Abstract
This study examined the social zeitgeber theory, which suggests that affective symptoms are caused by life events disrupting vulnerable individuals’ social and biological rhythms. Undergraduate participants were selected based on a two phase screening process, including a semi-structured diagnostic interview. The final sample consisted of 101 bipolar spectrum participants and 100 demographically matched normal controls. Participants who completed up to three follow-up visits, approximately every four months, as part of a longitudinal study were included in the current study. Life events did not predict social rhythm regularity and social rhythm regularity inconsistently predicted affective symptoms. However, life events, particularly social rhythm disruption (SRD) events, did predict depressive symptoms and episodes, and less consistently predicted hypo(manic) symptoms and episodes. Thus, the current study obtained mixed support for the social zeitgber theory.
Keywords: Bipolar disorder, life events, social rhythms, affective symptoms
Introduction
Despite its public health significance and high prevalence (4.4% of a nationally representative U.S. sample were affected by a bipolar spectrum disorder; Merikangas et al., 2007), bipolar disorder is understudied compared to other mental health disorders (Hyman, 2000). Even fewer studies have focused on bipolar spectrum disorders (i.e., Bipolar II, Cyclothymia). This is particularly surprising given that bipolar spectrum disorders are more prevalent than Bipolar I disorder in the community and mental health clinics and typically persist for years without the symptom free periods observed in more severe bipolar disorders (Depue et al., 1981; Merikangas et al., 2007). Moreover, 15 to 50% of cyclothymic individuals may subsequently develop Bipolar I or II disorders (APA, 2000; Shen, Alloy, Abramson, & Grandin, in press). Thus, the primary aim of the current study was to better understand mood episodes in a bipolar spectrum sample.
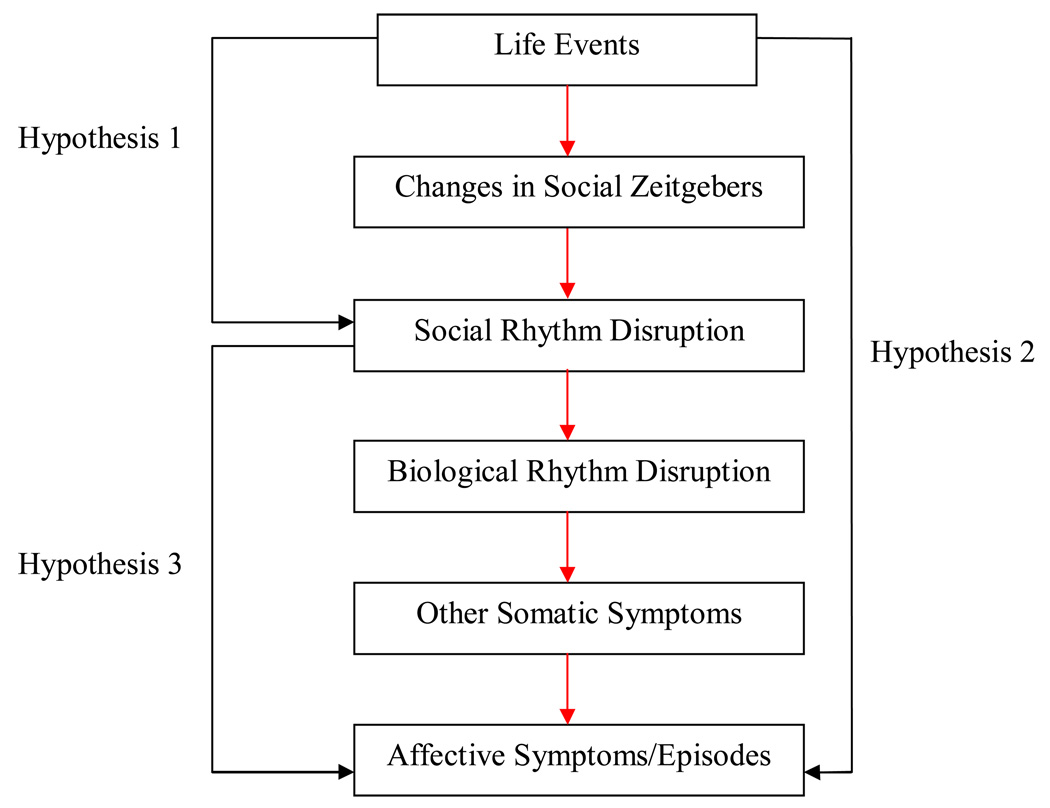
Much evidence indicates that life events precede the onset of both depressive and hypomanic/manic mood symptoms and episodes (see Alloy et al., 2005; Johnson, 2005; Johnson & Roberts, 1995; Paykel, 2001 for reviews). Several theories have been proposed to explain how life stress may precipitate bipolar mood episodes (Alloy et al., 2005; Johnson, 2005; Johnson & Roberts, 1995), although the pathway(s) by which life stress triggers affective symptoms remains unclear. Ehlers, Frank, and Kupfer (1988) initially proposed the social zeitgeber theory to explain unipolar depressive symptoms. This theory states that depressive symptoms arise as a consequence of life events disturbing social zeitgebers (external/social cues that function to entrain biological rhythms), which, in turn, derail social rhythms (e.g., meal times, getting out of bed) and biological rhythms (e.g., sleep-wake cycle, temperature and cortisol rhythms). According to this theory, disruptions in these rhythms influence somatic symptoms, which, in vulnerable individuals, lead to depression (see Figure 1) (Ehlers, Frank, & Kupfer, 1988).
Figure 1.
The Social Zeitgeber Theory and Main Hypotheses of the Current Study.
