Abstract
Uric acid, despite being a major antioxidant in the human plasma, both correlates and predicts development of obesity, hypertension, and cardiovascular disease, conditions associated with oxidative stress. While one explanation for this paradox could be that a rise in uric acid represents an attempted protective response by the host, we review the evidence that uric acid may function either as an antioxidant (primarily in plasma) or pro-oxidant (primarily within the cell). We suggest that it is the pro-oxidative effects of uric acid that occur in cardiovascular disease and may have a contributory role in the pathogenesis of these conditions.
Keywords: Uric acid, redox homeostasis, metabolic syndrome, cardiovascular disease
Uric acid is a final enzymatic product in the degradation of purine nucleosides and free bases in humans and Great Apes. The pathway of purine catabolism in humans is shortest among vertebrates because about 8–20 million years ago during primate evolution the activity of urate oxidase (uricase, an enzyme catalyzing conversion of uric acid to allantoin) was lost in a two-step mutation process.[1,2] In other mammals, the last enzymatic product of purine degradation chain is allantoin, which is excreted in the urine. Lower vertebrates (e.g., fish) have enzymes that further degrade allantoin to allantoic acid and glyoxylic acid and finally to urea. As a consequence, humans have to cope with relatively higher levels of uric acid in the blood (200–400 μM) and are prone to hyperuricemia and gout.[3]
According to a hypothesis championed in the early eighties by Ames et al.,[4] the silencing of the uricase gene with an increase in the blood level of uric acid provided an evolutionary advantage for ancestors of Homo sapiens. This hypothesis was based on in vitro experiments which showed that uric acid is a powerful scavenger of singlet oxygen, peroxyl radicals (RO·2) and hydroxyl radicals (·OH). Urate circulating in elevated concentrations was proposed to be one of the major antioxidants of the plasma that protects cells from oxidative damage, thereby contributing to an increase in life span of our species and decreasing the risk for cancer.
On the other hand, a vast literature on the epidemiology of cardiovascular disease, hypertension, and metabolic syndrome overwhelmingly shows that, at least among modern Homo sapiens, a high level of uric acid is strongly associated and in many cases predicts development of hypertension,[5–7] visceral obesity,[8–10] insulin resistance,[8,11,12] dyslipidemia,[8,11–13] diabetes type II,[11] kidney disease,[6] and cardiovascular and cerebrovascular events.[6,14] Despite the proposed beneficial role of uric acid, hyperuricemic patients have a higher rate of cardiovascular and all-cause mortality in comparison to subjects with normal levels of uric acid.[15] Although the pathogenesis of these diseases is extremely complex and incompletely understood, it is clear that oxidative stress and oxidative modifications of proteins and lipids is, paradoxically, common for all of them.[16–20] Together with the known ability of uric acid to become pro-oxidant under certain conditions discussed below, this creates the urate oxidant-antioxidant paradox, which, we believe, can be resolved by the analysis of the effects of uric acid in various physicochemical circumstances, in different compartments of the human organism and at the different levels of biological organization.
