Abstract
Objective
N-methyl-D-aspartate (NMDA) is a powerful cerebrovascular dilator in vivo. Cortical spreading depression (CSD) has recently been shown to contribute to the pial arteriolar dilation in mice. Our main aim was to examine the participation of CSD in the overall cerebrovascular response to NMDA in the rat.
Methods
Anesthetized Wistar rats (eight weeks old) were equipped with a closed cranial window to allow topical application of NMDA (10−5–10−3 M) to the parietal cortex. Cortical blood flow (CoBF) under and outside the cranial window was simultaneously monitored by using a two-channel laser-Doppler flowmeter. CSDs were detected by recording the changes in the cortical DC potential.
Results
Concentrations of 10−4 and 10−3 M of NMDA evoked single CSDs associated with rapid, transient hyperemia, followed by a sustained, but reduced, increase in CoBF. The latency and magnitude of the CoBF responses were dose dependent. The higher dose resulted in shorter latency (100 ± 5* vs. 146 ± 11 seconds, *P < 0.05; mean ± standard error of the mean) and larger overall flow response (77 ± 12* vs. 28 ± 3% from baseline) under, but not outside, the cranial window.
Conclusions
NMDA elicits dose-dependent increases in CoBF that are composed of CSD-dependent and -independent components in rats.
Keywords: glutamate, AMPA, kainate, cerebral blood flow, laser-Doppler flowmetry, piglet
INTRODUCTION
Glutamatergic neurotransmission is ubiquitous in the brain and plays a key role in neurovascular coupling via activation of ionotropic [N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA), and kainate] and metabotropic receptors [8,21,27,45]. Activation of NMDA receptors has been shown to elicit dose-dependent cerebrovascular dilation in vivo in numerous species, including rats, rabbits, and newborn pigs [10,16,19,32]. It has been hypothesized that the stimulation of NMDA receptors on neurons and the consequent membrane depolarization/Ca++ influx result in the production of vasodilator mediators, such as nitric oxide (NO), and ultimately leads to cerebral vascular relaxation, thereby adjusting the cortical blood flow (CoBF) to the local metabolic activity [4,14,16,21].
The concept of NMDA-receptor activation contributing to the generation and propagation of cortical spreading depression (CSD) is well established; synthetic antagonists of NMDA receptors are potent inhibitors of CSD development in numerous experimental models [22,29,31,37]. CSD is a self-propagating wave of cellular depolarization in the cerebral cortex that is typically evoked by localized trauma, excess neuronal firing, potassium chloride, or neurotransmitter application and is associated with short-lived, but dramatic, increases in CoBF. The underlying mechanisms of this cerebral hyperemia are very complex and probably require the contribution of diverse cell types within the neurovascular unit as well as a wide range of vasoactive factors, depending on age, species, and metabolic status of the cerebral cortex [8].
Because NMDA dilates pial arterioles via species-and age-dependent mechanisms and is implicated in CSD initiation and propagation, it is possible that, under some circumstances, NMDA-evoked CSDs contribute to the overall vascular responses. The recent findings of Ayata and Moskowitz [2] support this concept by providing evidence that, in mice, NMDA-induced pial arteriolar dilation is, at least partially, mediated by CSD. These researchers also suggest that, in some former studies where electrophysiological analyses were not performed, the apparent dose-dependent nature of the pial arterial responses was due to the increasing prevalence of CSDs in conjunction with the increasing concentration of NMDA. It is unclear, however, whether a similar complex interaction between CSD and the direct effects of NMDA occurs in other animal models.
Most data on NMDA-induced cerebrovascular changes have been acquired through studies on pial arteriolar diameters, which may or may not fully represent the overall response of the cerebral vasculature. We have shown previously that pial arterial responses most likely underestimate CoBF responses, and that in some situations the changes in diameter of pial arterioles may not accurately reflect alterations in resistance occurring in parenchymal or large arteries [9,11,40]. The aim of our study was to critically examine the effect of NMDA on CoBF, using laser-Doppler flowmetry, and to test whether CSD-related cortical hyperemia contributes to the overall NMDA-induced cerebrovascular response in the rat. To further clarify the glutamatergic cerebrovascular responses, we also determined if glutamate, AMPA, or kainate induced cortical hyperemia/CSD similar to NMDA, and if coapplication of glutamate or AMPA with NMDA would modify the NMDA-induced CoBF response by affecting CSD. Additionally, we studied the effect of NMDA on CoBF in newborn pigs, since NMDA-induced cortical hyperemia has not been reported in this species, although dose-dependent NMDA-induced pial arteriolar vasodilation has been extensively studied. Because the newborn pig is resistant to KCl-induced CSDs [15], NMDA-induced cerebrovascular changes are likely not confounded by CSDs in this subject. Therefore, use of piglets will allow us to compare the NMDA-evoked CSD-independent cerebral hyperemia across experimental models.
MATERIALS AND METHODS
Animals
Adult Wistar rats (male, eight weeks old, body weight 270–320 g, n = 57) and newborn pigs (male, one to three days old, body weight 1.6–2.4 kg, n = 9) were used in our experiments. Animals were maintained and used in compliance with the principles set forth by the Animal Care and Use Committee of Wake Forest University Health Sciences.
Surgical Procedures
Rats were anesthetized with pentobarbital [Nembutal; Hospira Inc., Lake Forest, Illinois, USA; 60–70 mg/kg intraperitoneally (i.p.) for induction and 25 mg/kg intravenously (i.v.) for maintenance]. This anesthetic is known to have no influence on CSD generation and propagation [26,28]. The surgical sites were treated with lidocaine (2%; Abbott Laboratories, North Chicago, Illinois, USA) locally for analgesia. Rats were intubated via tracheotomy and mechanically ventilated with room air and supplemental oxygen. Ventilation parameters were adjusted to maintain normal end-tidal CO2 levels (~40 mmHg), which were continuously monitored by a capnometer (Micro-Capnometer; Columbus Instruments, Columbus, Ohio, USA). The right femoral artery and vein were cannulated to measure arterial blood pressure and to administer drugs and fluids. A water-circulating heating mat was used to maintain the body temperature at ~37°C, which was monitored with a rectal probe. Arterial blood-gas tensions and pH were determined to verify the end-tidal pCO2 levels.
