Abstract
The increased demand for the lanthanides in commercial products result in increased production of lanthanide containing ores, which increases public exposure to the lanthanides, both from various commercial products and from production wastes/effluents. This work investigates lanthanide (La, Ce, Pr, Nd, Eu, Gd and Lu) binding properties of self-assembled monolayers on mesoporous silica supports (SAMMS™), that were functionalized with diphosphonic acid (DiPhos), acetamide phosphonic acid (AcPhos), propionamide phosphonic acid (Prop-Phos), and 1-hydroxy-2-pyridinone (1,2-HOPO), from natural waters (river, ground and sea waters), acid solutions (to mimic certain industrial process streams), and dialysate. The affinity, capacity, and kinetics of the lanthanide sorption, as well as regenerability of SAMMS materials were investigated. Going from the acid side over to the alkaline side, the AcPhos- and DiPhos-SAMMS maintain their outstanding affinity for lanthanides, which enable the use of the materials in the systems where the pH may fluctuate. In acid solutions, Prop-Phos- and 1,2-HOPO-SAMMS have differing affinity along the lanthanide series, suggesting their use in chromatographic lanthanide separation. Over 95% of 100 µg/L of Gd in dialysate was removed by the Prop-Phos-SAMMS after 1 min and 99% over 10 min. SAMMS can be regenerated with an acid wash (0.5 M HCl) without losing the binding properties. Thus, they have a great potential to be used as in large-scale treatment of lanthanides, lanthanide separation prior to analytical instruments, and in sorbent dialyzers for treatment of acute lanthanide poisoning.
Keywords: Lanthanides, Removal, Dialysis, Mesoporous silica, Sorbent, Water, Acid
1. Introduction
Lanthanide-based materials are gaining increasing importance, both in terms of research activity, and in terms of commercial products [1]. For example, the electroluminescent properties of various lanthanide complexes have made them popular as emitters in electroluminescent devices [2–6]. Lanthanide-based reagents have also become increasing popular in organic synthesis [7,8], as well as asymmetric catalysis [9,10]. Lanthanides are an important component of plastic fiber optic lasers [11], ionic conducting oxides (a key component of fuel cells) [12], MOCVD of oxides used in microelectronics [13], and even liquid crystals and surfactants [14].
This increased demand for the lanthanides means two things—increased production of lanthanide containing ores (i.e. mining), and increased public exposure to the lanthanides, both from various commercial products and from production wastes/effluents. Increased mining activity has been shown to result in increased rare earth releases and mobility, whether this mining is of the rare earth materials themselves [15], of other metals [16–19] or even coal [20,21]. In regions with high levels of rare earth elements (REE), elevated levels of REE are found in humans [15,22], and other organisms [23]. Metabolism and toxicity of rare earth compounds was previously reviewed [24]. Recent toxicology studies of the rare earth chlorides have shown that as a group they tend to have similar toxicities, and are similar to CdCl2, which has long been considered toxic [25].
Historically, lanthanide separations have commonly focused on purification of the raw ore (e.g. [26,27]), or separation of the lanthanides from spent nuclear fuels (e.g. [7,28]). These studies commonly focused on ligand design and solvent extraction studies (e.g. [29,30]), solid-phase extraction (e.g. [31,32]), or chromatography (e.g. [33,34]). As a general statement, these methods are not amenable to large-scale treatment of contaminated ground water or drinking water. Purification of water contaminated with lanthanides requires the ability to selectively remove the lanthanides in the presence of common, harmless ions like Ca and Mg. Therefore, it may be of value to design and build a chemically selective sorbent material, capable of selectively sequestering the lanthanides from waste streams, ground water and drinking water, without the need for any additional reagents, solvents, or treatment processes.
Self-assembled monolayers on mesoporous supports (SAMMS™) are versatile functional materials. Simply by varying the terminal functionality of the monolayer interface, it has been possible to tailor the affinity of these nanoporous sorbent materials to very effectively capture “soft” heavy metals [35–38], oxometallate anions [37,39–43], radioiodine [44,45], and cesium [46]. Of particular relevance to this discussion is the demonstrated ability of certain “flavors” of SAMMS to bind the rare earth actinides [47,48] and lanthanides [49,50]. However, it is important to recognize that the previous work focused on design and synthesis of the materials. Also sorption characterization has been done in simple laboratory buffers, and matrix effects (in particular complexing anions) are known to have a significant impact on lanthanide speciation, and therefore chemical behavior.
This manuscript summarizes our studies using these nanoporous sorbent materials to sequester lanthanides from acidic streams (to mimic industrial wastes), natural waters, and dialysate, the latter two contain strong complexing anions for lanthanides, including carbonate [51], phosphate [52], and sulfate [53]. Successful capturing of lanthanides from these matrices will provide a complete strategy for managing of lanthanides from lowering discharge levels (e.g. waste water treatment), lowering environmental exposure levels (e.g. lanthanide removal from drinking and natural waters), to treatment of lanthanide poisoning (e.g. with sorbent dialysis).
2. Experimental
2.1. Sorbent materials
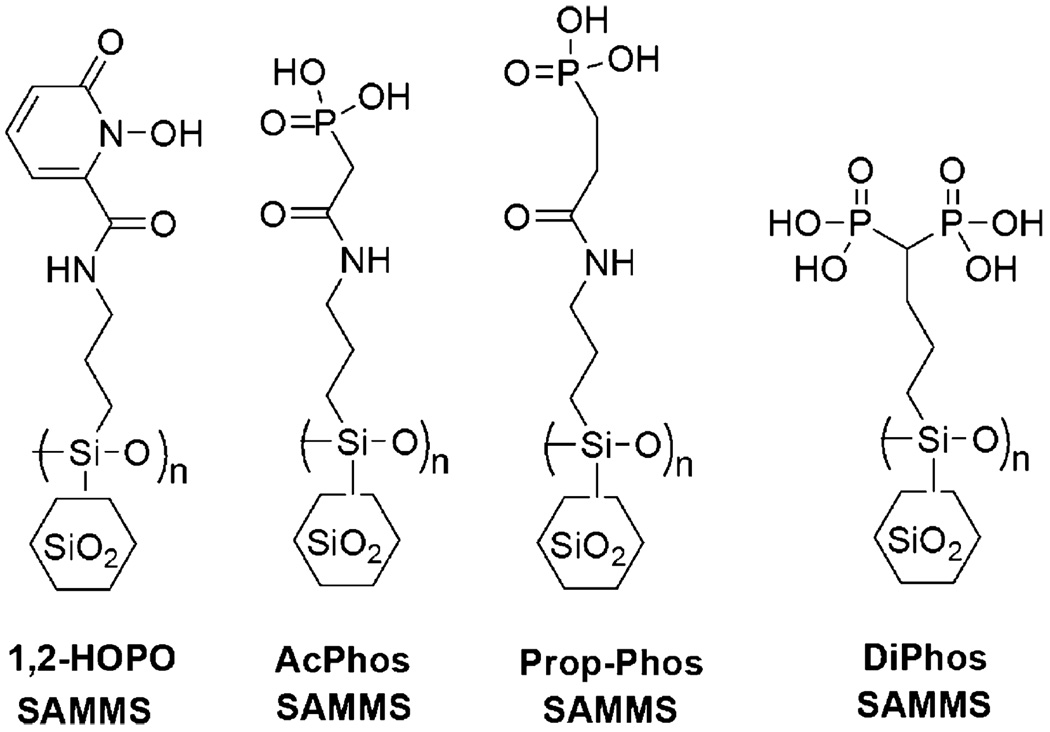
Synthesis and characterization of functional mesoporous silica materials were described elsewhere, including those functionalized with acetamide phosphonic acid (AcPhos) [49], propionamide phosphonic acid (Prop-Phos) [49], and 1-hydroxy-2-pyridinone (1,2-HOPO) [50]. The chemical structures of the SAMMS materials are presented in Fig. 1. For comparison, high surface area activated carbon (Darco KB-B, Sigma–Aldrich) were also tested along with the SAMMS materials.
