Abstract
Background
Human Neutrophil Peptide 1 (HNP-1) is a defensin with antibacterial activity secreted by various cells as a component of the innate immune host defense. NOD2 is a cytoplasmic protein that recognizes bacterial derived muramyl dipeptide, and is involved in bacterial clearance. The aim of the present study was to investigate the relationship between antibacterial activity of NOD2 and HNP-1 expression in epithelial cell lines.
Methods
Gentamicin protection assay using Salmonella Typhimurium was performed in Caco-2 cells. mRNA level was determined by quantitative RT-PCR and defensin expression was assessed by Western Blot and ELISA. Nuclear Factor-κB activation was assessed using pIV luciferase and renilla plasmids. NOD2 mutant was generated by site-directed mutagenesis.
Results
Among the defensins tested, only HNP-1 expression is induced in colonic epithelial model HCT116 cells after MDP-LD stimulation. HNP-1 secretion is significantly increased after MDP-LD stimulation in the cell supernatant of intestinal epithelial cells expressing endogenous NOD2, but not in cells which lack endogenous NOD2 expression. HNP-1 is required for NOD2 dependent NF-κB activation after MDP-LD stimulation since hnp-1 siRNA transfection abrogated response to MDP-LD stimulation. The antibacterial function of NOD2 against S. Typhimurium was impaired when expression of HNP-1 was blocked by siRNA.
Conclusions
HNP-1 secretion depends on NOD2 stimulation by MDP-LD and contributes to antibacterial activity in intestinal epithelial cells expressing endogenous NOD2, but not NOD2 3020insC mutant associated with increased susceptibility to Crohn’s disease.
Keywords: Crohn’s disease, NOD2, HNP-1, invasive bacteria
INTRODUCTION
The human intestinal tract contains a wide variety of microbial flora. Experimental rodent models of chronic immune-mediated colitis conclusively implicate commensal enteric bacteria and genetically determined defects in bacterial killing by innate immune cells are found in a subset of patients with Crohn’s disease (for a review, (1)). When the mucosal barrier is breached, invading pathogens are commonly contained and ultimately eliminated by the host immune system. Innate immunity represents a first line of host defense that is rapidly mobilized following the detection of microbial invasion. Human cells detect bacteria by pattern recognition molecules that confer responsiveness towards bacterium-derived products (2). Bacterial cell wallcomponents such as lipopeptides and peptidoglycan (PG) are among those products which elicit innate immune response. These molecules trigger immune responses by interactions with members of the family of Toll-like receptors at the cell membrane and NACHT-LRR proteins (NLRs) in the cytosol (3–5). NOD2, a member of the NLR family, responds to intracellular localized muramyl-dipeptide (MDP), the minimal bioactive component of bacterial PG (6). Of note, mutations in the nod2 gene are linked to the onset of Crohn’s disease, aninflammatory disorder of the gastrointestinal tract (7–9).
NOD2 (CARD15) is a member of the Apaf/CARD family of cytosolic proteins involved in apoptosis (10). This protein contains 2 caspase activating recruitment domains (CARDs), a central nucleotide binding domain (NBD), and a leucine-rich repeat (LRR) region. NOD2 is expressed by macrophages and dendritic cells as well as intestinal epithelial cells (IEC) and can activate Nuclear Factor kappa B (NF-κB) following intracellular recognition of muramyl-dipeptide (MDP) from Gram positive and Gram negative bacteria (6, 10). Sensing of MDP is mediated through theLRR domain, leading to downstream signaling by a homophilic interaction of the CARD of RIP2 with the CARDs of NOD2 (6, 11). Several groups have endeavored to identify and to characterize proteins which interact directly with NODs in order to better understand their functional effects and mechanisms of action. These efforts have previously highlighted the roles of GRIM-19 (12), transforming growth factor beta-activated kinase1 (TAK1) (13), Ipaf (14), Erbin (15, 16) as well as centaurin β1 (17) in the regulation of NOD2-mediated NF-κB activation.