ANTIOXIDANT FUNCTION OF URIC ACID AND ITS LIMITATIONS
The ability of urate to scavenge oxygen radicals and protect the erythrocyte membrane from lipid oxidation was originally described by Kellogg and Fridovich,[21] and was characterized further by Ames et al.[4] Although these experiments defined a paradigm, they addressed effects of uric acid under specific conditions in which exogenously added uric acid protected cells from oxidants, which were also added exogenously to aqueous incubation media. This kind of condition is undoubtedly relevant to a variety of physiological situations when circulating uric can scavenge reactive radicals released into the blood by deleterious reactions, such as autoxidation of hemoglobin or peroxide production by macrophages.[4] However, even in the plasma urate can prevent lipid peroxidation only as long as ascorbic acid is present.[22]
One of the major sites where the anti-oxidant effects of uric acid have been proposed is in the central nervous system, particularly in conditions such as multiple sclerosis, Parkinson’s disease, and acute stroke.[23–26] While chronic elevations in uric acid are associated with increased stroke risk,[27,28] acute elevations in uric acid may provide some anti-oxidant protection. For example, uric acid protects cultured rat hippocampal neuronal cells from oxidative stress,[29] and administration of uric acid 24 hours prior to middle artery occlusion also attenuated brain injury induced by acute ischemia in rats.[29]
Uric acid administration is also beneficial in the mouse model of experimental allergic encephalomyelitis (EAE)[25] that has been proposed to have similarities to multiple sclerosis. In EAE uric acid was found to block peroxynitrite (ONOO−) mediated nitrosylation of neuronal proteins and to block the increase in the blood brain barrier resulting in less infiltration of leukocytes.[25] Interestingly, ascorbate also blocked nitrotyrosine formation but did not block the blood brain barrier and did not provide protection.[26] One possibility is that the protective effects of uric acid may not be due to direct scavenging of peroxynitrite in neurons by urate but may be due to other effects of uric acid to block the blood brain barrier, such as by decreasing the level of endothelial nitric oxide, because nitric oxide inhibitors decreased EAE severity,[30] while uric acid decreased bioavailability of nitric oxide in endothelial cells.[31]
In addition to neurons, the administration of uric acid decreased neutrophil infiltration and injury of the liver during hemorrhagic shock.[32] These findings suggest that an ability of uric acid to prevent acute activation of proinflammatory cells in the blood by oxidants might be a beneficial mechanism by which uric acid manifests an overall antioxidant activity.
Considering the association of uric acid with high blood pressure and heart disease mentioned above, an important question is whether uric acid can provide an antioxidant protection for cells of the cardiovascular system. Some studies suggest this possibility. In the perfused guinea pig heart, the presence of physiologic concentrations of uric acid in the perfusate improved functional responses and functional stability of the heart impaired by the presence of oxidants.[33] Another study has identified among the products of urate-ONOO− interaction in human plasma a compound with an activity of an NO donor.[34] On the other hand, uric acid in vitro decreased NO bioavailability in bovine aortic endothelial cells[31] as well as adipocytes.[35] It still remains to be established, though, whether these effects have physiological significance and whether urate in general provides antioxidant protection to the heart in vivo.
Certain common components of the chemical milieu in the organism may affect the antioxidant ability of uric acid. As mentioned above, the presence of ascorbic acid in the plasma is required for the antioxidant effect of uric acid.[22] In a comprehensive study, Kuzkaya et al.[36] showed that uric acid is a unique scavenger of peroxynitrite in the extracellular space. However, uric acid cannot scavenge superoxide (O−2), and the presence of ascorbic acid and thiols is absolutely required for complete scavenging of peroxynitrite. Neither of these antioxidants alone can prevent reaction of peroxynitrite with tetrahydrobiopterin, which leads to uncoupling of NO synthase.[36] Other compounds, frequently present in biological fluids, have the opposite effect and can disable uric acid as an antioxidant. The presence of bicarbonate substantially inhibits the ability of uric acid to prevent tyrosine nitrosylation, a crucial mechanism of the oxidative damage of proteins in the cell.[37]
Uric acid is a powerful scavenger of carbon-centered and peroxyl radicals in the hydrophilic environment but loses an ability to scavenge lipophilic radicals and cannot break the radical chain propagation within lipid membranes.[38] At the same time, peroxynitrous radicals are extremely diffusible through the membrane,[39] and the hydrophobic environment is also more favorable for tyrosine nitration.[40,41] Thus, these physicochemical findings could explain why the antioxidant effects of uric acid are manifested only in the hydrophilic environment of biological fluids, such as plasma.