Fig. 1.
Chemical structures of SAMMS with various organic groups.
2.2. Test matrices
Batch metal sorption experiments were performed with natural waters, simulated acid wastes, and dialysate. The natural waters include river water (Columbia River, Richland, WA), sea water (Sequim Bay, WA), and ground water (Hanford, WA) which were used after filtering through a 0.45 µm cellulose membrane (MF-Millipore™). Simulated acid wastes include pH-adjusted 0.1 M nitrate (0.01 M HNO3/0.09 M HNO3, pH 1.82) and 0.1 M chloride (0.01 M HCl/0.09 M NaCl, pH 2.42). The dialysate (PrismaSate®, BGK4/2.5 from Gambo Inc., Lakewood, CO) consists of 2.5 mequiv./L of Ca2+, 1.5 mequiv./L of Mg2+, 140 mequiv./L of Na+, 4 mequiv./L of K+, 113 mequiv./L of Cl−, 3 mequiv./L of lactate, 32 mequiv./L of HCO3−, 110 mg/dL of glucose, and osmolarity of 300 mOSm/L.
2.3. Kd measurements
For Kd measurements, the test solutions were spiked with trivalent lanthanides (La, Ce, Pr, Nd, Eu, Gd and Lu) to obtain 50 µg/L each. After 30 min of incubation, it was aliquoted into 4.9 mL volumes in a 20 mL polypropylene vial. The solution was then spiked with 0.1 mL of a suspension of solid sorbent and deionized distilled (DI) water at liquid per solid ratio (L/S in the unit of mL/g throughout) of 100. This resulted in a final L/S of 5000. The sample was then shaken for 2 h at 160 rpm on an orbital shaker. After 2 h, the solution was removed by filtering thru 0.20-µm syringe Nylon-membrane filters and the filtrate was kept in 1 vol.% HNO3 prior to the analysis of lanthanides. The control was performed in the same fashion but without solid sorbent. The filtrates from the controls were analyzed against the starting solutions to ensure no lanthanide precipitation. The lanthanides were analyzed using an inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7500ce, Agilent Technologies, CA). All batch experiments were performed in triplicates and the averaged values were reported.
2.4. Sorption capacity
The sorption capacity of each material for lanthanides was measured in the same fashion as with the Kd, but only a single element was used and its concentration was varied in the solution until maximum sorption capacity was obtained. This was accomplished by using a large excess of the lanthanide to the number of binding sites on the sorbent materials (e.g. 0.1–5 mg/L of lanthanides at L/S of 100,000).
