Abstract
Background
Electromagnetic computer-assisted surgery (EM-CAS) can be affected by various metallic or ferromagnetic factors.
Questions/purposes
We determined to what extent metals interfere with accuracy and identified measures to prevent interference from occurring.
Methods
Using an EM-CAS system, we made six standard measurements of tibiofemoral position and alignment on a surrogate knee. A stainless steel mallet was positioned 10 cm from the stylus, and then 10 cm from the localizer to create errors attributable to electromagnetic interference. The experiment was repeated with bars of different metals placed 10 cm from the stylus.
Results
The maximum errors recorded with a mallet were: varus/valgus alignment, −2.7° and 2.4°; flexion/extension, −5.8° and 3.0°; lateral resection level, −3.1 and 7.5 mm; and medial resection level, −4.0 and 2.3 mm, respectively. The smallest errors were recorded with cylinders of titanium, cobalt-chrome alloy, and stainless steels. When moved more than 10 cm away from the stylus, errors became negligible.
Conclusions
The accuracy of EM navigation systems is affected substantially by the size, type, proximity, and shape of metal objects.
Clinical Relevance
Stainless steel objects, such as cutting blocks and trial prostheses, should be kept more than 10 cm from EM-CAS instruments to minimize error.
Introduction
EM technology provides a convenient alternative to traditional infrared (IR) technology for CAS navigation systems owing to the reduced size of the EM sensors and the elimination of the necessary IR technology. However EM technology has the drawback of being susceptible to interference caused by stray EM fields. In the operating room, the presence of metal tools in proximity to the EM emitter or stylus potentially can lead to errors in measurements of component position and alignment [16]. The magnitude of the error observed in the operative setting is highly variable and depends on the mass and elemental composition of metal objects in the operative field and the frequency of the EM field [3, 7, 14]. Although interference from stainless steel objects has been observed in the laboratory setting [11, 13], the relative magnitude of errors induced in EM navigation systems has not been documented for a range of metals that may be incorporated in devices and instruments in the operating room. Moreover, possible solutions to counter the interference caused by extraneous metal objects in the clinical setting have not been proposed or evaluated.
Interference in an EM system arises from two main sources: ferromagnetic and conductive. Metal objects with high iron content produce ferromagnetic interference in direct proportion to the mass of the object [7, 13, 16]. The second group includes materials conducting electric current which produce their own magnetic field. These same materials can interfere when they are not conducting electricity [3]. The EM field emitted by the navigation system induces eddy currents in the conductive material, which then produces a secondary magnetic field [13].
Commercial EM navigation systems attempt to eliminate interference from extraneous sources through the use of specialized computer routines that detect erroneous signals through analysis of the output of the EM sensors. Once interference is detected, the software is signaled and the CAS system will project a blank screen, thereby preventing the use of erroneous data. In practice, the computer routines controlling the navigation system must be set to block data from being displayed only when EM interference exceeds a threshold level. Below this level, interference is ignored by the system, potentially leading to errors in measurement without the knowledge of the operating surgeon. Although the accuracy of current EM-CAS technology has been evaluated in a range of settings, few studies have intentionally introduced sources of error, and no previous studies have directly assessed the risk of erroneous measurements in TKAs. In this study, nine metals (6061 aluminum, 260 brass, 110 copper, cast cobalt-chromium, 304 and 316 stainless steel, titanium, 1018 cold-rolled steel, and A-36 hot-rolled steel) were chosen to reflect common metal typically encountered in an OR or to comprise a composite structure of power equipment or disposables. If the surgeon is aware of scenarios producing greater error in navigation outcomes, modifications can be made to the surgeon’s technique or the setup in the OR. Additionally, at an industry level, metallurgic modifications to instruments and OR equipment can improve the accuracy of EM-CAS if materials known to generate clinically significant effects are avoided.
The purposes of our study were twofold: (1) to determine the magnitude of errors in critical measurements performed during TKA that occur through EM interference arising from surgical instruments placed in the vicinity of EM systems, and (2) to determine how the magnitude of errors generated in EM navigation systems varies with the metallurgic composition of the tool generating the interference [9].
