Abstract
Numerous reports during the last 60 years have reported a strong association between idiopathic nephrotic syndrome and atopic disorders. Idiopathic nephrotic syndrome can be precipitated by allergic reactions and has been associated with both aeroallergens (pollens, mold, and dust) and food allergies. Patients with idiopathic nephrotic syndrome also may show increased serum immunoglobulin E (IgE) levels. A review of the literature suggests that although some idiopathic nephrotic syndrome cases may be associated with allergies, evidence that it is a type of allergic disorder or can be induced by a specific allergen is weak. Rather, it is likely that the proteinuria and increased IgE levels in patients with idiopathic nephrotic syndrome are caused by increased levels of interleukin 13 observed in these patients. Recent studies suggest that interleukin 13, a known stimulator of IgE response, may mediate proteinuria in patients with minimal change disease because of its ability to directly induce CD80 expression on the podocyte.
Keywords: Atopy, nephrotic syndrome, minimal change disease
Idiopathic nephrotic syndrome in children is a clinical syndrome associated with a variety of glomerular lesions. Minimal change disease (MCD) is the most common cause of idiopathic nephrotic syndrome. MCD is often abrupt in onset. It can be dramatic in presentation, yet is one of the most rewarding diseases for a physician to manage because response to corticosteroids often is rapid and complete. Because kidney biopsy usually is not performed when the disease responds to corticosteroid therapy, the term MCD has become synonymous with steroid-sensitive nephrotic syndrome.
The mechanism(s) underlying the MCD pathogenesis are unknown, although it is believed to be immunologically mediated.1 Strong evidence suggests that it may be caused by a circulating factor, possibly T-cell related, that causes podocyte dysfunction resulting in massive proteinuria.2 However, there also have been numerous reports linking MCD with atopic disorders and increases in serum immunoglobulin E (IgE) levels. In this review, we discuss the evidence supporting the association of atopy and whether there may be a common underlying immune disorder that may predispose patients to both conditions.
ATOPY
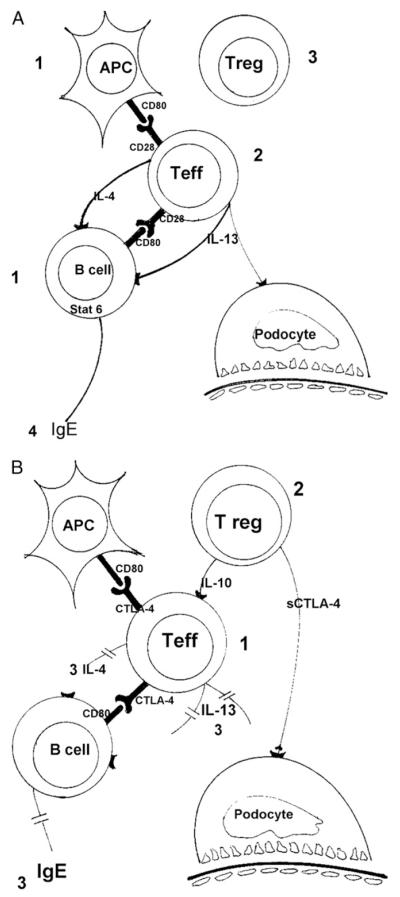
Atopy is a term used to describe IgE-mediated diseases. Persons with atopy have a hereditary predisposition to produce IgE antibodies to common allergens and often manifest with 1 or more atopic diseases (asthma, allergic rhinitis, and atopic eczema). Atopic patients mount an exaggerated immunologic response characterized by production of allergen-specific IgE antibodies and positive reactions to extracts of common aeroallergens on skin-prick tests. Type 2 helper T cells (TH2) from patients with atopy respond to allergens in vitro by expressing such cytokines as interleukin 4 (IL-4) and IL-133 (Fig 1A and B).
Figure 1.
(A) (1) Antigen-presenting cells (APCs) and activated B cells express CD80, which binds to CD28 on the (2) T effector (Teff) cellular membrane. In the absence of suppression by (3) T regulatory (Treg) cells, T effector cells release interleukin 4 (IL-4) and IL-13. These 2 cytokines trigger (4) the switch from immunoglobulin M (IgM) to IgE in the B cell. (B) T effector cells express cytotoxic T-lymphocyte antigen-4 (CTLA-4), which will compete with CD28 for CD80, resulting in (1) decreased activation of T effector cells. In addition, (2) T regulatory cells suppress T effector activation by releasing IL-10 and soluble CTLA-4. These combined events result in decreased production of IL-4, IL-13, and (3) IgE.
Early Reports of Atopy With MCD
In 1951, Fanconi et al4 were among of the first to associate atopy and nephrotic syndrome. Forty-three percent of their nephrotic patients showed signs of an “allergic diathesis.” They suggested that allergy could have a role in the pathogenesis of nephrotic syndrome. Since then, several studies have been reported linking atopy and nephrotic syndrome. In nephrotic patients, relapses have been described after exposure to allergens, including pollens,5-7 mold,8 poison oak,9 bee stings,10 and vaccinations. Serum IgE, which also occurs commonly in atopic patients, also commonly has increased levels in patients with MCD as opposed to other glomerular diseases.11 These findings have raised the possibility that atopy may have a role in the pathogenesis of MCD and allergens could be the triggering factor in the development of proteinuria. Is there evidence from controlled trials that atopic disorders are more common than expected in children with MCD?
