Abstract
The potential for directed differentiation of human induced pluripotent stem (iPS) cells to functional post-mitotic neuronal phenotypes is unknown. Following methods shown to be effective at generating motor neurons from human embryonic stem cells (hESCs), we found that once specified to a neural lineage, human iPS cells could be differentiated to form motor neurons with a similar efficiency as hESCs. Human iPS-derived cells appeared to follow a normal developmental progression associated with motor neuron formation and possessed prototypical electrophysiological properties. This is the first demonstration that human iPS-derived cells are able to generate electrically active motor neurons. These findings demonstrate the feasibility of using iPS-derived motor neuron progenitors and motor neurons in regenerative medicine applications and in vitro modeling of motor neuron diseases.
Introduction
Several groups have demonstrated the feasibility of reprogramming various types of human somatic cells to an embryonic state upon the introduction of pluripotency factors that yield induced pluripotent stem cells (iPS cells) [1–4]. The excitement surrounding iPS technology is predicated upon the potential to treat disease or injury with derivatives of patient-specific stem cells. In the more immediate term, their value may lie in the opportunity to model human diseases in vitro for which the etiology is unknown. Motor neurons are lost in many conditions, including spinal cord injury, amyotrophic lateral sclerosis (ALS), and Spinal Muscular Atrophy (SMA). A major therapeutic goal is to develop the means to functionally replace these cells, and to model their states of disease in vitro. Previous studies have outlined methods to derive functional motor neurons from human embryonic stem cells (hESCs) [5–7], and two recent studies applied similar methods to human iPS cell lines derived from patients with ALS and SMA [8, 9]. Before human iPS cells are utilized for regenerative medicine or to model motor neuron diseases, it is imperative to demonstrate that these cells can generate electrically active motor neurons with the characteristics of their natural counterparts.
Human iPS cells were capable of generating derivatives representing the three embryonic germ layers both in vitro in Embryoid Bodies (EBs) and in vivo teratoma assays ([1] and Fig S1). Here, we show that two established methods to derive motor neurons from murine and human embryonic stem cells can also be used to produce functionally mature motor neurons from human iPS cells[1]. First, an embryoid body (EB) differentiation protocol was used to enrich for motor neuron differentiation [10, 11]. Second, an adherent approach was employed to enable the characterization of the differentiated cells for their gene expression and electrophysiology [6, 7]. For both approaches, we directly compared the ability of different human iPS cell lines and HSF1, a stereotypical hESC line, to generate both motor neuron progenitors and fully differentiated motor neurons. The results of this study should provide a platform for future studies of human motor neuron diseases in vitro.
Materials and Methods
Immunostaining
Antibody staining was performed on 4% parformaldehyde fixed, cryosectioned embryoid bodies and cultured cells as previously described [12]. Antibodies used include: goat anti-Brn2 (sc-6029, Santa Cruz Biotechnology), goat anti-Choline Acetyl Transferase (ChAT, Millipore), rabbit anti-Hoxa3 and guinea pig anti-Hoxa5 (generously provided by J. Dasen and T. Jessell) [13]), goat anti-Hoxc6 (SC-46135, Santa Cruz Biotechnology), mouse anti-Hoxc8 clone C952-7E (MMS-266R, Covance), goat anti-Isl1 (AF1837, R&D Systems), mouse anti-Isl1 clone 39.4D5 (Developmental Studies Hybridoma Bank), mouse anti-Lim3 clone 4E12 (Developmental Studies Hybridoma Bank), mouse anti-Nkx6.1 clone F55A10 (Developmental Studies Hybridoma Bank), rabbit anti-Nkx6.1 (generously provided by S. Morton and T. Jessell)[14], guinea pig anti-Olig2 [13], mouse anti-Pax6 (Developmental Studies Hybridoma Bank), mouse anti-Pax7 (Developmental Studies Hybridoma Bank), mouse anti-Nestin (Neuromics), rabbit anti-Sox1, anti-Sox2, and anti-Sox3 (all generously provided by T. Edlund and J. Muhr) [15], rabbit anti-β-III tubulin (MRB-435P, Covance). The monoclonal antibodies obtained from the Developmental Studies Hybridoma Bank were developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Alexa488-, FITC-, Cy3- and Cy5-conjugated secondary antibodies were obtained from either Invitrogen or Jackson Immunoresearch. Fluorescence and DIC images were collected using a Zeiss Axioobserver microscope equipped with the Apotome optical imaging system, or a Zeiss LSM5 Exciter confocal imaging system. Images were processed using the Zeiss Axiovision and LSM Exciter software suites, and Adobe Photoshop CS2.