NOD2 mediated sensing of bacterial products, appears to facilitate control of bacterial infection. In vitro, NOD2 has been found to be involved in the bacterial clearance of Salmonella and Streptococcus species (18, 19), and nod2−/− knock-out mice display increased susceptibility to peroral Listeria monocytogenes infection, a finding paralleled by a decreased Paneth cell secretion of some antibacterial cryptidins (20). In contrast, NOD2 does not appear to play a role in the clearance of Yersinia pseudotuberculosis in a murine model (21). Interestingly, bacterial infection with commensal E. coli K-12 positively regulates nod2 mRNA transcription and NOD2 protein level in human IEC (22). In addition, MDPinduced a significant antibacterial peptide response in IEC with release of CCL20 from the apical pole. This was abolished in IEC expressing NOD2-L1007fs. Patients with ileal CD have been reported to exhibit decreased mRNA for two alpha-defensins, HD-5 and HD-6, in involved ileal mucosa compared to uninvolved ileal mucosa (23, 24). Although one study noted correlation of altered defense expression with mutant NOD2, another study found that reduction in HD-5 and HD-6 was not associated with NOD2 status and was instead dependent on the presence of inflammation (25). Together, these studies underscore the importance of NOD2 in bacterial clearance in the intestinal epithelium, and suggest that the CD-specific mutated NOD2-L1007fs can lead to a functionally impaired epithelial barrier.
Antimicrobial peptides including defensins have been recognized as an important component of mucosal immunity (26, 27). The defensins have microbicidal and cytotoxic properties. Vertebrate defensins comprise three subfamilies: α-defensins (HNP-1 to HNP-4, HD-5 and HD-6), β-defensins (HBD-1 to HBD-6) and θ-defensins. Six α-defensins have been reported in humans, among which human neutrophil peptide-1 (HNP-1), also designated as DEFA1, is predominantly expressed in normal bone marrow cells, neutrophils, NK and T cells (28, 29). However, HNP-1 expression is also found in epithelial cells in association with CD and UC (30). In this study, we examined the relationship between NOD2 activation and NOD2 dependent expression of HNP-1 in IEC with anti-bacterial activity.
MATERIALS AND METHODS
Cell culture and transfection
HEK293, SW480, HT-29, LS174, Caco-2, T84, Colo201 and HCT116 cells were obtained from the American Type Culture Collection (Manassas, VA) and grow until 90% to 100% confluent. Cells were cultured as described previously (18). Cells were transfected with a cationic lipid (Lipofectamine 2000; Invitrogen) according to the manufacturer’s protocols.
Expression plasmids
cDNA of HNP-1 was obtained from the above mentioned cell lines by PCR using Expand High Fidelity PCR system (Roche). PCR primers were forward: 5′-atgaggaccctcgccatccttgc-3′ and reverse: 5′-tcagcaatgcccagagtcttcc-3′. An expression plasmid-encoding Xpress-tagged HNP-1 mammalian expression vector (pCDNA4/HisMAX-HNP-1) was generated by RT-PCR from HCT116 cell cDNA library. The sequence of HNP-1 cDNA was confirmed using an ABI 3700 PRISM (Perkin Elmer) automated sequencer. An expression plasmid-encoding FLAG-tagged NOD2 (pCMV FLAG-NOD2) was previously generated (18). Two target sequences for hnp-1 siRNA (1: caggagaacgtcgctatggaa and 2: atggcctgctattgcagaata), for nod2 siRNA (1: gaacgtcatgctagaagaa and 2: ttgcagaagttagctctat) as well as control siRNA (aattctccgaacgtgtcacgt) were purchased from QIAGEN. Depletion of endogenous HNP-1 or NOD2 expression was confirmed by RT-PCR.
Assessment of hnp-1 mRNA
Total RNA from intestinal epithelial cell lines was extracted with TRIzol (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. For real-time polymerase chain reaction (RT-PCR), 1μg of total RNA was reverse transcribed using Super Script First-strand Synthesis System (Invitrogen). RT-PCR was performed in an ABI Prism 7000 Sequence Detector using SYBR Green JumpStart™ detection system. Briefly, 50 ng of the reversed transcribed cDNA was used for each PCR reaction with 200 nM of forward and reverse primers. Primers for HNP-1 were designed (forward: 5′-gcaagagctgatgaggttgctg-3′ and reverse 5′-ctggtagatgcaggttccatag-3′). The PCR program with specific primers was as follows: 94°C 1 min, 66°C 1 min and 72°C 1 min followed by 40 cycles and final extension at 72°C 10 min. PCR products were sequenced using ABI 3700 PRISM (Perkin Elmer Boston, MA) automated sequencer. Sequences were analyzed using NCBI BLAST software.