Atopic Diseases in Children With MCD and Their Families
Findings of atopic disorders in patients with MCD have varied widely (Table 1). In 1 of the first reported series, Thomson et al19 reported that 38% (15 of 40) of children with steroid-responsive nephrotic syndrome had asthma, eczema, or hay fever compared with 18% (7 of 40) of age-matched controls. Since then, other series have been reported, and most have confirmed an increase in prevalence of atopic disorders in patients with steroid-sensitive nephrotic syndrome compared with controls.12-15,17,18,20-22 However, the frequency has varied dramatically (from 10% to 50%), although most series suggest that 30% to 40% of children with steroid-sensitive nephrotic syndrome have some type of allergic disorder (hay fever, asthma, or atopic dermatitis). Interestingly, in some series, the prevalence of atopic diseases also was increased in first-degree relatives, with similar prevalence rates.12,18 Fewer studies have been performed in patients with biopsy-proven MCD, but a tendency for a greater prevalence of atopic disorders also was observed.17
Table 1.
Clinical and Histopathologic Data and Serum IgE Levels
| Nephrotic Group |
Controls |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Incidence of Allergy (P) |
Biopsy Findings |
Positive Skin Test |
Elevated IgE (P) | 1st-Degree Relatives With Allergy |
Incidence of Allergy |
Positive Skin Test (P) |
Elevated IgE | 1st-Degree Relatives With Allergy |
| Meadow & Sarsfield12 | 26/77 (<0.01*) | MCD | 52% (<0.01†) | 65% | 37/77 (<0.2∥) | 15/80 | 13/50 | 29/79 | |
| Salsano et al13 | 12/58 | SSNS‡ | SS, 36% | 51% | |||||
| Cheung et al14 | 17/38 | MCD | 7/20 (NS§) | ||||||
| Lin et al15# | 88/207 | 31/110 MCD | 43%-71%¶ | 17/31 MCD | |||||
| Tenbrock et al16#,‡‡ | 12/78 (NS**) | 79/84 MCD | 33/78 (positive skin test + elevated IgE) |
||||||
| Rebien et al17 | 7/42 (NS) | MCD | 21% | 18% | |||||
| Yap et al18 | 23/59 (<0.05*) | SSNS‡ | 30/59 (<0.05†) | 33/55 (<0.0001§) | 27/58 (<0.05∥) | 21/100 | 16/46 | 8/55 | 28/100 |
| Thomson et al19†† | 15/40 (<0.05*) | SSNS‡ | 21/56 | 16/50 | 7/40 | ||||
Abbreviations: IgE, immunoglobulin E; MCD, minimal change disease; NS, not significant; SS, steroid-sensitive; SSNS, steroid-sensitive nephrotic syndrome.
P compares the incidence of allergy between nephrotic patients and healthy controls.
P compares positive skin test results between nephrotic patients and healthy controls.
No renal biopsy data. All patients had SSNS.
P compares IgE serum levels between nephrotic patients and healthy controls.
P compares the incidence of allergy between first-degree relatives of nephrotic patients and healthy controls.
Two separate antigens were tested.
Historical controls were used.
Compared with German general population historical controls.
104 patients were studied; of these only 40 were age- and sex-matched to children who had undergone urological or orthopedic operations.
84 patients were studied; of these, 78 were classified as atopic/non-atopic. In reporting the biopsy findings, the authors did not differentiate between atopic/non-atopic.
IgE in Atopy and MCD
Many advances have been made in recent years for the pathogenesis of atopy. IgE synthesis by B cells requires 2 signals. The first signal is delivered by the cytokines IL-4 or IL-13 released by TH2 cells, which target the Cε gene for switch recombination. The second signal is delivered by interaction of the B-cell surface antigen CD40 with its ligand expressed on activated T cells.23 Therefore, patients with atopy typically present with increased serum IgE and serum IL-4 and IL-13 levels, although on repeated exposure to same allergen, patients also may have increased plasma levels of interferon γ.24
Patients with MCD also show increased IgE levels (Table 1). Most,13-15,18,22,25-28 but not all,17,29 studies have reported greater serum IgE levels in patients with MCD compared with controls. Not infrequently, increased IgE levels occur in the absence of other clinical findings of atopy.13,16 Cheung et al14 reported increased values during relapse with normal levels during remission.
One confounding issue is that corticosteroid therapy may affect serum IgE levels. Hydrocortisone, for example, can induce IgE production in purified B cells obtained from nonatopic donors in combination with IL-430 and selectively enhance spontaneous IgE in atopic patients.31 Interestingly, there is evidence that IgE level increases with the duration of nephrotic syndrome in patients with MCD.32
In summary, there clearly is evidence that steroid-responsive nephrotic syndrome, especially in children, is associated commonly with a history of atopy and increased serum IgE levels. However, there appears to be marked variability in the frequency of atopy and increased IgE levels among the various series. One reason for the variability in the frequency of atopy could relate to how atopy was defined because some studies defined atopy by using clinical history or questionnaires (which can be limited by recall), whereas others defined it by using total serum IgE level (which can increase for other reasons). Another confounder is the definition of normal versus increased serum IgE level15,25,26 and the potential confounding effects of corticosteroids. Clearly, additional studies could be of benefit, particularly because atopic disorders are increasing in industrialized nations.33
ROLE OF SPECIFIC ALLERGEN(S) IN PRECIPITATING RELAPSE OF NEPHROTIC SYNDROME IN PATIENTS WITH MCD
The observations that the onset of MCD can occur in association with allergic phenomena and patients with MCD have an increased prevalence of atopic disease have led to the question of whether a specific allergen could have a direct role in mediating this disease. If an allergen could precipitate nephrotic syndrome in patients with MCD, it would suggest that MCD could represent a type of allergic disorder. As mentioned, relapses in patients with MCD have been reported to follow exposure to inhaled allergens,6-8,34-37 foods,38-45 insect stings,46,47 and vaccinations48,49 (Table 2). However, how good is the evidence that a specific allergen may be responsible for inducing MCD?