Electrophysiology
Electrophysiology was performed at 20–23°C using standard whole-cell, current-clamp techniques. Patch pipettes [3–5 uM were filled with [in mM]: 140 potassium gluconate, 10 Na HEPES, 1 EGTA, 4 ATP-Mg, 0.3 GTP, pH 7.2 [adjusted with KOH]. Cells were bathed in [in mM]: 120 NaCl, 1.2 KH2PO4, 1.9 KCl, 26 NaHCO3, 2.2 CaCl2, 1.4 MgSO4, 10 D-Glucose, 7.5 Na HEPES [pH with NaOH to 7.2] equilibrated with 95% O2 and 5% CO2 and resting potentials were maintained at about −70mV. Graded current injections used durations of 0.5msec (in steps of 100pA) or 250msec (in steps of 20pA). Signals were sampled at 10kHz using a Digidata 1322A analog to digital converter, and acquired and stored on a computer hard drive using pClamp 6 software. Data were analyzed off-line using pClamp 6 (Clampfit).
Results
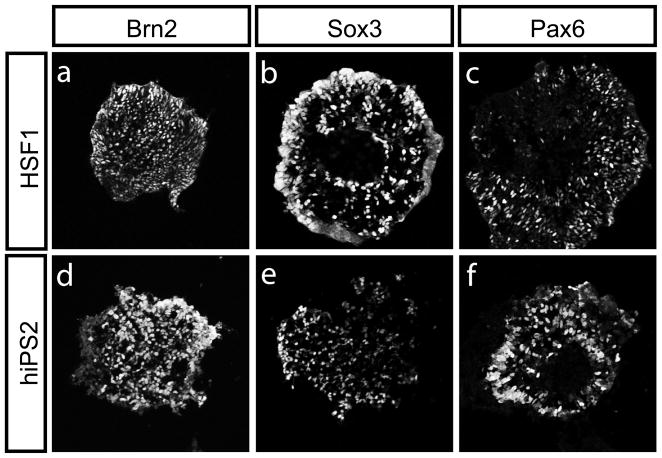
To demonstrate whether human iPS are able to differentiate down neural lineages to form motor neurons, we generated embryoid bodies (EBs) from human iPS cells (hiPS2) and hESCs (HSF1), as previously described [1]. The EBs were cultured for one week in hESC media lacking FGF2, and then treated for an additional week with Retinoic Acid (RA; 1 μM) and a Sonic Hedgehog pathway agonist (Purmorphamine, 1.5 μM) [16]. This method is known to both neuralize and ventralize EBs, as defined by the expression of ventral neural progenitor markers [11]. Both HSF1 and human iPS cells followed a standard course of development, serially differentiating from pluripotent cells to neural progenitors to fully differentiated motor neurons. As the EB protocol initiates specification in a somewhat stochastic manner, only a proportion of EBs from either HSF1 or iPS cells were specified to be neural, as demonstrated by immunostaining for the neural progenitor markers Brn2, Sox3 and Pax6 (Fig. 1a–f, Fig. S2).
Figure 1. hESC and human iPS-derived cells can both generate neural progenitors.