NF-κB activation assays
HEK293 cells were transfected with NOD2-DNA and/or HNP-1-DNA constructs, or with siRNA directed against hnp-1 and nod2, plus pIV luciferase reporter and Renilla plasmids. At the same time, 1μg of MDP-LD (Sigma) was added. After a 24-h period, luciferase activity was measured using the dual luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer’s instructions and normalized relative to Renilla activity.
Bacterial invasion assays
Invasion assays were performed with Salmonella enterica serovar Typhimurium. Briefly, monolayers were seeded in 24-well tissue culture plate with 105 cells/well and incubated for 20 hours. Monolayers were infected in 1 ml of the cell culture medium without antibiotics and with heat-inactivated fetal calf serum at a multiplicity of infection (MOI) of 10 bacteria/epithelial cell. After 2 h at 37°C, monolayers were washed two times with phosphate-buffered saline (PBS; pH 7.2). Fresh cell culture medium containing 100 μg/ml of gentamicin (Sigma) was added for 1 h to kill extracellular bacteria. Epithelial cells were lysed with 1% Triton X-100 in deionized water. After diluting with PBS at the 1:10 dilutions, the cell lysates were plated on a Luria-Bertani agar plate for culture of intracellular bacteria. Plates were cultured at 37°C overnight to determine the number of colony-forming units (CFUs) recovered from the lysed monolayer.
Defensins secretion
HNP-1 secretion was quantified by ELISA using 96-well immunoplates coated and incubated with 50 μl rabbit anti-HNP-1 antibody diluted 1:500 to 1 μg/ml in 0.05 M carbonate buffer, pH 9.6 at 4°C for 20 h. Blocking was performed with 200 μl of 1% bovine serum albumin in PBS for 10 min at room temperature. After washing three times with 200 μl of PBS containing 0.1% Tween 20, 100 μl per well of cell culture supernatants and serial dilutions of HNP-1 recombinant protein in dilution buffer were incubated for 30 min at room temperature. Plates were washed three times with PBS containing 0.1% Tween 20 and incubated for 30 min at room temperature with 50 μl of biotinylated rabbit anti-HNP-1 antibody diluted 1:2500 (0.2 μg/ml) in PBS containing 0.01% Tween 20. After 3 washes with PBS containing 0.1% Tween, plates were filled with 50 μl/well of Streptavidin-POD diluted 1:10000 in PBS + 0.01% Tween 20 (Roche Diagnostics, Mannheim, Germany). The plates were then incubated for 30 min at room temperature, washed three times as described above, and incubated with the development agent 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS; Roche Diagnostics) for 15 min at room temperature in the dark. Absorbance was measured at 405 nm with multichannel photometer.
Statistical analysis
For analysis of the significance of differences in NF-κB, invasion, and mRNA levels, Student’s t-test was used for comparison of two groups of data. All experiments were repeated at least four times. A p value <0.05 was considered to be statistically significant.
ETHICAL CONSIDERATIONS
None to address.
RESULTS
Expression of HNP-1 is increased in colonic epithelial cells after MDP-LD stimulation
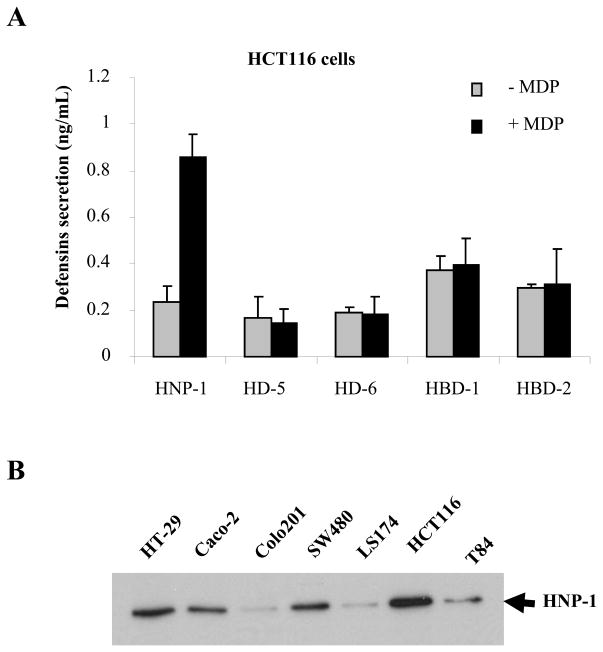
We analyzed the amounts of secreted human β-defensins HBD-1 and HBD-2, human α-defensins HD-5, HD-6, and HNP-1 without or after MDP-LD stimulation to activate NOD2 pathway using the HCT116 intestinal epithelial cell line which expresses endogenous NOD2. HCT116 intestinal epithelial cells (IEC) did not secrete α-defensins HD-5, HD-6 or β-defensins HBD-1 and HBD-2 either at baseline or following stimulation with MDP-LD (Fig. 1A). In contrast, MDP-LD stimulation of HCT116 cells significantly increased HNP-1 secretion in the cell supernatant as assessed by ELISA. Protein expression of HNP-1 was assessed by Western Blot in several human intestinal epithelial cells (SW480, HT-29, Caco-2, T84, Colo-205, HCT116). HNP-1 protein levels were high in HT-29, Caco-2, SW480 and HCT116 cell lines, and low in Colo201, LS174 and T84 cell lines (Fig. 1B).