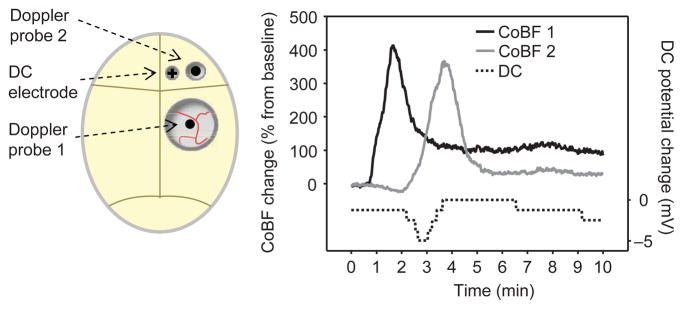
The heads of the rats were fixed in a stereotaxic frame, and a closed cranial window was inserted in the following manner. An incision was made on the scalp, and the soft tissues were retracted from the calvaria. A circular craniotomy was made over the right parietal cortex (~4 mm in diameter; the center of the window was 3 mm lateral and 4 mm caudal to the bregma), and then the dura was carefully removed. A ~2-mm-high rim was formed around the craniotomy, using bone wax, and was stabilized with cyanoacrylate. Two plastic tubes were inserted into the rim as input and output ports to enable drug administration and flushing of the brain surface. The window was sealed with Parafilm (American National Can, Greenwich, Connecticut, USA), through which a laser-Doppler probe (referred to as probe 1) was guided onto the surface (Figure 1). The window was filled with artificial cerebrospinal fluid (aCSF; composition in mg/L: 220 KCl, 7714 NaCl, 665 dextrose, 251.4 CaCl2, 61.9 MgCl2, and 2066.6 NaHCO3; bubbled with 5% CO2 in N2 and maintained at 37–38°C). Two additional holes were drilled under saline cooling over the right frontal cortex close to each other, one for a second laser-Doppler probe (probe 2) and one for a DC electrode, to verify the presence of possible CSDs. The distance between the two Doppler probes (7–8 mm) was measured to calculate CSD velocity. The dura was also removed under probe 2. The Doppler probes were mounted on micromanipulators and positioned so that they touched the surface of the brain, avoiding larger pial vessels. CoBF was monitored by using a two-channel laser-Doppler flowmeter (Periflux 4001 master; Perimed, Stockholm, Sweden). An Ag-AgCl electrode (1 mm in diameter) was connected to a DC amplifier (DAM 50, Differential Amplifier; World Precision Instruments, Sarasota, Florida, USA) for the measurement of DC potentials. A reference platinum electrode (Grass; Astro-Med, West Warwick, Rhode Island, USA) was fixed in the neck muscles.
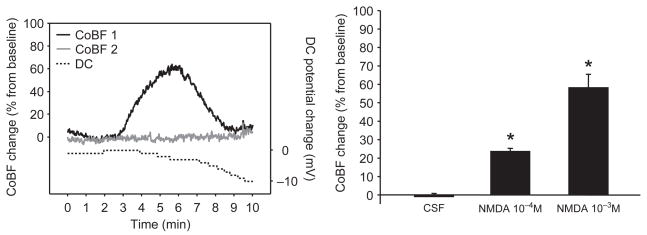
Figure 1.
Experimental design and representative recordings of cortical blood flow (CoBF) and direct current (DC) potential in the rat. On the left side, schematic figures show the position of the two laser-Doppler probes and the DC electrode during the experiments. The cranial window, where the drugs were applied, was placed over the parietal cortex, and additional holes were drilled anteriorly to the window. The first Doppler probe was placed under the window. The second probe and the DC electrode were positioned at a distant site, to detect the spread of depolarization and related cortical blood flow (CoBF) changes. In the presented experiment, NMDA (10−3 M) applied under the window evoked characteristic CoBF changes at the site of application (CoBF 1). Similar CoBF change was detected after a short delay outside the window (CoBF 2), accompanied by a negative DC-potential shift. The calculated rate of propagation between the two sites was consistent with that of cortical spreading depression. On the time axis, the 0 time point indicates the beginning of drug application onto the brain surface. The stimulus was removed 10 minutes later, when the window was flushed with artificial cerebrospinal fluid.
Piglets were anesthetized and underwent surgery, as previously described [10], with some modifications. Anesthesia was induced with pentobarbital (50–60 mg/kg, i.p.) and minimally supplemented by α-chloralose only when needed (in four of nine animals; Sigma, St. Louis, Missouri, USA; 5–10 mg/kg, i.v.). α-chloralose is known to have no effect on CSD, similar to barbiturates [28]. A catheter was inserted in the right femoral artery to monitor blood pressure and blood chemistry. Drugs and fluids were administered through a second catheter placed in the right femoral vein. The animals were intubated via tracheotomy and mechanically ventilated with room air. The ventilation rate (~30 breaths/min) and tidal volume (~20 mL) were adjusted to maintain the end-tidal CO2 level and arterial blood-gas parameters in the physiological range. The end-tidal CO2 level was monitored continuously, using a capnometer (Micro-Capnograph CI240; Columbus Instruments). A water-circulating heating pad was used to maintain the body temperature at ~37°C; core temperature was monitored with a rectal probe. The cranial window consisted of a stainless steel ring with three needle ports for drug administration, and aCSF flushing and was covered with Parafilm to facilitate the placement of Doppler probe 1 and the DC electrode onto the brain surface. For DC potential measurement, the same types of recording and reference electrodes were used as in the rat experiments, with appropriate changes in placement. according to the morphology of the piglet. Following surgery, the closed window was filled with aCSF [10]. For the placement of Doppler probe 2, a hole was drilled 5–8 mm anterior to the window (so that the maximal distance was 10 mm between the two probes) and the dura was removed.