URIC ACID AS A PRO-OXIDANT AND PROINFLAMMATORY FACTOR
Initial experiments by Ames et al.[4] showed that when urate at physiological concentration (300 μM) was exposed to singlet oxygen, it rapidly degraded (55% in 5 minutes), and the authors suggested that the end product of this reaction was allantoin. Subsequent studies demonstrated that the chemistry of urate oxidation by radicals is much more complicated. Uric acid can form free radicals in a variety of radical-forming systems[42] including the pathophysiologically relevant interaction with peroxynitrite.[43] Urate-derived radicals identified so far represent different degrees of degradation of the urate molecule, from the urate anion, in which the radical site is located on the five-membered ring of the purine structure,[42] to carbon-centered radicals[36,44] such as aminocarbonyl[36,44] formed after breakdown of the purine structure due to the ONOO− attack. At least some of these products can be cytotoxic.[43] Urate at the concentration 500 μM amplified oxidation of LDL and liposomes by peroxynitrite[44] suggesting that aminocarbonyl or other radicals formed from uric acid can propagate oxidative damage. Uric acid can also oxidize LDL in the presence of copper ions (Cu+ and Cu++) and lipid hydroperoxides.[45] The mechanism of these reactions is complex and remains incompletely understood.[45]
Thus, uric acid can become a pro-oxidant by forming radicals in reactions with other oxidants, and these radicals seem to target predominantly lipids (LDL and membranes) rather than other cellular components. At the same time, the hydrophobic environment created by lipids is unfavorable for the antioxidant effects of uric acid,[38] and oxidized lipids can even convert uric acid into an oxidant.[45] Given this background and association of hyperuricemia with obesity one may infer that uric acid has a direct effect on the adipose tissue, and this effect might have a redox-dependent component.
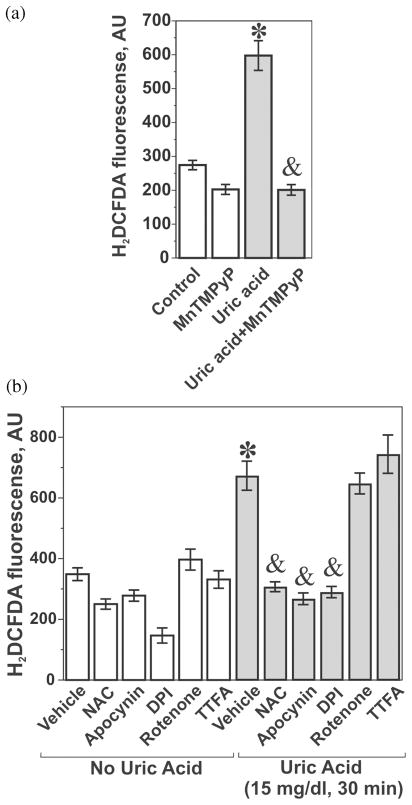
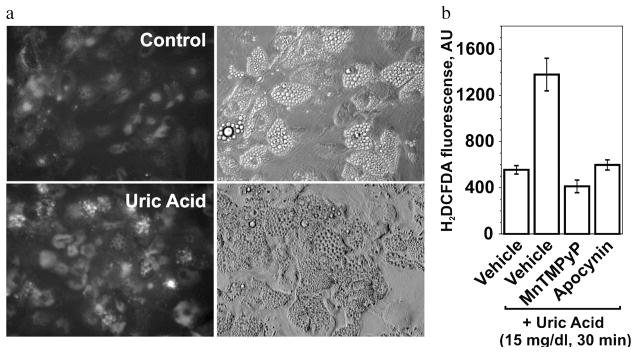
Well-established model of adipocytes differentiated in vitro from the mouse embryonic fibroblast cell line 3T3-L1[46,47] were used to characterize the effects of uric acid. An interesting phenotypic feature of differentiated adipocytes is a substantial uptake rate for urate, an expression of the urate transporters of OAT family URAT1 and OAT4, and high basal level of production of reactive oxygen species (ROS).[35] An increase in the oxidant production in adipocytes is an accompanying factor and a consequence of the lipid accumulation during differentiation.[17] We have found that the inclusion of uric acid in the media during the differentiation process or short-term incubation with differentiated adipocytes resulted in additional generation of ROS (Figure 1A and Sautin et al.[35]). This effect was blocked by the cell-permeable mimetic of superoxide dismutase MnTMPyP, which is a specific superoxide scavenger demonstrating that reactive oxygen intermediates induced by uric acid in adipocytes depend on superoxide generation (Figure 1A). The effect of uric acid was attenuated by the general antioxidant N-acetyl-cysteine as well as by apocynin and diphenylene-iodonium (DPI), inhibitors of NADPH oxidase, while rotenone and TTFA, blockers of the mitochondrial electron-transport chain, were without effect (Figure 1B). Uric acid