Abstract
Cells are mechanical as well as chemical machines, and much of the energy they consume is used to apply forces to each other and to the extracellular matrix around them. The cytoskeleton, the cell membrane, and the macromolecules composing the extracellular matrix form networks that in concert with the forces generated by the cell create dynamic materials with viscoelastic properties unique to each tissue. Numerous recent studies suggest that the forces that cells create and are subjected to, as well as the mechanical properties of the materials to which they adhere, can have large effects on cell structure and function that can act in concert with or override signals from soluble stimuli. This brief review summarizes recent studies of the effects of substrate mechanics on cell motility, differentiation, and proliferation, and discusses possible mechanisms by which a cell can probe the stiffness of its surroundings.
Keywords: mechanotransduction, substrate stiffness, cell mechanics
INTRODUCTION
Normally functioning organs in healthy organisms generally have well-defined mechanical properties characterized by elastic moduli that fall within a narrow range that depends on tissue type and the age and health of the organism. Studies of cells in vitro are by definition done on materials that are many orders of magnitude stiffer, but this stiffness difference has often been relatively neglected compared to the biochemical and genetic requirements for cells to survive and function. Recent developments in producing biocompatible materials and in understanding how cells react to environmental stimuli have enabled numerous demonstrations that cells can be exquisitely sensitive to changes in the mechanical properties of their substrates even when their chemical environment is held constant. One result of such studies is a reemergence of interest in mechanosensing and in the concept that changes in tissue stiffness that occur in such pathologic states as fibrosis and cancer are not merely epiphenomena of the disease, but might be causally related to its progression or resistance to treatments.
Mechanosensing has two major aspects, which are often studied or considered separately. Cells often respond specifically to forces applied to them from outside. Perhaps the most obvious example is hearing, in which acoustic waves lead to movement of stereocilia on the hair cell, thereby imposing forces on and deformation of proteins that regulate ion flux through the membrane, ultimately triggering the biochemical processes that lead to the perception of sound. A similar, although less well characterized mechanism is presumed to account for the sense of touch. The other aspect of mechanosensing relies not on forces applied from the outside, but on those generated by the cell itself. This brief review will focus on recent reports that specific cellular functions or structures depend on the mechanical, or more specifically, on the elastic properties of the material on which or in which they are attached.
HISTORICAL PERSPECTIVE
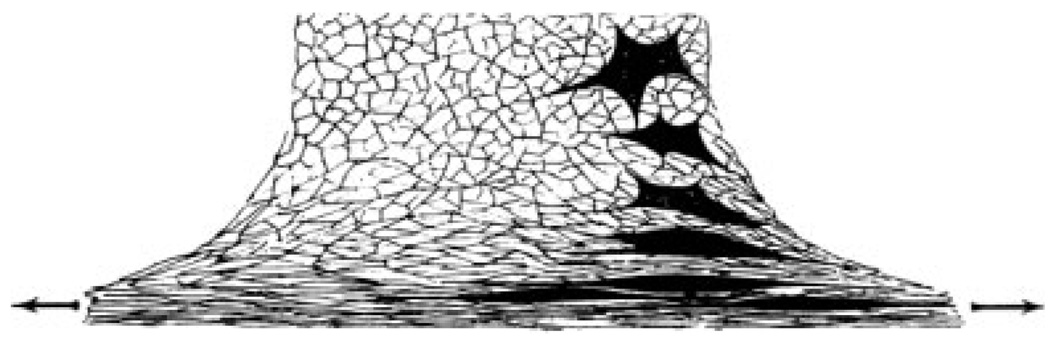
It has been known for centuries that live tissues are often in a state of internal tension, but aside from processes such as muscle contraction, a physiological function for such tension has not been obvious. The recent activity in cell mechanics and mechanotransduction builds on a long but sporadic history of studying the physical properties of cells and tissues as possible determinants of their biological functions. In the 1920s, pioneering studies showed that the shapes of mesenchymal cells varied depending on the concentration of clots formed by diluted blood plasma in which the cells were embedded. Such studies and the observation that the cells pulled on the fibrin strands within the gel were interpreted as evidence for “the dependence of cell shape and cell movement on the physical structure of the medium” [Weiss and Garber, 1952]. Figure 1 shows a drawing of fibroblasts isolated from heart and grown in matrices formed by clotting blood plasma and subjected to varying degrees of stress imposed on the matrix. This image shows the striking reorganization of cell shape from relatively polygonal and multi-armed in unperturbed clots to highly elongated and oriented in the direction of stress. Even in the absence of external stress the density of the plasma clot had a significant effect on cell morphology. Figure 2 shows that the axial ratio of both the whole cell and its nucleus changes with increasing clot density, suggesting that the cell probes some aspect of the clot structure and responds by altering its morphology.