Protocol
After reaching a stable baseline CoBF, the vehicle (aCSF) was superfused through the window to provide an experimental control for drug application in all animals. In rats, NMDA (group 1A; n=8) or glutamate (group 1B; n=10) (both drugs were purchased from Sigma, dissolved in aCSF) were administered topically at 10−5, 10−4, and 10−3 M concentrations for 10 minutes, interrupted by 10-minute-long drug-free periods when the surface was flushed with aCSF. To examine reproducibility, 10−4 or 10−3 M (n = 6) of NMDA was applied on the surface twice (group 2). In group 3 (n = 11), glutamate (10−3 M) was applied for 10 minutes prior to the coapplication of 10−4 and 10−3 M of NMDA. In group 4 (n = 8), the CoBF responses to AMPA (10−5 and 10−4 M, dissolved in 0.1 M of NaOH to 2 × 10−2 M and further diluted with aCSF; Acros Organics, Morris Plains, New Jersey) were characterized. In group 5 (n = 8), NMDA (10−4 and 10−3 M) was coapplied with AMPA (10−4 M) after a 10-minute incubation period with this latter agonist. In group 6 (n = 6), the brain surface was treated with 10−5 and 10−4 M of kainate (Sigma, dissolved in aCSF) for 10 minutes.
In piglets, the effects of topical NMDA (10−4 and 10−3 M, group 7A; n = 5) on CoBF were determined. The brain surface was exposed to NMDA for 10 minutes, after which this stimulus was removed with aCSF. DC potential and CoBF recording continued for an additional 20 minutes. In group 7B, CoBF responses to glutamate (10−4 and 10−3 M, n = 4) were evaluated similarly. In five animals, at least 30 minutes after the removal of the highest dose of NMDA/glutamate, we attempted to evoke CSDs by using repeated pinpricks (26-G needle, 1–2 mm in depth) as a mechanical stimulation. CoBF and DC potential were recorded in a 30-minute period following each pinprick.
CoBF, DC potential, blood pressure, and end-tidal CO2 were recorded continuously by PC-based software (IOX; EMKA Technologies, Falls Church, Virginia, USA). At the end of the experiments, the anesthetized animals were euthanized with an i.v. injection of saturated KCl solution. At this time, a biological zero for the laser-Doppler signal was determined. Data were analyzed offline.
Data Analysis
CoBF was recorded in arbitrary perfusion units. The biological zero was subtracted from the absolute values, and CoBF data were normalized to the average flow of five minutes prior to drug application. We evaluated CoBF responses in two ways, depending upon the experimental protocol and whether a CSD occurred during the application of drugs. In situations in which a CSD was absent, we determined the maximal CoBF response occurring over a one-minute interval. In situations in which a CSD occurred, we determined the maximal increase or peak CoBF response, the latency between the beginning of drug application and peak CoBF, and integrated CoBF responses within different time intervals.
Data were analyzed by one- or two-way ANOVA for repeated measures or by one-way ANOVA, followed by Tukey’s exact test, where appropriate. Reproducibility of NMDA-induced CoBF responses (group 2) was tested with the one-sample t-test. To compare the frequency of CSD occurrence between groups, Fisher’s exact test was used. A value of P < 0.05 was considered significant. Data are represented as mean ± standard error of the mean; n reflects the number of animals.
RESULTS
Body temperature, arterial blood pressure, end-tidal CO2 level, and blood gases were maintained within the normal range and did not vary significantly among different groups or during experiments. For example, in group 1A, rectal temperature was 37.1 ± 0.1°C, mean arterial blood pressure was 106 ± 5 mmHg, and while ventilation parameters were set to reach the normal end-tidal CO2, which was approximately 5%, arterial oxygen tension was 147 ± 5 mmHg, arterial carbon dioxide tension was 40 ± 1 mmHg, and pH was 7.39 ± 0.01. Also, raw baseline laser-Doppler flowmetry data (in arbitrary perfusion units; PUs) were similar among groups and did not shift considerably over the course of experiments. Baseline perfusions under the window were 163 ± 16 and 147 ± 14 PU in group 1A and 1B, respectively. Flushing of the brain surface with aCSF did not affect the CoBF significantly.
NMDA Induces CSD and Dose-Dependent CoBF Increase in the Rat
Topical NMDA at 10−5 M did not change the local CoBF significantly (5 ± 3%) and did not evoke any CSDs (0/8 trials). However, after the application of higher concentrations of NMDA (10−4–10−3 M), a rapid onset, but transient hyperemia, was observed, followed by a reduced, but sustained, elevation of CoBF under the cranial window (recorded by probe 1). Subsequently, a negative direct current (DC) potential shift (5–10 mV) and a characteristic CoBF increase were detected at the distant registration site (probe 2), confirming the induction of a CSD by NMDA (Figure 1).