induced similar effects on ROS production in human subcutaneous primary adipocytes (Figure 2A). The superoxide scavenger MnTMPyP and apocynin attenuated the effect of uric acid (Figure 2B) suggesting an involvement of NADPH oxidase-dependent superoxide. In subsequent experiments we showed that: (1) 3T3-L1 adipocytes express several isoforms of NADPH oxidase including NOX4 (predominant form), NOX3 and the classical phagocyte type NADPH oxidase NOX2 (gp91phox); (2) uric acid increased the enzymatic activity of NADPH oxidase; and (3) the mechanism of NOX activation involves translocation of regulatory subunits p67phox and p40phox from the cytoplasm to membranes with assembly of the active holoenzyme on the membrane.[35] On the other hand, activation of NOX4, a predominant NADPH oxidase in our adipocyte model, generally does not require translocation of cytoplasmic subunits;[48] therefore, the mechanism of activation of NOX4 by uric acid remains to be determined. These experiments demonstrate that uric acid can become a pro-oxidant by engaging an intracellular system of superoxide generation by NADPH oxidases. This family of enzymes represents one of the major sources of reactive oxygen species and oxidative stress in many cell types and tissues. Hormones, cytokines, and activators of the innate immunity response activate different isoforms of this enzyme depending on the cell type and the agonist. NOX catalyzes the reaction between molecular oxygen and NADPH associated with transport of electrons across the membrane and yields superoxide, which is converted into other oxygen radicals. All these ROS become involved in the redox-dependent signal transduction, immune defense and/or oxidative damage.[49–52]
FIGURE 1.
Uric acid induces production of reactive oxygen species in mouse adipocytes. Differentiated mouse adipocytes were treated with 15 mg/ml uric acid for 30 minutes with or wirhout 25 μM Mn(II) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP), N-acetyl cysteine (10 mM), apocynin (200 μM) and diphenylene-iodonium (DPI, 10 μM), rotenone (100 μM), and thenoyltrifluoroacetone (TTFA, 100 μM) followed by the measurement of ROS with the ROS-specific fluorescent probe 5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (CM-H2DCFDA) as described in details in (33). (A) Effect of uric acid is blocked by the superoxide scavenger MnTMPyP; (B) Effect of uric acid is blocked by inhibitors of NADPH oxidase apocynin and DPI but not the inhibitors of mitochondrial respiratory chain rotenone and TTPA. *— the effect of uric acid is significant (P < 0.05, nonparametric U-test); & —the effect of the inhibitor is significant (P < 0.05, U-test, n = 3). (Reproduced from (33)—used with the permission from the American Physiological Society)
FIGURE 2.
Uric acid induces production of reactive oxygen species in human subcutaneous primary adipocytes. Treatment with uric acid and inhibitors were performed as described in Figure 1. (A) ROS-induced fluorescence is shown in the left panels; images of the adipocytes by differential interference contrast (DIC) are shown in the right panels. (B) The effect of uric acid is blocked by the superoxide scavenger MnTMPyP and an inhibitor of NADPH oxidase apocynin. *— the effect of uric acid is significant (P < 0.05, nonparametric U-test); and — the effect of the inhibitor is significant (P < 0.05, U-test, n = 3).