2.5. Sorption kinetics
The kinetics of lanthanide sorption was performed in the same fashion as with the equilibrium studies except that 1 mL of well-mixed aliquot was removed and filtered at 1, 2, 5, 10, 30, 60 min, 1, 8, and 24 h and the initial sample volume was increased to 50 mL to minimize the change in L/S.
2.6. Regeneration
Six cycles of Gd adsorption and desorption on Prop-Phos-SAMMS were performed to assess its ability to be regenerated and its effectiveness after the regeneration. For the adsorption step, 10 mL of 230 µg/L of Gd in dialysate was passed through the bed of 0.01 g Prop-Phos-SAMMS at the flow rate of 2 mL/min and the filtrate was collected for Gd and Si analysis. For the regeneration step, 40 mL of 0.5 MHCl was passed thru the same bed at 4 mL/min and the filtrate was collected for Gd and Si analysis. The cycles were repeated for six times.
3. Results and discussion
The adsorption of lanthanides by sorbent materials was evaluated as follows.
3.1. Adsorption affinity
The affinity of a sorbent for a target species was frequently represented with the distribution coefficient (Kd) (in the unit of mL/g throughout), which is simply a mass-weighted partition coefficient between the solid phase and the liquid supernatant phase as follows:
| (1) |
where Co and Cf are the initial and final concentrations in the solution of the target species determined by ICP-MS, V is the solution volume in mL, and M is the mass in gram of the sorbent. In general, Kd values of ~103 mL/g are considered good and those above 5 × 104 mL/g are outstanding [47]. Higher affinities (Kd values) are absolutely keys for the capture of trace level constituents. Dynamic affinities (large differences in Kd with changing analyte or conditions) are the basis for selective separations.
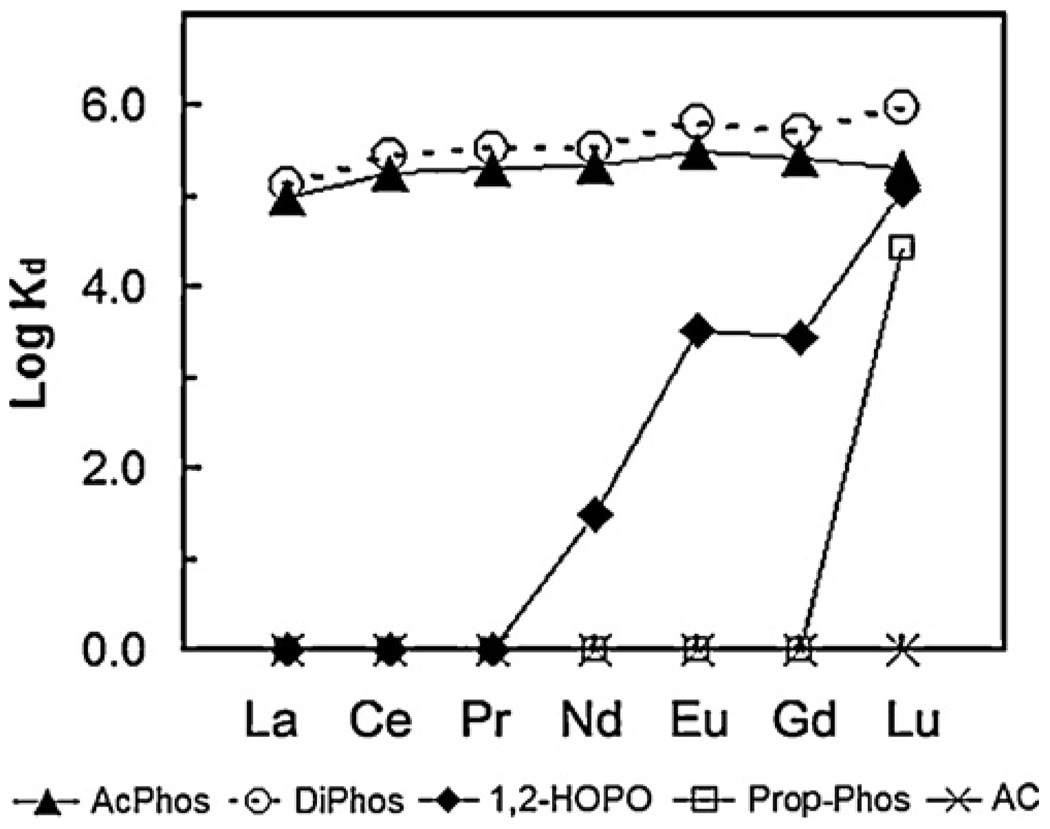
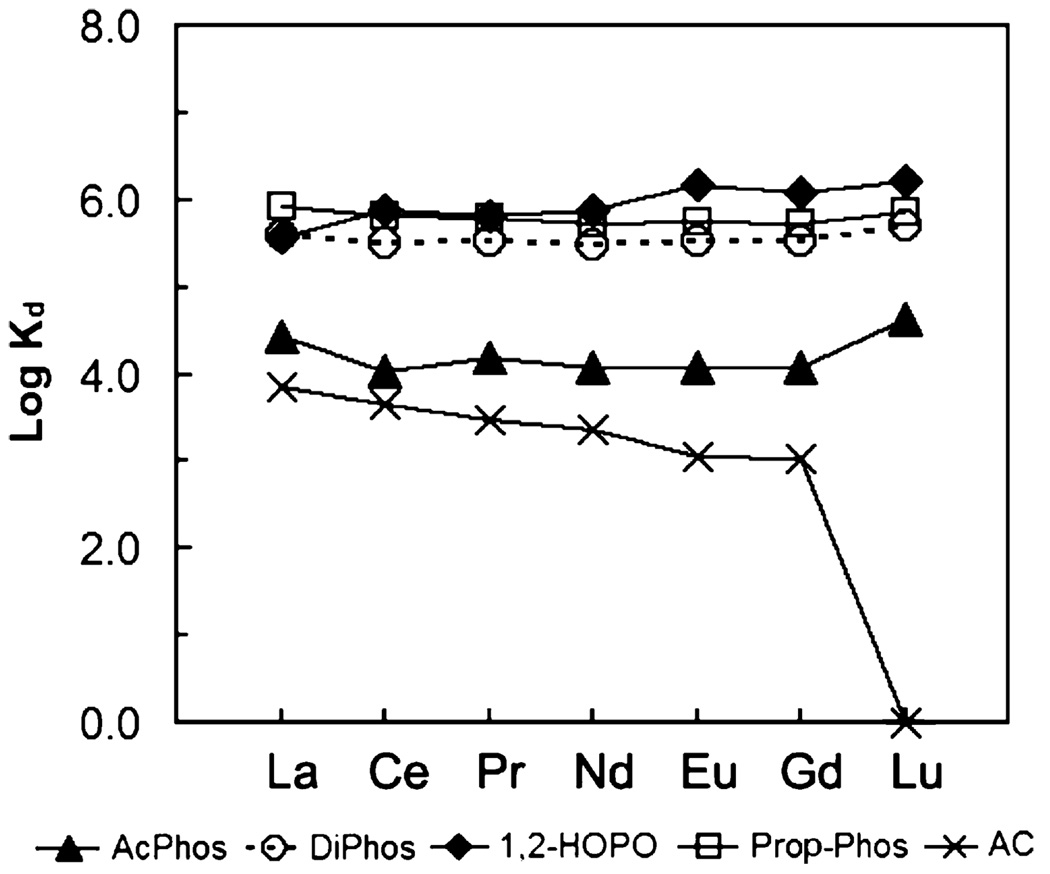
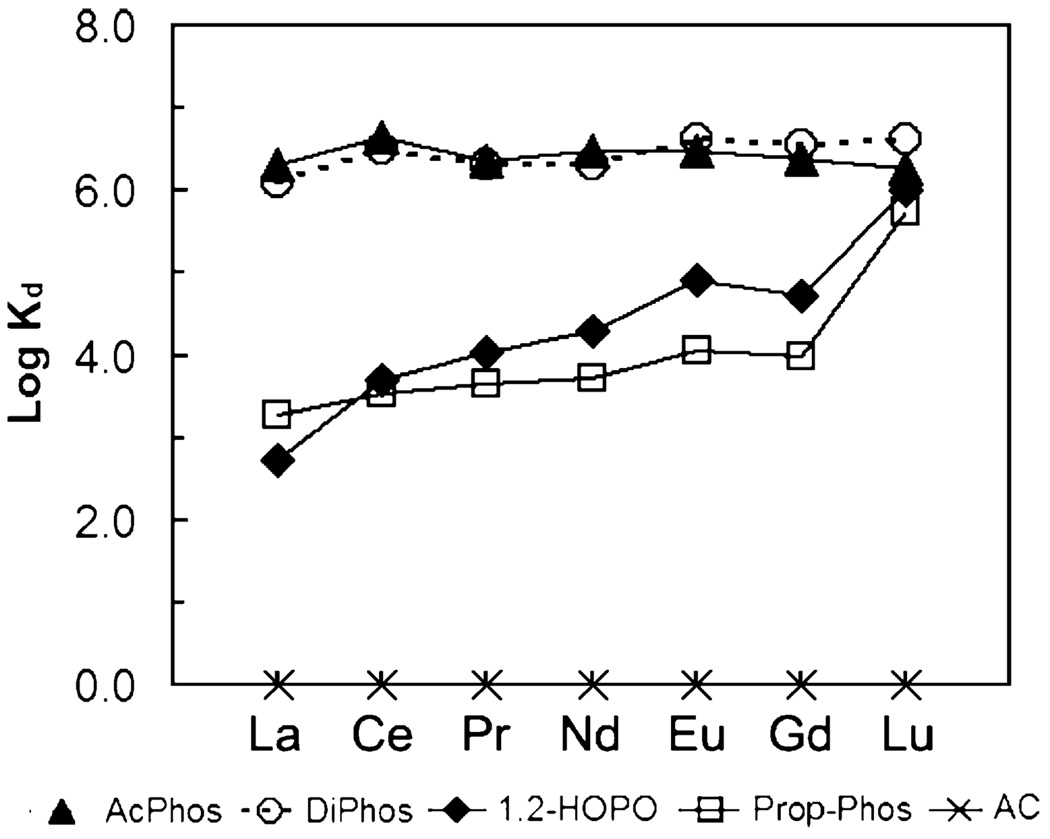
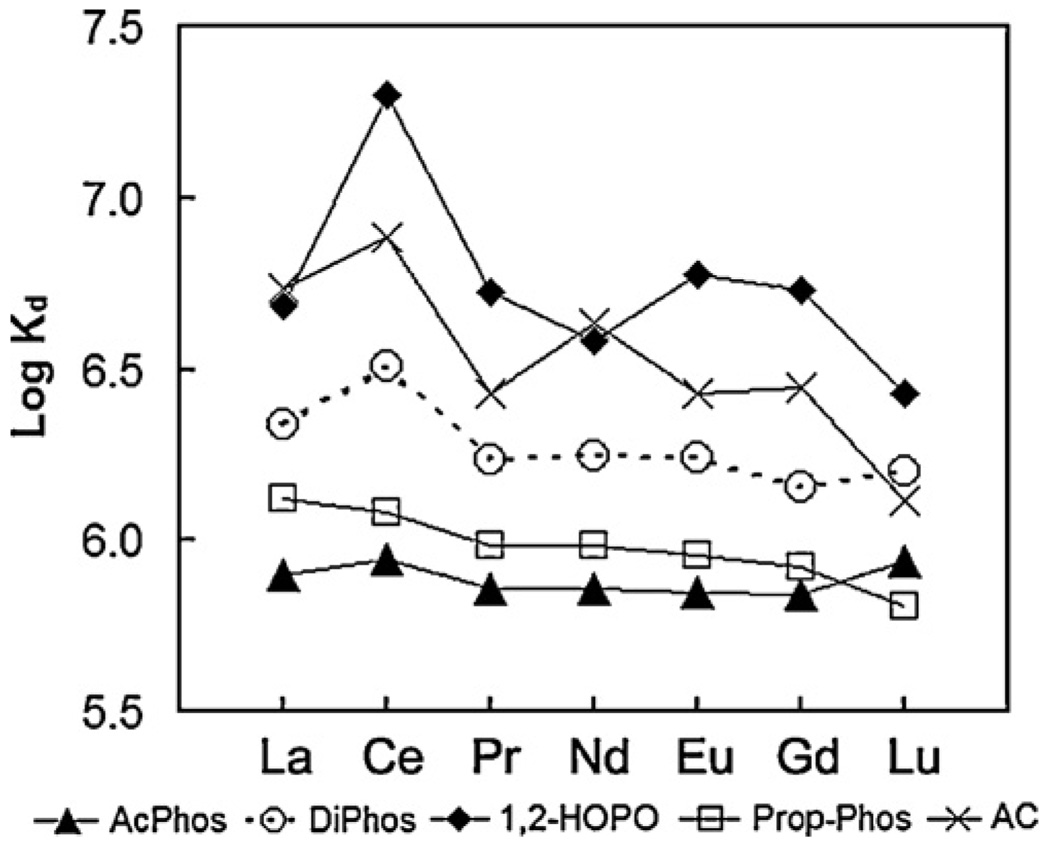
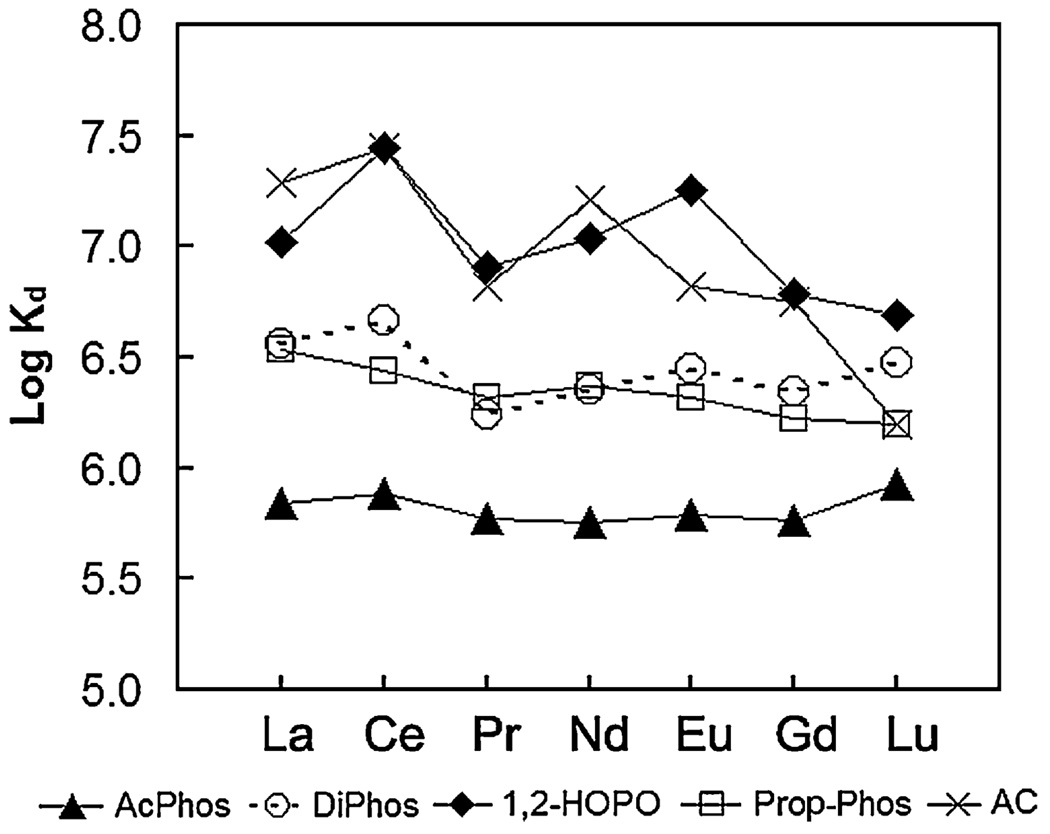
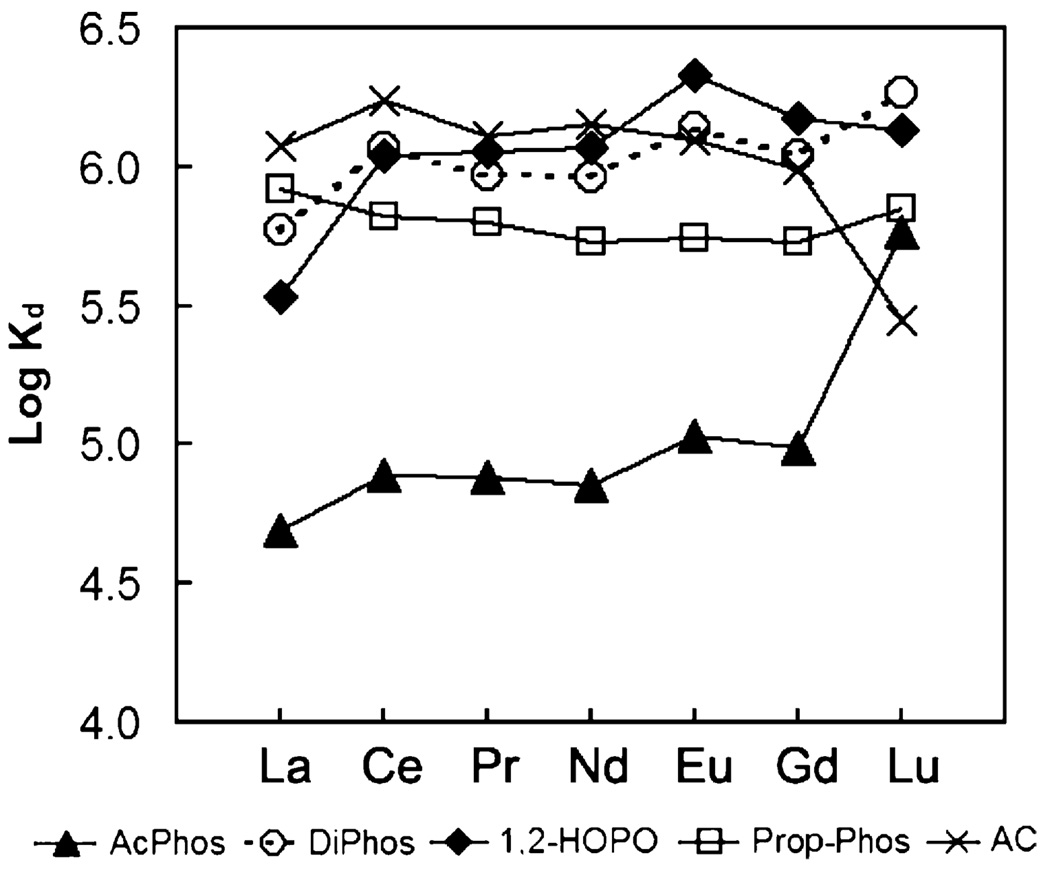