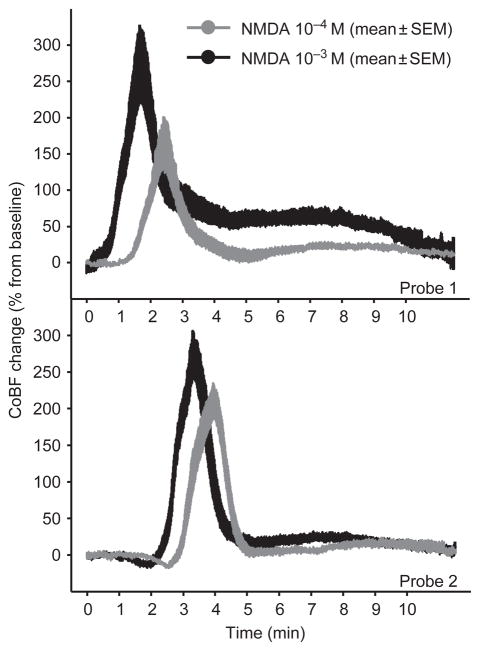
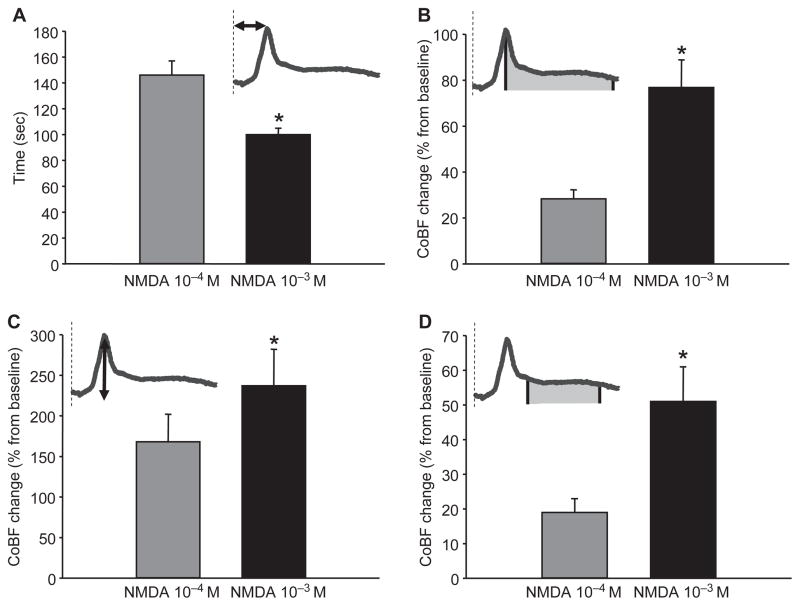
The CoBF responses to 10−4 and 10−3 M of NMDA were significantly different (Figure 2, upper panel). The higher dose of NMDA evoked CSD-related hyperemia with a significantly shorter latency (i.e., the time from the beginning of drug application to the maximal elevation in the flow) than the lower concentration (Figure 3A). The total CoBF increase (expressed as the integrated average of eight minutes after the flow maximum), the amplitude of the first, rapid CoBF increase (referred to as a peak), and the second, sustained CoBF increase or wave (defined as a five-minute period starting one minute after the peak) all showed prominent dose dependency (Figure 3B–3D) at the site of NMDA application. However, at the distant recording site, features of the CoBF responses were not concentration dependent (Figure 2, lower panel). The total CoBF responses detected by probe 2 were 21 ± 5 and 36 ± 8% (P = 0.094), the peak CoBF increases were 213 ± 21 and 278 ± 28% (P = 0.058), and the magnitude of the waves were 10 ± 3 and 21 ± 6% (P = 0.102) after the 10−4 and 10−3 M NMDA doses, respectively.
Figure 2.
The effect of topical NMDA on cortical blood flow (CoBF) in rats. CoBF was recorded under and away from the cranial window (marked as probes 1 and 2, respectively). NMDA evoked a rapid, cortical spreading depression (CSD)-related hyperemia (peak) followed by a sustained, but reduced, increase in CoBF (wave) that was concentration dependent only under the cranial window and not at the distant site. The CoBF data are represented as mean ± standard error of the mean (SEM) (SEM plotted as curve thickness; n = 8). The intervals between the 0 time point and the peaks represent the average latency (without error) from the beginning of drug application in both cases.
Figure 3.
NMDA evoked dose-dependent cortical blood flow (CoBF) increases in rats. The higher dose of NMDA (10−3 M) induced cortical spreading depression–related hyperemia with a significantly shorter latency than the lower (10−4 M) concentration (A). The magnitude of the total CoBF response (calculated as the average CoBF change of eight minutes after the maximal or peak value) was also dependent on the dose of NMDA applied (B). A similar difference was evident when analyzing its components; the peak (C), and the following, sustained CoBF increase or wave (measured during a five-minute period starting one minute after the peak; D). The dashed lines on the schematic icons show the beginning of NMDA application, whereas the arrows and filled areas determine the represented features of the flow response. Data are mean±standard error of the mean (n = 8; *P < 0.05) via one-way repeated measures analysis of variance and Tukey’s test.
The time intervals between the two peaks detected at the two sites were similar: 91±6 and 98±7 seconds when administering NMDA at 10−4 and 10−3 M concentrations, respectively (P = 0.24). The calculated velocities of 5.1±0.4 and 4.8±0.4 mm/min for 10−4 and 10−3 M, respectively, are consistent with published rates of CSD propagation [20], and confirmed that the detected CSD spread from the initiation site within the cranial window.
We demonstrated that repeated application of the same concentration of NMDA (group 2) induced remarkably similar CoBF elevations, which verified that dose-dependent responses seen at the site of the drug application were not due to hypersensitivity caused by the previously induced CSD. The ratios of second to first CoBF responses with NMDA application were 0.96±0.09, 1.04±0.11, and 1.05±0.12 for the peak, total, and wave values, respectively, which were not significantly different from the expected value of 1.
Glutamate Does Not Elicit CSD or CoBF Increase
In contrast to NMDA application, administration of glutamate (10−5–10−3 M) failed to increase CoBF significantly (maximal change was 9±2% at 10−3 M; P = 0.25) or to evoke a CSD (0/10 trials at each concentration). The CoBF and the DC potential were constant at the distant registration site following glutamate application.
AMPA Does Not Elicit CSD but Increases CoBF
AMPA did not evoke CSD but increased CoBF by 6.8±1.7 and 17±5%* (expressed as average CoBF change of one minute at maximal rate of hyperemia; *P < 0.05) for 10−5 and 10−4 M on AMPA, respectively (group 4). The CoBF increase was gradual, reaching the maximum from the fourth to fifth minutes of application. However, CoBF did not change at the distant registration site and the DC potential remained constant.