Figure 1.
HNP-1 is secreted by IEC after MDP stimulation. (A) HCT116 intestinal epithelial cells were stimulated for 24 h with or without 1μg of MDP-LD and secretion of HD-5, HD-6, HBD-1, HBD-2 and HNP-1 measured by ELISA. (B) HNP-1 protein assessed by Western Blot. *p<0.05
HNP-1 expression is up-regulated by NOD2 stimulation in colonic epithelial cell lines
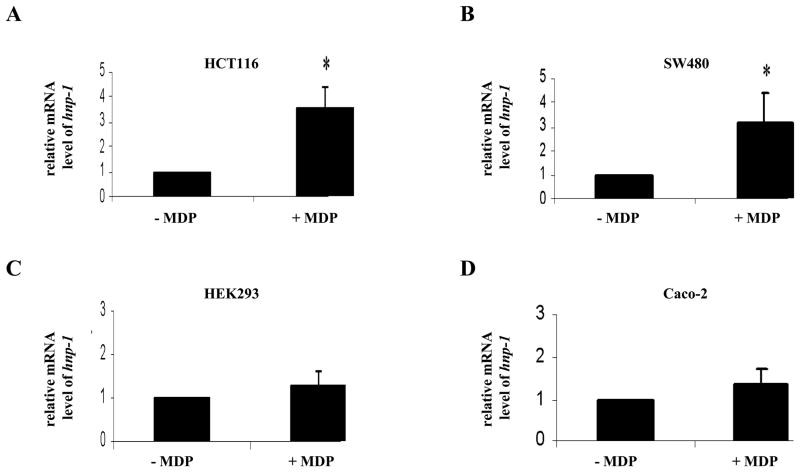
To investigate whether NOD2 regulates HNP-1 expression, we analyzed the effect of MDP-LD stimulation on hnp-1 mRNA expression in HCT116 and SW480 cell lines which express endogenous NOD2. After a 24-h stimulation period with 1μg of MDP-LD, hnp-1 mRNA was statistically significantly up-regulated 3.5 fold and 3.0 fold respectively in HCT116 and SW480 cell lines compared to non stimulated cells (Fig. 2A and B). In contrast, MDP-LD stimulation had no effect on hnp-1 mRNA expression by the HEK293 and Caco-2 cell lines which lack endogenous NOD2 expression (Fig. 2C and D). These results suggest that NOD2 stimulation increases HNP-1 expression.
Figure 2.