Materials and Methods
We performed two sets of experiments to explore the potential impact of metallic interference on the accuracy of EM-navigated TKAs. In the first set, we determined the magnitude of errors induced by placement of a surgical tool in proximity to the emitter and detectors of an EM-CAS system. By using a plastic (phenolic) surrogate model of the hip to ankle, all machined knee surfaces could be predetermined so as to have an exacting model with known values to measure inaccuracies of the CAS system in response to metal influence. This allowed us to assess the configuration of metal object and EM equipment that led to the greatest loss of accuracy, and the relative effect of metallic interference on each of the parameters measured during a TKA. These observations formed the basis for the second set of experiments in which the metallurgic composition of a standard rod was changed while its location in the operative field remained constant.
To test the accuracy of an EM navigation system in the presence of metallic objects, typical measurements obtained during a standard TKA were measured in the AP and sagittal planes.
We tested the accuracy of EM navigation by introducing a heavy mass instrument such as a stainless steel mallet to create a metallic interference. We then looked at usual customary measured angles and distances used in TKA under CAS guidance such as varus, valgus, flexion, extension, and distance separation. To ensure a high degree of accuracy, the surfaces of each block at the indexed positions were machined using a numerically controlled (CNC) milling machine and validated through measurements performed with a coordinate measurement machine (CMM) before the experiments. This allowed the true value of each dimensional and angular parameter to be determined with accuracies better than ± 0.1 mm and ± 0.1°, respectively.
Both phases of the experiment were conducted using the surrogate knee (Fig. 1). In the first experiment, the customary intraoperative measurements performed during a standard TKA were acquired with an imageless EM navigation system (Medtronic StealthStation® Treon™ Plus, Denver, CO, and AxiEM™, Denver, CO, USA). In each experiment, mini-EM detectors (dynamic reference frames) were attached to the phenolic blocks simulating the femur and the tibia. The operating software of this system included routines specifically designed to suspend data collection when extraneous interference was detected. The knee surrogate was placed in the typical set positions used during TKA, and the spatial coordinates of fiducial points representing standard anatomic landmarks were digitized as reference points for the navigation system. These landmarks included the center of the femoral head, distal and posterior edges of the medial and lateral femoral condyles, medial and lateral epicondyles, center of the distal femur, anterior femoral notch, Whiteside’s line, medial and lateral tibial plateaus, center of the proximal tibia, tibial tubercle, and medial and lateral malleoli. All parameters were recorded at the subdegree and submillimeter levels through modification of the data display software. The measurement procedure was repeated five times after returning the surrogate to the neutral position.
Fig. 1.
The surrogate knee model used in our study is shown. The surrogate knee is machined from a phenolic block measured to precise known angles of the cut surfaces and resection depths of ± 0.1° and ± 0.1 mm respectively. This makes an extremely accurate platform for detecting errors in CAS values.
All measurements were performed in an isolated, metal-free environment to minimize EM interference from uncontrolled sources. The surrogate knee was mounted on a phenolic sheet supported by a wooden work table specially constructed without metallic fasteners. The table was 4 feet tall, which was equidistant from metal reinforcement in the laboratory floor and ceiling. Based on the spatial coordinates of the digitized landmarks, the system defined the geometric relationship of the tibia and femur in terms of alignment in varus/valgus and flexion/extension. The separations of the resected surfaces of the medial and lateral condyles also were calculated. A common stainless steel surgical mallet then was introduced to the surgical field to evaluate the influence of EM interference on accuracy of the navigation system. The mallet was placed in two standardized locations: (1) 10 cm from the EM measuring instrument (stylus used for acquisition of discrete points), and (2) 10 cm from the magnetic field emitter or localizer. Minimum and maximum distortion values were recorded for each measured value with the mallet placed in both standardized locations.
In the second experiment, we evaluated the magnitude of errors induced by standard rods of different metallic alloys when placed in the proximity of the EM navigation system. Rods of fixed geometry (15 cm in length, 1.5 cm in diameter) were machined from nine different metals: 6061 aluminum, 260 brass, 110 copper, cast cobalt-chromium (Co-30Cr-7Mo), 304 stainless steel, 316 stainless steel, titanium, 1018 cold-rolled steel, and A-36 hot-rolled steel. These materials were selected because of their common use in orthopaedics and industry, in hand power equipment, and building construction and reinforcement. Four additional TKA simulations were performed on the surrogate knee using the same EM navigation system (Medtronic). EM trackers were attached, anatomic landmarks were recorded, and control measurements were noted in the same manner as for the previous experiment. During navigation of the distal femora, each metal bar was placed in four standardized positions within 10 cm of the stylus (adjacent to the proximal and distal corners of the device handle, and proximal and distal to the center of the device handle). Measurements then were made of the same parameters defining alignment of the knee and the extension space (varus/valgus, flexion/extension, and medial/lateral resection).