Uric acid induced an activation of protein kinase p38 in adipocytes (Figure 3), as well as in other cell types,[53,54] and our experiments showed that superoxide and active NADPH oxidase were required for this activation because apocynin and MnTMPyP abrogated the effect (Figure 3). These data confirm that intracellular signaling induced by uric acid in adipocytes is, indeed, redox-dependent.
FIGURE 3.
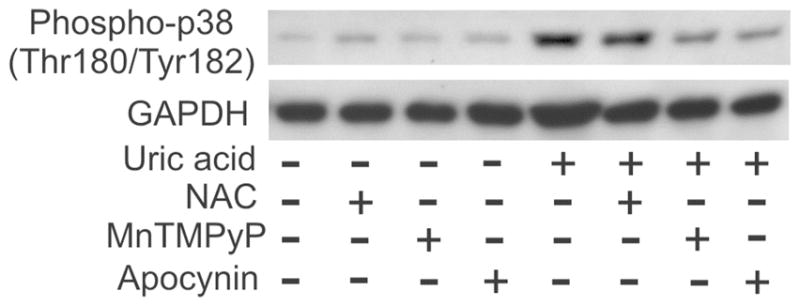
Activation of p38 kinase in adipocytes in response to uric acid is mediated by NADPH oxidase- and superoxide-dependent redox mechanism. Adipocytes were treated with 15 mg/dl uric acid for varying periods of time with or without antioxidants or an inhibitor of NADPH oxidase. Activated (phosphorylated p38 was detected by immunoblotting with phosphospecific (Thr180/Tyr182) p38 antibody and reprobed with GAPDH antibody as a control of equal protein loading/transfer. Reproduced from (33) —used with the permission from the American Physiological Society)
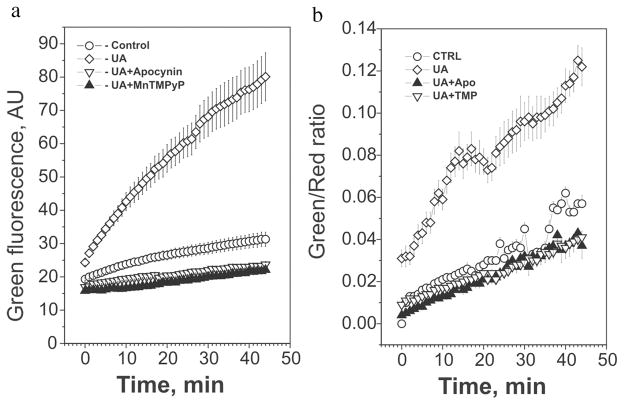
Addition of uric acid to the medium induced rapid oxidation of lipids detected by ratiometric live imaging of the oxidized lipids with oxidation-sensitive lipid-specific fluorescent probe C11-BODIPY 581,591 that accumulates in the cellular lipids and shifts fluorescence from red to green upon lipid peroxidation (Figure 4). The presence of MnTMPyP and apocynin completely prevented this effect of uric acid demonstrating an involvement of NOX-dependent superoxide. Uric acid also induced a moderate increase in tyrosine nitrosylation of several proteins in adipocytes suggesting formation of peroxynitrite.[35] Differentiated adipocytes produce simultaneously significant amounts of superoxide and nitric oxide,[35] which can rapidly interact with each other yielding ONOO−. As discussed earlier, the hydrophobic environment in the adipocyte is unfavorable for antioxidant effects of uric acid but is favorable for the diffusion of peroxynitrous radicals in the hydrophobic phase, which, in its turn, favors tyrosine nitrosylation. However, the biological significance of the effect of uric acid to induce protein nitrosylation remains unclear.
FIGURE 4.