This theory was, in part, derived from the substantial evidence that depressed individuals have irregular biological rhythms (e.g., Thase, Jindal & Howland, 2002). Recent findings suggest that the theory may also apply to (hypo)manic episodes, and thus, to bipolar disorder as well (for a review, see Grandin, Alloy, & Abramson, 2006). Specifically, Malkoff-Schwartz and colleagues (1998; 2000) reported that life events associated with social rhythm disruptions were more likely to be present prior to manic episodes than control periods in a bipolar I sample. Moreover, social rhythm irregularity predicted a shorter time to onset of major depressive and hypomanic/manic episodes in a prospective study (Shen et al., in press). Finally, Interpersonal and Social Rhythm Therapy (IPSRT), an intervention designed to maintain regular daily rhythms and manage potential precipitants of rhythm dysregulation, was shown to buffer against future bipolar episodes in a Bipolar I sample (Frank et al., 2005).
This research suggests that individuals with mood disorders may exhibit less social rhythm regularity and that social rhythm disruptions may contribute to affective symptoms in these individuals. However, the findings to date are somewhat mixed and several of these studies are limited by cross-sectional designs, small sample sizes, and short assessment periods (Grandin et al., 2006). Thus, the current study is a prospective, longitudinal examination of three of the hypothesized pathways of the social zeitgeber theory in a sample of bipolar spectrum and demographically matched normal control participants. Specifically, we hypothesized that: (1) life events would predict participants’ social rhythm regularity; (2) life events, particularly social rhythm disruption (SRD) events, would predict participants’ affective symptoms; and (3) social rhythm irregularity would predict participants’ affective symptoms (see Figure 1).
Methods
Participants
Participants for this study were recruited from the Temple University (TU) site of the Longitudinal Investigation of Bipolar Spectrum (LIBS) Project (Alloy et al., in press; Shen et al., in press) and were selected based on a two phase screening process. In Phase I, approximately 7,000 students at TU completed the revised General Behavior Inventory (GBI) (Depue, Krauss, Spoont, & Arbisi, 1989). Students who met the initial GBI screening criteria were invited to participate in Phase II (n = 2737), which consisted of a semi-structured diagnostic interview using an expanded Schedule for Affective Disorders and Schizophrenia – Lifetime interview (SADS-L) (Endicott & Spitzer, 1978). Students meeting the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) criteria (APA, 1994) or Research Diagnostic Criteria (RDC) (Spitzer, Endicott, & Robins, 1978) for Bipolar II or Cyclothymia were invited to participate in the study. These individuals were categorized as bipolar spectrum participants (n = 137). Individuals who did not meet criteria for any disorder in their lifetime were recruited as normal controls (n = 127) and matched on a case-by-case basis to bipolar participants on age, sex, and ethnicity.
The final sample for this study consisted of 201 students (131 female, 70 male) and ranged in age from 18–24 (mean = 19.8 ± 1.8 years). A higher proportion of females than males (65% and 35%, respectively) in this study is consistent with prior research indicating a preponderance of women in Bipolar II samples (Depue et al., 1989; Amsterdam, Brunswick & O'Reardon, 2002; Cassano, Akiskal, Savino, Musetti, & Perugi, 1992), given that our sample of bipolar spectrum individuals received mostly Bipolar II diagnoses (64 Bipolar II, 37 Cyclothymia/BiNOS). The ethnic composition of the final sample was diverse: 54% Caucasian, 25% African-American, 4% Hispanic, 5% Asian, 12% Other. Given that normal controls were matched to the bipolar spectrum individuals, the two groups did not differ significantly on gender, ethnicity, or age.
Measures
Self Report Screening Inventory (Phase I)
The GBI (Depue et al., 1981; 1989) is a self-report questionnaire used to identify potential bipolar spectrum and normal participants for Phase II of the study. The GBI has good internal consistency (α’s = 0.90–0.96), test-retest reliability (r’s = 0.71–0.74), adequate sensitivity (0.78) and high specificity (0.99) for bipolar spectrum conditions (Depue et al., 1981; 1989). The revised GBI (Depue et al., 1989) has 73 items, each of which captures either a depressive (D scale), hypo(manic) or biphasic (HB scale) symptom. The GBI has been extensively validated in college, psychiatric outpatient, and offspring of Bipolar I patient samples (Depue et al., 1981; 1989). The LIBS project used the case-scoring method and cut-off scores recommended by Depue et al. (1989) to identify potential bipolar (D ≥ 11; HB ≥ 13) and normal (D < 11; HB < 13) participants. These criteria were based on Depue et al.’s (1989) findings and a pilot study in which high and low GBI students, using these cutoffs, were validated against diagnoses derived from SADS-L interviews (Alloy et al., in press).
Diagnostic Screening Interview (Phase II)
An expanded SADS-L diagnostic interview (Endicott & Spitzer, 1978) was used to assess the occurrence, duration, and severity of symptoms related to mood, anxiety, substance abuse, eating, psychotic, and other disorders over participants’ lifetime. The expansion of the original SADS-L is described elsewhere (Alloy et al., in press; Francis-Raniere, Alloy, & Abramson, 2006). This modified version of the SADS-L has yielded kappas ≥ .95 for major depression diagnoses and ≥ .90 for all unipolar depressive diagnoses based on 80 jointly rated interviews (Alloy & Abramson, 1999; Alloy et al., 2000). An inter-rater reliability study based on 105 jointly rated SADS-L interviews for the LIBS project yielded kappas > .96 for bipolar diagnoses (Alloy et al., in press).