Glutamate and AMPA Reduce the Frequency of NMDA-induced CSDs but Do Not Affect the CSD-Independent Component of NMDA-induced CoBF Increases
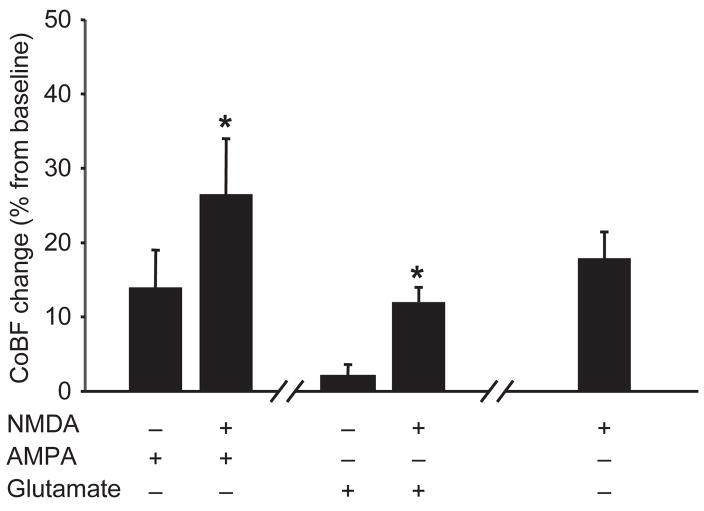
Glutamate was able to limit NMDA-induced CSD development (group 3) when it was coapplied with 10 −4 M (CSD failed to occur in 5 of 11 animals; *P < 0.05) but not with 10−3 M of NMDA (CSD occurred in all 11 animals). Despite the suppression of CSD expression with glutamate coapplication in the five animals, NMDA at 10−4 M still increased the CoBF significantly (Figure 4) to a level consistent with the post-CSD wave when only NMDA was applied (Figures 2–4).
Figure 4.
Cortical spreading depression (CSD)-independent NMDA-evoked cortical blood flow (CoBF) increases in rats. Topical glutamate (10−3 M) and AMPA (10−4 M) administration prevented the NMDA (10−4 M)-induced CSD generation in 5 of 11 and 8 of 8 cases, respectively. Despite blocked CSD formation, coapplication of 10−4 M NMDA still resulted in a significant increase in CoBF, as compared with AMPA or glutamate application alone (expressed as average CoBF change of one minute at maximal rate of hyperemia). The CoBF increase was consistent with the post-CSD wave when only NMDA was applied (represented as the average increase of CoBF over a one-minute interval, three minutes after the peak, time-matched with CSD-independent CoBF increases). Data are mean±standard error of the mean; (n = 5–8; *P < 0.05) via one-way repeated measures analysis of variance and Tukey’s test.
Coapplication of AMPA also interfered with CSD induction by NMDA (group 5). AMPA (10−4 M) prevented the initiation of CSDs in all eight animals when coapplied with 10−4 M (*P < 0.05), and prevented occurrence of CSD only in one of eight animals when coapplied with 10−3 M of NMDA. Despite the absence of CSD, coapplication of 10−4 M of NMDA still resulted in a significant increase in CoBF, as compared with 10−4 M of AMPA alone (Figure 4).
Kainate Does Not Elicit CSD but Increases CoBF
Kainate (group 6) increased CoBF by 36±6* and 114±16%* (10−5 and 10−4 M, respectively; *P < 0.05) but did not induce CSD in rats. However, in contrast to AMPA and NMDA, CoBF did not return to baseline, even after repeated aCSF flushing. Consequently, due to the magnitude and the prolonged nature of the CoBF response, we did not coapply kainate with NMDA.
NMDA Elicits Dose-Dependent CoBF Increase without CSD in the Piglet
Topical application of NMDA (group 7A) produced concentration-dependent increases in CoBF, as recorded under the cranial window (Figure 5). The pattern of the CoBF responses was not consistent with those seen in the rats during NMDA application (Figure 5), and we did not detect significant CoBF changes at the second, distant registration site outside the window (3±2 and 2±0.5% to 10−4 and 10−3 M NMDA; P=0.7 and 0.9, respectively). The DC potential decreased gradually (by 5–10 mV) and returned to the baseline level in approximately two to three minutes after removal of the NMDA with aCSF. Local activation of cortical neurons with “pinpricks,” using a sterile needle, although sufficient to cause CSD in other models, also failed to initiate CSD (0/5 animals) or significantly change CoBF in piglets (3.2±1.2% in response to the first pinprick; P=0.5). In group 7B, we did not detect the presence of CSDs during glutamate application, and the increase in CoBF (8.3±1.4 and 9.5±1.1% to 10−4 and 10−3 M of glutamate) was not significant (P=0.35 and 0.3).
Figure 5.
A representative recording of cortical blood flow (CoBF) and direct current (DC) potential and NMDA-evoked dose-dependent cortical blood flow (CoBF) increases in newborn pigs. Right panel: NMDA (10−3 M) application onto the cortex elicited a transient CoBF increase (CoBF 1) and a prolonged DC potential deflection; however, propagation of the CoBF response was not detected with the distant probe (CoBF 2). On the time axis, the 0 time point indicates the beginning of drug application onto the brain surface. The stimulus was removed 10 minutes later, when the window was flushed with artificial cerebrospinal fluid. Left panel: Average CoBF change per minute was calculated during a 10-minute period of drug application. Bars represent the maximal CoBF changes within the examined time interval. Artificial cerebrospinal fluid did not produce significant CoBF changes, while topical NMDA (10−4 and 10−3 M) increased CoBF in a concentration-dependent manner. Data are mean±standard error of the mean (n=5; *P < 0.05) via one-way repeated measures analysis of variance and Tukey’s test.