Both HSF1 and hiPS2-derived EBs were grown for 5–7 days in the presence of retinoic acid (RA) (1 μM, Sigma) and the Shh pathway agonist Purmorphamine (1.5 μM, Calbiochem) and generated EBs full of neural progenitors as judged by immunostaining for Brn2, Sox3, and Pax6 (a-f). Similar results were obtained using a different Shh pathway agonist (HhAg1.3, 500 nM, Curis, data not shown).
We did observe marked differences in the efficiencies by which HSF1 and hiPS cells underwent specification down the neural lineage (Fig. S2). Because of the well-described variation of differentiation potentials amongst pluripotent stem cell lines [17], it is unclear whether this finding reflects an inherent difference between embryonic and induced pluripotent stem cells. However, within those EBs that were specified as neural, the percentage of cells expressing neural progenitor markers Brn2, Sox3 and Pax6 was similar whether the EBs were derived from HSF1 or human iPS cells (Fig. 1a–f and data not shown). These findings suggest that both HSF1 and human iPS-derived cells can be directed to form comparable neural progenitors.
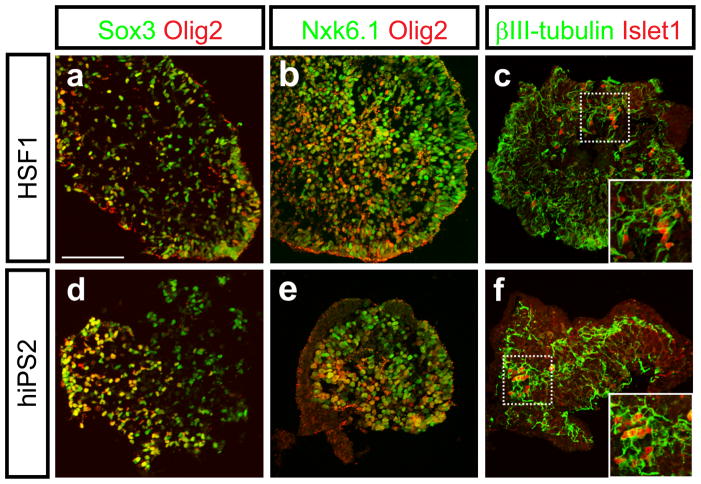
After another week in the presence of RA and Shh pathway agonists, along with neurotrophic factors known to promote motor neuron survival (CNTF 20 ng/ml, BDNF and GDNF, 10 ng/ml each), the EBs were fixed, cryosectioned, and immunostained for Sox3 and the motor neuron progenitor markers Nkx6.1 and Olig2. In the EBs that expressed markers of neural progenitors, the extent of labeling with Nkx6.1 and Olig2 antibodies was similar between HSF1 and human iPS-derived cells (Fig. 2b and e), and the percentage of Sox3+ cells that expressed Olig2 was comparable (59.1% ± 7.07% for HSF1 and 57.6 ± 9.88% for human iPS-derived cells). Further analysis was conducted with a combination of markers known to be specific to differentiated motor neurons. Within EBs that were specified towards a neural fate and expressed markers of motor neuron progenitors (Nkx6.1 and Olig2), a small but significant number of Islet1 and βIII- tubulin double-positive neurons were observed (Fig. 2c and f). The physical limitations of the EB differentiation method precluded detailed functional analysis of these cells, but these results together provide evidence that both HSF1 and human iPS cells can be induced to generate differentiated motor neurons.
Figure 2. The directed differentiation of hESC and human iPS-derived cells recapitulates the stereotypical progression associated with motor neuron formation.
After 8–10 more days in the presence of RA, Shh pathway agonists and neurotrophic factors, both HSF1 and human iPS-derived EBs contained Sox3+, Nkx6.1+ and Olig2+ motor neuron progenitors (a, b, d, e). Both HSF1 and human iPS cells were further able to produce differentiated Islet1 and βIII-tubulin-positive motor neurons within these EBs (c and f). Scale bar: a–f, 100μm.