Table 2.
List of Potential Allergens Implicated in the Cause of Atopy/Minimal Change Disease
| Allergen | References |
|---|---|
| Inhaled allergens | |
| Tree and grass pollens | 6-8, 19, 21, 37, 39, 50 |
| Ragweed pollens | 8 |
| Fungi (molds) | 8 |
| House dust | 21, 35, 39 |
| Food allergens | |
| Cow’s milk | 38-40, 42-44 |
| Ovalbumin | 39, 40 |
| Pork | 41 |
| Fish, chicken, gliadin, beef | 40 |
| Vaccination | |
| Hepatitis B vaccine | 51 |
| Measles vaccine | 52 |
| Others | |
| Insect stings | 46 |
| Bee stings | 10, 47 |
| Poison oak | 9 |
Inhaled Allergens and Nephrotic Syndrome
Inhaled allergens triggering relapses of nephrotic syndrome in patients with MCD have been suggested by anecdotal reports and by the finding of seasonal variability in the onset and relapse of MCD. The list of triggering aeroallergens includes tree and grass pollens,6,7,19 rag-weed pollens,8 fungi,8 and house dusts.35 Given the frequency of grass pollen allergy, the isolated case reports do not provide convincing evidence of the relationship. Hardwicke et al,6 who first described the association between exposure to pollen and nephrotic syndrome, was unable to find another case in more than 300 subsequent patients with this condition.6
Some caution is required when evaluating the role of aeroallergens in triggering relapses in patients with nephrotic syndrome. First, most aeroallergens have been identified only by using the skin-prick test. The danger of ascribing a specific allergen by using skin-prick test as the triggering agent for MCD is that most patients with allergies often show a positive skin-prick test result to more than 1 antigen.12 Second, no “challenge” to induce MCD by exposing the participant to the suspected allergen has been attempted. Third, some studies incriminate allergens based on their seasonality, such as relapses in spring with pollen exposure6-8 or in fall coinciding with the peak incidence of mite allergens in house dust.53 However, Meadow and Sarsfield12 examined a series of 72 children with MCD and found no evidence for seasonal relapse. In their series, the initial episodes of nephrotic syndrome were distributed throughout the year.
If aeroallergens were the cause of MCD, therapies for treating the allergies might be expected to reduce the frequency of relapse. In this regard, skin desensitization to specific allergens was followed by prolonged remission in 1 patient described by Hardwicke et al6 and another by Reeves et al.7 In contrast, skin desensitization was unsuccessful to control relapses in 2 patients reported by Florido et al.34
Disodium cromoglycate is known for its proved efficacy for preventing relapse in patients with extrinsic asthma and hay fever. It stabilizes mast cells and prevents their degranulation on exposure to allergens.54 In the series of patients reported by Florido et al,34 only 3 of 20 patients achieved prolonged remission after cromoglycate was administered. Two controlled studies of the use of this drug in patients with relapsing nephrotic syndrome also have been published. Trompeter et al50 studied 21 children with at least 3 relapses of nephrotic syndrome. Patients were randomly allocated in a double-blind controlled study to cromoglycate or placebo for 16 weeks, together with a gradual reduction in maintenance prednisone dosage, with complete discontinuation by week 8. At 16 weeks, 5 of 10 patients in the placebo group and 9 of 11 in the disodium cromoglycate group had experienced relapse. In the other controlled study12 of the use of cromoglycate in patients with nephrotic syndrome, the 5 patients receiving cromoglycate had a combined total of 33 weeks in remission, whereas the 5 patients in the control group had 40 weeks in remission. Trials with other mast cell–stabilizing drugs (nivimedone and doxantrazole) also have failed to show a beneficial effect on prolonging remission in patients with relapsing nephrotic syndrome.12,55
Thus, although allergens occasionally have been implicated in triggering nephrotic syndrome in patients with MCD, evidence that blocking the specific allergic agent may prevent relapse is weak. This suggests that the atopic response is associated with the immune abnormality in patients with MCD, rather than having a causal role.
Food Allergens and Nephrotic Syndrome
Food allergens as triggering factors in the pathogenesis of MCD38-45 also have been suggested based on case reports. The potential role of these allergens in nephrosis has been insinuated by the presence of positive skin or radioallergosorbent test (RAST) test results for cow’s milk, fish, chicken, gliadin, or ovalbumin in individual patients.
In many cases, patients may not have had true MCD because some patients had steroid resistance42,43 or multiple relapses38 and were maintained during the trial at the same previous dose of steroids found to be not effective. Some reports also included patients younger than 1 year, making MCD less likely, and some included kidney biopsy specimens that showed a pathological state other than MCD.