NOD2 activation by MDP-LD increased hnp-1 mRNA level. hnp-1 mRNA level was measured using quantitative RT-PCR and normalized for gapdh mRNA after 24 h of stimulation with 1μg of MDP-LD in NOD2 expressing HCT116 and SW480 cells (A,B) and non-NOD2 expressing cell lines HEK293 and Caco-2 (C,D). *p<0.05
HNP-1 is involved in NOD2-mediated NF-κB activation following MDP-LD stimulation
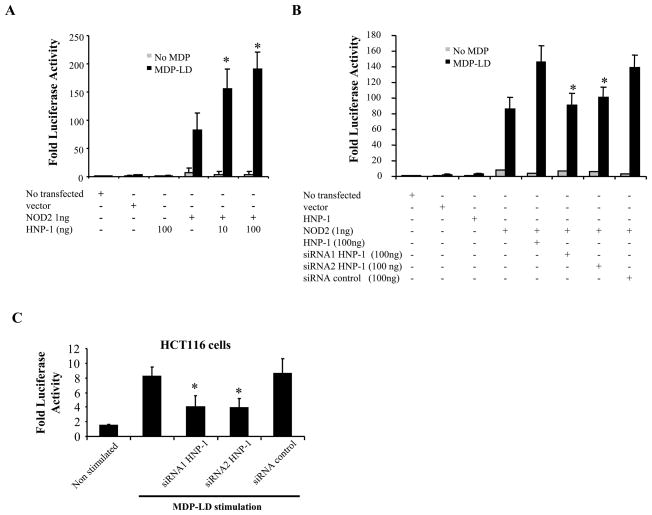
In order to determine whether HNP-1 expression influenced NOD2-mediated NF-κB activation, varying concentrations of constructs encoding HNP-1 and NOD2 were transiently transfected into HEK293 cells. Subsequent activation of NF-κB was measured using the NF-κB-driven luciferase reporter assay after a 24-h period of MDP-LD stimulation. HNP-1 expression enhanced NF-κB activation after MDP-LD stimulation in a concentration-dependent manner in HEK293 cells transfected with NOD2-encoding DNA (Fig. 3A). To confirm these results, NOD2 was overexpressed in HEK293 cells and HNP-1 expression was blocked using specific hnp-1 siRNA approach. After MDP-LD stimulation, NF-κB activation was significantly reduced in HEK293 cells overexpressing NOD2 and HNP-1 and transfected with hnp-1 siRNA compared to non transfected cells or cells transfected with control siRNA (Fig. 3B). This observation was confirmed by knocking-down endogenous HNP-1 expression in intestinal epithelial cells HCT116. NF-κB activation following endogenous NOD2 stimulation with MDP-LD in HCT116 cells was significantly decreased after hnp-1 siRNA transfection compared to non transfected cells or cells transfected with control siRNA (Fig. 3C). Together, these results indicate that HNP-1 is required for NOD2 NF-κB activation pathway.
Figure 3.
HNP-1 is required for NOD2-induced NF-κB pathway activation. (A) NF-κB luciferase assay was performed on HEK293 cells transfected with DNA encoding NOD2 (1ng), DNA encoding HNP-1 at different concentrations (10–100ng), NF-κB luciferase and Renilla reporter plasmids as well as empty vector. Cells were stimulated with 1 μg of MDP-LD for 18 h followed by measurement of luciferase and normalized with Renilla in cell lysates. *p<0.05 (B) Effect of specific siRNA against HNP-1 on ligand NOD2-dependent NF-κB activation. HEK293 cells were transiently transfected with the above mentioned plasmids and two different siRNA proven to knock down HNP-1 (100ng) as well as empty vector as control. *p<0.05 (C) HCT116 cells expressing endogenous NOD2 were transfected with two differents siRNA against HNP-1 and siRNA control and stimulated 36 h later with 1μg of MDP. NF-κB activation was measured 18 h after MDP stimulation as described above. *p<0.05
NOD2 activation enhances HNP-1 secretion by colonic epithelial cells
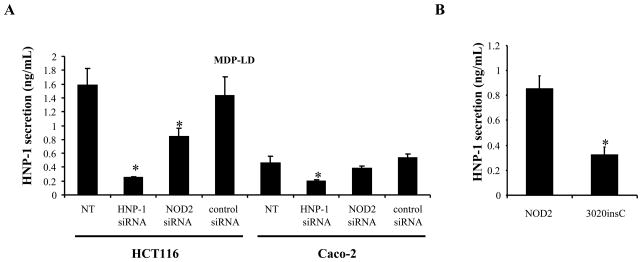
The role of NOD2 in mediating HNP-1 secretion was investigated in HCT116 and Caco-2 intestinal epithelial cells. Cells were stimulated with MDP-LD and transfected with specific siRNA directed against hnp-1 or nod2 genes and secretion of HNP-1 in the cell supernatant was assessed by ELISA. As control, hnp-1 siRNA significantly decreased HNP-1 secretion in the cell supernatant of both HCT116 and Caco-2 cells (Fig. 4A). Nod2 siRNA significantly decreased HNP-1 secretion only in HCT116 cells expressing endogenous NOD2, and had no effect on HNP-1 secretion by Caco-2 cells which lack endogenous NOD2. These results suggest that HNP-1 expression by IEC is NOD2 dependent. To assess the impact of mutation in NOD2 on HNP-1 peptide secretion, Caco-2 cells were transiently transfected with DNA constructs encoding wild-type NOD2 or the NOD2 3020insC (also named NOD2-L1007fs) mutant associated with CD and measured HNP-1 release in response to MDP-LD stimulation. HNP-1 secretion was significantly lower in response to MDP-LD when NOD2 3020insC mutant was overexpressed in Caco-2 compared to Caco-2 cells overexpressing wild-type NOD2 (Fig. 4B). Collectively, these results indicate that NOD2, but not NOD2 3020insC mutant, mediated defensin secretion by IEC.