Prospective Diagnostic Measure
An expanded SADS – Change interview (exp-SADS-C) (Spitzer & Endicott, 1978) assessed the presence or absence of affective episodes and symptoms throughout each four-month prospective assessment. The exp-SADS-C was expanded to allow the derivation of DSM-IV as well as RDC diagnoses (Alloy et al., in press; Francis-Raniere et al., 2006). In addition, depressive and (hypo)manic symptoms from the SADS-C were counted at each follow-up visit to yield a symptom count for each participant. Inter-rater reliability (Francis-Raniere et al., 2006) for the exp-SADS-C in joint ratings of 60 interviews for the LIBS Project was good (k > .80). In a validity study, participants dated their symptoms on the exp-SADS-C with at least 70% accuracy compared with daily symptom ratings made over a 4-month interval (Francis-Raniere et al., 2006).
Life Events
The Life Event Scale (LES) used in the LIBS project is an expansion of the earlier 134-item LES (Alloy & Clements, 1992; Needles & Abramson, 1990) to include positive events as well as negative ones. The expanded LES contains 193 events and was designed to reduce ambiguous and redundant events as well as exclude items that reflected obvious symptoms of depression or hypo(mania). Studies have found that the LES has good reliability and validity (Francis-Raniere et al., 2006; Metalsky & Joiner, 1992; Needles & Abramson, 1990).
At each four-month follow-up, after participants completed the LES, they were interviewed by a trained research assistant (who was blind to the participants’ group status) as a reliability and validity check on the occurrence and dates of the events reported on the LES. A recent study found that participants correctly recalled 100% of the major events using the LES followed by this Life Event Interview (LEI), when compared to a daily life event list created by the participant prospectively over a month (Alloy & Abramson, 1999). The inter-rater reliability of 40 LEIs yielded an average correlation of .89 between interviewers for the dating of these events (Francis-Raniere et al., 2006). The impact of events was rated on a 5-point scale from 0 (no/slight impact) to 4 (extreme impact). Events associated with a moderate, major, or extreme negative impact for the participant as rated by the interviewer (a rating of a 2, 3 or 4) were classified as “negative events.”
The LEI also assesses the extent to which a life event disrupted participants’ daily routine. This rating scale ranges from 1 (little to no effect) to 4 (marked effect) and relies on the degree of disruption in the sleep-wake cycle and was modeled after the first standardized assessment of such social rhythm disruption (SRD) events (Frank et al., 1999). Events rated as SRD events had a score of a 2, 3 or 4 on this scale, similar to the guidelines used by other studies (Malkoff-Schwartz et al., 1998; 2000). A severe SRD event had a score of a 3 or 4 on this scale. The LEI also measures the total amount of sleep lost due to an event. This rating scale ranges from 1 (little or no sleep loss), or losing less than an hour of sleep, to 5 (extreme sleep loss), or losing ≥ 7 hours of sleep. Life events associated with at least an hour of sleep loss (or a rating of a 2, 3, 4, or 5 on the LEI) were categorized as “sleep loss” events.
Lifestyle Regularity
The Social Rhythm Metric (SRM) (Monk, Flaherty, Frank, Hoskinson, & Kupfer, 1990) was designed to quantify participants’ typical daily social rhythm patterns, and therefore, targets activities that only occur habitually or regularly. This measure consists of 17 daily activities, 15 specified (i.e., “Get out of Bed”, “Have lunch,” “Physical Exercise”) and 2 individualized write-in items. The daily version of the SRM was found to be moderately consistent (i.e., r = 0.44) and valid (e.g., participants on vacation have considerably lower SRM scores) in a group of 50 healthy controls (Monk et al., 1990; Monk, Kupfer, Frank, & Ritenour, 1991). Other evidence for the validity of the SRM is derived from a study that found SRM scores are correlated positively with other indices of social rhythm stability (Monk, Petrie, Hayes, & Kupfer, 1994).
For this study, a modified version of the SRM (M-SRM) was used to assess the frequency with which participants performed activities at approximately the same time (± 45 minutes) over each month of the follow-up period. A regular activity was defined as occurring within 45 min of the same time, every day. A score of 0 (“activity was performed regularly two or less times per week”), 1 (“activity was performed regularly three or more times per week”), or 2 (“activity was performed regularly every day per week”) was assigned to each item of the M-SRM. An M-SRM score was calculated by averaging across the scores for each item of every month of the follow-up period. Thus, higher M-SRM scores represent a higher frequency of activities performed regularly.
Procedure
After the two phase screening process, participants were invited back for follow-up visits every 4 months. Only the first three follow-up visits (i.e., F1, F2, F3) for each participant were included in this study. At each follow-up visit, participants completed the M-SRM and LES and were also interviewed with the exp-SADS-C and the LEI. These visits were conducted by two separate interviewers, one to administer the exp-SADS-C and the other to administer the LEI, in order to minimize potential interviewer bias. Participants were paid $50 for each follow-up visit, which took approximately 3 hours.
Results
Preliminary Analyses
Group Equivalency
Demographic variables (age, sex, ethnicity) were examined to confirm the equivalency of the control and bipolar groups. The groups did not differ significantly on sex (χ21, N=201 = 0.06, p = .81), age (χ28, N=201 = 4.47, p = .88), or ethnicity coded as a dichotomous variable (i.e., non-white versus white) (χ21, N=201 = .54, p = .97). The means and standard deviations of all study variables are presented in Tables 1 and 2.
Table 1.