Different types of dietary manipulations to remove suspected food allergens have been attempted. Dietary interventions have included the use of elemental diets (100% free amino acid liquid diet), limited exclusion diets (diet avoiding specific foods according to skin, RAST, and histamine release test results), or oligoantigenic diets (a diet that allows patients to eat only 4 or 5 food items without restriction of calories or proteins). An inconsistent response has been observed to each of these diets. Some patents initially showed improvement in proteinuria, whereas others failed to respond when the proposed food allergen was removed.38,42 Of patients who responded to dietary changes, some experienced a relapse, whereas others remained in remission after the offending allergen was reintroduced.
Use of Elemental Diets
One of the better studies examining the effects of dietary removal of a suspected food allergen was reported by Sandberg et al.38 These investigators described 5 children with frequently relapsing MCD in whom sensitization to cow’s milk was documented by means of oral challenge and skin testing. After relapse occurred (diagnosed as > 2+ proteinuria), an elemental diet was administered in the absence of corticosteroid treatment. Proteinuria decreased to protein less than 500 mg/24 h within the next 7 days in all participants. One participant reexposed to raw milk (30 g) redeveloped massive proteinuria within 3 days. In contrast to the initial response to the elemental diet, when patients experienced relapse, remission subsequently was achieved by only 3 patients by subsequent withdrawal of cow’s milk. Although these participants showed increased diuresis with resolution of edema, a decrease in urinary protein excretion, and improvement in serum albumin levels, it is unclear whether the effect of the elemental diet was caused by removal of an allergen or the elemental diet resulted in decreased total protein and intake. Placement of nephrotic patients on a low-protein diet also can result in a decrease in urinary protein excretion with some improvement in serum albumin level.56 Of interest, serum IgE concentrations were within the normal range in all patients.
Lagrue et al40 examined the effect of various diets in 42 patients with steroid-dependent or steroid-resistant disease with idiopathic nephrotic syndrome (18 MCD, 15 focal segmental glomerulosclerosis, and 9 mesangial proliferative glomerulonephritis). The investigators tested possible allergy to 10 different food items by using skin sensitivity tests, RAST, and histamine release test. Six patients with MCD, 9 patients with focal segmental glomerulosclerosis, and 5 patients with mesangial proliferative glomerulonephritis tested positive for 1 or more food proteins. Patients usually were maintained on a corticosteroid regimen before the trial. Diets were provided for 14 days. Use of the elemental diet (similar to that prescribed by Sandberg et al38) resulted in a transient remission in only 1 of 7 participants (histopathologic characteristics of nephrotic syndrome not defined), and the patient later required glucocorticoid therapy.
Limited Diets
Lagrue et al40 also administered a limited diet to 27 patients, with 7 of them achieving complete remission. However, many of these patients also were receiving glucocorticoid therapy, which confounds results. In another study,43 6 of 17 children with steroid-resistant nephrotic syndrome experienced remission in response to a milk-free diet. Unfortunately, in this study, all participants also received full doses of steroids during the dietary trial, making it unclear whether the remissions may have been related to use of the immunosuppressive therapy.
Oligoantigenic Diet
Oligoantigenic diets also have been tried. Genova et al42 reported that 6 of 12 patients with MCD experienced remission; however, some participants required up to 8 months on the diet before achieving remission. Because MCD has its own spontaneous remission rate, the lack of controls makes this report difficult to interpret. Thus, although the use of various diets to treat patients with MCD is interesting, they often are confounded by the concurrent use of prednisone and lack of a control group. In addition, the investigators did not correlate skin sensitivity test results with response to the diet and, as described by the investigators, some patients were not able to maintain remission despite maintaining strict adherence to their diet. In conclusion, although dietary restriction of milk and other proteins has resulted in some tantalizing responses in some patients with MCD associated with nephrotic syndrome, better controlled studies are required before conclusions can be drawn.
ALTERNATIVE VIEWS OF THE ROLE OF ALLERGY IN MCD
It is evident that some patients with MCD may present with nephrotic syndrome after an allergen exposure, and many patients with MCD have increased serum IgE levels (Fig 2). Nevertheless, as noted, treatment of the allergy appears to result only rarely in remission, and usually this has been done in isolated case reports. In recent years, it has become evident that patients with MCD may be prone to allergy because of an underlying immunologic system that predisposes them to both disorders.
Figure 2.