Uric acid induces NADPH oxidase-dependent lipid oxidation in mouse adipocytes. Adipocytes were treated with 15 mg/ml uric acid with or without superoxide scavenger MnTMPyP and an inhibitor of NADPH oxidase apocynin as indicated in Figure 1. Cells were loaded with the oxidation-sensitive lipid probe C11-BODIPY581,591 followed by washing cells to remove unincorporated probe. Detection of lipid peroxidation by live imaging was started immediately after addition of uric acid. (A) Green fluorescence representing oxidized probe was detected every 60 seconds for 100 ms after addition uric acid. (B) Ratiometric analysis of lipid oxidation: ratio of green/red fluorescence measured every 60 seconds is shown. The values for 12–20 different cells (mean ± SD) are shown.
An important unanswered question is the upstream mechanism responsible for the activation of NADPH oxidase in response to uric acid and whether this effect is specific for adipocytes. We can speculate that this effect might be related to the recently discovered biological role of uric acid as a molecular signal, which alerts the immune system of dying cells.[55] This response is mediated by activation of the IL-1 receptor coupled with the myeloid differentiation primary response protein 88 (MyD88) involved in innate immunity.[56] This mechanism could be relevant to adipocytes because of the functional and developmental proximity of adipocytes and macrophages that allows conversion of one phenotype into the other.[57,58]
The pro-oxidative effect of uric acid mediated by NOX-dependent superoxide and redox signaling might be important for overall balance of endocrine activity in adipose tissue. Oxidative stress induced by obesity is an important factor in shifting the balance of adipose-specific hormones and cytokines (adipokines) from the normal phenotype facilitating insulin sensitivity and maintaining vascular homeostasis towards a phenotype characterized by low-grade inflammation, insulin resistance, and dyslipidemia.[17,59] The proposed mechanism, which involves urate-induced oxidative stress in adipocytes, is summarized in Figure 5.
FIGURE 5.
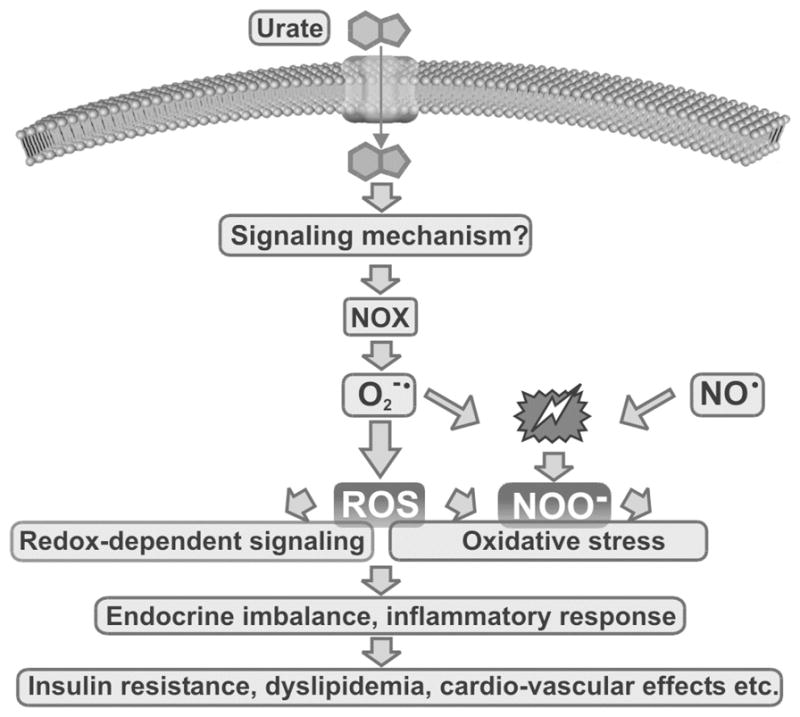
A model for urate-induced oxidative stress in adipocytes.