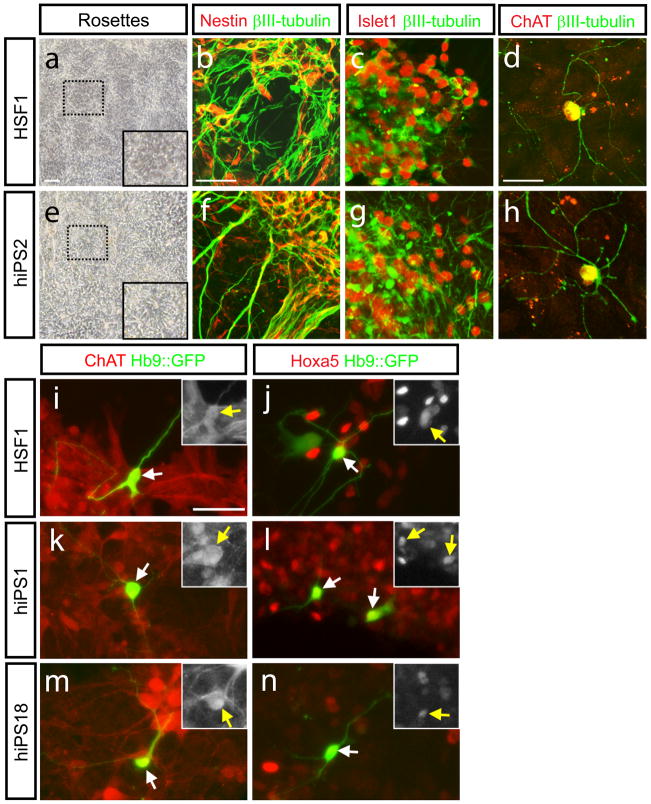
To enable a physiological characterization of these iPS-derived motor neurons, we employed another method of directed differentiation using previously described adherent conditions [6,7]. Neural rosettes generated from HSF1 and human iPS1, 2 and 18, were mechanically isolated, and then re-plated onto laminin-coated dishes in medium containing RA (1μM) and Shh (200ng/ml). After a week, neurotrophic factors were added (BDNF, CNTF, and GDNF; 10ng/ml each), the Shh concentration was lowered (50ng/ml), and the cells were allowed to differentiate for 3–5 weeks. Both HSF1 and human iPS-derived cells followed the expected course of differentiation, from Nestin-positive neuronal progenitors (Fig. 3b and f), to mature motor neurons (βIII-tubulin, Choline acetyl transferase (ChAT) and Islet1-positive, Fig. 3c–h). In both HSF1 and human iPS derived βIII-tubulin-positive cells, similar percentage of Islet1-positive cells was detected (28.2% ± 5.7% for HSF1, 33.6% ± 12% for human iPS2)(Fig. 3c and g), suggesting again that once specified to a neuronal fate, human iPS–derived cells and HSF1-derived cells are equally efficient at generating motor neurons in these conditions.
Figure 3. Neurons derived from human iPS cells and hESCs express several motor neuron marrkers.