Means and Standard Deviations of Symptoms and Social Rhythm Regularity by Group
| Control Group Mean (SD) |
Bipolar Group Mean (SD) |
F (df) | |
|---|---|---|---|
| F1 HYPO Symptoms | .26 (.64) | 1.58 (2.16) | 32.99 (1,189)** |
| F2 HYPO Symptoms | .23 (.69) | 1.34 (2.11) | 22.71 (1,174)** |
| F3 HYPO Symptoms | .28 (.81) | 1.21 (2.03) | 14.45 (1,156)** |
| F1 DEP Symptoms | 1.46 (1.89) | 6.58 (4.72) | 97.10 (1, 189)** |
| F2 DEP Symptoms | 1.91 (2.67) | 5.35 (4.37) | 39.98 (1, 173)** |
| F3 DEP Symptoms | 1.65 (1.93) | 5.16 (4.62) | 39.21 (1, 155)** |
| F1 M-SRM Score | 19.67 (5.28) | 17.77 (5.87) | 5.05 (1,171)* |
| F2 M-SRM Score | 19.87 (5.57) | 17.88 (6.07) | 4.11 (1,139)* |
| F3 M-SRM Score | 20.19 (5.50) | 17.94 (6.27) | 4.63 (1,127)* |
Note. The degrees of freedoms (df) vary for each variable due to participant attrition and incomplete data. F1 = First Follow-up visit. F2 = Second Follow-up visit. F3 = Third Follow-up visit. HYPO = Hypo(manic) symptoms from the SADS-C interview. DEP = Depressive symptoms from the SADS-C interview. M-SRM = Modified-Social Rhythm Metric.
p < .05,
p < .01
Table 2.
Means and Standard Deviations of Life Event Categories by Group
| Categories of Events |
Control Group Mean (SD) |
Bipolar Group Mean (SD) |
F (df) |
|---|---|---|---|
| F1 Total | 24.40 (13.96) | 39.88 (26.26) | 25.84 (1,190)** |
| F2 Total | 20.89 (14.80) | 27.24 (17.82) | 6.61 (1,173)* |
| F3 Total | 22.69 (20.94) | 27.65 (25.31) | 1.81 (1,157) |
| F1 Negative | 7.17 (6.44) | 19.67 (17.75) | 42.22 (1,193)** |
| F2 Negative | 6.32 (7.23) | 12.31 (11.24) | 17.64 (1, 173)** |
| F3 Negative | 4.90 (5.11) | 11.45 (12.24) | 18.84 (1,153)** |
| F1 SRD | 2.34 (2.79) | 7.86 (9.72) | 28.34 (1,190)** |
| F2 SRD | 2.66 (3.97) | 4.64 (6.21) | 6.36 (1,173)* |
| F3 SRD | 2.12 (3.32) | 5.17 (6.22) | 14.45 (1,153)** |
| F1 Sleep Loss | 1.95 (2.13) | 7.11 (9.25) | 28.15 (1,190)** |
| F2 Sleep Loss | 1.76 (3.12) | 3.44 (5.60) | 6.21 (1,176)* |
| F3 Sleep Loss | 1.14 (1.50) | 4.35 (5.63) | 23.32 (1,153)** |
Note. The degrees of freedom (df) vary for each variable due to participant attrition and incomplete data. F1 = First Follow-up visit. F2 = Second Follow-up visit. F3 = Third Follow-up visit. SRD = Social Rhythm Disruption.
p < .05,
p < .01
We conducted analyses of variance (ANOVAs) to determine whether the groups differed in each follow-up period on M-SRM scores as well as (hypo)manic (HYPO scores) and depressive (DEP scores) symptoms obtained from the SADS-C interview. As expected, the bipolar group reported higher HYPO and DEP scores, but less social rhythm regularity, at each follow-up visit compared to the control group (see Table 1). We also conducted ANOVAs for each category of life events at each of the follow-up visits to determine potential differences between the groups (see Table 2). As expected, the bipolar group reported more total life events, as well as specific categories of life events (i.e., negative, SRD, sleep loss), than the control group at each of the follow-up visits with one exception. At F3, the two groups did not differ significantly in the total number of events experienced (see Table 2).
Analyses with Demographic Variables and Study Variables of Interest
We conducted ANOVAs to determine whether any demographic characteristics were associated with any study variable over the follow-up period. We found that ethnicity was significantly associated with M-SRM scores at F1 (F1,151 = 7.81, p = .01), F2 (F1,123 = 13.56, p < .01), and F3 (F1,111 = 7.42, p = .01), such that the non-white group reported significantly higher scores than the white group. Thus, when M-SRM scores were used as the dependent variables in regression analyses, ethnicity was entered as a predictor. Age and gender were not significantly related to M-SRM, DEP, and HYPO scores at any of the follow-up visits (all p’s> .05). We also found that total life events at F1 (F1,186 = 5.23, p = .02) and negative events at F1 (F1,188 = 5.24, p = .02) were significantly associated with age. Thus, when these variables were the dependent variables in regression analyses, age was entered as a predictor. Ethnicity and gender were not significantly related to any category of life events at any of the follow-up visits (all p’s> .05).
Main Analyses
Hypothesis 1: Life Events and Social Rhythm Regularity
Linear regression analyses were conducted to examine whether the total number of events, as well as each category of life events (i.e., negative, SRD, sleep loss), predicted M-SRM scores (see Table 3). There was not a significant association of total number or any category of life events at the first follow-up visit (F1) with M-SRM scores at the second follow-up visit (F2) or of the total number or any category of events at F2 with M-SRM scores at F3 (see Table 3). Additionally, there were no significant group X event (i.e., total, negative, SRD, sleep loss) interactions for each of these analyses (all p’s > .05). These findings suggest that life events did not significantly predict social rhythm regularity.