Figure 4.
Secretion of HNP-1 after MDP stimulation is abrogated with NOD2 3020insC mutant CD associated. (A) Effect of hnp-1 or nod2 siRNA on human HNP-1 peptide release in HCT116 and Caco-2 cells 18 h after MDP-LD stimulation. (B) Caco-2 cells were transfected with DNA encoding NOD2 wild-type and DNA encoding the NOD2 3020insC CD associated mutant. After transfection, cells were stimulated with 1 μg of MDP-LD and HNP-1 peptide secretion was measured in the cell culture supernatants 18 h later by ELISA. *p<0.05
NOD2 exerts anti-bacterial activity after MDP-LD stimulation by inducing HNP-1 secretion
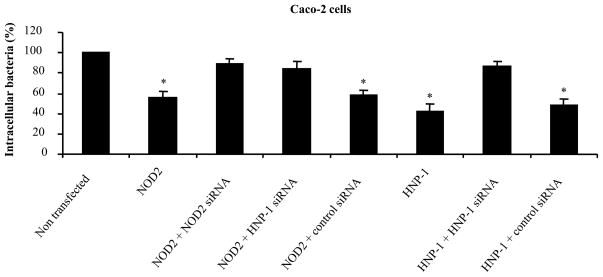
To determine whether NOD2-mediated HNP-1 secretion plays a role in the control of intracellular bacterial survival, we assessed the intracellular viability of S. Typhimurium in Caco-2 cells. After a 2-h incubation period with the epithelial monolayer, the percentage of intracellular bacteria was statistically significantly decreased in Caco-2 cells expressing HNP-1 or NOD2 protein compared to non-transfected cells (Fig. 5). Moreover, knocking-down HNP-1 production using hnp-1 siRNA in Caco-2 cells overexpressing NOD2 abrogated antibacterial function of NOD2 against S. Typhimurium (Fig. 5). These results suggest that NOD2 exerts antibacterial activity after MDP-LD stimulation through induced HNP-1 secretion whereas NOD2 mutants associated with CD were unable to exert this function.
Figure 5.
NOD2 exerts antibacterial activity against Salmonella Typhimurium via induction of HNP-1 secretion by Caco-2 cells. Caco-2 cells were transfected with DNA encoding HNP-1 or NOD2 (1ng), and with siRNA raised against HNP-1 or NOD2. Invasive bacteria were quantified after a 2 h infection period at a multiplicity of infection of 10, followed by 1 h of gentamicin treatment. Results are expressed as viable intracellular bacteria relative to those recovered from non transfected Caco-2 cells, taken as 100%. * p<0.05
DISCUSSION
It is increasingly clear that commensal microbiota play a critical role in the pathogenesis of inflammatory bowel disease. The recognition of bacterial products by NOD2 activates signal transduction pathways via nuclear factor kappa B (NF-κB) as a part of the innate immune response (31, 32). Mutations in the nod2/card15 gene, encoding an intracellular pattern recognition receptor, are strongly associated with ileal CD (7, 8, 33). Patients with ileal CD manifest decreased expression of HD-5 and HD-6 by Paneth cell (34). Some studies associate NOD2 mutations with this deficiency (23, 24). Because intestinal epithelial cells play a role as the barrier to luminal microorganisms, we investigated whether the expression of defensins contribute to the function of NOD2 in intestinal epithelial cells.
In the present study, we find that the expression of human neutrophil peptide-1 (HNP-1) is up-regulated in HCT116 intestinal epithelial cells after NOD2 stimulation by MDP-LD and HNP-1 is involved in NOD2-mediated NF-κB activation. This effect appears to be selective: no association with other alpha defensins expressed in human colonic epithelial cells was found. Previous studies have reported that α-defensins are highly expressed in Paneth cells from ileum in human (35) and mouse (also named cryptdins) (20). In this study, endogenous expression of HNP-1 was found in colonic epithelial cell lines expressing endogenous NOD2. Human neutrophil peptides (1 to 3) were originally isolated as endogenous peptide antibiotics from the primary granules of human PMNs (36). However, HNP are also expressed by some surface epithelial cells.