Fig. 2–Fig 7 show the distribution coefficients of lanthanides on various sorbent materials, measured in 0.1 M NO3− (pH 1.82), 0.1 M Cl− (pH 2.42), filtered river water, filtered ground water, filtered sea-water, and dialysate, respectively. The specific behavior of SAMMS in various matrices can be explained as follows.
Fig. 2.
Distribution coefficients (Kd) of lanthanides on various sorbents measured in 0.1 M HNO3/NaNO3 (pH 1.82), initial [lanthanide] of ~50 ppb (each), L/S of 5000 mL/g.
Fig. 7.
Distribution coefficients (Kd) of lanthanides on various sorbents measured in dialysate (pH 8.33), initial [lanthanide] of ~50 ppb (each), L/S of 5000 mL/g.
3.2. Kd in acid solutions
Industrially, lanthanides are commonly processed and handled in acid solutions, and so we started off by studying these sorbents in acidic solutions to address any effluents (or waste) arising from these process streams. In both acidic nitrate and chloride systems, the AcPhos-SAMMS and DiPhos-SAMMS outperformed 1,2-HOPO-SAMMS and Prop-Phos-SAMMS due to the higher acidity of the AcPhos and DiPhos ligands. Both materials can effectively bind all lanthanides (Kd ~ 105 to 106) covering the entire range of ionic radii from La to Lu. On the other hand, phenols and carboxylic acids (the two main classes of ligands in activated carbon, AC) are fully protonated in the acid solutions, resulting in virtually no affinity (Kd ~ 0) of AC for all lanthanides. Being weaker ligands in acid solutions, the 1,2-HOPO and Prop-Phos ligands may also be affected by competing anions in the solutions. This is evidenced with nitrates which form stronger complexes with lighter lanthanides (La to Gd) than the heavier lanthanides like Lu [54], and bind less favorably to SAMMS. As a result, the Kd of lanthanides on 1,2-HOPO-SAMMS and Prop-Phos-SAMMS increased along the lanthanide series and was highest with Lu which forms the weakest complexes with nitrates (Fig. 2). The same holds true with chloride system (Fig. 3) but to a lesser extent since chloride is a much weaker ligand for lanthanides than is nitrate. The differing affinity (Kd) for differing lanthanides of 1,2-HOPO-SAMMS and Prop-Phos-SAMMS suggests that they can be used to chromatographically separate lanthanides for both analytical and larger scale lanthanide separation processes.