Fig. 1.
Effect of regionally varying tension on the organization of a fibrin network and, through it, on the morphology and orientation of enclosed cells. From [Weiss, 1959].
Fig. 2.
Dependence of the cell and nuclear axial ratio on the concentration of plasma clots in which cardiac fibroblasts are grown. From [Weiss and Garber, 1952].
As soon as cytoskeletal filaments could be visualized by fluorescence in cells, it became apparent that not only the shape of the cell, but also the structure and assembly of the cytoskeleton depended on whether cells were grown on glass slides or on softer collagen gels. Fibroblasts grown on glass, where their morphologies could be optimally visualized, were more spread but not as elongated as they were in vivo or when grown in 3D collagen matrices [Tomasek et al., 1982]. Remarkable images of single cells grown on square adhesive islands showed the formation of the actin filament bundles that had become known as stress fibers along the diagonal of the cell [Marek et al., 1982], and when grown in collagen gels, fibroblasts acquired stress fibers as they applied force to the lattice, but once the matrix was released from its constraints and the cells relaxed, the stress fibers rapidly disappeared even though the cell remained in the same medium, bound to the same collagen fibers [Farsi and Aubin, 1984]. When grown on flexible films, fibroblasts were seen to spread better as the film stiffness increased [Keese and Giaever, 1991].
SEPARATING MATRIX STIFFNESS FROM OTHER ENVIRONMENTAL SIGNALS
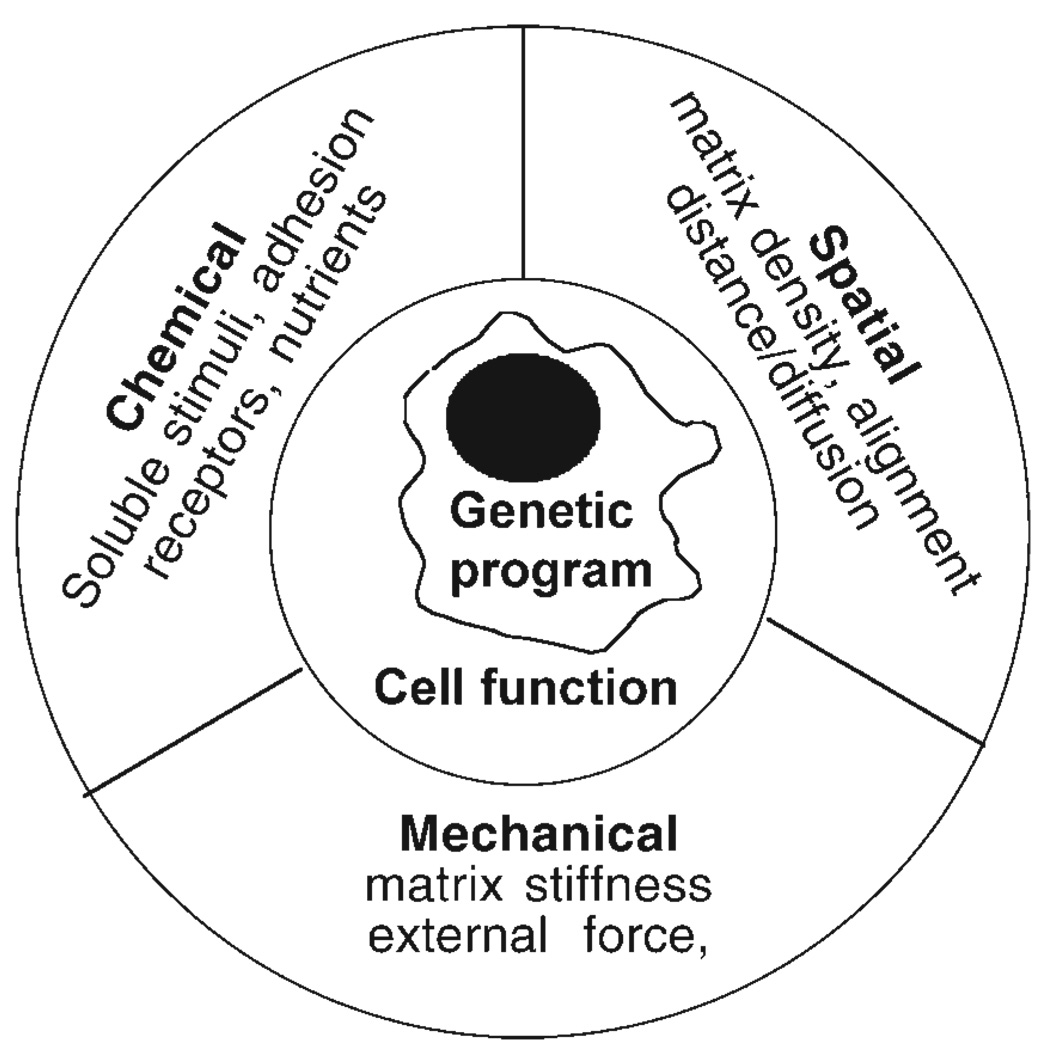
Whereas physical factors were long known to affect a cell’s response to its genetic and chemical context, the precise physical features that can be transduced into the intracellular signals governing cell response remain to be completely defined. The studies showing effects of fibrin gel density on fibroblast structure (Figs. 1 and 2) concluded that the orientation of fibrin fibers that occurs when external or cell-generated stresses are applied to networks of these stiff fibers was the main factor causing the changes in fibroblast morphology from mostly polygonal to highly elongated. In other words, spatial characteristics arising from matrix remodeling, rather than the forces or gel stiffness that control deformation were the stimuli to which the cell responded. Since nearly all native extracellular matrices are composed of open meshworks of stiff fibers, changes in matrix stiffness inevitably coincide with changes in fiber density, thickness, and orientation, all of which alter the spatial relationships of activated adhesion receptors and therefore can affect the cell by spatial rather than mechanical signals. Even before it was possible to uniquely specify which of several possible physical effects elicited specific cellular responses, the shape and function of cells and the tissues they form had been proposed to be controlled by a constellation of chemical, mechanical, and spatial or topographical inputs as well as by the genetic program of the cell, as outlined in Fig. 3.
Fig. 3.
Schematic diagram of factors determining cell fate. Adapted from [Schwarz and Bischofs, 2005].