DISCUSSION
The major findings of our experiments were: 1) NMDA induced dose-dependent CoBF increases in both species studied; 2) NMDA-evoked CSDs contributed to the CoBF response in adult rats; 3) stimulation of cortical AMPA- and kainate-receptors also increased CoBF in rats without generating CSD; 4) glutamate failed to generate CSD-related CoBF changes; 5) glutamate and AMPA reduced the occurrence of NMDA-induced CSDs, thus unveiling CSD-independent vasoactivity of NMDA; and finally, 6) NMDA-induced hyperemia was independent of CSDs in piglets. After the elimination of the CSD-dependent component of the CoBF response to NMDA in rats, the degree of cortical hyperemia to NMDA was similar in rats and piglets.
NMDA evokes well-described dose-dependent pial arteriolar dilation in vivo [10,12,32,16,17,19]; however, many studies failed to show NMDA-induced vasodilation in isolated cerebral resistance vessels [16,24,41]. NMDA-induced vasodilation is abolished by NMDA-receptor antagonist MK-801 and attenuated by inhibitors of neuronal nitric-oxide synthase (nNOS) [4,16,18,32]. These findings suggest that neuronal NMDA-receptor stimulation and subsequent Ca++ influx/membrane depolarization would activate nNOS, leading to vascular smooth muscle relaxation by NO [8,14,21,35,44]. In addition to the unequivocal role of NO, the participation of other species-specific factors, such as adenosine [25], carbon monoxide (CO) [30], ATP-sensitive potassium (KATP), and/or calcium-activated potassium (KCa) channels [36] has been proposed.
Ayata and Moskowitz [2] suggested an alternative mechanism of NMDA-induced pial arteriolar vasodilation in mice. Using electrophysiological recordings and vessel-diameter measurements, they demonstrated the involvement of CSD in the complex vascular responses evoked by topical application of as little as 50 μM of NMDA. However, there are some limitations of that study. First, responses of small arterioles (approximately 25 μm in diameter) were tested that may not represent responses of other resistance blood vessels during CSD. As recently described, different vascular segments of the cerebral circulation are exposed to varying influences from dilator and constrictor stimuli during CSD [7]. Second, pial arteriolar diameter may not accurately reflect the overall response of the cerebral vasculature to CSD [9,11,40]. And, third, the CSD component of the overall responses to NMDA was not removed, so that the direct effects of NMDA could not be conclusively determined.
Our study provides novel evidence that NMDA-evoked CSD is a major component of the overall cerebral vascular response to higher (100 μM to 1 mM) doses of NMDA in the rat model; however, detailed analysis of the response to NMDA revealed non–CSD-dependent characteristics as well. CoBF increases were dose dependent, which might be explained by a faster, deeper penetration of the drug at the higher dose and/or by a more extensive receptor occupation. In the present study, we also demonstrated the CSD-independent cerebrovascular effect of AMPA and the inhibitory potential of AMPA-receptor activation on NMDA-induced CSDs. Importantly, even in the absence of CSDs, the CSD-independent increase in CoBF upon NMDA application remained intact. Similar to AMPA-receptor activation, kainate application also increased CoBF without producing CSDs. The dramatic, sustained CoBF effects of topical kainate prevented the exploration of any potential modulatory influence of kainate on NMDA-induced CoBF and CSD responses in the rat. We previously described the pronounced, prolonged effects of kainate on pial arterioles in piglets [5].
Unlike NMDA, AMPA, or kainate, the application of the natural neurotransmitter, glutamate, failed to significantly increase CoBF or to induce CSDs in the rat. This apparent discrepancy is likely caused by experimental conditions. The peak concentration of glutamate in the synaptic cleft during neurotransmission is unknown, but is estimated at about 1.1 mM in hippocampal glutamatergic synapses [13]. In our study, glutamate and its analogs were applied with a single application in a small volume (~15–20 μL) under the cranial window to minimally interfere with the laser-Doppler flowmetry signal that is very sensitive to movement. The discrepancy between the cerebrovascular effects of glutamate and NMDA/AMPA/kainate may be due to the well-known difference in the clearance of these drugs from the extracellular space [34]. In our experiments, glutamate concentration may not have stayed high enough or reached the appropriate cell layers in the stimulated cortex because of degradation and neuronal/glial uptake to reproduce the cerebrovascular effects of the selective analogues. Further, the difference between glutamate and NMDA in facilitating CSDs has been described by other researchers [29,33]. These results further indicate that the effect of glutamate is complex, resulting not only from NMDA receptor stimulation, but from the summation of different, and sometimes opposing, actions via multiple receptors and vasoactive substances [8,27]. The complex action of glutamate is further suggested by its efficacy to reduce the incidence of NMDA-induced CSDs. Glutamate has two more ionotropic (AMPA and kainate) and numerous metabotropic receptors. It is possible that the activation of one or more of them acts antagonistically in terms of CSD initiation. The AMPA receptor can be an important site of this effect, since AMPA produced a similar inhibition in the present study. AMPA is able to antagonize NMDA-induced changes in rat cortical networks [1]. Conceivably, AMPA-receptor activation may have antagonized CSD initiation by NMDA, as well. Importantly, glutamate or AMPA did not affect the CSD-independent component of the CoBF response to NMDA, suggesting that not the direct interaction with the NMDA receptor, but the subsequent electrophysiological sequence triggering a CSD, was impaired by glutamate/AMPA.
Our findings in the piglet provide novel data on the integrated CoBF responses to the activation of cortical neuronal NMDA receptors in this important experimental model and confirm our earlier results concerning the dose-dependent nature of responses to NMDA, based upon measurements of pial arteriolar diameters in this species [3,6,10,12,15,36]. The hemodynamic changes are not the consequence of CSD-related events, but rather represent a direct, specific effect on cortical neurons [8,14]. While CSD has been shown to occur in the mature brains of all mammalian species studied, the neonatal brain appears to be relatively resistant to generation of CSD [39]. For example, in the rat, it is difficult to elicit CSD-like events before postnatal days 9–10 [38], and in the piglet, we have not been able to induce CSD, even with mechanical means (i.e., pinpricks), during the first days of life. While the inability of the neonatal brain to produce CSDs is not completely understood, it seems likely that factors such as lack of maturity of individual neuronal and glial components and their incomplete integration with each other, as well as different expression of ion channels and neurotransmitter receptors, are responsible for this phenomenon [42]. In a recent study, Girouard et al. [23] found that the cortical application of 40 μM of NMDA increased CoBF in mice by 20–25% without inducing CSD. Additionally, they confirmed earlier reports that cerebral vascular dilation to NMDA is dependent on NO derived from neuronal NOS [4,18]. The amount of the increase in CoBF observed in mice, following NMDA application by Girouard et al. [23], is similar to the non-CSD component of the CoBF response that we observe in rats and to the CoBF responses we see in piglets.