Fig. 3.
Distribution coefficients (Kd) of lanthanides on various sorbents measured in 0.1M HCl/NaCl (pH 2.42), initial [lanthanide] of ~50 ppb (each), L/S of 5000 mL/g.
3.3. Kd in natural waters
All sorbents (SAMMS and AC) offer large Kd values (>50,000) for all lanthanides, suggesting that they are outstanding for capture of lanthanides from natural waters. Note that the Kd in natural waters was measured in filtered waters. When river waters are not filtered, the humic substances often present in river waters are readily bond to activated carbon [55] and may cause fouling. As a result, the activated carbon will become less effective metal binder. On the other hand, fouling of SAMMS materials have not been observed even in protein-rich samples like human plasma and blood (unpublished data).
For light lanthanides (La to Gd), in filtered river water (FRW) the order of binding affinity is 1,2-HOPO~AC > DiPhos > Prop-Phos >AcPhos (see the data in Fig. 4); in filtered ground water (FGW) the order is 1,2-HOPO~AC > DiPhos~Prop-Phos >AcPhos (see Fig. 5); and in filtered sea water (FSW) the observed binding affinity is 1,2-HOPO~AC~DiPhos~Prop-Phos >AcPhos (as summarized in Fig. 6). In these “natural” aqueous environments the affinities of the lighter lanthanides clearly show a significant dependence upon the sorbent ligand chemistry. For the heavy lanthanide Lu, the Kd of the five sorbents are similar with 1,2-HOPO- and DiPhos-SAMMS having the highest Kd. For all sorbents, Kd values in the three natural waters decreases as follows: river water ~ ground water > sea water, clearly showing the negative effect of ionic strength (which is highest in sea water) on the sorption properties of the materials.
Fig. 4.
Distribution coefficients (Kd) of lanthanides on various sorbents measured in river water (pH 7.98), initial [lanthanide] of ~50 ppb (each), L/S of 5000 mL/g.
Fig. 5.
Distribution coefficients (Kd) of lanthanides on various sorbents measured in ground water (pH 8.07), initial [lanthanide] of ~50 ppb (each), L/S of 5000 mL/g.
Fig. 6.
Distribution coefficients (Kd) of lanthanides on various sorbents measured in sea water (pH 7.68), initial [lanthanide] of ~50 ppb (each), L/S of 5000 mL/g.
3.4. Kd in dialysate
In light of severe lanthanide toxicity from the recent studies [24,25], in addition to their potential use for limiting lanthanide exposure (by removal of lanthanides from acidic streams and natural waters), SAMMS materials having high affinity and selectivity for lanthanides may be used in sorbent dialysis for treatment of lanthanide poisoning. In conventional hemodialysis, the removal rate of a toxin is determined by the concentration gradient of the toxin as well as its diffusion coefficient. The normal dialysis duration is 3 h. If the dialyzer is used with sorbent to remove the toxins that have been cleared into the dialysate, the dialysate can be recycled, which is an important step toward developing of personal and portable dialysis devices. The current state-of-the-art sorbent dialyzers still rely on activated carbon for cation removals, which is not yet optimal for lanthanide clearance. We have found that in dialysate (pH 8.33), the affinity of lanthanides for sorbents decreased as follows: 1,2-HOPO~Prop-Phos~DiPhos >AcPhos >AC. In carbonate-rich dialysate (pH 8.33), lanthanides (noted as HLn for heavy lanthanides and LLn for light lanthanides) are likely be present as follows [56]: HLn(CO3)2− > LLn(CO3)2− > LLnCO3+ > HLnCO3+≫Ln3+. Unlike ligands on SAMMS, which bind to lanthanides via strong multidentate chelation reaction, the ligands of the activated carbon (e.g. car-boxylates, phenols, etc.) undergo a less ordered, more random coordination with anionic lanthanide–carbonate complexes. When compared with filtered sea water (Fig. 6), which also has as high an ionic strength as the dialysate, the bicarbonate, lactate and glucose components of the dialysate did not significantly affect the binding of all lanthanides on the 1,2-HOPO-, Prop-Phos- and DiPhos-SAMMS: the Kd ~ 106 in both FSW and dialysate. However, for AC, the Kd drops from 106 to 103.5 for light Lanthanides and to 0 for Lu. This drop in binding affinity is felt to be due to less effective binding of the lanthanide–carbonate complexes by AC, especially for Lu which forms stronger dicarbonato complexes than the lighter lanthanides [56,57].
In short, the greatest advantage of SAMMS over activated carbon is in challenging matrices such as in acidic streams (e.g. industrial waste effluents, nuclear wastes, and mine waters), in high-organic-content natural waters where fouling of AC is severe, and in high carbonate solutions (e.g. dialysate). We further explore the properties of SAMMS including adsorption kinetics and capacity in the challenging matrices.
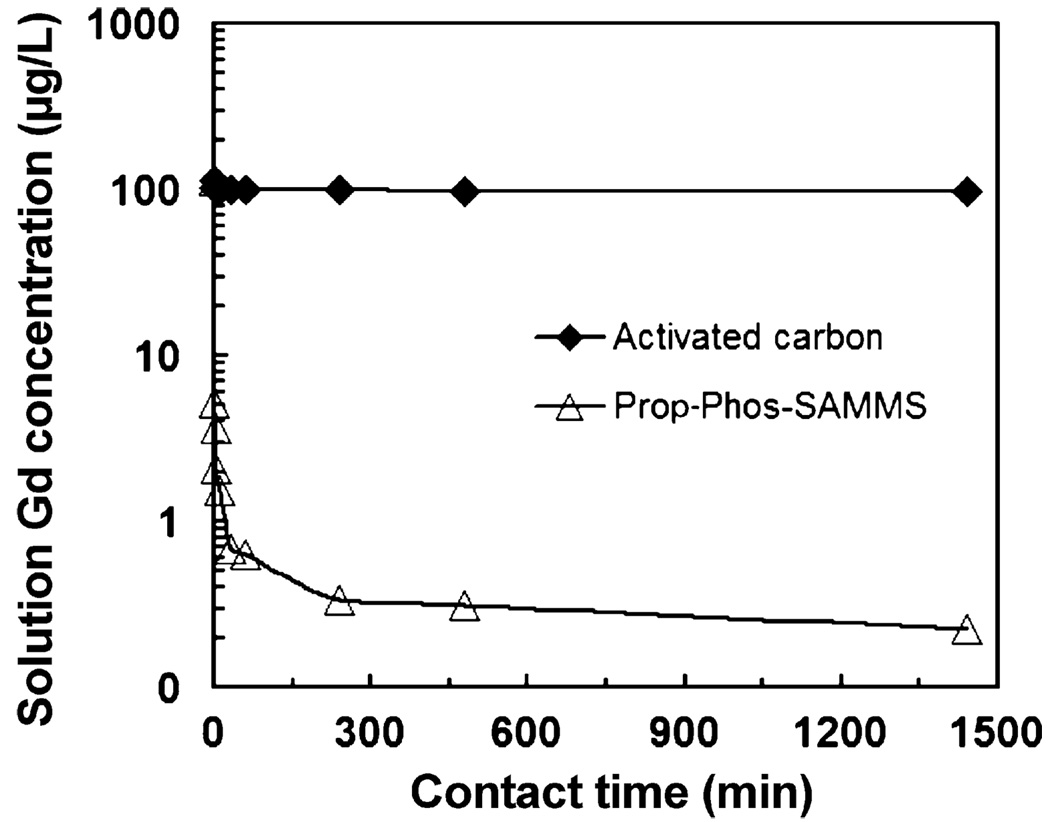
3.5. Adsorption kinetics
Fast sorption kinetics is highly beneficial for deployment of sorbent materials both in large-scale water treatment and sorbent dialysis devices. Fig. 8 shows the sorption kinetics of Gd in dialysate measured on Prop-Phos-SAMMS and activated carbon. Even in a dialysate containing high concentration of chloride, carbonate, glucose and lactate, over 95% of 100 ppb Gd was removed by the Prop-Phos-SAMMS after 1 min (and 99% after 10 min). The fast kinetics is similar to what previously reported for plutonium in carbonate-free matrices on Prop-Phos-SAMMS [47] and 1,2-HOPO-SAMMS [48]. This rapid sorption rate is owed to rigid structure and suitable pore sizes of the SAMMS, which allow easy access of Gd (likely present as Gd-carbonate complexes) to the binding sites inside the pores. From 4 to 24 h of contact, the extent of sorption remains steady, suggesting no significant degradation of the materials in the dialysate. Note that due to the limited affinity of activated carbon for Gd in dialysate (Fig. 7), only ~ 15% of Gd could be removed by the activated carbon after 24 h.
