Abstract
BACKGROUND
The PI3K/Akt/mTOR pathway plays a critical role in the growth and progression of colorectal cancer (CRC). The purpose of our study was twofold: 1) to determine the expression levels of several key components of this pathway including p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 in CRCs, and 2) to correlate the expression of these proteins with cancer stage and location (left- vs. right-sided).
STUDY DESIGN
Immunohistochemistry for p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 was performed on normal colon and CRCs from 154 patients.
RESULTS
All proteins investigated were significantly overexpressed in CRCs compared to matched normal colonic tissue from the same patient (p<0.0001). The PI3K pathway component proteins were moderately correlated across normal and malignant colon tissues; correlations tended to be stronger in normal tissues as compared to the same correlations in cancers. Expression levels of p85α were significantly higher in Stage IV cancers than in Stage I–III cancers (p = 0.0005); interestingly, p85α expression was also significantly increased in the adjacent normal colonic mucosa of patients with Stage IV CRC compared with earlier stages (p=0.003). Finally, expression of Akt1, Akt2, and p-p70S6KThr389 was higher in left-sided CRCs compared with CRCs in the right colon (p = 0.007, p = 0.0008, and p = 0.04, respectively).
CONCLUSIONS
The PI3K/Akt/mTOR pathway components, p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389, are highly overexpressed in CRCs thus providing the rationale for targeting this pathway therapeutically in CRC patients. The increased expression of p85α in the adjacent normal mucosa of Stage IV patients suggests an important field defect, which may contribute to the growth and progression of these cancers.
Keywords: mTOR, p70S6K, PI3K, Akt1, Akt2, colorectal cancer
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States (1). When localized to the mucosa and submucosa of the bowel wall (Stage I), the five-year survival rate approaches 100% after surgical resection; however, metastasis to lymph nodes (Stage III) results in a precipitous decrease in five-year survival (2). Systemic metastasis (Stage IV) is associated with a five-year survival that is less than 5%. Despite recent advances in the treatment regimens for CRC, the underlying mechanisms regulating the growth and progression of CRCs are not entirely known. A better understanding of the signaling pathways responsible for these processes will facilitate development of novel therapeutic strategies and further enhance patient survival.
The phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling axis plays a critical role in the proliferation, resistance to apoptosis, angiogenesis and metastasis that is central to the development and maintenance of CRCs (3–6). Heterodimeric Class I PI3Ks are composed of a Src homology-2 domain-containing regulatory subunit (p85) and a 110-kDa catalytic subunit (p110) (4). The effects of PI3K on tumor growth and progression are thought to be mediated by Akt, a downstream effector of PI3K (7). PI3K-generated D3-phosphorylated phosphoinositides bind to the pleckstrin homology (PH) domain of both protein kinase B (PKB/Akt) and phosphoinositide-dependent kinase-1 (PDK-1) and induce their translocation to the plasma membrane where PDK-1 phosphorylates the Akt kinase at the Threonine 308 residue and activates it (4). The Akt kinase family is composed of three members: Akt1, Akt2 and Akt3. All three isoforms are structurally homologous and share similar mechanisms of activation but exhibit distinct features (4). Akt is overexpressed in a number of cancers, including colon, pancreatic, ovarian, and some steroid hormone-insensitive breast cancers (6, 8, 9). Moreover, it has been reported that Akt phosphorylation in human CRCs correlates with cell proliferation and apoptosis inhibition, as well as with different clinicopathologic parameters such as invasion grade, vessel infiltration, metastasis to lymph nodes, and tumor stage (10, 11). We have previously shown that targeted inhibition of upstream PI3K/Akt pathway components decreases growth, increases apoptosis, increases sensitivity to chemotherapy and decreases the metastatic capability of CRCs (9, 12–15).
A variety of downstream targets are regulated by Akt including mTOR, which promotes protein translation, growth, metabolism and angiogenesis (16). mTOR exists in two distinct functional complexes: mTORC1 and mTORC2. Previous studies have shown that mTOR is a direct substrate for the Akt kinase and identified Serine 2448 as the Akt target site on mTOR (16). However, recent evidence suggests that p70 S6 kinase (S6K) also regulates phosphorylation of this residue in response to both mitogen- and nutrient-derived stimuli (17). Furthermore, mTORC1 positively regulates phosphorylation of S6K (at the Threonine 389 residue) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1), which together control protein translation (16). In contrast, mTORC2 directly phosphorylates the Serine 473 residue in the C-terminal hydrophobic motif of Akt, which is necessary for full activation of the latter (16). We have recently shown that the mTORC1/mTORC2 proteins, mTOR, Raptor and Rictor are highly overexpressed in CRC tissues (18). Moreover, expression of Rictor was found to correlate with pAktSer473 expression in CRCs derived from the same patient. Finally, we showed that targeted inhibition of the downstream mTORC2 component, Rictor, resulted in growth inhibition and induced apoptosis in both rapamycin-sensitive and rapamycin-resistant CRCs, suggesting that selective targeting of mTORC2 may represent a novel therapeutic strategy for treatment of CRC patients.