CONCLUSIONS
In summary, NMDA elicits dose-dependent CoBF increases in rats and piglets. Although previous data obtained by pial arteriolar diameter measurements suggest many superficial similarities in the cerebrovascular responses to NMDA, our results show that the underlying mechanisms are quite different between these two models. In rats, NMDA-evoked CSD has a substantial, but not exclusive, contribution to the cerebrovascular response. In piglets, however, the CoBF increase seems to be solely the result of the local activation of neuronal NMDA receptors without the confounding effect of CSDs.
Acknowledgments
This study was supported by grants from the National Institutes of Health (HL30260, HL65380, and HL77731; Bethesda, Maryland, USA), from the National Scientific Research Fund of Hungary (OTKA, K68976 and K63401), and by the National Bureau of Research and Development (NKTH RET-08/2004). FD was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. The authors gratefully thank Nancy Busija, MA, for editing the manuscript for this article.
Footnotes
Declaration of interest: The authors report no financial conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Addae JI, Evans SM, Ali N, Stone TW. NMDA-induced changes in a cortical network in vivo are prevented by AMPA. Brain Res. 2000;869:211–215. doi: 10.1016/s0006-8993(00)02233-2. [DOI] [PubMed] [Google Scholar]
- 2.Ayata C, Moskowitz MA. Cortical spreading depression confounds concentration-dependent pial arteriolar dilation during N-methyl-D-aspartate superfusion. Am J Physiol Heart Circ Physiol. 2006;290:H1837–H1841. doi: 10.1152/ajpheart.01102.2005. [DOI] [PubMed] [Google Scholar]
- 3.Bari F, Errico RA, Louis TM, Busija DW. Differential effects of short-term hypoxia and hypercapnia on N-methyl-D-aspartate–induced cerebral vasodilatation in piglets. Stroke. 1996;27:1634–1639. doi: 10.1161/01.str.27.9.1634. discussion, 1639–1640. [DOI] [PubMed] [Google Scholar]
- 4.Bari F, Errico RA, Louis TM, Busija DW. Interaction between ATP-sensitive K+ channels and nitric oxide on pial arterioles in piglets. J Cereb Blood Flow Metab. 1996;16:1158–1164. doi: 10.1097/00004647-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Bari F, Louis TM, Busija DW. Kainate-induced cerebrovascular dilation is resistant to ischemia in piglets. Stroke. 1997;28:1272–1276. doi: 10.1161/01.str.28.6.1272. discussion, 1277. [DOI] [PubMed] [Google Scholar]
- 6.Bari F, Nagy K, Guidetti P, Schwarcz R, Busija DW, Domoki F. Kynurenic acid attenuates NMDA-induced pial arteriolar dilation in newborn pigs. Brain Res. 2006;1069:39–46. doi: 10.1016/j.brainres.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol. 2008;86:379–395. doi: 10.1016/j.pneurobio.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-D-aspartate in cerebral cortex. Brain Res Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busija DW, Heistad DD, Marcus ML. Continuous measurement of cerebral blood flow in anesthetized cats and dogs. Am J Physiol. 1981;241:H228–H234. doi: 10.1152/ajpheart.1981.241.2.H228. [DOI] [PubMed] [Google Scholar]
- 10.Busija DW, Leffler CW. Dilator effects of amino-acid neurotransmitters on piglet pial arterioles. Am J Physiol. 1989;257:H1200–H1203. doi: 10.1152/ajpheart.1989.257.4.H1200. [DOI] [PubMed] [Google Scholar]
- 11.Busija DW, Marcus ML, Heistad DD. Pial artery diameter and blood-flow velocity during sympathetic stimulation in cats. J Cereb Blood Flow Metab. 1982;2:363–367. doi: 10.1038/jcbfm.1982.37. [DOI] [PubMed] [Google Scholar]
- 12.Busija DW, Wei M. Altered cerebrovascular responsiveness to N-methyl-D-aspartate after asphyxia in piglets. Am J Physiol. 1993;265:H389–H394. doi: 10.1152/ajpheart.1993.265.1.H389. [DOI] [PubMed] [Google Scholar]
- 13.Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 14.Domoki F, Perciaccante JV, Shimizu K, Puskar M, Busija DW, Bari F. N-methyl-D-aspartate–induced vasodilation is mediated by endothelium-independent nitric oxide release in piglets. Am J Physiol Heart Circ Physiol. 2002;282:H1404–H1409. doi: 10.1152/ajpheart.00523.2001. [DOI] [PubMed] [Google Scholar]
- 15.Domoki F, Veltkamp R, Bari F, Louis TM, Busija DW. Cerebrovascular reactivity remains intact after cortical depolarization in newborn piglets. Pediatr Res. 1999;45:834–837. doi: 10.1203/00006450-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circ Res. 1993;72:476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM, Breese KR. Dilatation of cerebral arterioles in response to N-methyl-D-aspartate: role of CGRP and acetylcholine. Brain Res. 1994;640:93–97. doi: 10.1016/0006-8993(94)91860-0. [DOI] [PubMed] [Google Scholar]
- 18.Faraci FM, Brian JE., Jr 7-nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilatation in response to N-methyl-D-aspartate. Stroke. 1995;26:2172–2175. doi: 10.1161/01.str.26.11.2172. discussion, 2176. [DOI] [PubMed] [Google Scholar]
- 19.Faraci FM, Heistad DD. Responses of cerebral arterioles to N-methyl-D-aspartate and activation of ATP-sensitive potassium channels in old rats. Brain Res. 1994;654:349–351. doi: 10.1016/0006-8993(94)90499-5. [DOI] [PubMed] [Google Scholar]