These measurements were compared with the true values determined in the initial runs. All the data were assessed using Welch’s t-test with α = 0.05 (twin-tailed) to accommodate variance and sample size. We determined the statistical significance of differences between measurements made with the EM system, with and without distortion of the electromagnetic field through the presence of the metal rods of different metallurgic compositions. Changes in alignment of the surrogate, in varus/valgus and flexion/extension, and width of the medial and lateral joint spaces were evaluated using Welch’s t-test, a version of Student t-test, modified for the presence of unequal variances.
Results
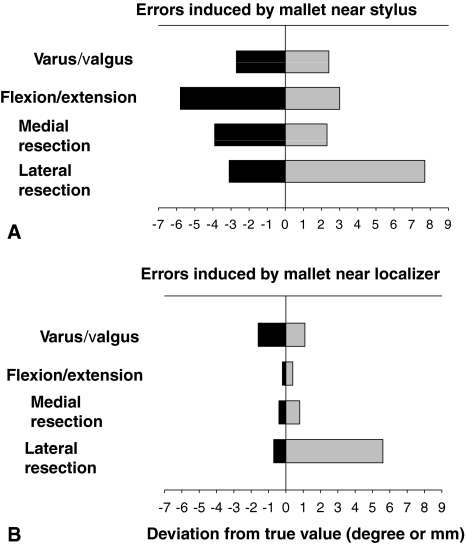
In the first experiment, placing the stainless steel surgical mallet within 10 cm of the EM stylus caused the varus/valgus angle measurement to vary over a range of 5.1°, from 2.7° less than the target value to 2.4° greater than the target value (Fig. 2A). Similarly, the range of deviations in the recorded angle of flexion/extension of the surrogate knee varied over a range of 8.8°, from 5.8° less than the true value to 3.0° greater than the true value. Similar variations were observed for the medial and lateral widths of the resection space between the femur and tibia. With repetition of the experimental measurements, the medial gap width varied from 4.0 mm less than to 2.3 mm greater than the target value, whereas the lateral gap width varied from 3.1 mm less than to 7.7 mm greater than the target value. This corresponds to total ranges of error of 6.3 mm and 10.8 mm, respectively (Fig. 2A). When the mallet was placed within 10 cm of the EM field localizer, the varus/valgus angle deviated from 1.6° less than to 1.1° greater than the target value, a total range of 2.7° (Fig. 2B). With the mallet near the localizer, the flexion/extension angle deviated from 0.2° less than to 0.4° greater than the true value with a range of 0.6°. Variation was noted from 0.4 mm less than to 0.8 mm greater than this value for the medial resection and 0.7 mm less than to 5.6 mm greater than the target value for the lateral resection. This gave total ranges in error from medial to lateral of 1.2 mm and 6.3 mm, respectively. When the mallet was placed greater than 10 cm from either the stylus or the localizer, the measurement errors became negligible.
Fig. 2A–B.
A graph shows the results of metal EM interference created when placing a mallet near the (A) stylus or (B) localizer. Four degrees of potential varus error or 6 mm of potential joint line over-resection error is possible. Additionally, there is a potential error of 7° excessive extension. A potential of 8o of excessive hyperextension in a cut is possible from this interference. The metal interference, when near the localizer, created much less error, but it was in the realm of 6°. Measurable units are presented for varus/valgus and flexion/extension angles (degrees) or lateral/medial resection depth (mm), respectively. These values point out the gravity of the possible undetected errors which may occur while using CAS around metal objects causing interference.