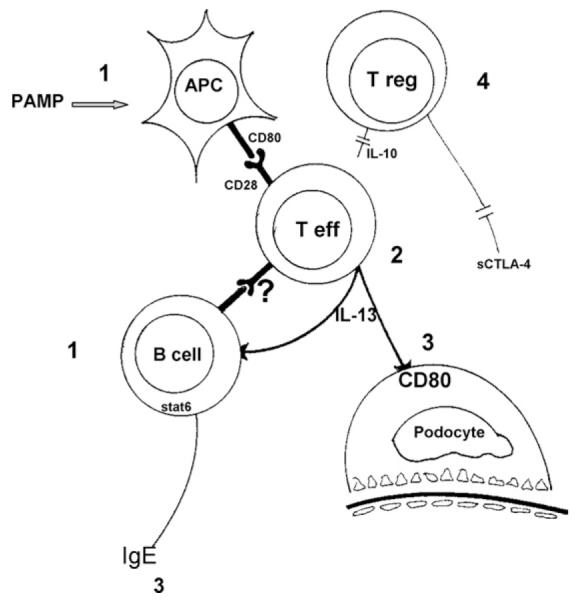
In patients with minimal change disease, activation of T effector (T eff) cells is by (1) antigen-presenting cells (APCs) after exposure to microbial pathogen-associated molecular patterns (PAMP). It is unknown whether activated B cells have a role (?). (2) Activated T effector cells release cytokines, including interleukin 13 (IL-13). IL-13 will induce the switch from immunoglobulin M (IgM) to IgE in B cells and expression of (3) CD80 by podocytes. A postulated defect in (4) T regulatory (T reg) cell function will allow T effector cells to continue secreting the pathogenetic cytokine.
Production of IgE is driven primarily by 2 cytokines, IL-4 and IL-13. In this regard, Kimata et al57 were the first to report that although spontaneous IL-4 production by T cells was increased in patients with atopy, IL-13 production by T cells was increased in patients with MCD. Subsequent studies have found that MCD is associated with increased IL-13 levels in urine57 and serum,58 with increased spontaneous production of IL-13 messenger RNA in isolated T cells59 and increased IL-13 production by T cells after stimulation14,59 compared with patients in remission or controls. Furthermore, intracellular expression of IL-13 by T cells correlated directly with serum IgE level.14 In contrast, studies examining IL-4 expression have been conflicting. Thus, although T cells have been reported to express high levels of IL-4 either spontaneously60 or after stimulation,61-63 other studies59,64,65 have not confirmed these findings.
More recent studies have implicated IL-13 as a potential mediator of MCD. Podocytes express IL-13 receptors66,67 and, in response to IL-13 binding, CD80 (also known as B7.1).67,68 CD80 is a transmembrane protein normally found on dendritic cells that have a key role in T-cell costimulation. Reiser et al68 have shown that induction of CD80 by podocytes results in proteinuria in mice with glomerular epithelial cell foot-process fusion, and Reiser and Mundel69 have suggested that CD80 expression could be a mechanism for MCD. Consistent with this observation, Lai et al67 reported that IL-13–overexpressing rats develop nephrotic syndrome with features consistent with MCD.
We also found that urinary CD80 levels are increased in patients with MCD during relapse and return to normal after remission.70 We also have preliminary evidence that the source of the CD80 is the podocyte because we found that by using immunohistochemical staining, CD80 was expressed by podocytes in kidney biopsy specimens from patients with MCD in relapse (E.H. Garin et al, unpublished data).
Although these studies incriminate IL-13 in the nephrotic syndrome observed in patients with steroid-sensitive MCD, there are reports that serum IL-13 levels increase after remission58 despite a decrease in expression by isolated T cells.14,59 In this regard, we have postulated that podocyte expression of CD80 may continue unless expression is turned off by soluble cytotoxic T-lymphocyte antigen-4 (CTLA-4).70 In our preliminary studies, CD80/CTLA-4 ratio is increased in patients with MCD during relapse, with levels returning to the normal range in remission.70 If true, MCD might be considered a 2-step disease in which there is initial stimulation of CD80 on podocytes by IL-13 or other cytokines followed by inadequate silencing of CD80 by insufficient release of soluble CTLA-4.
In conclusion, allergies are common in patients with MCD, but there is little evidence that they have a direct pathogenic role in this disorder. More likely, the underlying immune system in these individuals predisposes them to both disorders. IL-13 has been found to be increased in patients with MCD. IL-13 is known to induce a switch from IgM to IgE in B cells and induce CD80 expression by podocytes. Concomitantly, increased CD80 expression by podocytes is associated with proteinuria. Additional studies are needed to elucidate the role of IL-13 and CD80 in MCD. In addition, these studies could result in novel therapies (such as the use of soluble CTLA-4 IgG) that specifically target CD80 or factors stimulating podocyte CD80 expression.
ACKNOWLEDGEMENTS
Support: None.
Financial Disclosure: None.