CONCLUSION
In conclusion, uric acid is involved in a complex reaction with several oxidants and may have some protective effects under certain conditions. On the other hand, uric acid cannot scavenge all radicals, with superoxide as an example. Uric acid is an antioxidant only in the hydrophilic environment, which is probably a major limitation of the antioxidant function of uric acid. Reactions of uric acid with oxidants may also produce other radicals that might propagate radical chain reaction and oxidative damage to cells. In addition, uric acid itself and/or downstream radicals can engage, as a biologically active proinflammatory factor, intracellular oxidant production via the ubiquitous NADPH oxidase-dependent pathway resulting in redox-dependent intracellular signaling and, in some conditions, oxidative stress. In our opinion, these considerations taken together may explain the oxidant-antioxidant paradox.
Acknowledgments
Supported by NIH grants HL-68607 (R.J.J.), grant from the American Heart Association 0755595B (Y.S.) and generous funds from Gatorade.
Footnotes
Conflict of Interest: Dr. Johnson has patent applications related to the lowering of uric acid as a means for treating cardiovascular disease and obesity via the University of Florida and University of Washington.
References
- 1.Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 2.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RJ, Kang DH, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. In: Uric acid in Cardiovascular and Renal Disease. Semin Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alper AB, Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–38. doi: 10.1161/01.HYP.0000150783.79172.bb. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sanchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16:1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 8.Bonora E, Targher G, Zenere MB, Saggiani F, Cacciatori V, Tosi F, Travia D, Zenti MG, Branzi P, Santi L, Muggeo M. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord. 1996;20:975–980. [PubMed] [Google Scholar]
- 9.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 10.Ogura T, Matsuura K, Matsumoto Y, Mimura Y, Kishida M, Otsuka F, Tobe K. Recent trends of hyperuricemia and obesity in Japanese male adolescents, 1991 through 2002. Metabolism. 2004;53:448–453. doi: 10.1016/j.metabol.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 12.Zavaroni I, Mazza S, Fantuzzi M, Dall’Aglio E, Bonora E, Delsignore R, Passeri M, Reaven GM. Changes in insulin and lipid metabolism in males with asymptomatic hyperuricaemia. J Intern Med. 1993;234:25–30. doi: 10.1111/j.1365-2796.1993.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara M, Chiba H, Maruoka S, Katayose S. Elevated serum leptin concentrations in women with hyperuricemia. J Atheroscler Thromb. 2002;9:28–34. doi: 10.5551/jat.9.28. [DOI] [PubMed] [Google Scholar]
- 14.Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150. doi: 10.1161/01.hyp.34.1.144. [DOI] [PubMed] [Google Scholar]
- 15.Tomita M, Mizuno S, Yamanaka H, Hosoda Y, Sakuma K, Matuoka Y, Odaka M, Yamaguchi M, Yosida H, Morisawa H, Murayama T. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10:403–409. doi: 10.2188/jea.10.403. [DOI] [PubMed] [Google Scholar]
- 16.Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am J Physiol Endocrinol Metab. 2004;287:E1178–1188. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 19.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 20.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellogg EW, 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977;252:6721–6728. [PubMed] [Google Scholar]
- 22.Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaro S, Soy D, Obach V, Cervera A, Planas AM, Chamorro A. pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke. 2007;38:2173–2175. doi: 10.1161/STROKEAHA.106.480699. [DOI] [PubMed] [Google Scholar]
- 24.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 25.Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitsin SV, Scott GS, Mikheeva T, Zborek A, Kean RB, Brimer CM, Koprowski H, Hooper DC. Comparison of uric acid and ascorbic acid in protection against EAE. Free Radic Biol Med. 2002;33:1363–1371. doi: 10.1016/s0891-5849(02)01048-1. [DOI] [PubMed] [Google Scholar]