A UNIQUE ROLE FOR MATRIX STIFFNESS
A direct demonstration that matrix stiffness, rather than a spatial cue could direct cell structure and motility was achieved by using thin films [Keese and Giaever, 1991] or hydrogels formed by crosslinked polyacrylamide [Pelham and Wang, 1997]. Polyacrylamide is a highly flexible uncharged hydrophilic polymer that has little or no affinity for cells or their transmembrane proteins and forms gels only at concentrations where the distance between polymer strands is a few nanometers and for which stiffness depends on the density of crosslinks formed by bisacrylamide. The rubberlike elasticity of polyacrylamide gels makes it possible to design a series of gels with large differences in stiffness governed by small mole fractions of crosslinker, while maintaining a constant network topography, governed by the total polyacrylamide concentration. Moreover, the rheology of polyacrylamide gels is nearly perfectly rubberlike and affine, meaning that it is nearly perfectly elastic, its stiffness does not depend on the extent of its deformation, and it remains approximately isotropic even when strained, since the highly flexible chains can accommodate significant displacements of the ends without significant changes in chain orientation. Since neither cells nor the adhesion proteins they secrete can bind well to the surface of polyacrylamide, cell adhesion can be varied independently of stiffness by altering the concentration and type of adhesion protein that is covalently linked to its surface. Similar uniform flexible surfaces have been produced using other polymers such as silicone rubber sheets [Wipff et al., 2009] and polydimethylsiloxane gels [Cheng et al., 2009], and each system has its advantages and optimal stiffness range.
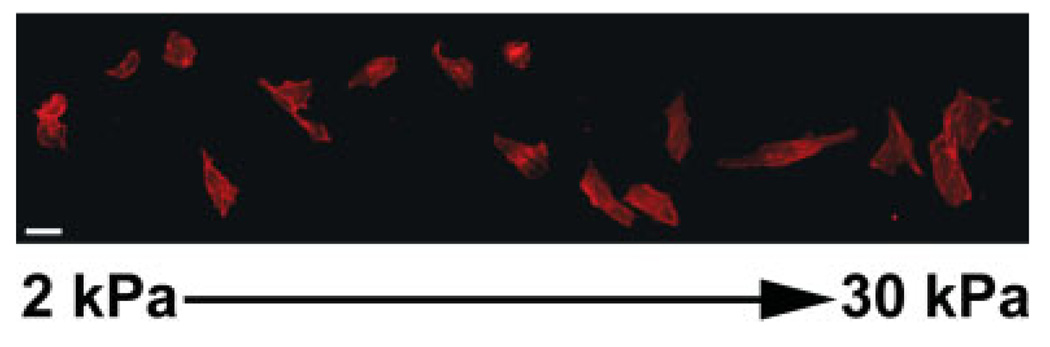
Studies using rheologically and spatially uniform substrates have shown that mechanical effects such as the elastic resistance of the material to deformation by a cell have effects on cell structure, motility, function, and gene expression that cannot be overcome by other stimuli and that presumably work in concert with other influences to determine the phenotype of both normal and abnormal cells in vivo [Wells, 2008b]. Figure 4 shows one example in which a polyacrylamide gel with a gradient of crosslinker, and therefore a gradient of stiffness, is produced by microfluidics methods, laminated with collagen as an adhesive ligand and then used to culture A7 melanoma cells. The morphology of these cells covers a spectrum of shapes that range from nearly spherical on the soft part of the gel to polygonal and well-spread, with a much larger adherent area on the stiffer side of the gel. In this system, the adhesion protein density and the culture medium are identical for the whole sample, and the only apparent variable is the stiffness of the material to which the cells adhere. Loss of filamin from these cells nearly completely eliminates the stiffness-induced changes in spread area, demonstrating that this response is tightly controlled by the cell and requires connection of the membrane to a crosslinked cytoskeleton [Byfield et al., 2009].
