aided Surg. 2007;12:225–232. doi: 10.1080/10929080701536046. [DOI] [PubMed] [Google Scholar]
- 18.Zwingmann J, Konrad G, Kotter E, Südkamp NP, Oberst M. Computer-navigated iliosacral screw insertion reduces malposition rate and radiation exposure. Clin Orthop Relat Res. 2009;467:1833–1838. doi: 10.1007/s11999-008-0632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]