The purpose of our current study was twofold: 1) to determine the expression levels of several key components of this pathway including p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 in CRC patient tissues, and 2) to correlate the expression of these proteins with the stage of cancer, location of cancer (left- vs. right-sided) and age of patients. Here we show that all of the aforementioned proteins are significantly overexpressed in CRCs compared to matched normal colonic tissue from the same patient. Moreover, expression levels of p85α were significantly higher in Stage IV tumors than earlier stage tumors; interestingly, this effect was also noted for adjacent normal colonic tissues comparing Stages I–III and Stage IV CRC patients. Finally, expression of Akt1, Akt2, and p-p70S6KThr389 was more prominent in left-sided CRCs than right-sided CRCs. Our findings provide further evidence in support of targeting this pathway as a therapeutic strategy for treatment of CRC patients.
METHODS
Antibodies & Tissue Arrays
All antibodies for immunohistochemistry were obtained from Cell Signaling Technology (Beverly, MA, USA) except for p85α, which was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Tissue microarrays of normal and malignant colonic tissues were purchased from AccuMax. Five copies each of four distinct tissue arrays were used for these experiments (A203; numbers I, II, V, and VI) except that one slide each was omitted from p-S6KThr389 and p-mTORSer2448 staining (II and V, respectively). Array A203 (II) contained data from liver metastases, but only the colon data was utilized for this analysis. Each of these arrays includes two tumor cores, pathological and patient characteristics for all samples and 5–12 normal specimens per slide.
Immunohistochemistry
Paraffin embedded colorectal cancer tissue arrays were deparaffinized in xylene and rehydrated in descending ethanol series. Immunostaining was performed using DAKO EnVision Kit (Dako Corp., Carpinteria, CA) as we have described previously (18). Briefly, antigen retrieval was performed by boiling slides in citrate buffer for 10 min. Slides were blocked and incubated overnight at 4°C with monoclonal antibodies diluted in the range of 1:50 to 1:150 in 0.05M Tris-HCL + 1% BSA against p85α, Akt1, Akt2, p-S6KThr389 and p-mTORSer2448. After 3 washes with TBST, the sections were incubated for 30 min with secondary antibody labeled with peroxidase, then washed 3 times with TBST. Lastly, peroxidase substrate DAB was added for staining. All sections were counterstained with hematoxylin and observed by light microscopy. For negative controls, primary antibody was omitted from the above protocol.
All array slides were scanned at 40X with a digital scanner and visualized using the software viewer, Aperio ImageScope (v.8.2.5.1263). Two independent researchers graded each array manually according to a semi-quantitative eight-tier system. This system assesses the percentage of positive cells (<10% of cells staining brown=0; 10–25% of cells stain positive=1; 25–50% of cells stain positive=2; 50–75% of cells stain positive=3; and >75% of cells stain positive=4) and intensity of staining (no brown staining=0; lightest brown from entire array=1; low intermediate=2; high intermediate=3; and darkest brown stain from entire array=4). The intensity and percentage scores are added to give a final immunoreactivity score ranging from 0 to 8. To compensate for minor differences in staining intensity of arrays stained at different times, the ‘high’ and ‘low’ intensity for each array was set before grading by scanning the entire image for most and least intense.
Statistical Analysis
Four immunohistochemistry (IHC) scores for each patient based on duplicate tumor spots and grading by two authors were averaged for analysis. Immunohistochemistry scores for each biomarker ranged from 0 – 8 (total score for intensity staining and percent positive staining) and were summarized descriptively for each biomarker, between normal and tumor tissues, and by tumor stage, tumor location, patient gender and age. Correlations between markers were calculated using the Pearson's correlation coefficient along with tests for significance. Paired analysis based on the t-test was employed to assess differential expression in biomarker IHC scores in the subset of patients with matched tumor and normal tissues. Univariate comparisons of p85α, Akt1, Akt2, p-S6KThr389 and p-mTORSer2448 protein IHC expression levels for each tumor and patient characteristics were assessed using one-way analysis of variance (combination of tumor stage and tissue array sets) or two-sample t-test (for tumor location, gender, age). All tests were assessed at the 0.05 level of significance. Statistical computations were carried out using statistical software, SAS Release 9.2.
RESULTS
Clinicopathologic Characteristics
A total of 308 malignant and 35 non-malignant colon tissues were studied. These samples were derived from a total of 154 patients; duplicate tumor core samples were present from 154 patients and adjacent normal tissues from a subset (n=35) of these patients (Table 1). Four of the normal colonic tissue samples do not have the corresponding cancer tissues (non-matched). The number of patients with Stage IV disease was highest (n=65) since two of the four arrays used for this analysis were designed specifically for studying metastatic disease. Seventy-three percent of cancers were from the rectum and left colon and 27% from the right colon. Sixty-two percent of the patients were male while 38% were female. The age of patients ranged from 26 to 87 years old. Age (p = 0.027) but not gender and tumor location was significantly different across cancer stage. Specific information about race or ethnicity was not available from the array manufacturer.
Table 1.