REFERENCES
- 1.Shalhoub RJ. Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 2.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 3.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 4.Fanconi G, Kousmine C, Frisch B, Knecht W. Prognosis of the nephrotic syndrome. Helv Pediatr Acta. 1951;6:219–224. [PubMed] [Google Scholar]
- 5.Kark RM, Pirani CL, Pollak VE, Muehrcke RC, Blainey JD. The nephrotic syndrome in adults: A common disorder with many causes. Ann Intern Med. 1958;49:751–754. doi: 10.7326/0003-4819-49-4-751. [DOI] [PubMed] [Google Scholar]
- 6.Hardwicke J, Soothill JF, Squire JR, Holti G. Nephrotic syndrome with pollen hypersensitivity. Lancet. 1959;1:500–502. doi: 10.1016/s0140-6736(59)91026-8. [DOI] [PubMed] [Google Scholar]
- 7.Reeves WG, Cameron JS, Johansson SG, Ogg CS, Peters DK, Weller RO. Seasonal nephrotic syndrome. Description and immunological findings. Clin Allergy. 1975;5:121–137. doi: 10.1111/j.1365-2222.1975.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 8.Wittig HJ, Goldman AS. Nephrotic syndrome associated with inhaled allergens. Lancet. 1970;1:542–543. doi: 10.1016/s0140-6736(70)90770-1. [DOI] [PubMed] [Google Scholar]
- 9.Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548–560. doi: 10.1016/0002-9343(48)90105-3. [DOI] [PubMed] [Google Scholar]
- 10.Rytand DA. Onset of the nephrotic syndrome during a reaction to bee sting. Stanford Med Bull. 1955;13:224–233. [PubMed] [Google Scholar]
- 11.Schulte-Wissermann H, Gortz W, Straub E. IgE in patients with glomerulonephritis and minimal-change nephrotic syndrome. Eur J Pediatr. 1979;131:105–111. doi: 10.1007/BF00447472. [DOI] [PubMed] [Google Scholar]
- 12.Meadow SR, Sarsfield JK. Steroid-responsive and nephrotic syndrome and allergy: Clinical studies. Arch Dis Child. 1981;56:509–516. doi: 10.1136/adc.56.7.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salsano ME, Graziano L, Luongo I, Pilla P, Giordano M, Lama G. Atopy in childhood idiopathic nephrotic syndrome. Acta Paediatr. 2007;96:561–566. doi: 10.1111/j.1651-2227.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheung W, Wei CL, Seah CC, Jordan SC, Yap HK. Atopy, serum IgE, and interleukin-13 in steroid-responsive nephrotic syndrome. Pediatr Nephrol. 2004;19:627–632. doi: 10.1007/s00467-004-1438-8. [DOI] [PubMed] [Google Scholar]
- 15.Lin CY, Lee BH, Lin CC, Chen WP. A study of the relationship between childhood nephrotic syndrome and allergic diseases. Chest. 1990;97:1408–1411. doi: 10.1378/chest.97.6.1408. [DOI] [PubMed] [Google Scholar]
- 16.Tenbrock K, Schubert A, Stapenhorst L, et al. Type I IgE receptor, interleukin 4 receptor and interleukin 13 polymorphisms in children with nephrotic syndrome. Clin Sci (Lond) 2002;102:507–512. [PubMed] [Google Scholar]
- 17.Rebien W, Muller-Wiefel DE, Wahn U, Scharer K. IgE mediated hypersensitivity in children with idiopathic nephrotic syndrome. Int J Pediatr Nephrol. 1981;2:23–28. [PubMed] [Google Scholar]
- 18.Yap HK, Yip WC, Lee BW, et al. The incidence of atopy in steroid-responsive nephrotic syndrome: Clinical and immunological parameters. Ann Allergy. 1983;51:590–594. [PubMed] [Google Scholar]
- 19.Thomson PD, Stokes CR, Barratt TM, Turner MW, Soothill JF. HLA antigens and atopic features in steroid-responsive nephrotic syndrome of childhood. Lancet. 1976;2:765–768. doi: 10.1016/s0140-6736(76)90600-0. [DOI] [PubMed] [Google Scholar]
- 20.Yap HK, Han EJ, Heng CK, Gong WK. Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatr Nephrol. 2001;16:1049–1052. doi: 10.1007/s004670100024. [DOI] [PubMed] [Google Scholar]
- 21.Trompeter RS, Barratt TM, Kay R, Turner MW, Soothill JF. HLA, atopy, and cyclophosphamide in steroid-responsive childhood nephrotic syndrome. Kidney Int. 1980;17:113–117. doi: 10.1038/ki.1980.13. [DOI] [PubMed] [Google Scholar]
- 22.Tain YL, Chen TY, Yang KD. Implication of serum IgE in childhood nephrotic syndrome. Pediatr Nephrol. 2003;18:1211–1215. doi: 10.1007/s00467-003-1269-z. [DOI] [PubMed] [Google Scholar]
- 23.Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000;105(2 pt 2):S547–S558. doi: 10.1016/s0091-6749(00)90059-9. [DOI] [PubMed] [Google Scholar]
- 24.Smart JM, Kemp AS. Increased Th1 and Th2 allergen-induced cytokine responses in children with atopic disease. Clin Exp Allergy. 2002;32:796–802. doi: 10.1046/j.1365-2222.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- 25.Takei T, Koike M, Suzuki K, et al. The characteristics of relapse in adult-onset minimal-change nephrotic syndrome. Clin Exp Nephrol. 2007;11:214–217. doi: 10.1007/s10157-007-0484-5. [DOI] [PubMed] [Google Scholar]