- 27.Mazza A, Pessina AC, Pavei A, Scarpa R, Tikhonoff V, Casiglia E. Predictors of stroke mortality in elderly people from the general population. The CArdiovascular STudy in the ELderly. Eur J Epidemiol. 2001;17:1097–1104. doi: 10.1023/a:1021216713504. [DOI] [PubMed] [Google Scholar]
- 28.Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34:1951–1956. doi: 10.1161/01.STR.0000081983.34771.D2. [DOI] [PubMed] [Google Scholar]
- 29.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Danilov AI, Jagodic M, Wiklund NP, Olsson T, Brundin L. Effects of long term NOS inhibition on disease and the immune system in MOG induced EAE. Nitric Oxide. 2005;13:188–195. doi: 10.1016/j.niox.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsukada K, Hasegawa T, Tsutsumi S, Katoh H, Kuwano H, Miyazaki T, Yamamoto Y. Effect of uric acid on liver injury during hemorrhagic shock. Surgery. 2000;127:439–446. doi: 10.1067/msy.2000.104486. [DOI] [PubMed] [Google Scholar]
- 33.Becker BF, Reinholz N, Ozcelik T, Leipert B, Gerlach E. Uric acid as radical scavenger and antioxidant in the heart. Pflugers Arch. 1989;415:127–135. doi: 10.1007/BF00370582. [DOI] [PubMed] [Google Scholar]
- 34.Skinner KA, White CR, Patel R, Tan S, Barnes S, Kirk M, Darley-Usmar V, Parks DA. Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J Biol Chem. 1998;273:24491–24497. doi: 10.1074/jbc.273.38.24491. [DOI] [PubMed] [Google Scholar]
- 35.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classical antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:584–596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 36.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann NY Acad Sci. 2002;962:242–259. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 38.Muraoka S, Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. 2003;93:284–289. doi: 10.1111/j.1600-0773.2003.pto930606.x. [DOI] [PubMed] [Google Scholar]
- 39.Khairutdinov RF, Coddington JW, Hurst JK. Permeation of phospholipid membranes by peroxynitrite. Biochemistry. 2000;39:14238–14249. doi: 10.1021/bi001270x. [DOI] [PubMed] [Google Scholar]
- 40.Bartesaghi S, Ferrer-Sueta G, Peluffo G, Valez V, Zhang H, Kalyanaraman B, Radi R. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids. 2007;32:501–515. doi: 10.1007/s00726-006-0425-8. [DOI] [PubMed] [Google Scholar]
- 41.Bartesaghi S, Valez V, Trujillo M, Peluffo G, Romero N, Zhang H, Kalyanaraman B, Radi R. Mechanistic studies of peroxynitrite-mediated tyrosine nitration in membranes using the hydrophobic probe N-t-BOC-L-tyrosine tert-butyl ester. Biochemistry. 2006;45:6813–6825. doi: 10.1021/bi060363x. [DOI] [PubMed] [Google Scholar]
- 42.Maples KR, Mason RP. Free radical metabolite of uric acid. J Biol Chem. 1988;263:1709–1712. [PubMed] [Google Scholar]
- 43.Vasquez-Vivar J, Santos AM, Junqueira VB, Augusto O. Peroxynitrite-mediated formation of free radicals in human plasma: EPR detection of ascorbyl, albumin-thiyl and uric acid-derived free radicals. Biochem J. 1996;314 (Pt 3):869–876. doi: 10.1042/bj3140869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372:285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- 45.Bagnati M, Perugini C, Cau C, Bordone R, Albano E, Bellomo G. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: a study using uric acid. Biochem J. 1999;340 (Pt 1):143–152. [PMC free article] [PubMed] [Google Scholar]
- 46.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 47.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu RevCell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 48.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 49.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 50.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 51.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes–prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 53.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 54.Kang DH, Park SK, Lee IK, Johnson RJ. Uric Acid-Induced C-Reactive Protein Expression: Implication on Cell Proliferation and Nitric Oxide Production of Human Vascular Cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 55.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 56.Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 58.Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, et al. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 59.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]