Clinical and Pathologic Characteristics of Specimens Used for Immunohistochemistry Analysis.
| No. of patients with cancer tissues (matching normals) |
Right/left colon | Female/male | Average age, y (range) |
|
|---|---|---|---|---|
| Stage I | 7 (1) | 0/7 | 3/4 | 67 (62–86) |
| Stage II | 39 (4) | 10/24 | 17/22 | 63 (40–86) |
| Stage III | 43 (7) | 12/28 | 20/23 | 57 (28–87) |
| Stage IV | 65 (23) | 14/37 | 19/46 | 57 (26–78) |
| Total | 154 (35) | 36/96 | 59/95 | 59 (26–87) |
Expression and Correlation of PI3K/Akt/mTOR components in normal and cancer samples from CRC patients
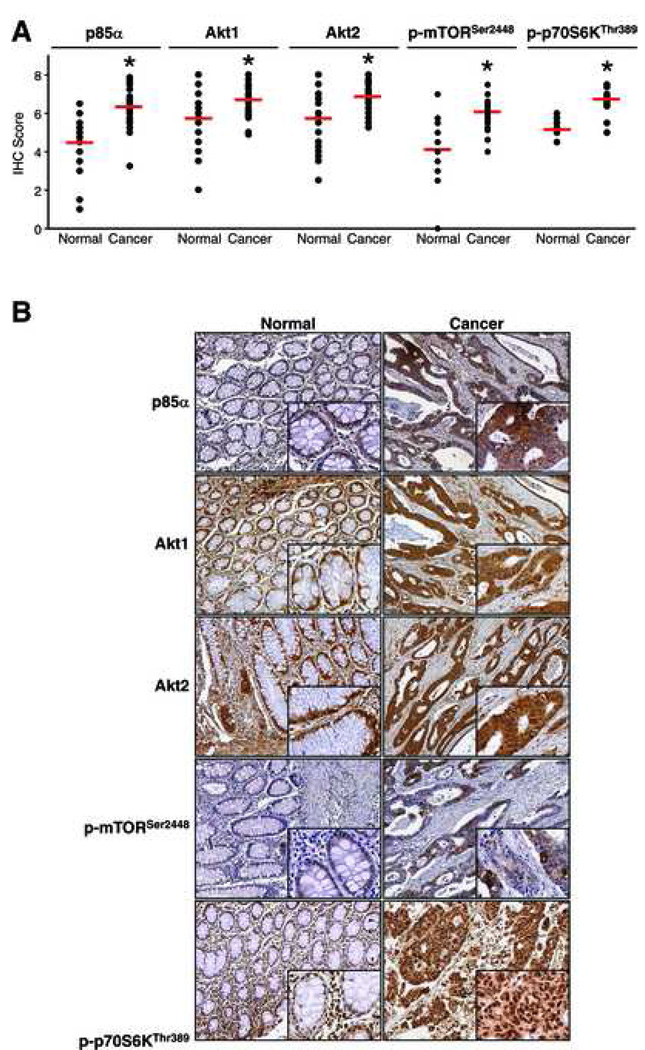
To determine whether PI3K/Akt/mTOR pathway proteins are overexpressed in CRCs, we examined CRCs and adjacent normal colonic tissue for expression of p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389. Each sample was assigned an IHC immunoreactivity score ranging from 0–8 as described above. Data analysis is shown in Fig. 1A along with representative patient samples for each protein in Fig. 1B. Immunohistochemical analyses showed cytoplasmic staining of all five proteins studied with nuclear staining also uniformly observed with p-S6KThr389. Akt1, Akt2, and p85α generally stained homogeneously throughout the epithelial elements, whereas p-mTORSer2448 tended to demonstrate variable intensity, even within a 1-mm tissue core. All of the proteins investigated in this study were significantly overexpressed in CRCs compared to the matched normal colonic tissue from the same patient (p<0.0001) (Table 2 and Fig. 1). Average IHC scores for normal colon tissues ranged from 4.1– 5.7 for all proteins studied whereas tumor IHC scores averaged 6.1–6.8.
Figure 1.
Immunohistochemical analysis of normal and malignant colonic tissues. (A) Comparison of IHC scores for p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 in normal and malignant colonic tissues derived from 154 patients (*p<0.05 vs normal). (B) Representative images showing staining pattern and intensity for p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 in normal and malignant colonic tissues (panel=100X; inset=400X).
Table 2.
Comparison of IHC scores between matched normal and CRC samples.
| Biomarker | Normal (mean ± SD) |
Cancer (mean ± SD) |
Difference (cancer – normal) (mean ± SD) |
p Value (paired t- test) |
|---|---|---|---|---|
|
p85α (n = 31) |
4.5 ± 1.4 | 6.3 ± 0.9 | 1.8 ± 1.5 | <0.0001 |
|
Akt1 (n = 31) |
5.7 ± 1.3 | 6.7 ± 0.8 | 1.0 ± 1.1 | <0.0001 |
|
Akt2 (n = 31) |
5.7 ± 1.4 | 6.8 ± 0.8 | 1.1 ± 1.4 | <0.0001 |
|
p-mTORSer2448 (n = 23) |
4.1 ± 1.4 | 6.1 ± 0.9 | 2.0 ± 1.7 | <0.0001 |
|
p-S6KThr389 (n = 16) |
5.2 ± 0.4 | 6.7 ± 0.7 | 1.5 ± 0.9 | <0.0001 |
We found that IHC scores for the various proteins were moderately correlated across both normal and malignant colorectal tissues (Table 3). In general, the correlations tended to be stronger in normal tissues as compared to the same correlation in tumor tissues. The correlation between Akt2 and p85α was stronger (correlation coefficient, r = 0.64; p <0.0001) than that between Akt1 and p85α (r = 0.38; p = 0.027) in normal tissues as well as cancers. The correlation between IHC scores for Akt1 and Akt2 was strong (r = 0.83 and r = 0.50; p<0.0001 for normal tissues and cancers, respectively). p-p70S6KThr389 was correlated with Akt1 in normal mucosa and cancers, and with Akt2 in cancers only. Surprisingly, there was no significant correlation between p-mTORSer2448 and p-S6KThr389 in normal or tumor tissues. In summary, p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 are overexpressed in CRCs; IHC scores for each of these proteins are moderately correlated across both normal and malignant colonic tissue.