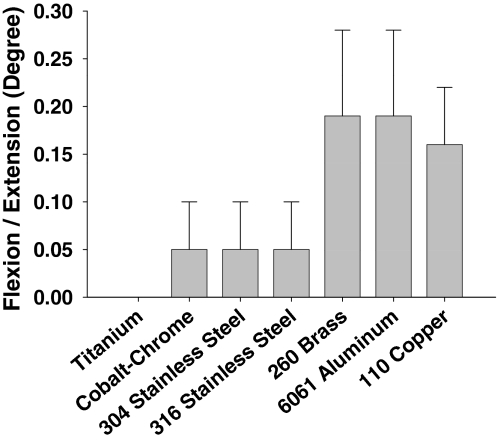
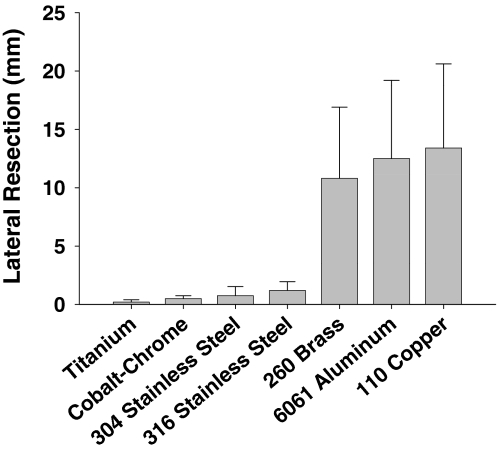
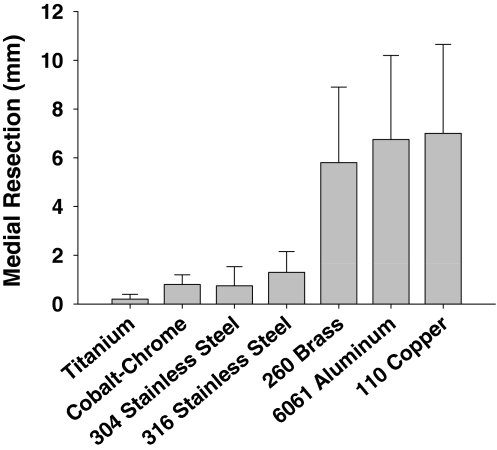
In the second experiment, rods fabricated from the two steel alloys (1018 cold-rolled steel and A-36 hot-rolled steel) caused sufficient field distortion for the automatic override to disable the navigation system causing no measurements to be displayed. The remaining seven alloys significantly affected the accuracy and precision of the EM measurements of the varus/valgus (Δ = 4.8; p = 0.0031) and flexion/extension (Δ = 6.1; p = 0.0008) alignment of the surrogate, and the medial (Δ = 7.2; p = 0.0003) and lateral (Δ = 5.7; p = 0.0012) gap widths (Figs. 3–6). Additional analysis showed, in each of the four parameters, the alloys were stratified into two groups. Measurements made in the presence of titanium, cobalt-chrome, and 304 and 316 stainless steel were more accurate and more precise than measurements made in the presence of 260 brass, 6061 aluminum, and 110 copper (Table 1; Figs. 3–6).
Fig. 4.
A graph shows the root mean square error and standard deviation in flexion/extension (degrees) measurement of seven of the alloys: titanium; cobalt-chrome; 304 and 316 stainless steels; 260 brass; 6061 aluminum; and 110 copper, respectively. Aluminum surprisingly exhibits a large influence, especially as it is weak in much of the operating room equipment such as leg holders and hand drills.
Fig. 5.
A graph shows the root mean square error and standard deviation in lateral resection (mm) measurement of seven of the alloys: titanium; cobalt-chrome; 304 and 316 stainless steels; 260 brass; 6061 aluminum; and 110 copper, respectively. Although some metals show little effect, these are very influential in inducing errors.
Fig. 3.
A graph shows the root mean square error and standard deviation in varus/valgus (degrees) measurement of seven of the alloys: titanium; cobalt-chrome; 304 and 316 stainless steels; 260 brass; 6061 aluminum; and 110 copper, respectively. Although some metal has no effect, some very common metals such as aluminum exert a profound effect.
Fig. 6.
A graph shows the root mean square error and standard deviation in medial resection (mm) measurement of the seven alloys.
Table 1.
Statistical significance of differences in the RMS errors and standard deviations
| Parameter | T-test (p value) | |
|---|---|---|
| RMS error | Standard deviation | |
| Varus/valgus | 0.0041 | 0.0026 |
| Flexion/extension | 0.0004 | 0.0530 |
| Lateral resection | 0.0028 | 0.0014 |
| Medial resection | 0.0018 | 0.0007 |
Discussion
Before EM systems can become a realistic alternative to established IR systems for surgical navigation, concerns regarding the potential effects of interference from ferromagnetic and electrically conductive sources must be addressed and resolved. The objectives of this study were first to determine if interference is a valid concern in the operative arena and, if so, which metals cause the greatest distortion. Second, once these sources of error are known, are there guidelines identifiable to minimize EM interference generated by commonly used surgical instruments? This study provides a glimpse into sources of error that can be avoided and the expected magnitude of that error.