Fig. 4.
Morphology of A7 melanoma cells on a stiffness gradient gel. A7 cells plated on a collagen coated stiffness gradient gel with stiffness increasing from 2 kPa at the left side of the image to 30 kPa at the right side of the image.
PHYSIOLOGICAL AND PATHOLOGICAL CONSEQUENCES OF STIFFNESS SENSING
A suggestion from the limited number of quantitative studies of the range over which cells respond to stiffness in vitro and of the viscoelastic parameters of tissues in vivo is that cells adopt their physiologically relevant morphology when cultured on a substrate with the same stiffness as their native tissue environment [Engler et al., 2008]. As the matrix stiffness deviates from some optimal stiffness range, cells begin to behave abnormally. In vivo, changes in adult tissue stiffness usually occur by one of two mechanisms: injury or disease. In the case of injury a provisional fibrin matrix is deposited and contracted first by platelets [Burstein and Lewi, 1952] and later by myofibroblasts [Majno et al., 1971]. A key step in wound healing is degradation and replacement of the provisional matrix with a more integrated scar tissue and several recent studies report increased release of matrix metalloproteases [Karamichos et al., 2008] and ECM proteins [Schlunck et al., 2008] on stiff substrates compared with softer ones.
Tissue stiffening also occurs in several pathological conditions including cancer and fibrosis. Though in most cases it is not yet clear what role stiffening plays in disease progression, new studies have begun to parse this process in liver fibrosis. Recent work suggests a two stage process for stiffening, which begins with the existing collagen matrix being stiffened by lysyl oxidase crosslinking [Georges et al., 2007]. In culture a stiff substrate in combination with TGF beta signaling drives the differentiation of portal fibroblasts into myofibroblasts [Arora et al., 1999; Li et al., 2007] which have been shown to deposit a fibrotic matrix [Wells, 2008a] and thus may contribute to later stage stiffening. These findings demonstrate how changes in matrix stiffness can facilitate differentiation from a quiescent phenotype to a remodeling phenotype which then further stiffens the matrix creating a positive feedback loop that promotes disease progression.
MECHANICAL EFFECTS ON CELL PROLIFERATION
Two recent reports show a dependence of cell cycle rate on substrate stiffness. One found decreased proliferation of dermal fibroblasts in less dense, more compliant 3D collagen matrices compared to denser, stiffer gels [Hadjipanayi et al., 2008]. The other reported almost complete suppression of proliferation when mesenchymal stem cells were cultured on or between ligand coated, soft, synthetic gels confirming that a drop in proliferation could occur independent of a change in ligand density [Winer et al., 2009]. These results with primary cells confirm an earlier study, which found that soft substrates suppressed proliferation of normal 3T3 fibroblasts but not H-ras-transformed 3T3 fibroblasts [Wang et al., 2000], In contrast, other transformed cells, such as HMT3522 S-1 mammary epithelial cells, retain proliferation sensitivity to substrate compliance [Paszek et al., 2005]. In another study with clonally-derived bone marrow multipotent cells, proliferation was insensitive to matrix stiffness until the cells were chemically induced to differentiate into osteoblasts [Hsiong et al., 2008]. This result, when combined with the results of other studies suggests that mesenchymal stem cells are likely a heterogeneous population when it comes to their responsiveness to substrate mechanics.
Although a link between proliferation and substrate stiffness has been repeatedly demonstrated it is not known where in the cell cycle the cells on soft substrates have become trapped. For instance there are no reports of DNA content in compliance-arrested cells. What has been shown is that mammary epithelial cells on compliant soft substrates do not phosphorylate ERK in response to growth factor stimulation [Paszek et al., 2005] and ERK activation is required for mid G1 induction of cyclin D [Klein et al., 2008]. Studies to shed light on this connection have many implications for cell function and dysfunction in vivo [Assoian and Klein, 2008].