Fig. 8.
Adsorption kinetics of Gd on SAMMS and activated carbon in dialysate (pH 8.33), initial [Gd] of ~100 ppb, L/S of ~5000 mL/g.
3.6. Adsorption capacity
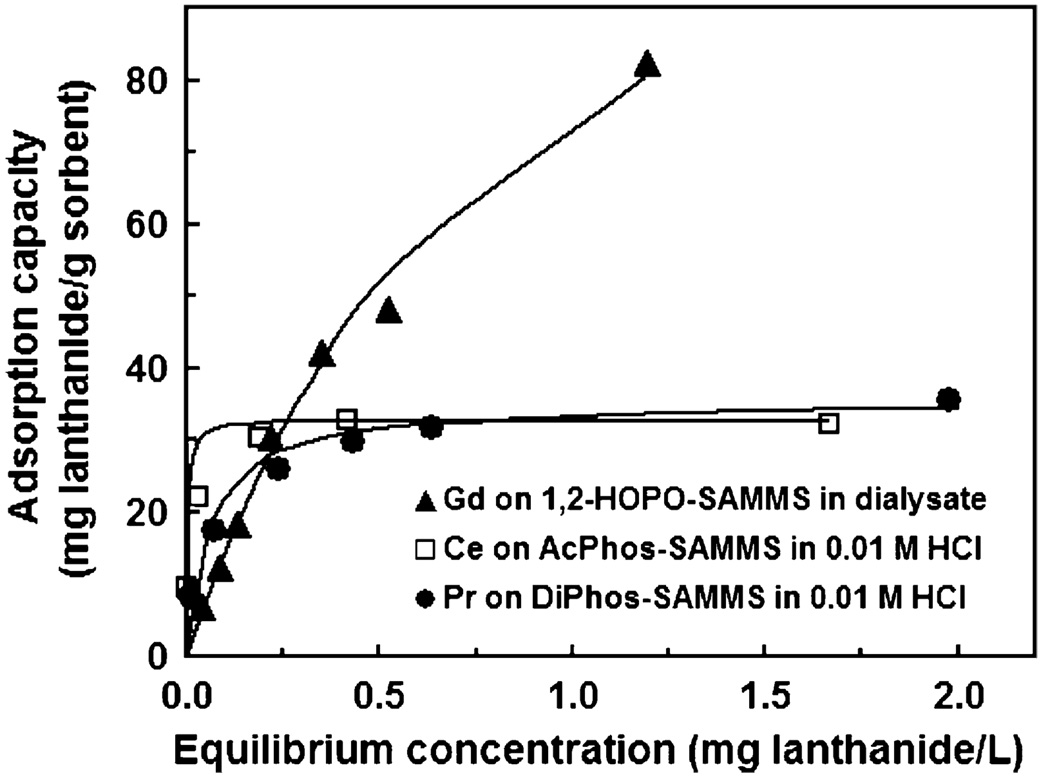
Fig. 9 shows the adsorption isotherms of Ce and Pr in 0.01 M HCl (pH 2.4) measured on AcPhos-SAMMS and DiPhos-SAMMS, respectively. Also shown is the adsorption isotherm of Gd on 1,2-HOPO-SAMMS in dialysate. These materials were selected for the isotherm measurements because of their high affinity (Kd) for the lanthanides in these matrices. All isotherms were measured at L/S of 105 mL/g. All three data sets are represented well by Langmuir adsorption model (R2 > 0.99) suggesting monolayer adsorption without precipitation of the lanthanides out of the solutions at these conditions. The Langmuir model estimates the maximum sorption capacity in 0.01M HCl to be 35.5 mg Pr/g DiPhos-SAMMS, 33.0 mg Ce/g AcPhos-SAMMS, and in dialysate to be 135 mg Gd/g 1,2-HOPO-SAMMS.
Fig. 9.
Adsorption isotherms of Ce, Pr, and Gd on SAMMS materials measured in 0.01 M HCl for Ce and Pr and dialysate for Gd at L/S of 105 mL/g.
3.7. Regeneration
After capturing lanthanides, the ability to release lanthanides and regenerate the sorbent materials is important for many reasons: it allows the cost-effective use of the materials either in field applications or devices (e.g. in sorbent dialyzers) and it enables the preconcentration of lanthanides from large sample volume into a small specimen prior to analytical instruments in order to improve the detection limits or the separation of the lanthanide species. The smaller Kd values in acidic solutions compared to in neutral solutions suggest that SAMMS can be regenerated by a pH swing (i.e. an acid wash). We tested the hypothesis by passing 0.5 M HCl to strip Gd adsorbed on Prop-Phos-SAMMS and then re-exposing the SAMMS to fresh Gd in dialysate and measuring the adsorption and desorption of Gd per gram of SAMMS. Results shown in Table 1 suggest that rapid acid washing with 0.5 M HCl (at 4 mL/min on 0.01 g bed) could remove from 84 to 93% of adsorbed Gd. Longer contact time between the acid and SAMMS should result in near-complete desorption. Repeating the cycle 6 times revealed no loss in binding affinity and the extent of Gd sorption remained the same. This easy and efficient regeneration could make Prop-Phos-SAMMS a viable strategy for many types of selective lanthanide separation needs, ranging from industrial processing to medical dialysis.
Table 1.
Adsorption and desorption of Gd on Prop-Phos-SAMMS.
| Cycle | mg Gd adsorbed/ga | mg Gd desorbed/gb | Recovery (%) |
|---|---|---|---|
| 1 | 0.227 | 0.195 | 86.0 |
| 2 | 0.227 | 0.207 | 91.2 |
| 3 | 0.230 | 0.194 | 84.7 |
| 4 | 0.229 | 0.213 | 93.2 |
| 5 | 0.230 | 0.199 | 86.1 |
| 6 | 0.228 | 0.191 | 83.5 |
10mL of 230 ppb Gd3+ in dialysate (pH 8.33) passing through 0.01 g SAMMS at 2 mL/min.
40 mL of 0.5 M HCl passing through 0.01 g SAMMS at 4 mL/min.
4. Conclusion
This work investigates lanthanide binding ability of nanoporous silica materials which may potentially lead to various applications associated with lanthanide management strategies from reducing their environmental exposure levels, their monitoring, to the treatment of acute lanthanide poisoning. AcPhos- and DiPhos-SAMMS not only can remove lanthanides in acidic streams (where they were originally designed to operate), but they are also very effective in alkaline solutions consisting of complexing anions (e.g. natural waters). This allows flexible use of the materials in systems where the pH may fluctuate (e.g. chemical process and mining effluents). Phos-Phos- and 1,2-HOPO-SAMMS have differing affinities along the lanthanide series, thus they may be used for chromatographic lanthanide separation (e.g. for collection of valuable lanthanides or for their preconcentration prior to analytical devices). The two SAMMS materials are also ideal for regenerative sorbent dialysis since they offer high affinity for lanthanides in dialysate, while allowing regeneration of the materials using acid wash.