Table 3.
Correlation Coefficients Between Immunohistochemistry Scores for PI3K/Akt/mTOR Pathway Components in Normal and Cancer Samples
| Biomarker | Normal | Cancer | ||
|---|---|---|---|---|
| Correlation coefficient* |
p Value | Correlation coefficient* |
p Value | |
| p85α | ||||
| Akt1 | 0.38 | 0.027 | 0.21 | 0.008 |
| Akt2 | 0.64 | <0.0001 | 0.31 | <0.0001 |
| p-mTORSer2448 | 0.67 | 0.0002 | 0.24 | 0.01 |
| p-S6KThr389 | −0.21 | 0.40 | 0.30 | 0.0003 |
| Akt1 | ||||
| Akt2 | 0.83 | <0.0001 | 0.50 | <0.0001 |
| p-mTORSer2448 | 0.50 | 0.01 | 0.05 | 0.58 |
| p-S6KThr389 | 0.47 | 0.05 | 0.28 | 0.0009 |
| Akt2 | ||||
| p-mTORSer2448 | 0.65 | 0.0004 | 0.07 | 0.50 |
| p-S6KThr389 | 0.18 | 0.48 | 0.39 | <0.0001 |
| p-mTORSer2448 | ||||
| p-S6KThr389 | 0.17 | 0.64 | 0.01 | 0.90 |
Pearson correlation coefficients indicate moderate correlations between biomarkers in both normal and tumor tissues.
p85α is overexpressed in normal and cancer tissue from Stage IV CRC patients in comparison with Stage I–III CRC patients
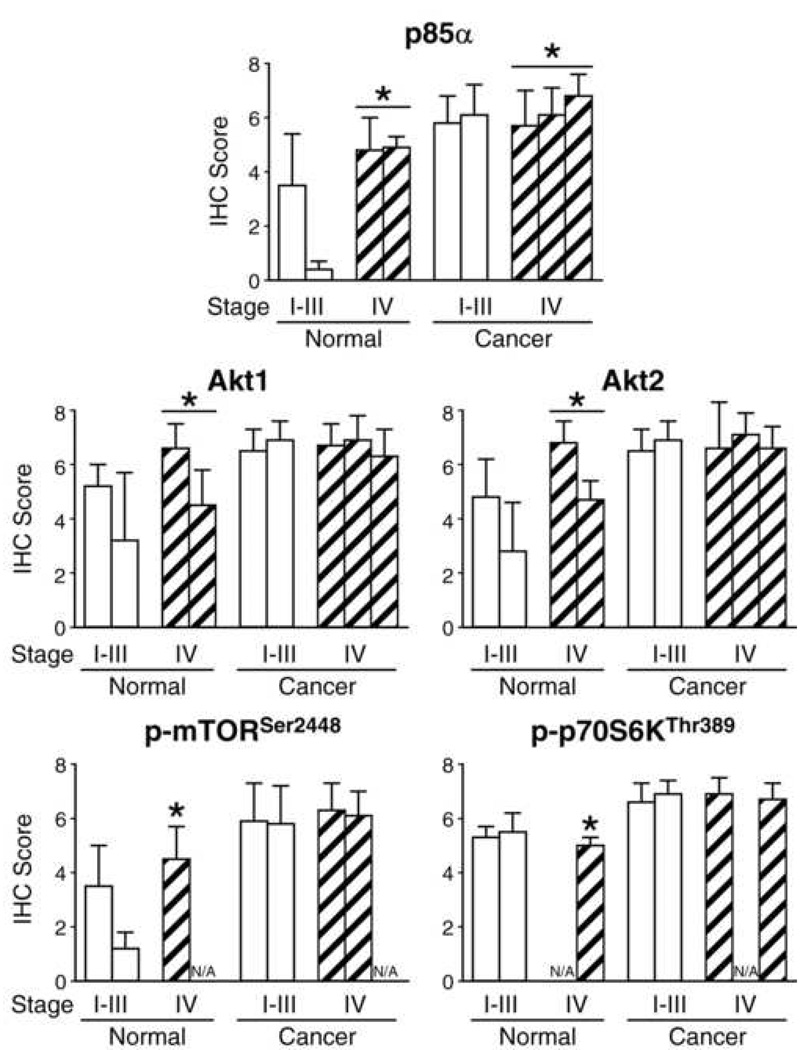
In order to determine whether expression of PI3K/Akt/mTOR pathway proteins is increased along with tumor stage, we compared IHC scores between stages in normal mucosa and cancers (Table 4 and Fig. 2). There were no significant differences in any of the five proteins comparing normal tissues and cancers between Stages I, II, and III (data not shown). Therefore, we present the data from Stages I–III together as compared with scores from Stage IV tumor and non-malignant tissues from the corresponding stage patients. Even though there was variability in the average scores classified by stage amongst the array sets, the IHC scores for p85α were significantly higher in Stage IV tumors than earlier stages (p = 0.0005). This expression pattern was not noted for Akt1, Akt2, p-S6KThr389 or p-mTORSer2448. Interestingly, the increased expression of p85α was also noted for non-malignant colonic tissues when comparing IHC scores between early stages and Stage IV patients (p=0.003); this pattern was also noted for Akt2 (p=0.004), p-mTORSer2448 (p=0.01), p-S6KThr389 (p=0.0442) and a similar trend was present for Akt1 (p=0.054). In summary, expression of p85α was significantly higher in Stage IV tumors than earlier stages, while expression of p85α, Akt2, p-mTORSer2448 and p-p70S6KThr389 was significantly higher in adjacent normal mucosa from Stage IV cancer patients than earlier stages.