Table 3.
Categories of Life Events Predicting to Subsequent Social Rhythm Scores
| Categories of Events |
Social Rhythm Scores | ||
|---|---|---|---|
| ΔR2 | B | t (df) | |
| F2 Total | .01 | −.02 | −.84 (131) |
| F3 Total | .01 | −.05 | −1.36 (117) |
| F2 Negative | .00 | −.02 | −.38 (131) |
| F3 Negative | .02 | −.10 | −1.71 (116) |
| F2 Sleep Loss | .00 | .08 | .71 (129) |
| F3 Sleep Loss | .00 | −.11 | −.71 (117) |
| F2 SRD | .01 | .11 | 1.13 (129) |
| F3 SRD | .01 | −.13 | −1.16 (117) |
Note. The degrees of freedom (df) vary for each variable due to participant attrition and incomplete data. F1 = First Follow-up visit. F2 = Second Follow-up visit. F3 = Third Follow-up visit. SRD = Social Rhythm Disruption events. ΔR2 = Change in the proportion of variance accounted for in the model by the main effect.
Hypothesis 2: Life Events and Affective Symptoms/Episodes
Linear regression analyses were conducted to examine whether the total number of events, as well as each category of life events (i.e., negative, SRD, sleep loss), significantly predicted subsequent bipolar symptoms. We found that each category of life events predicted DEP scores at the next follow-up such that participants who reported more life events also had more depressive symptoms (see Table 4). In contrast, there was a less consistent predictive association of life events and HYPO scores. Specifically, there was only a significant predictive relationship of the total number of events, negative events, and sleep loss events at F2 with HYPO scores at F3 (see Table 4). However, there were no significant group X event category interactions in predicting to DEP or HYPO scores, suggesting that the life event-depressive and hypo(manic) symptom relationships were consistent across both groups (all p’s > .05).
Table 4.
Categories of Life Events Predicting to Depressive and (Hypo)manic Symptoms
| Categories of Events |
Depressive Symptoms | (Hypo) Manic Symptoms | ||||
|---|---|---|---|---|---|---|
| ΔR2 | B | t (df) | ΔR2 | B | t (df) | |
| F2 Total | .03 | .03 | 2.45 (163)** | .00 | .00 | .19 (159) |
| F3 Total | .06 | .06 | 3.50 (146)** | .04 | .02 | 2.44 (148)* |
| F2 Negative | .08 | .09 | 4.33 (165)** | .01 | −.10 | 1.16 (161) |
| F3 Negative | .08 | .12 | 4.14 (145)** | .05 | .04 | 2.94 (145)** |
| F2 Sleep Loss | .07 | .23 | 4.43 (163)** | .02 | .05 | 1.81 (159)^ |
| F3 Sleep Loss | .05 | .19 | 3.23 (146)** | .03 | .06 | 2.11 (146)* |
| F2 SRD | .07 | .19 | 3.88 (163)** | .01 | .02 | .94 (159) |
| F3 SRD | .05 | .17 | 3.14 (147)** | .01 | .03 | 1.27 (145) |
Note. HYPO = Hypo(manic) symptoms from the SADS-C interview. F1 = First Follow-up F2 = Second Follow-up visit. F3 = Third Follow-up visit. SRD = Social Rhythm Disruption events. ΔR2 = Change in the proportion of variance accounted for in the model by the main effect.
p < .07,
p < . 05,
p < .01
We also performed Cox regression survival analyses to examine the length of time to the bipolar individuals’ first (hypo)manic and depressive episodes as a function of the number of life events reported prior to this episode. We entered group (Bipolar II vs. Cyclothymic/BiNOS), age, gender, and ethnicity on the first step and the number of events prior to bipolar individuals’ first episode (or the number of events over the study duration for bipolar participants who did not experience an episode during the study duration) on the second step. We found that the total number of life events significantly predicted time to bipolar individuals’ first depressive, but not to their first (hypo)manic, episode (see Table 5). Thus, bipolar participants who experienced more life events had a shorter time to onset of their first depressive episode. Negative, SRD, and sleep loss events did not significantly predict time to depressive or (hypo)manic episodes (see Table 5).
Table 5.
Cox Regression Survival Analyses Predicting Time to Affective Episodes as a Function of Life Events for Bipolar Spectrum Participants
| Categories of Events |
Time to Depressive Episode | Time to (Hypo) manic Episode | ||||
|---|---|---|---|---|---|---|
| B (SE) | Wald | p | B (SE) | Wald | p | |
| Total | −.01 (.00) | 5.69 | .02 | −.00 (.00) | 2.24 | .14 |
| Negative | −.01 (.01) | 1.91 | .17 | −.00 (.00) | .70 | .40 |
| SRD | −.01 (.01) | .79 | .38 | −.01 (.01) | 1.33 | .25 |
| Sleep Loss | .00 (.01) | .04 | .84 | −.00 (.01) | .02 | .90 |
Note. F1 = First Follow-up visit. F2 = Second Follow-up visit. F3 = Third Follow-up visit. SRD = Social Rhythm Disruption.
Next, we conducted matched t-tests to compare the number of (hypo)manic and depressive symptoms before a SRD event versus after the SRD event. These analyses included 118 SRD events (29 reported by normal and 89 reported by bipolar participants). We found that the bipolar participants reported significantly more depressive symptoms after than before a SRD event (t88 = −2.77, p < .01, d =.17), but not more (hypo)manic symptoms(t88 =−.56, p > .05, d = .06). Participants in the control group reporting SRD events did not experience significant change in their depressive symptoms or their (hypo)manic symptoms from before to after an SRD event (all p’s > .05).