We acknowledge limitations in this study. First, we assumed no magnetic fields were created outside the controlled interference of the test metals. These metals were not magnetized. However, multiuse jigs, cutting blocks, and provisional implants may become magnetized after heavy use [13]. This may induce higher levels of interference if not demagnetized on a regular basis. We did not demagnetize between runs nor check field strength of ferric metal test bars; however, all instruments were new and unused. Also, if magnetic conditions existed, they would exert the same effect for all runs. Second, ours was a laboratory study, not an in vivo experiment.
Plasticity of cartilage and bone potentially may lead to errors in reading, not only from undulations in cut surfaces, but also deformation induced from excessive force when taking measurements. The absence of human tissue is made up for by the consistency of the planar firm plastic surrogate model, where much greater accuracy of measures should be possible. Third, this is a bench study rather than a real intraoperative study when either surgeon-induced errors may occur or varying patient anatomy may make consistent measures impossible. The limitation is made up for by one consistent model of angles and distances where in this isolated environment, true instantaneous errors can be discerned from known values as opposed to postoperative radiographs where interpretive and rotational errors could induce errors.
Finally, although a mallet placed 10 cm from measurement trackers may seem contrived, one can imagine pinning a cutting block with pins and inadvertently holding the hammer while taking a final reading. These spurious scenarios are what make up limitations in studies just as they might in an operative environment.
In the first study, a common stainless steel mallet altered accuracy in varus and valgus by as much as 4° and 2°, respectively. Errors in the width of the resection space were even larger, ranging from greater than 6 mm medially and 11 mm laterally. Ferromagnetic interference is proportional to the mass and the geometry or shape of the interfering object [3]. The distortion effect also is dependent on the relative positions of the interfering instrument, the EM stylus and the EM field emitter, which we also observed. We found the mallet could create a deviation from true values by a margin of several degrees. The error was greatest when the mallet was near the stylus rather than the emitter by factors of 1.7 (lateral joint opening) and 14.6 (flexion/extension angle). These levels of error rapidly return the surgeon to the same level of accuracy seen in traditional, nonnavigated surgery, thereby defeating the purpose of CAS: to improve accuracy. This 4° in angular measure, or if not corrected, 7° error of geometrically calculated error of resection, would result in a major undetected error if not caught.
The clinical significance of errors of this magnitude can be deduced from numerous reports of early failures in TKA secondary to malalignment of the tibial and femoral components in the coronal plane. Previous authors have shown that varus/valgus malalignment of only ± 3° with respect to the mechanical axis leads to failure rates of 12% to 22% at only 2 years postoperatively [1–6, 8, 10, 12, 15]. Although there is no evidence that errors of accuracy in flexion of 5.8° may not be of clinical significance for failure, this degree of malalignment in the sagittal plane may impair ROM if manifested in hyperextension of the femoral or tibial components. The most disturbing scenario would be if a surgeon were to take an elevation reading of the 7.7-mm error and not check the CAS after resection, the resultant angular error could exceed 7° malalignment leading to early failure or, at the least, a poor clinical result.
In the second study, standard steel alloys interfered with the EM system. Because of the substantial degree of distortion, the EM system detected this aberration and the protective circuit disruption activated. More striking, however, were the differing interference values caused by copper, brass, and aluminum. Therefore, these metals may be potential sources of error unless they are kept outside the 10-cm radius. However, cobalt-chromium, titanium, and 304 and 316 stainless steels did not significantly alter measurements taken with the EM system even when placed at distances inside 10 cm and as close as 1 cm from the stylus. Thus, the interference from these metals is generally negligible, and therefore presumed safe when used with the EM surgical navigation system tested. However, if placed closer than 1 cm, cobalt-chromium alloy, titanium, and surgical stainless steels can and will cause significant interference. Although there appears to be little danger in the typical methods correctly used in the OR for the typical TKA, this answers the question of the implication of massive cutting blocks made of stainless steel as a potential area of error in the first study. The second study explains why provisional femoral components fabricated from cobalt-chromium and tibial trays made from titanium alloys do not cause significant errors when used in conjunction with EM-CAS systems.