SUBSTRATE STIFFNESS EFFECTS ON TRACTION FORCES AND CELL MOTILITY
A number of recent results now suggest that a cell’s ability to apply traction forces to its substrate is an important factor that determines how the cell moves, how it organizes its cytoskeleton, grows or divides, and what kinds or amount of proteins it secretes into the extracellular space. These mechanically-dictated cellular responses appear to be important for tissue- and organism- level processes including pattern formation during development, wound healing after injury, and maintenance of organ integrity.
The first studies of cell migration on polyacrylamide gels [Pelham and Wang, 1997] showed that fibroblasts migrated much more slowly on stiff gels (0.06 µm/min) than on soft substrates (0.55 µm/min). However, directed movement is more persistent on stiffer substrates so that when presented with a boundary between soft and stiff materials, fibroblasts tend to move toward the stiffer regions, a process termed “durotaxis” [Lo et al., 2000]. A systematic study of fibroblast durotaxis showed that when the cell approaches the boundary from the soft side, the protrusion accelerates and enters the stiff region of the gel. When the leading edge of the cell approaches a soft region of the gel from the stiff side, the cell stops when it senses the softer gel, and then either moves parallel to the boundary of stiff and soft substrates, or reorients itself to move away from the boundary. Similar effects are seen in three dimensions using a compressed collagen matrix [Hadjipanayi et al., 2009]. Briefly, in these experiments, adult human dermal fibroblasts were embedded in a wedge shaped collagen matrix in such a way that when the matrix is compressed, the cells are uniformly distributed throughout and the side of the construct that has more compressed collagen has been measured to be stiffer. After 6 days in culture with mitomycin C to prevent cell division, there is a significant difference between the number of cells on the stiff side of the construct and on the soft side of the construct. These data suggest that the cells are migrating preferentially to the stiff side of the matrix. Cell migration was also studied on two dimensional surfaces where areas of stiff and compliant substrate were micropatterned in order to measure preferential migration [Gray et al., 2003]. After 24 hours, NIH-3T3 fibroblasts accumulated in the stiffer regions in the gel. In addition, whereas previous studies had shown that durotaxis only occurred without cell-cell contact, this study showed that cells preferentially migrated towards the stiffer substrates regardless of whether or not they were in contact with another cell.
Not all cells durotax, and neurons extend processes farther and faster as substrate stiffness is lowered to the values characteristic of normal CNS tissue [Flanagan et al., 2002; Georges et al., 2006; Kostic et al., 2007; Chan and Odde, 2008; Jiang et al., 2008; Saha et al., 2008]. Differences in the motility of different cell types on stiffness gradients might be relevant for patterning and other processes that involve cell sorting in tissues [Georges et al., 2006].
The processes of durotaxis and substrate stiffness sensing requires that cells generate and transmit forces to their substrates. First quantified by measuring wrinkles produced by cells cultured on flexible silicone films [Harris et al., 1980], these traction forces were later localized to the focal adhesions [Balaban et al., 2001] and found to be a function of matrix compliance [Dembo and Wang, 1999]. Just as neurons extend larger, more branched neurites on soft matrices, they also apply greater traction forces and display slower retrograde actin flow on substrates softer than 1 kPa [Chan and Odde, 2008]. Strong traction forces modulated by either adhesion density or substrate stiffness also induce scattering of kidney epithelial cells by straining the cell-cell adhesions [de Rooij et al., 2005].
INTRACELLULAR SIGNALS SENSITIVE TO SUBSTRATE MECHANICS
Integrin attachment to the substrate mediates signaling down the MAP kinase and other pathways [Schlaepfer et al., 1994; Parsons, 1996; Coppolino and Dedhar, 2000; Yee et al., 2008] and provides a reasonable hypothesis for how substrate stiffness might regulate intracellular signaling as substrate stiffness sensing occurs through the same ECM-integrin-actin connection [Discher et al., 2005]. One example of this interconnection is that preosteoblasts cultured on stiff gels had higher alkaline phosphatase activity and increased expression of osteocalcin and bone sialoprotein than those cells cultured on soft gels. These increases were mitigated when an inhibitor of the MAPK cascade was added to the induction media even though the effectiveness of the inhibitor also decreased with increasing substrate stiffness [Khatiwala et al., 2007].