We also conducted matched t-tests to compare the number of life events prior to bipolar mood episodes to the number of events experienced during control periods for each bipolar participant that experienced an episode. Control periods were episode-free periods that occurred 1 year after or 1 year before the bipolar mood episode to be as comparable as possible with respect to season of the year. If this was not possible (because the 1 year before or after the episode also contained an episode), then the control period was chosen to be an episode-free period either 4 weeks after the bipolar episode or 4 weeks prior to the 8-week period before the bipolar episode. Bipolar individuals who experienced a depressive episode during the study experienced more SRD events within the 8 weeks prior to onset of the depressive episode than during 8 week control periods (t32 = −3.42, p < .01, d= .59). In contrast, the number of total, as well as negative, events within the 8 weeks prior to onset of a depressive episode did not significantly differ from the number of total or negative events reported during control periods (t32 =−.62, p > .05, d = .19; t32 = −1.06, p > .05, d = .26, respectively). With respect to (hypo)manic episodes, we found that there were no significant differences between the pre-episode and control periods in the number of total life events and negative events (t16 = .90, p > .05, d = .25; t16 = 1.43, p > .05, d= .39, respectively). There was a marginally significant difference between the number of SRD events prior to hypo(manic) episodes versus control periods (t16 = 2.01, p = .07, d =.49), but it was not in the expected direction. Specifically, the number of SRD events prior to hypo(manic) episodes was less than the number of SRD events reported during control periods.
Finally, we conducted matched t-tests to examine whether the number of life events reported prior to bipolar mood episodes differed significantly from the number of events reported by bipolar participants who did not experience a bipolar mood episode over the study duration. Episodic (bipolar individuals who had an episode during the follow-up period) and non-episodic (bipolar individuals who did not have an episode during the follow-up period) bipolar participants were matched based on gender, ethnicity, and age in that hierarchical order. We found that there were no significant differences between the episodic and non-episodic bipolar participants on the total number of events, or negative events, reported prior to depressive episodes or (hypo)manic episodes (all p’s > .05, d < .29). In contrast, episodic bipolar spectrum participants did experience more SRD events prior to depressive episodes than non-episodic bipolar spectrum participants (t30 = −3.91, p < .05, d = .70). However, this relationship did not hold for the number of SRD events reported prior to hypo(manic) episodes (t16 = 1.17, p = .26, d = .28).
Hypothesis 3: Social Rhythm Regularity and Affective Symptoms/Episodes
Linear regression analyses were conducted to examine whether participants’ M-SRM scores significantly predicted bipolar symptoms at the next follow-up. We found that less social rhythm regularity at F2 was associated with greater depressive symptoms at F3 (t117 = −2.60, p < .01); however, this relationship did not hold for M-SRM scores at F1 predicting to DEP scores at F2. There were no significant relationships of social rhythm regularity (M-SRM scores) with subsequent HYPO scores (all p’s > .05). There were also no group X M-SRM score interactions in predicting bipolar symptoms (all p’s > .05).
We also conducted Cox regression survival analyses to examine the length of time to the bipolar spectrum participants’ first (hypo)manic and depressive episodes in the study as a function of their M-SRM scores over the study duration. We entered group, age, gender, and ethnicity on the first step and M-SRM scores on the next step. Participants’ social rhythm regularity did not significantly predict the time to first depressive episode (Wald = .61, p = .43) or first (hypo)manic episode (Wald = 2.44, p = .12). Thus, retrospective report of social rhythms did not predict the time to bipolar participants’ affective episodes.
Discussion
The primary goal of this study was to examine the social zeitgeber theory (Ehlers et al., 1988; Ehlers, Kupfer, Frank, & Monk, 1993) as a potential explanation for affective symptoms and episodes in individuals with bipolar spectrum disorders. Specifically, we investigated three causal associations postulated by this theory: 1) whether the occurrence of life events, and particular types of events, predict social rhythm regularity; 2) whether life events were associated with, and temporally predicted, affective symptoms and episodes; and 3) whether social rhythm regularity predicted affective symptoms and episodes (see Figure 1). Overall, we obtained mixed support for the theory.
Contrary to the social zeitgeber theory, the present findings suggested that life events were not associated with an individual’s social rhythm regularity (Hypothesis 1). These findings may be limited in that they relied on the retrospective report of social rhythm regularity. The use of objective measures of social rhythm regularity (i.e., actigraphy) or daily assessments of rhythms would have been preferable, but difficult to do in a long-term longitudinal study such as this (Jones, Hare, & Evershed, 2005). Further, given that this is the first study to examine this association in a bipolar sample (Grandin et al., 2006), there is no empirical context in which to evaluate these results. However, one study found that bipolar participants rated life events as social rhythm disruptive and most (83%) of these events were also rated as disruptive by the study investigators as well (Malkoff-Schwartz et al., 1998). These data suggest that objective raters found bipolar individuals’ life events to be strongly linked to social rhythm disruptions.
Consistent with the social zeitgeber theory, we found that life events prospectively predicted bipolar symptoms and mood episodes as well as the time to onset of their first depressive episode (Hypothesis 2). However, most notably providing strong support for the theory, there was a significant increase in depressive symptoms from before to after a SRD event for both normal and bipolar individuals. Bipolar individuals also experienced more SRD events (but not more total or negative events) before depressive episodes compared to equivalent control periods. Further, bipolar individuals who had a depressive episode onset experienced more SRD events (but again, not more total or negative events) before their depressive episodes as compared to other bipolar individuals who did not have a depressive episode onset. These findings suggest that SRD events may have more of an impact than other negative life events on depressive symptoms. Other studies have concluded that manic episodes were particularly likely to be precipitated by SRD events (Malkoff-Schwartz et al., 1998; 2000), but this is the first study to find that bipolar spectrum individuals’ depressive symptoms and episodes may also be particularly vulnerable to, or perhaps triggered by, SRD events.