Acknowledgements
The work was partially supported by Laboratory Directed Research and Development (LDRD) program at PNNL, National Institute of Environmental Health Sciences (NIEHS), grant# R21 ES015620, and National Institute of Allergy and Infectious Diseases (NIAID), grant# R01 AIO74064. A portion of the research was performed using EMSL, a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research and located at PNNL. The authors thank Jarupa Kanlayanatham, Dr. Worapon Kiatkittipong, Dr. Joongjai Panpranot, Dr. Daniel J. Gaspar, Dr. Charles Timchalk, Dr. William J.Weber, and Dr. Karla Thrall for their contributions.
References
- 1.The entire issue of Chem. Rev. 2002;102(6):1805–2476. was dedicated to lanthanide complexes, their chemistry, and applications. [Google Scholar]
- 2.Evans RC, Douglas P, Winscom CJ. Coordination complexes exhibiting room-temperature phosphorescence: evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord. Chem. Rev. 2006;250:2093–2126. [Google Scholar]
- 3.Gawryszewska P, Sokolnicki J, Legendziewicz J. Photophysics and structure of selected lanthanide compounds. Coord. Chem. Rev. 2005;249:2489–2509. [Google Scholar]
- 4.Kalinowski J, Mezyk J, Meinardi F, Tubino R, Cocchi M, Virgili D. Electric field and charge induced quenching of luminescence in electroluminescent emitters based on lanthanide complexes. Chem. Phys. Lett. 2008;453:82–86. [Google Scholar]
- 5.Yu JB, Zhou L, Zhang HJ, Zheng YX, Li HR, Deng RP, Peng ZP, Li ZF. Efficient electroluminescence from newlanthanide (Eu3+, Sm3+) complexes. Inorg. Chem. 2005;44:1611–1618. doi: 10.1021/ic0485561. [DOI] [PubMed] [Google Scholar]
- 6.Zheng XL, Liu Y, Pan M, Lu XQ, Zhang JY, Zhao CY, Tong YX, Su CY. Bright blue-emitting Ce3+ complexes with encapsulating polybenzimidazole tripodal ligands as potential electroluminescent devices. Angew. Chem. Int. Ed. 2007;46:7399–7403. doi: 10.1002/anie.200702401. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T. An assessment of multistage counter current extraction of TRU from spent molten salt into liquid metal. II. Effect of stage efficiency, scrub stage, and TRU concentration. J. Nucl. Sci. Technol. 2008;45:79–83. [Google Scholar]
- 8.Molander GA, Romero JAC. Lanthanocene catalysts in selective organic synthesis. Chem. Rev. 2002;102:2161–2185. doi: 10.1021/cr010291+. [DOI] [PubMed] [Google Scholar]
- 9.Inanaga J, Furuno H, Hayano T. Asymmetric catalysis and amplification with chiral lanthanide complexes. Chem. Rev. 2002;102:2211–2225. doi: 10.1021/cr010444t. [DOI] [PubMed] [Google Scholar]
- 10.Shibasaki M, Yoshikawa N. Lanthanide complexes in multifunctional asymmetric catalysis. Chem. Rev. 2002;102:2187–2209. doi: 10.1021/cr010297z. [DOI] [PubMed] [Google Scholar]
- 11.Kuriki K, Koike Y, Okamoto Y. Plastic optical fiber lasers and amplifiers containing lanthanide complexes. Chem. Rev. 2002;102:2347–2356. doi: 10.1021/cr010309g. [DOI] [PubMed] [Google Scholar]
- 12.Adachi G-Y, Imanaka N, Tamura S. Ionic conducting lanthanide oxides. Chem. Rev. 2002;102:2405–2430. doi: 10.1021/cr0103064. [DOI] [PubMed] [Google Scholar]
- 13.Jones AC, Aspinall HC, Chalker PR. Molecular design of improved precursors for the MOCVD of oxides used in microelectronics. Surf. Coat. Technol. 2007;201:9046–9054. [Google Scholar]
- 14.Binnemans K, Gorller-Walrand C. Lanthanide-containing liquid crystals and surfactants. Chem. Rev. 2002;102:2303–2345. doi: 10.1021/cr010287y. [DOI] [PubMed] [Google Scholar]
- 15.Tong S-L, Zhu W-Z, Gao Z-H, Meng Y-X, Peng R-L, Lu G-C. Distribution characteristics of rare earth elements in children's scalp hair from a rare earths mining area in southern China. J. Environ. Sci. Health A-Toxic/Hazard. Subst. Environ. Eng. 2004;39:2517–2532. doi: 10.1081/ese-200026332. [DOI] [PubMed] [Google Scholar]
- 16.Bechtel A, Ghazi AM, Elliott WC, Oszczepalski S. The occurrences of the rare earth elements and the platinum group elements in relation to base metal zoning in the vicinity of Rote Faule in the Kupferschiefer of Poland. Appl. Geochem. 2001;16:375–386. [Google Scholar]
- 17.Gammons CH, Wood SA, Jonas JP, Madison JP. Geochemistry of the rare-earth elements and uranium in the acidic Berkeley Pit lake, Butte, Montana. Chem. Geol. 2003;198:269–288. [Google Scholar]
- 18.Protano G, Riccobono F. High contents of rare earth elements (REES) in stream waters of a Cu-Pb-Zn mining area. Environ. Pollut. 2002;117:499–514. doi: 10.1016/s0269-7491(01)00173-7. [DOI] [PubMed] [Google Scholar]
- 19.Shumilin E, Rodríguez-Figueroa G, Sapozhnikov D. Lanthanide contamination and strong positive europium anomalies in the surface Sediments of the Santa Rosalia copper mining region, Baja California Peninsula, Mexico. Bull. Environ. Contam. Toxicol. 2005;75:308–315. doi: 10.1007/s00128-005-0754-4. [DOI] [PubMed] [Google Scholar]
- 20.Cravotta CA. Dissolved metals and associated constituents in abandoned coal-mine discharges Pennsylvania, USA. Part 1. Constituent quantities and correlations. Appl. Geochem. 2008;23:166–202. [Google Scholar]
- 21.Hower JC, Ruppert LF, Eble CF. Lanthanide, yttrium, and zirconium anomalies in the Fire Clay coal bed, Eastern Kentucky. Int. J. Coal Geol. 1999;39:141–153. [Google Scholar]
- 22.Zhang H, Feng J, Zhu WFL, Liu C, Xu S, Shao P, Wu D, Yang W, Gu J. Chronic toxicity of rare-earth elements on human beings—Implications of blood biochemical indices in REE-high regions, South Jiangxi. Biol. Trace Elem. Res. 2000;73:1–17. doi: 10.1385/BTER:73:1:1. [DOI] [PubMed] [Google Scholar]
- 23.Weltje L, Heidenreich H, Zhu WZ, Wolterbeek HT, Korhammer S, de Goeij JJM, Markert B. Lanthanide concentrations in freshwater plants and molluscs, related to those in surface water, pore water and sediment. A case study in The Netherlands. Sci. Total Environ. 2002;286:191–214. doi: 10.1016/s0048-9697(01)00978-0. [DOI] [PubMed] [Google Scholar]
- 24.Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds, Environ. Health Perspect. 1996;104:85–95. doi: 10.1289/ehp.96104s185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipi R, Nesmerak K, Rucki M, Roth Z, Hanzlikova I, Tichy M. Acute toxicity of rare earth elements and their compounds. Chem. Listy. 2007;101:793–798. [Google Scholar]
- 26.Awadalla FT, Habashi F. Recovery of uranium and lanthanides from calcium hydrogen phosphate [Ca(H2PO4)2]-calcium nitrate-water and Ca(H2PO4)2-calcium chloride-water systems. Ind. Eng. Chem. Res. 1989;28:1101–1103. [Google Scholar]
- 27.Rabie KA. A group separation and purification of Sm, Eu and Gd from Egyptian beach monazite mineral using solvent extraction. Hydrometallurgy. 2007;85:81–86. [Google Scholar]