Another signaling pathway affected by substrate stiffness is calcium regulation by RhoA and its effecter RhoA kinase (ROCK). Myocytes on collagen coated polyacrylamide apply the greatest contractile force and have the largest calcium stores and transient calcium peaks when cultured on intermediate stiffness gels [Jacot et al., 2008]. In hMSCs the frequency and magnitude of cytosolic Ca2+ oscillations decreased with substrate stiffness. Unlike other stiffness-regulated effects, neither cytoD, nocodazole or blebbistatin had a significant effect on the Ca2+ oscillations. Instead RhoA activity was substantially reduced on soft gels, and ROCK inhibited the frequency but not magnitude of the calcium oscillations [Kim et al., 2009].
The signals mediating differentiation of bone marrow-derived mesenchymal stem cell (MSC) are also dependent on substrate compliance [Engler et al., 2006]. MSCs on the stiffest substrates expressed early markers of osteogenesis, whereas these cells on intermediate stiffness gels expressed myogenic markers and cells on the softest gels expressed neuronal markers. Another study reported that stiffness regulation of lineage specific markers depended on which ECM ligand was attached to the substrate. When the substrate was coated with collagen I, expression of the myogenesis marker MyoD was highest in MSCs cultured on the stiffest gels; however, if the substrate was coated by collagen IV or fibronectin, expression was highest on intermediate stiffness gels [Rowlands et al., 2008]. Subsequent studies have demonstrated that stiffness alone is not sufficient to fully differentiate cells, but appropriate stiffness matching enhances the differentiation rate when chemical induction factors are added [Hsiong et al., 2008; Saha et al., 2008; Winer et al., 2009]. Control of differentiation by substrate stiffness has not only been seen in MSCs but also in neural stem cells which differentiated into neurons on soft substrates and glia on stiff substrates when cultured in media that promoted both lineages [Saha et al., 2008].
HOW DO CELLS SENSE STIFFNESS?
In principle, the cell’s mechanism of stiffness measurement has to be analogous to the way in which rheometers measure stiffness. In the simplest case, the stiffness of a material that is perfectly elastic and does not undergo viscous flow, stiffness is quantified by an elastic modulus: a ratio of stress to strain, or equivalently, a measure of the amount of force per area needed to achieve a given amount of deformation. Since there are two different quantities needed to define stiffness: stress and strain, or equivalently, force per area and deformation, cells, like rheometers can either apply a defined amount of stress and measure the resulting strain, or else they can measure the amount of stress that is required to achieve a given amount of strain. In other words, they can be stress-controlled or strain-controlled.
It might appear simpler for cells to function as controlled stress devices, in which case they would activate a set number of motors per area at the membrane/ECM interface and then detect strain by the amount of deformation imposed on some structure such the folded domains of proteins inside or outside the cell or in the lipid bilayer. An equivalent strategy for stress-controlled measurement would be to produce a given amount of force per area with motors and then detect the amount of work done (by e.g., ATP hydrolysis) until the resulting strain produced an elastic resistance equal to the active force generated. However, most of the limited number of measurements of the tractions forces that fibroblasts [Ghosh et al., 2007] or epithelial (MDCK) cells [Saez et al., 2005] apply to the surfaces of gels or pillars with different stiffness show that the softer the gel or the more flexible the pillar array, the smaller the traction force, and that therefore these cells might function as strain-controlled devices. It seems probable that different cells employ different strategies, and even that cells can switch from one mode to the other depending on the conditions.
WHERE IS THE STIFFNESS SENSOR AND WHAT MOLECULES ARE NECESSARY?
Almost certainly the structure that transduces mechanical information into the chemical messengers that orchestrate cell functions resides at the cell membrane and involves proteins or other molecules within the extracellular matrix, transmembrane receptor, intracellular proteins that link these receptors to the cytoskeleton and to motor proteins and possibly even to the nuclear matrix and chromatin [Wang et al., 2009]. The adhesion complexes formed at the tips of filopodia would appear to be particularly good places at which a stiffness sensor could be placed.
There is no reason a prior why a single mechanism would account for the divergent mechanical responses of different cells, and there is now abundant evidence that different cell types have distinct responses to different ranges of substrate stiffnesses and that their responses depend strongly on the type of adhesion receptors by which they engage their substrate [Georges and Janmey, 2005]. A plausible model for stiffness sensing involves a number of proteins or perhaps other macromolecules linked in series that are subject to the same tension when a force applied by the cell is transmitted to its link to the extracellular matrix. Plausible candidates for these elements have been identified by changes in mechanosensing when specific genes are deleted, overexpressed, or mutated. Such studies identify candidate gene products involved in the response, but might not necessarily identify the unique factor that turns a force into a biochemical change.