Intestinal epithelial cells are part of the first line of defense against enterovirulent microbial pathogens. The results presented here suggest that HNP-1 may play a protective role in intestinal host defense against pathogenic bacteria. Expression of HNP-1 in Caco-2 cells decreased the intracellular survival of invasive Salmonella. Given that the overall transfection efficiency in Caco-2 cells was 30–40%, the reduction in colony-forming units suggests that HNP-1 is highly effective in controlling intracellular survival in those cells expressing the transfected protein. Consistent with these findings, the intracellular survival of S. Typhimurium was also decreased in Caco-2 cells expressing increased endogenous HNP-1 and siRNA against HNP-1 restored invasive activity of S. Typhimurium in Caco-2 cells. Of note, siRNA against NOD2 resulted in higher Salmonella survival compared to those cells transfected with siRNA against HNP-1 suggesting that the antibacterial activity of NOD2 depends at least in part to HNP-1 expression, although other mediators could also contribute to the antibacterial activity (37–40). Besides, the antimicrobial activity of HNP expressed by purified epithelial cells was previously demonstrated using a defensin sensitive phoP mutant of S. Typhimurium (30).
NOD2 has been identified as an intracellular receptor of the innate immune system. It is involved in the recognition of pathogen associated molecular patterns present in the bacterial cell wall but little is known about its role during the host response towards pathogenic bacteria. We have previously shown that NOD2, but not the NOD2 3020insC mutant associated with CD, protects intestinal epithelial cells against Salmonella infection (18). GRIM-19 directly binds NOD2 and acts as a component of the innate immune mucosal response by modulating NF-κB activation via NOD2. In fact, decreased expression of GRIM-19 using specific siRNA in HEK293 overexpressing NOD2 decreased NF-κB activation in response to MDP-LD. Importantly, NF-κB activation correlates with a decrease in the number of viable intracellular S. Typhimurium, suggesting that NOD2 anti-bacterial activity is dependent on NF-κB activation (12). In the present study, we demonstrate that MDP stimulation induces HNP-1 secretion only in IEC expressing endogenous NOD2, suggesting that NOD2 activation positively regulate hnp-1 gene expression and/or HNP-1 secretion. However, epithelial cells lacking expression of endogenous NOD2 failed to induce endogenous expression and elevated production of HNP-1 upon MDP treatment, indicating that HNP-1 expression is mediated by NOD2. These results parallel findings in a previous study demonstrating NOD2 mediated induction of human beta defensin 2 (41). This observation provides additional insight into the mechanisms through which NOD2 protects Caco-2 cells against Salmonella infection. Protective immunity mediated by NOD2 recognition of bacterial muramyl-dipeptide is abolished in nod2-deficient mice, which are susceptible to bacterial infection via the oral route but not through intravenous or peritoneal delivery (20).
Finally, HNP-1 secretion was significantly lower in response to MDP-LD when NOD2 3020insC mutant was overexpressed in Caco-2 cells compared to Caco-2 cells overexpressing wild-type NOD2 suggesting that NOD2 wild-type but not NOD2 mutant (3020insC) can mediate HNP-1 secretion by intestinal epithelial cells. We suggest that invasive bacteria can release MDP which stimulates the NOD2 pathway in intestinal epithelial cells. As a consequence, the transcription of hnp-1 is increased and the intestinal epithelial cells released HNP-1 having antibacterial activity. Alteration of defensins expression in patients harboring NOD2 mutations could have deleterious effects and can shift the balance toward inflammation. NOD2 is a critical regulator of bacterial immunity within the intestine, and alteration in this function could underlie the fundamental impact of NOD2 mutations on CD susceptibility.
Supplementary Material
Acknowledgments
Funding source: This work was supported by grants from National Institutes of Health (DK069344 and DK43351). J.K.Y.F. was supported by postdoctoral fellowships from Mexican and American Gastroenterological Associations (Jon Isenberg International Scholar Award); Harvard Foundation in Mexico (Harvard University Award); the National Research System, CONACYT; and the program “Incubacion de talentos” FUNSALUD, Mexico.
We thank Ian Rosenberg and Katheryn Devaney for technical assistance and helpful discussions.