Unfortunately, these relationships with SRD events did not hold for (hypo)manic symptoms and episodes in the present study (Hypotheses 2). The failure to replicate previous findings that SRD events predict (hypo)manic symptoms and episodes (Malkoff-Schwartz et al., 1998; 2000) may be due to low variability of hypomanic symptoms in the current study. Specifically, the bipolar spectrum group in the current study only experienced an average of 1 to 2 (hypo)manic symptoms in each follow-up period, which may not have allowed for enough power to detect significant associations (see Table 2). This explanation seems particularly likely given that many of the nonsignificant findings involving hypo(manic) symptoms were in the expected direction. Alternatively, the association of (hypo)manic symptoms and life events has only been consistently documented in samples with severe forms of bipolar disorder (i.e., Bipolar I) (Malkoff-Schwartz et al., 1998; 2000). Thus, perhaps individuals with less severe forms of bipolar disorder (i.e., Bipolar II, Cyclothymia) are less vulnerable to life stress, or perhaps specifically, socially disruptive life stress.
Unexpectedly, we did not find that lower social rhythm regularity predicted increased depressive or (hypo)manic symptoms, nor the time to onset of bipolar participants’ first depressive or (hypo)manic episode (Hypothesis 3). These null findings are particularly surprising, as a recent study found that participants’ trait-like social rhythm regularity scores (assessed at baseline) significantly predicted time to their first depressive and (hypo)manic episode onset (Shen et al., in press). The contradiction in these findings may be because the current study had a 50% smaller sample size. It is also possible that the retrospective self-report of social rhythms in the current study, over long time intervals, limited the results. For example, a post-hoc analysis of our data showed that the bipolar individuals reported nearly twice as many SRD and sleep loss events (18.30% and 15.44% of their total number of life events, respectively) than the normal controls (10.44% and 7.58% of their total number of life events, respectively) over the study duration. These data suggest a relationship between social rhythm disruptions and affective symptoms and indicate that self-reported social rhythms may be biased compared to interview based scoring of social rhythm disruption events. Further, although several studies, including this one, found that bipolar individuals tend to have low social rhythm scores compared to normal controls (i.e., Ashman et al., 1999; Jones, 2005; Szuba, Yager, Guze, Allen, & Baxter, 1992), this is the first study to prospectively assess the association between social rhythms and affective symptoms in a bipolar spectrum sample.
In conclusion, the current study yielded mixed support for the social zeitgeber theory. There was a consistent prospective association of life events and mood symptoms and episodes. Specifically, there seemed to be a unique relationship between SRD events and depressive symptoms and episodes. However, there was not a consistent association between life events and social rhythm scores or between social rhythm scores and mood symptoms and episodes. Several explanations were proposed to explain these null findings. Yet, the findings with SRD events, such as the increase of depressive symptoms from before to after an SRD event, offer quite promising support for the social zeitgeber theory. Thus, we believe that this evaluation of the social zeitgeber theory highlights the importance of continuing research in this area.
Contributor Information
Louisa G. Sylvia, Psychology Department, Temple University
Lauren B. Alloy, Psychology Department, Temple University
Joanna A. Hafner, Psychology Department, Temple University
Marisa C. Gauger, Psychology Department, Temple University
Katrina Verdon, Psychology Department, Temple University
References
- Alloy LB, Abramson LY. The Temple-Wisconsin Cognitive Vulnerability to Depression (CVD) Project: Conceptual background, design, and methods. Journal of Cognitive Psychotherapy: An International Quarterly. 1999;13:227–262. [Google Scholar]
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, Kim RS, Lapkin JB. The Temple-Wisconsin Cognitive Vulnerability to Depression (CVD) Project: Lifetime history of Axis I psychopathology in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2000;109:403–418. [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Urosevic S, Walshaw PD, Nusslock R, Neeren AM. The psychosocial context of bipolar disorder: Environmental, cognitive, and developmental risk factors. Clinical Psychology Review. 2005;25:1043–1075. doi: 10.1016/j.cpr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Clements CM. Illusion of control: Invulnerability to negative affect and depressive symptoms after laboratory and natural stressors. Journal of Abnormal Psychology. 1992;101:234–245. doi: 10.1037//0021-843x.101.2.234. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME. Behavioral Approach System (BAS) and Behavioral Inhibition System (BIS) sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders. doi: 10.1111/j.1399-5618.2007.00547.x. (in press) [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Rev. Washington DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Ed.-Text Rev. Washington DC: Author; 2000. [Google Scholar]
- Amsterdam JD, Brunswick DJ, O'Reardon J. Bipolar disorder in women. Psychiatric Annals. 2002;32:397–404. [Google Scholar]