Neural rosettes formed after 8–10 days in adherent culture (a and e). After mechanical dissection of rosettes by both HSF1 and hiPS2, Nestin-positive neural progenitors remain while differentiated neurons expressingβIII-tubulin are formed (b and f). Confocal imaging demonstrates the generation of cells double stained for definitive markers of motor neurons including βIII-tubulin and Islet1 (c and g), or βIII-tubulin and ChAT (,d and h). Late-stage differentiated neurons from were transiently transfected with a reporter indicative of Hb9 expression (Hb9::GFP; i-n). Staining with an antibody recognizing ChAT demonstrates the specificity of the reporter for mature motor neurons (i, k, m). Co-staining with antibody against Hoxa5 demonstrates a rostral cervical character of both HSF1 and hiPS-derived motor neurons (j, l, n). Insets in panels i-n show the single channel stains for either ChAT or Hoxa5 in the Hb9::GFP-positive cells indicated by the arrows. Scale bars: a and e: 200μm, 2b,c,f, and g: 70μm, 2d and h: 50μm, i-n: 50μm
To further demonstrate that this differentiation protocol generates spinal cord neurons, cells from both HSF1 and human iPS cells were also stained with markers of various regions of the spinal cord and a reporter specific for activity of Hb9 (Mnx1 or Hlxb9), which encodes for a transcription factor specifically expressed by mature motor neurons [6]. This Hb9-driven GFP reporter was transfected into HSF1 and human iPS derived cells to enable the identification and targeting of motor neurons in which Hb9 was transcriptionally active [6]. Activity of this reporter tightly correlated with markers characteristic of rostral cervical motor neurons such as Hoxa5 and ChAT in both HSF1 and hiPS-derived cells (Fig. 3)[11, 12].
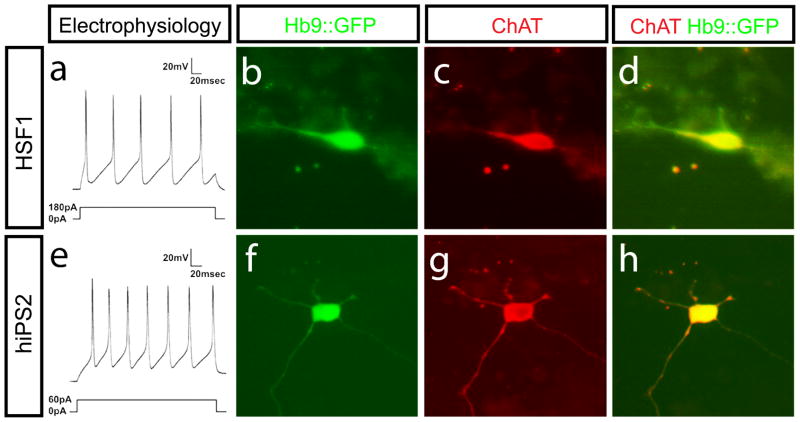
Lastly, to establish the phenotypic maturation of the human iPS-derived neurons we studied their electrophysiological properties. It is well established that the firing of repetitive action potentials in response to current injection is typical of the behavior of adult, vertebrate motor neurons [10] and that this repetitive firing develops as function of maturation [18]. The excitability of HSF1 and human iPS-derived motor neurons was assayed by whole cell patch clamping in the current clamp mode. Action potentials were recorded 48 to 62 days after plating. Upon application of current to either hESC or human iPS-derived neurons with Hb9::GFP activity, roughly half responded with single action potentials, while the other half responded with repetitive action potentials (Figure 4a and e). After recordings were made, the neurons were fixed and analyzed for Hb9::GFP expression and ChAT staining to confirm that those cells that generated a typical motor neuron response to electrical stimulation also possessed cholinergic properties (Fig 4b–h). Identical results were achieved with motor neurons derived from three independent human iPS cell lines and were indistinguishable from motor neurons derived from HSF1 (Fig. S3). Together, these results demonstrate the general feasibility of the generating electrophysiologically active motor neurons from human iPS cells.
Figure 4. Neurons derived from human iPS cells and hESCs display mature motor neuron characteristics.
Whole cell patch clamp recordings from Hb9::GFP expressing hESC and human iPS-derived neurons show repetitive firing after stimulation (a and e). Results shown are representative of recordings made from at least 20 cells derived from both hESCs and human iPS. Imaging of cells fixed after electrophysiological recordings show that these cells expressing the Hb9::GFP reporter also contained ChAT (b-d and f-h).