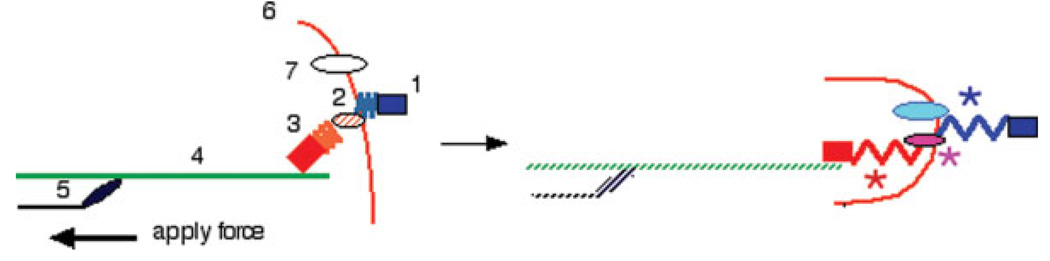
From a mechanical perspective, a minimal set of proteins that might function as a mechanical transducer capable of detecting stiffness changes is shown in Fig. 5. Many extracellular matrix proteins such as laminin, fibronectin, and collagen are large flexible macromolecules that are deformed by pN forces [Gee et al., 2008]. These are in turn linked to transmembrane protein receptors such as integrins that also undergo conformational changes in response to force [Friedland et al., 2009; Puklin-Faucher and Sheetz, 2009]. On the intracellular face of the plasma membrane lie numerous large flexible proteins such as talin, filamin, and many others that mediate the linkage between integrins and either the actin or the intermediate filament networks. These proteins have been proposed as candidates for mechanotransduction because they often exhibit changes in ligand binding or other functions when they are subjected to forces [del Rio et al., 2009] and their deletion can render a cell insensitive to stiffness differences to which it would otherwise respond [Zhang et al., 2008; Byfield et al., 2009]. The cytoskeletal filaments are then linked either directly, for actin, or indirectly, for IFs, to motor proteins such as myosins, each of which can apply pN forces to the filament to create tension that propagates through the filament and its linker proteins to the ECM. Activation of motor proteins places all elements linked in series to the same tension, and in this case, the softest element will deform the most. If molecular deformation or unfolding exposes a new active site or otherwise alters protein function, then cellular signals can be initiated by the same biochemical pathways that are engaged by chemical ligands. Recent studies of neuronal filopodia have revealed the importance of molecular clutches that determine the duration and extent to which motor protein-generated forces are transmitted through actin filaments onto the cell membrane and the ECM [Chan and Odde, 2008]. It has also been proposed that the mechanical linkage proceeds deeper into the cell, and could initiate transcriptional activation within the nucleus even in the absence of soluble signals generated in the cytoplasm [Wang et al., 2009].
Fig. 5.
Schematic diagram of structures potentially involved in mechanotransduction during stiffness sensing. In the resting cell, the ECM is linked to the cell interior by linkages among ECM proteins (1), transmembrane adhesion receptors (2), one or more proteins (3) that bind transmembrane proteins to cytoskeletal filaments (4) to which forces can be applied by motors (5). The resulting tension can deform any of these elements in series or can be transmitted through the membrane (6) to affect enzymes and other proteins (7) that are physically linked to it. The motor-generated force and the transmitted tension can potentially unfold extracellular proteins to expose a new receptor activating site (*), activate a transmembrane receptor (*), unfold an intracellular protein active site (*), recruit proteins to regions of increased membrane curvature, or transmit force through the cytoskeleton to an interior target such as the nucleus. The tension can also change the stability of motor-filament or clutch protein-filament binding through activation of catch bonds or slip-bonds.
FUTURE DIRECTIONS
In the broadest sense, the mechanical interaction between a cell and the substrate to which it adheres is beginning to be seen as a significant factor that helps determine cell structure and function. Some proteins and intracellular signals that mediate this mechanical response are beginning to be identified, but a complete model for stiffness sensing remains to be developed. In particular, very little is known about the physical properties of a putative stiffness sensor: how much force does the cell apply in order to probe stiffness, how long does it probe before deciding whether the substrate is softer or stiffer than some set point, how much movement does the cell need to achieve before significant molecular rearrangements occur? These and many other features of stiffness sensing and mechanotransduction in general remain to be revealed.
CONCLUSION
The concept that cell and environmental mechanics might be a cause and not just the effect of the complex processes that produce biological function is increasingly supported by a range of studies, but is still relatively untested. In some studies such as those outlined here, the mechanical properties of the extracellular matrix or substrate can dominate chemical or spatial cues to determine cell fate. Whether further studies will confirm that manipulating intracellular or extracellular mechanics can cause a normal cell to malfunction or a malignant cell to revert or die remains to be seen.