Table 4.
Comparison of Immunohistochemistry Scores by Colorectal Cancer Stage
| Stage | Array slide |
n | IHC Scores | |||||
|---|---|---|---|---|---|---|---|---|
| p85α | Akt1 | Akt2 | p-mTORser2448 | p-S6KThr389 | ||||
| Normal | I–III | 1 | 8 | 3.5±1.9 | 5.2±0.8 | 4.8±1.4 | 3.5±1.5 | 5.3±0.4 |
| I–III | 4 | 4 | 0.4±0.3 | 3.2±2.5 | 2.8±1.8 | 1.2±0.6 | 5.5±0.7 | |
| IV | 2 | 15 | 4.8±1.2 | 6.6±0.9 | 6.8±0.8 | 4.5±1.2 | n/a | |
| IV | 3 | 8 | 4.9±0.4 | 4.5±1.3 | 4.7±0.7 | n/a | 5.0±0.3 | |
| P value* | 0.0029 | 0.0544 | 0.0037 | 0.0097 | 0.0442 | |||
| Cancer | I–III | 1 | 40 | 5.8±1.0 | 6.5±0.8 | 6.5±0.8 | 5.9 ±1.4 | 6.6±0.7 |
| I–III | 4 | 49 | 6.1±1.2 | 6.9±0.7 | 6.9±0.7 | 5.8±1.4 | 6.9±0.5 | |
| IV | 1 | 5 | 5.7±1.3 | 6.7±0.8 | 6.6 ±1.7 | 6.3±1.0 | 6.9±0.6 | |
| IV | 2 | 15 | 6.1±1.0 | 6.9±0.9 | 7.1 ±0.8 | 6.1±0.9 | n/a | |
| IV | 3 | 45 | 6.8±0.8 | 6.3±1.0 | 6.6 ±0.8 | n/a | 6.7±0.6 | |
| p Value* | 0.0005 | 0.1261 | 0.9700 | 0.2971 | 0.7703 | |||
p Value is for comparison between Stage IV and Stages I–III grouped together.
Figure 2.
Immunohistochemical analysis of normal and malignant colonic tissues grouped by stage of cancer. Comparison of IHC scores for p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 in normal and malignant colonic tissues grouped by stage of cancer: early (Stage I–III) versus late (Stage IV) and array sets (*p<0.05 versus normal).
Akt1, Akt2, and p-S6KThr389 are overexpressed in left-sided CRCs
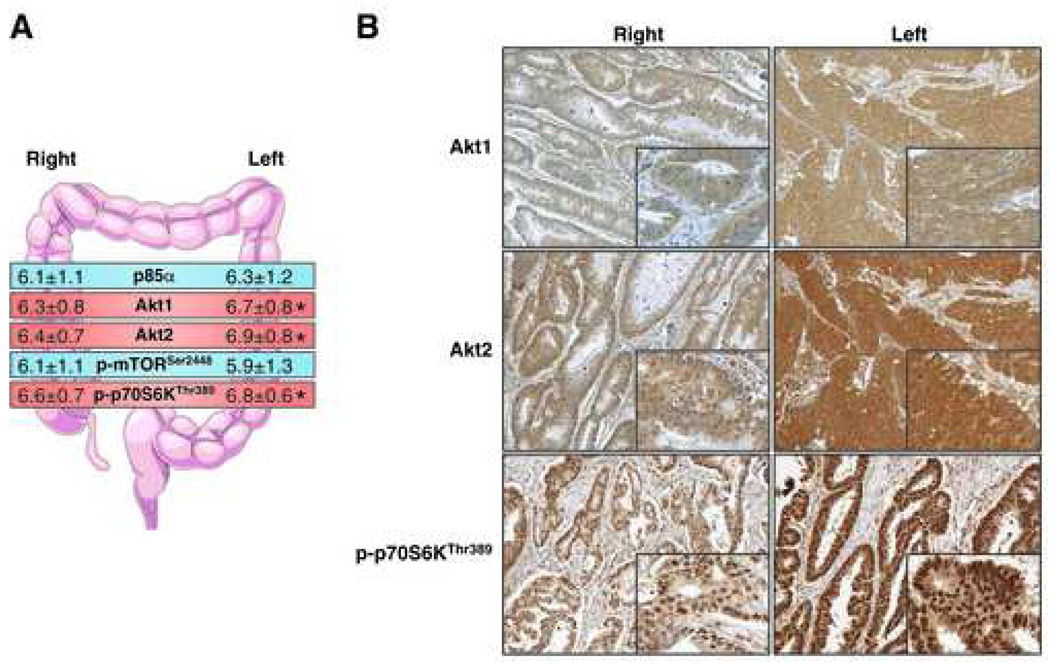
It has previously been demonstrated that left- and right-sided CRCs display different clinical and biological characteristics (19). Therefore, we further investigated differences in expression of the PI3K/Akt/mTOR proteins based on the location of the tumor (Table 5 and Fig. 3). Left-sided and rectal cancers were grouped together and compared to cancers of the right colon; tissues from indeterminate location were eliminated for this portion of the analysis. We found that expression of Akt1, Akt2, and p-S6KThr389 was more prominent in left-sided CRCs than right-sided CRCs (p = 0.007, p = 0.0008, and p = 0.04, respectively). However, since the absolute difference in scores was less than half a point on the 8-point scale, the clinical significance of this finding has yet to be determined.