Discussion
A primary objective of hESC and human iPS cell technology is to be able to generate relevant cell types to enable the repair of tissue damage and in vitro modeling of human disease processes. Here, we demonstrate the successful generation of electrically active motor neurons from multiple human iPS cell lines and provide evidence that these neurons are molecularly and physiologically indistinguishable from motor neurons derived from hESCs. The generation of motor neurons from human iPS cells isolated from patients harboring ALS and SMA mutations has recently been described, though the electrophysiological activity of these motor neurons was not assessed [8, 9]. Demonstrating that human iPS cells can adopt this key hallmark of functional maturation is essential for any future application of human iPS cells in the study or treatment of motor neuron diseases. To our knowledge, our results present the first demonstration of the electrical activity of human iPS-derived neurons and further suggest the feasibility of using these cells to explore how changes in motor neuron activity contributes to the degeneration of these cells underlying these disorders [19, 20].
It remains unclear why the potential for the human iPS cell lines described here to appeared to undergo neural specification with a lower efficiency than HSF1 in these experiments. Marked variability in neuralization competence has been described among different lines of human ES cells [21], and it thus seems likely that such differences may be noted also in human iPS cells. More importantly, the data presented here demonstrate that, of the cells that were specified to become neural, human iPS-derived neural progenitors proved as competent at generating motor neurons as their HSF1-derived counterparts. These findings support the possibility that reprogrammed somatic cells might prove to be a viable alternative to embryo-derived cells in regenerative medicine. Finally, as the human iPS cells appeared to obey a normal developmental progression to mature, electrically active neurons, it seems possible that disease-specific somatic cells may be reprogrammed and utilized to model, and ultimately to treat a variety of human neurological disorders.
Supplementary Material
Acknowledgments
We thank J. Dasen, T. Edlund, T. Jessell, S. Morton, and J. Muhr for the generous gift of reagents. J.A.U. has support from the Department of Molecular and Medical Pharmacology at UCLA. B.G.N. was supported by grants from the March of Dimes Foundation, the Muscular Dystrophy Association, and the NINDS. H.I.K., S.K., M.W.P., and S.A.G. were supported by The Miriam and Sheldon Adelson Medical Research Foundation and the Adelson Program for Neural Regeneration and Repair. MWP was also supported by the David Vickter Foundation and by Robert and Louise Schwab. W.E.L. is supported by a CIRM grant (#RS1-00259-1) and a Basil O’Connor Starter Scholar Research Award from the March of Dimes.
Footnotes
Author contributions:
Karumbayaram S: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Novitch B: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support; Patterson M: collection and assembly of data, data analysis and interpretation; Umbach J: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Richter L: technical support, provision of study material; Lindgren A: collection and assembly of data; Conway A: collection and assembly of data; Clark A: collection and assembly of data, financial support; Goldman SA: data analysis and interpretation, manuscript writing, final approval of manuscript; Plath K: data analysis and interpretation, manuscript writing, final approval of manuscript; Wiedau-Pazos M: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support; Kornblum HI: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; Lowry WE: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, and financial support.
References
- 1.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 6.Singh Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Experimental neurology. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nature biotechnology. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 8.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science (New York, NY. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 9.Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2008 doi: 10.1038/nature07677. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 12.Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- 13.Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 14.De Marco Garcia NV, Jessell TM. Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity. Neuron. 2008;57:217–231. doi: 10.1016/j.neuron.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nature neuroscience. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 16.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nature biotechnology. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 18.Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. Journal of neurophysiology. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- 19.Balice-Gordon RJ, Smith DB, Goldman J, Cork LC, Shirley A, Cope TC, Pinter MJ. Functional motor unit failure precedes neuromuscular degeneration in canine motor neuron disease. Annals of neurology. 2000;47:596–605. [PubMed] [Google Scholar]
- 20.Bories C, Amendola J, Lamotte d’Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. The European journal of neuroscience. 2007;25:451–459. doi: 10.1111/j.1460-9568.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- 21.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.