Our data suggest EM interference is an important limitation of EM-based navigation systems that may compromise the accuracy and reproducibility of intraoperative measurements, despite the presence of software designed to detect interference effects and prevent erroneous measurements. As this is not described in any current operator manual or by implant representatives, it is incumbent of industry to make users aware of possible inaccurate situations and the appropriate means with which to avoid these errors. We also showed that substantial measurement errors occur only with specific metals when placed in close proximity to the EM field emitter or stylus. Therefore, it is reasonable that the prudent surgeon can expect reliable performance using EM navigation systems, provided that certain precautionary measures are taken. Although protective circuitry may prevent large field distortions from certain ferromagnetic sources, we do not recommend reliance on this feature to guarantee the accuracy of readings performed during surgery. We recommend that large metallic objects such as a mallet, be kept more than 10 cm from the stylus and surface being measured when taking navigated measurements during TKA. By taking these precautions, it is possible to reduce errors to negligible amounts when using EM-CAS systems for navigated measurements during TKA.
Acknowledgments
We thank Dr. L. Fang for editorial assistance and other FSOR staff who helped with this project. We also thank Dr. Rohan R. Wagle for technical assistance in performing the reported experiments.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Choong PF, Dowsey MM, Stoney JD. Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty. 2009;24:560–569. doi: 10.1016/j.arth.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Fang D, Ritter MA. Malalignment: forewarned is forearmed. Orthopedics. 2009;32: pii: orthosupersite.com/view.asp?rID = 42850. doi:10.3928/01477447-20090728-29. [DOI] [PubMed]
- 3.Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 suppl):39–43. doi: 10.1016/j.arth.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315–318. doi: 10.1097/00003086-200111000-00041. [DOI] [PubMed] [Google Scholar]
- 5.Gioe TJ, Killeen KK, Grimm K, Mehle S, Scheltema K. Why are total knee replacements revised?: analysis of early revision in a community knee implant registry. Clin Orthop Relat Res. 2004;428:100–106. doi: 10.1097/01.blo.0000147136.98303.9d. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709–714. doi: 10.1302/0301-620X.73B5.1894655. [DOI] [PubMed] [Google Scholar]
- 7.LaScalza S, Arico J, Hughes R. Effect of metal and sampling rate on accuracy of Flock of Birds electromagnetic tracking system. J Biomech. 2003;36:141–144. doi: 10.1016/S0021-9290(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 8.Longstaff LM, Sloan K, Stamp N, Scaddan M, Beaver R. Good alignment after total knee arthroplasty leads to faster rehabilitation and better function. J Arthroplasty. 2009;24:570–578. doi: 10.1016/j.arth.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Marmulla R, Hilbert M, Niederdellmann H. [Intraoperative precision of mechanical, electromagnetic, infrared and laser-guided navigation systems in computer-assisted surgery] Mund Kiefer Gesichtschir. 1998;2(suppl 1):S145–S148. doi: 10.1007/PL00014462. [DOI] [PubMed] [Google Scholar]
- 10.Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22:1097–1106. doi: 10.1016/j.arth.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Milne AD, Chess DG, Johnson JA, King GJ. Accuracy of an electromagnetic tracking device: a study of the optimal range and metal interference. J Biomech. 1996;29:791–793. doi: 10.1016/0021-9290(96)83335-5. [DOI] [PubMed] [Google Scholar]
- 12.Nafis C, Jensen V, von Jako R. Method for evaluating the compatibility of commercial electromagnetic (EM) micro sensors with surgical and imaging tables. GE Global Research. Niskayuna, NY: GE Healthcare. 2008;9:35–50.
- 13.Poulin F, Amiot LP. Interference during the use of an electromagnetic tracking system under OR conditions. J Biomech. 2002;35:733–737. doi: 10.1016/S0021-9290(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 14.Schicho K, Figl M, Donat M, Birkfellner W, Seemann R, Wagner A, Bergmann H, Ewers R. Stability of miniature electromagnetic tracking systems. Phys Med Biol. 2005;50:2089–2098. doi: 10.1088/0031-9155/50/9/011. [DOI] [PubMed] [Google Scholar]
- 15.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wagner A, Schicho K, Birkfellner W, Figl M, Seemann R, Konig F, Kainberger F, Ewers R. Quantitative analysis of factors affecting intraoperative precision and stability of optoelectronic and electromagnetic tracking systems. Med Phys. 2002;29:905–912. doi: 10.1118/1.1469625. [DOI] [PubMed] [Google Scholar]