TABLE 5.
Comparison of Immunohistochemistry Scores by Location within Colon: Right-versus Left-Sided
| n | IHC Scores | ||||||
|---|---|---|---|---|---|---|---|
| p85α | Akt1 | Akt2 | p-mTORser2448 | p-S6KThr389 | |||
| Normal | Right | 8 | 4.2±1.8 | 5.7±0.3 | 5.6±0.9 | 4.4±0.4 | 5.5±0.4 |
| Left | 22 | 3.7±2.0 | 5.5±1.6 | 5.4±1.8 | 3.5±1.9 | 5.0±0.4 | |
| p Value | 0.5507 | 0.7270 | 0.7990 | 0.2781 | 0.0589 | ||
| Cancer | Right | 36 | 6.1±1.1 | 6.3±0.8 | 6.4±0.7 | 6.1±1.1 | 6.6±0.7 |
| Left | 96 | 6.3±1.2 | 6.7±0.8 | 6.9±0.8 | 5.9±1.3 | 6.8±0.6 | |
| p Value | 0.3754 | 0.0071 | 0.0008 | 0.5520 | 0.0397 | ||
Figure 3.
Immunohistochemical analysis of CRC tissues grouped by location of cancer. (A) Comparison of IHC scores for p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 in CRC tissues based on tumors that were proximal (right-sided) or distal (left-sided) to the splenic flexure (*p<0.05). (B) Representative images showing staining pattern and intensity for Akt1, Akt2 and p-p70S6KThr389 in CRC tissues from right- and left-sided cancers (panel=100X; inset=400X).
p85α and p-mTORSer2448 are overexpressed in younger CRC patients
Finally, we investigated the effect of age on expression of these proteins in normal colon and CRC tissues. When age was examined as a variable in normal colon tissues, patients younger than the median age showed increased expression of p85α (mean = 4.9 vs. 3.4; p = 0.017) and p-mTORSer2448 (mean = 4.6 vs. 3.2; p = 0.029) compared to older patients (Table 6). There was a similar trend in Akt2 scores (p = 0.073). However, this age-related difference was not noted in the cancers. We also examined gender as a variable, but did not find any significant differences between males and females in our sample set (data not shown). In summary, normal colon from younger patients tends to exhibit increased expression levels of p85α and p-mTORSer2448 compared to older patients.
Table 6.
Comparison of Immunohistochemistry Scores by Age
| IHC Scores | ||||||
|---|---|---|---|---|---|---|
| p85α | Akt1 | Akt2 | p-mTORser2448 | p-S6K Thr389 | ||
| Normal | ||||||
| >Median | 20 | 3.4±2.0 | 5.4±1.3 | 5.1±1.6 | 3.2±1.6 | 5.3±0.5 |
| <Median | 15 | 4.9±0.3 | 5.8±1.6 | 6.1±1.4 | 4.6±1.2 | 5.1±0.4 |
| p Value | 0.017 | 0.441 | 0.073 | 0.029 | 0.512 | |
| Cancer | ||||||
| >Median | 73 | 6.2±1.0 | 6.5±0.9 | 6.7±0.7 | 6.0±1.2 | 6.7±0.6 |
| <Median | 81 | 6.2±1.2 | 6.6±0.8 | 6.8±0.9 | 5.8±1.4 | 6.8±0.6 |
| p Value | 0.834 | 0.521 | 0.569 | 0.586 | 0.318 | |
DISCUSSION
In this study, we determined the expression levels of several key components of the PI3K/Akt/mTOR signaling axis including p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 in CRC patient tissues and correlated their expression with the stage of cancer, location (left- vs. right-sided) and age of patients. We found that all five proteins are significantly overexpressed in CRCs compared to the matched normal colonic tissues. Moreover, expression levels of p85α were significantly higher in Stage IV cancers than earlier stages; interestingly, this effect was also noted for adjacent normal colonic tissues comparing Stages I–III and Stage IV CRCs. Finally, we show that expression of Akt1, Akt2, and p-p70S6KThr389 was more prominent in left-sided than right-sided CRCs.
Previous studies have also shown that overexpression of Akt isoforms is a much more frequent event than their gene amplification in human malignancies, suggesting transcriptional regulation of Akt during tumorigenesis (20). We examined the expression of specific Akt isoforms and found increased expression of both Akt1 and Akt2 in CRCs compared to normal colon. Moreover, we found a high degree of correlation in IHC scores between the two isoforms. Akt2 is the predominant isoform in ovarian, breast and pancreatic cancers, whereas Akt1 expression has mostly been detected in gastric cancer (21, 22). Unlike other tumor types such as breast cancer, activating mutations in Akt1 are very rare in CRC (<2%) (23, 24). Both isoforms are hypothesized to play specific roles in the growth and progression of cancer. Akt1 is believed to be important for PI3K-mediated cell proliferation in CRC, while we have recently shown that Akt2 is the more predominant isoform in CRCs and acts as a critical regulator of CRC metastasis (13).