- 26.Meadow SR, Sarsfield JK, Scott DG, Rajah SM. Steroid-responsive nephrotic syndrome and allergy: Immunological studies. Arch Dis Child. 1981;56:517–524. doi: 10.1136/adc.56.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groshong T, Mendelson L, Mendoza S, Bazaral M, Hamburger R, Tune B. Serum IgE in patients with minimal-change nephrotic syndrome. J Pediatr. 1973;83:767–771. doi: 10.1016/s0022-3476(73)80367-1. [DOI] [PubMed] [Google Scholar]
- 28.Mishra OP, Ibrahim N, Das U, Das BK. Serum immunoglobulin E in idiopathic nephrotic syndrome. J Trop Pediatr. 2004;50:149–152. doi: 10.1093/tropej/50.3.149. [DOI] [PubMed] [Google Scholar]
- 29.Barratt TM, Turner MW, Johansson SG. Urinary excretion of immunoglobulin E in the nephrotic syndrome and atopic eczema. Lancet. 1971;2:402–403. doi: 10.1016/s0140-6736(71)90116-4. [DOI] [PubMed] [Google Scholar]
- 30.Jabara HH, Ahern DJ, Vercelli D, Geha RS. Hydrocortisone and IL-4 induce IgE isotype switching in human B cells. J Immunol. 1991;147:1557–1560. [PubMed] [Google Scholar]
- 31.Kimata H, Lindley I, Furusho K. Effect of hydrocortisone on spontaneous IgE and IgG4 production in atopic patients. J Immunol. 1995;154:3557–3566. [PubMed] [Google Scholar]
- 32.Fuke Y, Endo M, Ohsawa I, et al. Implication of elevated serum IgE levels in minimal change nephrotic syndrome. Nephron. 2002;91:769–770. doi: 10.1159/000065049. [DOI] [PubMed] [Google Scholar]
- 33.Sibbald B, Rink E, D’Souza M. Is the prevalence of atopy increasing? Br J Gen Pract. 1990;40:338–340. [PMC free article] [PubMed] [Google Scholar]
- 34.Florido JF, Pena JM Díaz, Belchi J, Estrada JL, Ara MC García, Ojeda JA. Nephrotic syndrome and respiratory allergy in childhood. J Investig Allergol Clin Immunol. 1992;2:136–140. [PubMed] [Google Scholar]
- 35.Laurent J, Lagrue G, Belghiti D, Noirot C, Hirbec G. Is house dust allergen a possible causal factor for relapses in lipoid nephrosis? Allergy. 1984;39:231–236. doi: 10.1111/j.1398-9995.1984.tb02628.x. [DOI] [PubMed] [Google Scholar]
- 36.Thomson PD, Barratt TM, Stokes CR, Soothill JF, Turner MW. HLA typing and atopic features in steroid sensitive nephrotic syndrome of childhood. Monogr Allergy. 1977;11:60. [PubMed] [Google Scholar]
- 37.Williamson DA. Nephrotic syndrome associated with inhaled allergens. Lancet. 1970;1:778. doi: 10.1016/s0140-6736(70)91004-4. (ltr) [DOI] [PubMed] [Google Scholar]
- 38.Sandberg DH, Bernstein CW, McIntosh RM, Carr R, Strauss J. Severe steroid-responsive nephrosis associated with hypersensitivity. Lancet. 1977;1:388–391. doi: 10.1016/s0140-6736(77)92603-4. (ltr) [DOI] [PubMed] [Google Scholar]
- 39.Richards W, Olson D, Church JA. Improvement of idiopathic nephrotic syndrome following allergy therapy. Ann Allergy. 1977;39:332–333. [PubMed] [Google Scholar]
- 40.Lagrue G, Laurent J, Rostoker G. Food allergy and idiopathic nephrotic syndrome. Kidney Int Suppl. 1989;27:S147–S151. [PubMed] [Google Scholar]
- 41.Howanietz H, Lubec G. Idiopathic nephrotic syndrome, treated with steroids for five years, found to be allergic reaction to pork. Lancet. 1985;2:450. doi: 10.1016/s0140-6736(85)92772-2. [DOI] [PubMed] [Google Scholar]
- 42.Genova R, Sanfilippo M, Rossi ME, Vierucci A. Food allergy in steroid-resistant nephrotic syndrome. Lancet. 1987;1:1315–1316. doi: 10.1016/s0140-6736(87)90567-8. [DOI] [PubMed] [Google Scholar]
- 43.Sieniawska M, Szymanik-Grzelak H, Kowalewska M, Wasik M, Koleska D. The role of cow’s milk protein intolerance in steroid-resistant nephrotic syndrome. Acta Paediatr. 1992;81:1007–1012. doi: 10.1111/j.1651-2227.1992.tb12164.x. [DOI] [PubMed] [Google Scholar]
- 44.de Sousa JS, Rosa FC, Baptista A, Fonseca H, Sa G. Cow’s milk protein sensitivity: A possible cause of nephrotic syndrome in early infancy. J Pediatr Gastroenterol Nutr. 1995;21:235–237. doi: 10.1097/00005176-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 45.McCrory WW, Becker CG, Cunningham-Rundles C, Klein RF, Mouradin J, Reisman L. Immune complex glomerulopathy in a child with food hypersensitivity. Kidney Int. 1986;30:592–598. doi: 10.1038/ki.1986.226. [DOI] [PubMed] [Google Scholar]
- 46.Tareyeva IE, Nikolaev AJ, Janushkevitch TN. Nephrotic syndrome induced by insect sting. Lancet. 1982;2:825. doi: 10.1016/s0140-6736(82)92718-0. (ltr) [DOI] [PubMed] [Google Scholar]
- 47.Cuoghi D, Venturi P, Cheli E. Bee sting and relapse of nephrotic syndrome. Child Nephrol Urol. 1988;9:82–83. [PubMed] [Google Scholar]
- 48.Kuzemko JA. Measles vaccination and the nephrotic syndrome. Br Med J. 1972;4:665–666. doi: 10.1136/bmj.4.5841.665-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macario F, Freitas L, Correia J, Campos M, Marques A. Nephrotic syndrome after recombinant hepatitis B vaccine. Clin Nephrol. 1995;43:349. (ltr) [PubMed] [Google Scholar]