- Ashman SB, Monk TH, Kupfer DJ, Clark AH, Myers FS, Frank E, Leibenluft E. Relationship between social rhythms and mood in patients with rapid cycling bipolar disorder. Psychiatry Research. 1999;86:1–8. doi: 10.1016/s0165-1781(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Akiskal HS, Savino M, Musetti L, Perugi G. Proposed subtypes of Bipolar II and related disorders: With hypomanic episodes (or cyclothymia) and with hyperthymic temperament. Journal of Affective Disorders. 1992;26:127–140. doi: 10.1016/0165-0327(92)90044-7. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR, Arbisi P. General Behavior Inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. Journal of Abnormal Psychology. 1989;98:117–126. doi: 10.1037//0021-843x.98.2.117. [DOI] [PubMed] [Google Scholar]
- Depue RA, Slater J, Wolfstetter-Kausch H, Klein D, Goplerud E, Farr D. A behavioral paradigm for identifying persons at risk for bipolar depressive disorder: A conceptual framework and five validation studies (Monograph) Journal of Abnormal Psychology. 1981;90:381–437. doi: 10.1037//0021-843x.90.5.381. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. Archives of General Psychiatry. 1988;45:948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ, Frank E, Monk TH. Biological rhythms and depression: The role of zeitgebers and zeitstörers. Depression. 1993;1:285–293. [Google Scholar]
- Endicott J, Spitzer RA. A Diagnostic Interview: The Schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Flaherty J, Frank E, Hoskinson K, Richman J, Kupfer DJ. Social zeitgebers and bereavement. Paper presented at the 140th Annual Meeting of the American Psychiatric Association; Chicago. 1987. [Google Scholar]
- Francis-Raniere EL, Alloy LB, Abramson LY. Depressive personality styles and bipolar spectrum disorders: Prospective tests of the event congruency hypothesis. Bipolar Disorders. 2006;8:382–399. doi: 10.1111/j.1399-5618.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, Grochocinski V, Houck P, Scott J, Thompson W, Monk T. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Archives of General Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Frank E, Malkoff-Schwartz S, Sherrill JT, Anderson B. Social Rhythm Disruption (SRD) criteria. Pittsburgh: Western Psychiatric Institute and Clinic; 1999. [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: Review and evaluation. Clinical Psychology Review. 2006;26:679–-. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Goals for research on bipolar disorder: The view from NIMH. Biological Psychiatry. 2000;48:436–441. doi: 10.1016/s0006-3223(00)00894-5. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Life events in bipolar disorder: Towards more specific models. Clinical Psychology Review. 2005;25:1008–1027. doi: 10.1016/j.cpr.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Roberts JE. Life events and bipolar disorder: Implications from biological theories. Psychological Bulletin. 1995;117:434–449. doi: 10.1037/0033-2909.117.3.434. [DOI] [PubMed] [Google Scholar]
- Jones SE. Psychotherapy of bipolar disorder: a review. Journal of Affective Disorders. 2004;80:101–114. doi: 10.1016/S0165-0327(03)00111-3. [DOI] [PubMed] [Google Scholar]
- Jones SE, Hare DJ, Evershed K. Actigraphic assessment if circadian activity and sleep patterns in bipolar disorder. Bipolar Disorders. 2005;7:1–11. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson B, Sherril JT, Siegel L, Patterson D, Kupfer DJ. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episode. Archives of General Psychiatry. 1998;55:702–707. doi: 10.1001/archpsyc.55.8.702. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherril JT, Houck PR, Kupfer DJ. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychological Medicine. 2000;30:1005–1016. doi: 10.1017/s0033291799002706. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metalsky GI, Joiner TE. Vulnerability to depressive symptomatology: A prospective test of the diathesis-stress and casual mediation components of the hopelessness theory of depression. Journal of Personality and Social Psychology. 1992;63:667–675. doi: 10.1037//0022-3514.63.4.667. [DOI] [PubMed] [Google Scholar]
- Monk TH, Flaherty JF, Frank E, Hoskinson K, Kupfer DJ. The Social Rhythm Metric: an instrument to quantify the daily rhythms of life. Journal of Nervous and Mental Disease. 1990;178:120–126. doi: 10.1097/00005053-199002000-00007. [DOI] [PubMed] [Google Scholar]
- Monk TH, Kupfer DJ, Frank E, Ritenour AM. The social rhythm metric (M-SRM): Measuring daily social rhythms over 12 weeks. Psychiatry Research. 1991;36:195–207. doi: 10.1016/0165-1781(91)90131-8. [DOI] [PubMed] [Google Scholar]
- Monk TH, Petrie SR, Hayes AJ, Kupfer DJ. Regularity of daily life in relation to personality, age, gender, sleep quality, and circadian rhythms. Journal of Sleep Research. 1994;3:196–205. doi: 10.1111/j.1365-2869.1994.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Needles DJ, Abramson LY. Positive life events, attributional style, and hopelessness: Testing a model of recovery from depression. Journal of Abnormal Psychology. 1990;99:156–165. doi: 10.1037//0021-843x.99.2.156. [DOI] [PubMed] [Google Scholar]
- Paykel ES. The evolution of life events research in psychiatry. Journal of Affective Disorders. 2001;62:141–149. doi: 10.1016/s0165-0327(00)00174-9. [DOI] [PubMed] [Google Scholar]
- Shen GC, Alloy LB, Abramson LY, Grandin LD. Social rhythm regularity and the onset of affective episodes in bipolar spectrum individuals. Bipolar Disorders. doi: 10.1111/j.1399-5618.2008.00583.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RA, Endicott J. Biometrics Research. New York State Psychiatric Institute; 1978. Schedule for Affective Disorders and Schizophrenia – Change version. [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: Rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Szuba MP, Yager A, Guze BH, Allen EM, Baxter LR. Disruption of social circadian rhythms in major depression: a preliminary report. Psychiatry Research. 1992;42:221–230. doi: 10.1016/0165-1781(92)90114-i. [DOI] [PubMed] [Google Scholar]
- Thase ME, Jindal R, Howland RH. Biological aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York: Guilford Press; 2002. pp. 192–218. [Google Scholar]