- 20.Farkas E, Pratt R, Sengpiel F, Obrenovitch TP. Direct, live imaging of cortical spreading depression and anoxic depolarisation using a fluorescent, voltage-sensitive dye. J Cereb Blood Flow Metab. 2008;28:251–262. doi: 10.1038/sj.jcbfm.9600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fergus A, Lee KS. Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Res. 1997;754:35–45. doi: 10.1016/s0006-8993(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 22.Gill R, Andine P, Hillered L, Persson L, Hagberg H. The effect of MK-801 on cortical spreading depression in the penumbral zone following focal ischaemia in the rat. J Cereb Blood Flow Metab. 1992;12:371–379. doi: 10.1038/jcbfm.1992.54. [DOI] [PubMed] [Google Scholar]
- 23.Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, et al. NMDA-receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29:2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardebo JE, Wieloch T, Kahrstrom J. Excitatory amino acids and cerebrovascular tone. Acta Physiol Scand. 1989;136:483–485. doi: 10.1111/j.1748-1716.1989.tb08690.x. [DOI] [PubMed] [Google Scholar]
- 25.Iliff JJ, D’Ambrosio R, Ngai AC, Winn HR. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am J Physiol Heart Circ Physiol. 2003;284:H1631–H1637. doi: 10.1152/ajpheart.00909.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kitahara Y, Taga K, Abe H, Shimoji K. The effects of anesthetics on cortical spreading depression elicitation and c-fos expression in rats. J Neurosurg Anesthesiol. 2001;13:26–32. doi: 10.1097/00008506-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Kudo C, Nozari A, Moskowitz MA, Ayata C. The impact of anesthetics and hyperoxia on cortical spreading depression. Exp Neurol. 2008;212:201–206. doi: 10.1016/j.expneurol.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J Cereb Blood Flow Metab. 1992;12:223–229. doi: 10.1038/jcbfm.1992.32. [DOI] [PubMed] [Google Scholar]
- 30.Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol. 2006;291:H2897–H2904. doi: 10.1152/ajpheart.00722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrannes R, Willems R, De Prins E, Wauquier A. Evidence for a role of the N-methyl-D-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain Res. 1988;457:226–240. doi: 10.1016/0006-8993(88)90690-7. [DOI] [PubMed] [Google Scholar]
- 32.Meng W, Tobin JR, Busija DW. Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-D-aspartate receptors. Stroke. 1995;26:857–862. doi: 10.1161/01.str.26.5.857. discussion, 863. [DOI] [PubMed] [Google Scholar]
- 33.Obrenovitch TP, Zilkha E, Urenjak J. Evidence against high extracellular glutamate promoting the elicitation of spreading depression by potassium. J Cereb Blood Flow Metab. 1996;16:923–931. doi: 10.1097/00004647-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelligrino DA, Gay RL, 3rd, Baughman VL, Wang Q. NO synthase inhibition modulates NMDA-induced changes in cerebral blood flow and EEG activity. Am J Physiol. 1996;271:H990–H995. doi: 10.1152/ajpheart.1996.271.3.H990. [DOI] [PubMed] [Google Scholar]
- 36.Philip S, Armstead WM. NMDA dilates pial arteries by KATP and Kca channel activation. Brain Res Bull. 2004;63:127–131. doi: 10.1016/j.brainresbull.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Richter F, Bauer R, Lehmenkuhler A, Schaible HG. Spreading depression in the brainstem of the adult rat: electrophysiological parameters and influences on regional brainstem blood flow. J Cereb Blood Flow Metab. 2008;28:984–994. doi: 10.1038/sj.jcbfm.9600594. [DOI] [PubMed] [Google Scholar]
- 38.Richter F, Lehmenkuhler A, Fechner R, Manveljan L, Haschke W. Postnatal conditioning for spreading cortical depression in the rat brain. Brain Res Dev Brain Res. 1998;106:217–221. doi: 10.1016/s0165-3806(98)00018-2. [DOI] [PubMed] [Google Scholar]
- 39.Schade JP. Maturational aspects of EEG and of spreading depression in rabbit. J Neurophysiol. 1959;22:245–257. doi: 10.1152/jn.1959.22.3.245. [DOI] [PubMed] [Google Scholar]
- 40.Shibata M, Leffler CW, Busija DW. Prostanoids attenuate pial arteriolar dilation induced by cortical spreading depression in rabbits. Am J Physiol. 1991;261:R828–R834. doi: 10.1152/ajpregu.1991.261.4.R828. [DOI] [PubMed] [Google Scholar]
- 41.Simandle SA, Kerr BA, Lacza Z, Eckman DM, Busija DW, Bari F. Piglet pial arteries respond to N-methyl-D-aspartate in vivo but not in vitro. Microvasc Res. 2005;70:76–83. doi: 10.1016/j.mvr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T. Postsynaptic receptor mechanisms underlying developmental speeding of synaptic transmission. Neurosci Res. 2005;53:229–240. doi: 10.1016/j.neures.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Van Harreveld A, Stamm JS, Christensen E. Spreading depression in rabbit, cat, and monkey. Am J Physiol. 1956;184:312–320. doi: 10.1152/ajplegacy.1956.184.2.312. [DOI] [PubMed] [Google Scholar]
- 44.Yang ST, Chang HH. Nitric oxide of neuronal origin mediates NMDA-induced cerebral hyperemia in rats. Neuroreport. 1998;9:415–418. doi: 10.1097/00001756-199802160-00011. [DOI] [PubMed] [Google Scholar]
- 45.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]