- 28.Marsh SF. Separation of lanthanide fission products from nuclear fuels by extraction chromatography and cation exchange for isotope dilution mass spectrometric analysis. Anal. Chem. 1967;39:641–645. [Google Scholar]
- 29.Moore FL. Improved extraction method for isolation of trivalent actinide-lanthanide elements from nitrate solutions. Anal. Chem. 1966;38:510–512. [Google Scholar]
- 30.Yamada E, Freiser H. Mixed ligand chelate extraction of lanthanide ions in systems involving 7-(1-vinyl-3,3,6,6-tetramethylhexyl)-8-quinolinol, 8-quinolinol, and 1,10-phenanthroline. Anal. Chem. 1981;53:2115–2117. [Google Scholar]
- 31.Dutta S, Das AK. Separation of selected 4f and 5f metals by solid phase extraction: a review. J. Indian Chem. Soc. 2008;85:9–21. [Google Scholar]
- 32.Hubicka H. Investigation of polyacrylate anion-exchangers for separation of rare earth element complexes with EDTA. J. Rare Earths. 2002;20:31–35. [Google Scholar]
- 33.Campbell DO, Buxton SR. Rapid ion exchange separations. Chromatographic lanthanide separations using a high-pressure ion exchange method. Ind. Eng. Chem. Proc. Des. Dev. 1970;9:89–94. [Google Scholar]
- 34.Filer TD. Separation of the trivalent actinides from the lanthanides by extraction chromatography. Anal. Chem. 1974;46:608–610. [Google Scholar]
- 35.Chen X-B, Feng X-D, Liu J, Fryxell GE, Gong M-L. Mercury separation and immobilization using self-assembled monolayers on mesoporous supports (SAMMS) Sep. Sci. Technol. 1999;34:1121–1132. [Google Scholar]
- 36.Kemner KM, Feng X, Liu J, Fryxell GE, Wang L-Q, Kim AY, Gong M, Mattigod SV. Investigation of the local chemical interactions between Hg and self assembled monolayers on mesoporous supports. J. Synchrot. Radiat. 1999;6:633–635. doi: 10.1107/S090904959801560X. [DOI] [PubMed] [Google Scholar]
- 37.Fryxell GE, Liu J, Hauser TA, Nie Z, Ferris KF, Mattigod SV, Gong M, Hallen RT. Design and synthesis of selective mesoporous anion traps. Chem. Mater. 1999;11:2148–2154. [Google Scholar]
- 38.Mattigod SV, Fryxell GE. A thiol-functionalized nanoporous silica sorbent for removal of mercury from actual industrial waste. In: Fryxell GE, Cao G, editors. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors. Imperial College Press; 2007. pp. 275–284. [Google Scholar]
- 39.Kelly SD, Kemner KM, Fryxell GE, Liu J, Mattigod SV, Ferris KF. An X-ray absorption fine structure spectroscopy study of the interactions between contaminant tetrahedral anions to self-assembled monolayers on mesoporous supports. J. Phys. Chem. B. 2001;105:6337–6346. [Google Scholar]
- 40.Yoshitake H. Functionalization of periodic mesoporous silica and its application to the adsorption of toxic anions. In: Fryxell GE, Cao G, editors. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors. Imperial College Press; 2007. pp. 241–274. [Google Scholar]
- 41.Yoshitake H, Koiso E, Tatsumi T, Horie H, Yoshimura H. Preparation and characterization of polyamine-functionalized mesoporous silica. Chem. Lett. 2004;33:872–873. [Google Scholar]
- 42.Yoshitake H, Yokoi T, Tatsumi T. Adsorption of chromate and arsenate by amino-functionalized MCM-41 and SBA-1. Chem. Mater. 2002;14:4603–4610. [Google Scholar]
- 43.Yoshitake H, Yokoi T, Tatsumi T. Adsorption behavior of arsenate at transition metal cations captured by amino-functionalized mesoporous silicas. Chem. Mater. 2003;15:1713–1721. [Google Scholar]
- 44.Mattigod SV, Fryxell GE, Parker KE. Functionalized nanoporous sorbents for adsorption of radioiodine from groundwater and waste glass leachates. In: Fryxell GE, Cao G, editors. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors. Imperial College Press; 2007. pp. 111–122. [Google Scholar]
- 45.Mattigod SV, Fryxell GE, Serne RJ, Parker KE, Mann FM. Evaluation of novel getters for adsorption of radioiodine from groundwater and waste glass leachates. Radiochim. Acta. 2003;91:539–545. [Google Scholar]
- 46.Lin Y, Fryxell GE, Wu H, Engelhard M. Selective sorption of cesium using self-assembled monolayers on mesoporous supports (SAMMS) Environ. Sci. Technol. 2001;35:3962–3966. doi: 10.1021/es010710k. [DOI] [PubMed] [Google Scholar]
- 47.Fryxell GE, Lin Y, Fiskum S, Birnbaum JC, Wu H, Kemner K, Kelly S. Actinide sequestration using self-assembled monolayers on mesoporous supports. Environ. Sci. Technol. 2005;39:1324–1331. doi: 10.1021/es049201j. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y, Fiskum SK, Yantasee W, Wu H, Mattigod SV, Vorpagel E, Fryxell GE, Raymond KN, Xu J. Incorporation of hydroxypyridinone ligands into self-assembled monolayers on mesoporous supports for selective actinide sequestration. Environ. Sci. Technol. 2005;39:1332–1337. doi: 10.1021/es049169t. [DOI] [PubMed] [Google Scholar]
- 49.Fryxell GE, Wu H, Lin Y, Shaw WJ, Birnbaum JC, Linehan JC, Nie Z, Kemner K, Kelly S. Lanthanide selective sorbents: self-assembled monolayers on mesoporous supports (SAMMS) J. Mater. Chem. 2004;14:3356–3363. [Google Scholar]
- 50.Yantasee W, Fryxell GE, Lin Y, Wu H, Raymond KN, Xu J. Hydroxypyridinone functionalized self-assembled monolayers on nanoporous silica for sequestering lanthanide cations. J. Nanosci. Nanotechnol. 2005;5:527–529. doi: 10.1166/jnn.2005.096. [DOI] [PubMed] [Google Scholar]
- 51.Kawabe I. Hydration change of aqueous lanthanide ions and tetrad effects in lanthanide(III)–carbonate complexation. Geochem. J. 1999;33:267–275. [Google Scholar]
- 52.Byrne RH, Lee JH, Bingler LS. Rare-earth element complexation by PO4(3−) ions in aqueous solution. Geochim. Cosmochim. Acta. 1991;55:2729–2735. [Google Scholar]
- 53.Gu Z-M, Wang X-R, Cheng J, Wang L-S, Dai L-M. Effects of sulfate on speciation and bioavailability of rare earth elements in nutrient solution. Chem. Speciation Bioavail. 2000;12:53–58. [Google Scholar]
- 54.Andersson S, Eberhardt K, Ekberg C, Liljenzin J-O, Nilsson M, Skarnemark G. Determination of stability constants of lanthanide nitrate complex formation using a solvent extraction technique. Radiochim. Acta. 2006;94:469–474. [Google Scholar]
- 55.Abbtbraun G, Johannsen K, Kleiser M, Frimmel FH. Adsorption behavior of humic substances on activated carbon: comparison with the physical and chemical character of material from different origin. Environ. Int. 1994;20:397–403. [Google Scholar]
- 56.Tweed SO, Weaver TR, Cartwright I, Schaefer B. Behavior of rare earth elements in groundwater during flow and mixing in fractured rock aquifers: an example from the Dandenong ranges, southeast Australia. Chem. Geol. 2006;234:291–307. [Google Scholar]
- 57.Lee JH, Byrne RH. Complexation of trivalent rare earth elements (Ce, Eu, Gd, Tb, Yb) by carbonate ions. Geochim. Cosmoschim. Acta. 1993;57:295–302. [Google Scholar]