- 50.Trompeter RS, Thomson PD, Barratt TM, Soothill JF. Controlled trial of disodium cromoglycate in prevention of relapse of steroid-responsive nephrotic syndrome of childhood. Arch Dis Child. 1978;53:430–432. doi: 10.1136/adc.53.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Işlek I, Cengiz K, Cakir M, Küçüködük S. Nephrotic syndrome following hepatitis B vaccination. Pediatr Nephrol. 2004;14:89–90. [PubMed] [Google Scholar]
- 52.Kuzemko JA. Measles vaccination and the nephrotic syndrome. Br Med J. 1972;4:665–666. doi: 10.1136/bmj.4.5841.665-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toyabe S, Nakamizo M, Uchiyama M, Akazawa K. Circannual variation in the onset and relapse of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2005;20:470–473. doi: 10.1007/s00467-004-1780-x. [DOI] [PubMed] [Google Scholar]
- 54.Shin HY, Kim JS, An NH, Park RK, Kim HM. Effect of disodium cromoglycate on mast cell-mediated immediate-type allergic reactions. Life Sci. 2004;74:2877–2887. doi: 10.1016/j.lfs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Bluett NH, Chantler C, Hughes DT. Failure of doxantrazole in steroid-sensitive nephrotic syndrome. Lancet. 1977;1:809. doi: 10.1016/s0140-6736(77)93001-x. (ltr) [DOI] [PubMed] [Google Scholar]
- 56.Kaysen GA, Gambertoglio J, Jimenez I, Jones H, Hutchison FN. Effect of dietary protein intake on albumin homeostasis in nephrotic patients. Kidney Int. 1986;29:572–577. doi: 10.1038/ki.1986.36. [DOI] [PubMed] [Google Scholar]
- 57.Kimata H, Fujimoto M, Furusho K. Involvement of interleukin (IL)-13, but not IL-4, in spontaneous IgE and IgG4 production in nephrotic syndrome. Eur J Immunol. 1995;25:1497–1501. doi: 10.1002/eji.1830250604. [DOI] [PubMed] [Google Scholar]
- 58.Tain YL, Chen TY, Yang KD. Implications of serum TNF-beta and IL-13 in the treatment response of childhood nephrotic syndrome. Cytokine. 2003;21:155–159. doi: 10.1016/s1043-4666(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 59.Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: Evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol. 1999;10:529–537. doi: 10.1681/ASN.V103529. [DOI] [PubMed] [Google Scholar]
- 60.Adrogue HE, Borillo J, Torres L, et al. Coincident activation of Th2 T cells with onset of the disease and differential expression of GRO-gamma in peripheral blood leukocytes in minimal change disease. Am J Nephrol. 2007;27:253–261. doi: 10.1159/000101371. [DOI] [PubMed] [Google Scholar]
- 61.Cho BS, Yoon SR, Jang JY, Pyun KH, Lee CE. Up-regulation of interleukin-4 and CD23/FcepsilonRII in minimal change nephrotic syndrome. Pediatr Nephrol. 1999;13:199–204. doi: 10.1007/s004670050592. [DOI] [PubMed] [Google Scholar]
- 62.Neuhaus TJ, Wadhwa M, Callard R, Barratt TM. Increased IL-2, IL-4 and interferon-gamma (IFN-gamma) in steroid-sensitive nephrotic syndrome. Clin Exp Immunol. 1995;100:475–479. doi: 10.1111/j.1365-2249.1995.tb03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jian K. Increased production of interleukin 4 in children with simple idiopathic nephrotic syndrome. Chinese Med J. 1994;105:347–350. [PubMed] [Google Scholar]
- 64.Shimoyama H, Nakajima M, Naka H, et al. Up-regulation of interleukin-2 mRNA in children with idiopathic nephrotic syndrome. Pediatr Nephrol. 2004;19:1115–1121. doi: 10.1007/s00467-004-1569-y. [DOI] [PubMed] [Google Scholar]
- 65.Kaneko K, Tuchiya K, Fujinaga S, et al. Th1/Th2 balance in childhood idiopathic nephrotic syndrome. Clin Nephrol. 2002;58:393–397. doi: 10.5414/cnp58393. [DOI] [PubMed] [Google Scholar]
- 66.Parry RG, Gillespie KM, Mathieson PW. Effects of type 2 cytokines on glomerular epithelial cells. Exp Nephrol. 2001;9:275–283. doi: 10.1159/000052622. [DOI] [PubMed] [Google Scholar]
- 67.Lai KW, Wei CL, Tan LK, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18:1476–1485. doi: 10.1681/ASN.2006070710. [DOI] [PubMed] [Google Scholar]
- 68.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reiser J, Mundel P. Danger signaling by glomerular podocytes defines a novel function of inducible B7-1 in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol. 2004;15:2246–2248. doi: 10.1097/01.ASN.0000136312.46464.33. [DOI] [PubMed] [Google Scholar]
- 70.Garin EH, Diaz L, Mu W, Araya CE, Johnson RJ. Urinary CD80 excretion is increased in idiopathic minimal lesion nephrotic syndrome. J Am Soc Nephrol. 2009;20:260–266. doi: 10.1681/ASN.2007080836. [DOI] [PMC free article] [PubMed] [Google Scholar]