mTOR is a direct substrate for Akt kinase and Serine 2448 is the site on mTOR that is phosphorylated by the latter; deletion of this residue in the C-terminal regulatory region of the protein results in increased mTOR activity (16). More recent evidence suggests that S6K also regulates phosphorylation of this residue in response to both mitogen- and nutrient-derived stimuli (17). Furthermore, mTORC1 positively regulates phosphorylation of S6K at the Threonine 389 residue in response to nutrients and growth factors (16). Additionally, two strong feedback loops exist within this signaling axis, both emanating from mTORC1 and its downstream mediator, S6K. Firstly, activation of mTORC1 signaling strongly represses signaling upstream in the PI3K/Akt pathway primarily by S6K-dependent downregulation of IRS-1, thereby resulting in attenuated pAktSer473 (16, 25, 26). Secondly, inhibition of mTORC1 leads to the release of a S6K-PI3K-Ras dependent brake, which results in feedback activation of MAPK signaling and elevated p-ERKThr202/Tyr204 levels (27). Moreover, any changes in MAPK activity will subsequently modulate S6K phosphorylation (17). Given the complexity of inputs regulating the signaling patterns in this pathway, it is not surprising that we found no significant correlation between expression of p-mTORSer2448 and p-S6KThr389 in normal or tumor tissues. Baseline levels of pERKThr202/Tyr204 and pAktSer473 in these patient samples may provide an explanation for these paradoxical findings given the regulatory functions of these proteins in controlling p-S6KThr389 and subsequently p-mTORSer2448 levels in cancer cells.
In order to determine whether expression of the target proteins correlates with stage, we assessed their expression levels in normal and malignant tissues from Stage IV patients compared to Stage I–III patients. In general, the correlations tended to be stronger in normal tissues as compared to cancers. We found that the expression of p85α was significantly induced in Stage IV tumors compared to earlier stages. However, there were no significant differences in the expression of the other proteins investigated with regard to stage. Interestingly, the effect of significantly increased expression of p85α was also true for normal colonic tissues when comparing IHC scores between early stages and Stage IV patients; this result also held true for Akt2, p-mTORSer2448, p-S6KThr389 and a similar trend was present for Akt1. These findings may be explained by the “field effect” phenomenon. In the multi-step carcinogenesis model for CRC, genetic changes occur in a stepwise fashion such that a clone that has growth advantage proliferates, acquires further genetic aberrations and undergoes another selection for survival and growth, eventually resulting in cancer (28). According to this phenomenon, pre-cancerous “normal” appearing cells in proximity to neoplastic tissue also possess some but not all of the genetic aberrations that are present in cancer cells (29). Based on our results, we speculate that “normal” colon is presumably primed for aggressive malignant transformation by induction of expression of PI3K/Akt/mTOR pathway proteins even before the characteristic phenotypic changes of neoplasia take place. The differences in expression levels between the various stages are less obvious in tumor tissue as all target proteins are already expressed at maximal levels during early stages of progression, which masks any differences in their expression between early and late stages.
Field effects are of particular interest because they give insight into the early stages of cancer progression and provide potential biomarkers of cancer risk. Our findings indicate that p85α may be such a biomarker, although further validation is required. Specifically, it is unclear whether p85α expression in apparently normal colon is associated with an increased risk of CRC. Field effects are also important from a translational standpoint because the common practice of identifying markers of malignancy by comparing genomic or proteomic expression profiles of tumors to that of neighboring “normal” tissue will often overlook early changes that are already present in the surrounding “normal” areas. Finally, from a more practical standpoint, it is unclear how much of the large bowel would have to be sampled to sufficiently rule out a field effect in individuals without neoplastic lesions.
Normal left (distal to splenic flexure) and right (proximal to splenic flexure) colon differs in their embryological origin, expression of antigens, metabolism of glucose, polyamines and butyric acid, as well as in bile acid concentrations and composition and density of the normal flora of the gut (19, 30, 31). Furthermore, CRCs that arise on the left or right side exhibit significant differences in incidence, response to 5-fluorouracil treatment, gene expression and signal transduction patterns leading to transformation (19, 30, 31). For instance, left-sided CRCs display a significantly increased frequency of K-RAS and TP53 mutations in comparison to right-sided tumors (19, 30, 31). We investigated differences in expression of our five target proteins based on the location of the tumor (left- vs. right-sided) within the colon. We found that expression of Akt1, Akt2, and p-S6KThr389 was more significant in left-sided CRCs than right-sided CRCs. Although the absolute difference in scores was less than half a point on the 8-point scale employed, it is interesting to speculate that activation of the PI3K/Akt/mTOR pathway may be related to the higher incidence of mutations of the corresponding genes for PI3K/Akt/mTOR in left-sided CRCs.
K-Ras is one of the most frequently activated oncogenes in multiple cancer types, including colorectal cancer (32, 33). K-Ras, while originally associated with the activation of the Raf cascade, has since been linked to the activation of multiple effectors, including PI3K/Akt/mTOR signaling. K-Ras becomes constitutively active in CRC through mutations in codons 12, 13, 61 and 146 and has been shown to be mutated more often in left-sided CRCs (19, 30–33). Ras directly interacts with PI3K through unique epitopes in p110α which, when disrupted, significantly reduce the ability of oncogenic Ras to induce tumorigenesis (34). After initial tumor formation, the requirement for K-Ras signaling is reduced and partially replaced by a dependence on PI3K signaling for maintaining tumor growth (35). These findings may contribute to the higher levels of Akt/mTOR protein expression and activation seen in left-sided CRCs. Furthermore, the tumor suppressor p53 is a major checkpoint protein that is mutated in >50% of human cancers, including CRC (36). It has previously been shown that the p53 and mTOR signaling machineries can cross-talk and coordinately regulate cell growth, proliferation and death (36). Activation of p53 inhibits mTOR signaling and its downstream targets such as autophagy (37). Given that p53 mutations are more common in left-sided CRCs, this may contribute to the higher levels of mTORC1 activation, as evidenced by increased levels of p-S6KThr389, seen in left-sided CRCs.