References
- 1.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 4.Inohara Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 5.Kufer TA, Fritz JH, Philpott DJ. NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol. 2005;13:381. doi: 10.1016/j.tim.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Girardin SE, I, Boneca G, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 7.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 8.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber S, Rosenstiel P, Albrecht M, et al. Genetics of Crohn disease, an archetypal inflammatory barrier disease. Nat Rev Genet. 2005;6:376. doi: 10.1038/nrg1607. [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y, Inohara N, Benito A, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 11.Tanabe T, Chamaillard M, Ogura Y, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. Embo J. 2004;23:1587. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnich N, Hisamatsu T, Aguirre JE, et al. GRIM-19 interacts with nucleotide oligomerization domain 2 and serves as downstream effector of anti-bacterial function in intestinal epithelial cells. J Biol Chem. 2005;280:19021. doi: 10.1074/jbc.M413776200. [DOI] [PubMed] [Google Scholar]
- 13.Chen CM, Gong Y, Zhang M, et al. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J Biol Chem i. 2004;279:25876. doi: 10.1074/jbc.M400682200. [DOI] [PubMed] [Google Scholar]
- 14.Damiano JS, Oliveira V, Welsh K, et al. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem J. 2004;381:213. doi: 10.1042/BJ20031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald C, Chen FF, Ollendorff V, et al. A role for Erbin in the regulation of Nod2-dependent NF-kappaB signaling. J Biol Chem. 2005;280:40301. doi: 10.1074/jbc.M508538200. [DOI] [PubMed] [Google Scholar]
- 16.Kufer TA, Kremmer E, Banks DJ, et al. Role for erbin in bacterial activation of Nod2. Infect Immun. 2006;74:3115. doi: 10.1128/IAI.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto-Furusho JK, Barnich N, Xavier R, et al. Centaurin beta1 down-regulates nucleotide-binding oligomerization domains 1- and 2-dependent NF-kappaB activation. J Biol Chem. 2006;281:36060. doi: 10.1074/jbc.M602383200. [DOI] [PubMed] [Google Scholar]
- 18.Hisamatsu T, Suzuki M, Reinecker HC, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 19.Opitz B, Puschel A, Schmeck B, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 21.Meinzer U, Esmiol-Welterlin S, Barreau F, et al. Nod2 mediates susceptibility to Yersinia pseudotuberculosis in mice. PLoS ONE. 2008;3:e2769. doi: 10.1371/journal.pone.0002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begue B, Dumant C, Bambou JC, et al. Microbial induction of CARD15 expression in intestinal epithelial cells via toll-like receptor 5 triggers an antibacterial response loop. J Cell Physiol. 2006;209:241. doi: 10.1002/jcp.20739. [DOI] [PubMed] [Google Scholar]
- 23.Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A. 2005;102:18129. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simms LA, Doecke JD, Walsh MD, et al. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s disease. Gut. 2008;57:903. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann JA, Kafatos FC, Janeway CA, et al. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 27.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 28.Daher KA, Lehrer RI, Ganz T, et al. Isolation and characterization of human defensin cDNA clones. Proc Natl Acad Sci U S A. 1988;85:7327. doi: 10.1073/pnas.85.19.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086. [PubMed] [Google Scholar]
- 30.Cunliffe RN, Kamal M, Rose FR, et al. Expression of antimicrobial neutrophil defensins in epithelial cells of active inflammatory bowel disease mucosa. J Clin Pathol. 2002;55:298. doi: 10.1136/jcp.55.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckmann L, Karin M. NOD2 and Crohn’s disease: loss or gain of function? Immunity. 2005;22:661. doi: 10.1016/j.immuni.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Lala S, Ogura Y, Osborne C, et al. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 33.Radford-Smith G, Pandeya N. Associations between NOD2/CARD15 genotype and phenotype in Crohn’s disease--Are we there yet? World J Gastroenterol. 2006;12:7097. doi: 10.3748/wjg.v12.i44.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouellette AJ, Bevins CL. Paneth cell defensins and innate immunity of the small bowel. Inflamm Bowel Dis. 2001;7:43. doi: 10.1097/00054725-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Bevins CL, Martin-Porter E, Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut. 1999;45:911. doi: 10.1136/gut.45.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter EM, van Dam E, Valore EV, et al. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun. 1997;65:2396. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott MG, Hancock RE. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Rev Immunol. 2000;20:407. [PubMed] [Google Scholar]
- 39.Schroder JM. Epithelial antimicrobial peptides: innate local host response elements. Cell Mol Life Sci. 1999;56:32. doi: 10.1007/s000180050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D, Biragyn A, Hoover DM, et al. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 41.Voss E, Wehkamp J, Wehkamp K, et al. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.