REFERENCES
- Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154:871–882. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–352. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Burstein M, Lewi S. Platelets and structure of the plasma clot; the mode of action of platelets during retraction. C R Seances Soc Biol Fil. 1952;146(11–12):829–832. [PubMed] [Google Scholar]
- Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J. 2009;96 doi: 10.1016/j.bpj.2009.03.046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Steward RL, Jr, Leduc PR. Probing cell structure by controlling the mechanical environment with cell-substrate interactions. J Biomech. 2009;42:187–192. doi: 10.1016/j.jbiomech.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Dedhar S. 2000. Bi-directional signal transduction by integrin receptors. Int J Biochem Cell Biol. 32:171–188. doi: 10.1016/s1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:323–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(Pt 22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsi JM, Aubin JE. Microfilament rearrangements during fibroblast-induced contraction of three-dimensional hydrated collagen gels. Cell Motil. 1984;4:29–40. doi: 10.1002/cm.970040105. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Gee EP, Ingber DE, Stultz CM. Fibronectin unfolding revisited: modeling cell traction-mediated unfolding of the tenth type-III repeat. PLoS ONE. 2008;3:e2373. doi: 10.1371/journal.pone.0002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DS, Tien J, Chen CS. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. J Biomed Mater Res A. 2003;66:605–614. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2008;3:77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- Hadjipanayi E, Mudera V, Brown RA. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton. 2009;66:121–128. doi: 10.1002/cm.20331. [DOI] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85:145–156. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang FX, Yurke B, Firestein BL, Langrana NA. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann Biomed Eng. 2008;36:1565–1579. doi: 10.1007/s10439-008-9530-z. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Skinner J, Brown R, Mudera V. Matrix stiffness and serum concentration effects matrix remodelling and ECM regulatory genes of human bone marrow stem cells. J Tissue Eng Regen Med. 2008;2(2–3):97–105. doi: 10.1002/term.69. [DOI] [PubMed] [Google Scholar]
- Keese CR, Giaever I. Substrate mechanics and cell spreading. Exp Cell Res. 1991;195:528–532. doi: 10.1016/0014-4827(91)90406-k. [DOI] [PubMed] [Google Scholar]
- Khatiwala CB, Peyton SR, Metzke M, Putnam AJ. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J Cell Physiol. 2007;211:661–672. doi: 10.1002/jcp.20974. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Seong J, Ouyang M, Sun J, Lu S, Hong JP, Wang N, Wang Y. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218:285–293. doi: 10.1002/jcp.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EA, Campbell LE, Kothapalli D, Fournier AK, Assoian RK. Joint requirement for Rac and ERK activities underlies the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells. J Biol Chem. 2008;283:30911–30918. doi: 10.1074/jbc.M804537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A, Sap J, Sheetz MP. RPTPalpha is required for rigiditydependent inhibition of extension and differentiation of hippocampal neurons. J Cell Sci. 2007;120(Pt 21):3895–3904. doi: 10.1242/jcs.009852. [DOI] [PubMed] [Google Scholar]
- Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173:548–550. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- Marek LF, Kelley RO, Perdue BD. Organization of the cytoskeleton in square fibroblasts. Cell Motil. 1982;2:115–130. doi: 10.1002/cm.970020204. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci. 2009;122(Pt 2):179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037–C1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- Saez A, Buguin A, Silberzan P, Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys J. 2005;89:L52–L54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlunck G, Han H, Wecker T, Kampik D, Meyer-ter-Vehn T, Grehn F. Substrate rigidity modulates cell matrix interactions and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2008;49:262–269. doi: 10.1167/iovs.07-0956. [DOI] [PubMed] [Google Scholar]
- Schwarz US, Bischofs IB. Physical determinants of cell organization in soft media. Med Eng Phys. 2005;27:763–772. doi: 10.1016/j.medengphy.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, alpha-actinin, and myosin. Dev Biol. 1982;92:107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Weiss P. Cellular dynamics. Rev Mod Phys. 1959;31:11–20. [Google Scholar]
- Weiss P, Garber B. Shape and movement of mesenchyme cells as functions of the physical structure of the medium: contributions to a quantitative morphology. Proc Natl Acad Sci USA. 1952;38:264–280. doi: 10.1073/pnas.38.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008a;12:759–768. viii. doi: 10.1016/j.cld.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008b;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15:147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Majd H, Acharya C, Buscemi L, Meister JJ, Hinz B. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials. 2009;30:1781–1789. doi: 10.1016/j.biomaterials.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Yee KL, Weaver VM, Hammer DA. Integrin-mediated signalling through the MAP-kinase pathway. IET Syst Biol. 2008;2:8–15. doi: 10.1049/iet-syb:20060058. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]