In conclusion, we found that p85α, Akt1, Akt2, p-mTORSer2448 and p-p70S6KThr389 are significantly overexpressed in CRCs compared to the matched normal colonic tissues. Moreover, expression levels of p85α were significantly higher in Stage IV tumors than earlier stages; interestingly, this effect was also true for adjacent normal colonic tissues between early stages and Stage IV CRC patients. Finally, we show that expression of Akt1, Akt2, and p-p70S6KThr389 was more prominent in left-sided CRCs than right-sided CRCs. Our findings provide evidence in support of targeting the PI3K/Akt/mTOR pathway as a therapeutic strategy for treatment of CRC patients.
ACKNOWLEDGMENT
The authors would like to thank Karen Martin for manuscript preparation.
This work was supported by grants P20CA1530343 (UK SPORE in GI Cancer), RO1CA104748 and RO1DK48498 (BME).
ABBREVIATIONS
- CRC
Colorectal cancer
- PI3K
Phosphatidylinositol 3-kinase
- mTOR
Mammalian target of rapamycin
- RTK
Receptor tyrosine kinase
- PIP3
Phosphatidylinositol-(3,4,5)-phosphate
- PKB
Protein kinase B
- PH
Pleckstrin homology
- PDK-1
Phosphoinositide-dependent kinase-1
- S6K
p70 S6 kinase
- 4E-BP1
Eukaryotic initiation factor 4E binding protein 1
- IRS-1
Insulin receptor substrate 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at Southern Surgical Association 121st Annual Meeting, Hot Springs, VA, December 2009.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Griffin MR, Bergstralh EJ, Coffey RJ, et al. Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer. 1987;60:2318–2324. doi: 10.1002/1097-0142(19871101)60:9<2318::aid-cncr2820600934>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Nagata S, Iwasaki T, et al. Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1999;96:4874–4879. doi: 10.1073/pnas.96.9.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 5.Philp AJ, Campbell IG, Leet C, et al. The phosphatidylinositol 3'-kinase p85α gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- 6.Roy HK, Olusola BF, Clemens DL, et al. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 7.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 8.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Rychahou PG, Jackson LN, Silva SR, et al. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 843-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khaleghpour K, Li Y, Banville D, et al. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–248. doi: 10.1093/carcin/bgg195. [DOI] [PubMed] [Google Scholar]
- 11.Itoh N, Semba S, Ito M, et al. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- 12.Rychahou PG, Murillo CA, Evers BM. Targeted RNA interference of PI3K pathway components sensitizes colon cancer cells to TNF-related apoptosis-inducing ligand (TRAIL) Surgery. 2005;138:391–397. doi: 10.1016/j.surg.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Rychahou PG, Kang J, Gulhati P, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Li N, Wang X, et al. Augmentation of sodium butyrate-induced apoptosis by phosphatidylinositol 3'-kinase inhibition in the KM20 human colon cancer cell line. Clin Cancer Res. 2002;8:1940–1947. [PubMed] [Google Scholar]
- 16.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 18.Gulhati P, Cai Q, Li J, et al. Targeted inhibition of mTOR signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-09-1249. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 20.Altomare DA, Tanno S, De Rienzo A, et al. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2002;87:470–476. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]
- 21.Yuan ZQ, Sun M, Feldman RI, et al. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 22.Han Z, Wu K, Shen H, et al. Akt1/protein kinase B alpha is involved in gastric cancer progression and cell proliferation. Dig Dis Sci. 2008;53:1801–1810. doi: 10.1007/s10620-007-9824-2. [DOI] [PubMed] [Google Scholar]
- 23.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 24.Bleeker FE, Felicioni L, Buttitta F, et al. AKT1(E17K) in human solid tumours. Oncogene. 2008 doi: 10.1038/onc.2008.170. [DOI] [PubMed] [Google Scholar]
- 25.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 26.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 29.Giovannucci E, Ogino S. DNA methylation, field effects, and colorectal cancer. J Natl Cancer Inst. 2005;97:1317–1319. doi: 10.1093/jnci/dji305. [DOI] [PubMed] [Google Scholar]
- 30.Distler P, Holt PR. Are right- and left-sided colon neoplasms distinct tumors? Dig Dis. 1997;15:302–311. doi: 10.1159/000171605. [DOI] [PubMed] [Google Scholar]
- 31.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 32.Wistuba II, Behrens C, Albores-Saavedra J, et al. Distinct K-ras mutation pattern characterizes signet ring cell colorectal carcinoma. Clin Cancer Res. 2003;9:3615–3619. [PubMed] [Google Scholar]
- 33.Edkins S, O'Meara S, Parker A, et al. Recurrent KRAS codon 146 mutations in human colorectal cancer. Cancer Biol Ther. 2006;5:928–932. doi: 10.4161/cbt.5.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Ramjaun AR, Haiko P, et al. Binding of ras to phosphoinositide 3-kinase p110α is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Junttila MR, Evan GI. p53--a Jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]