Abstract
Approximately 80 million people worldwide are infertile, and nearly half of all infertility cases are attributed to a male factor. Therefore, progress in reproductive genetics becomes crucial for future diagnosis and treatment of infertility. In recent years, enormous progress has been made in this field. More than 400 mutant mouse models with specific reproductive abnormalities have been produced, and numerous human association studies have been discovered. However, the translation of basic science findings to clinical practice remains protracted, with only modest progress in the application of novel findings to clinical genetic testing and cures. To date, the most significant findings in male infertility remain numeric and structural chromosomal abnormalities and Y-chromosome microdeletions in infertile men. Thus, we anticipate that future genetic investigations will focus on infertile men with a normal somatic karyotype but with various spermatozoal defects, like insufficient production of spermatozoa (oligozoospermia), inadequate motility (asthenozoospermia), abnormal morphology (teratozoospermia), or combinations of these defects. Ultimately, basic advances in mammalian nonhuman reproduction will translate to clinical advances in human reproduction and testing for infertile humans, thereby helping to improve diagnostics and health care for infertile patients.
Keywords: Reproduction, mouse model, human male infertility
During the last 18 years, the Matzuk laboratory has been focused on deciphering the processes of normal and abnormal reproduction in mammals. Because fertility cannot be evaluated in a test tube, our laboratory has been using knockout and knockin transgenic mouse models to dissect mammalian transforming growth factor β(TGFβ) superfamily signaling pathways, study ovarian and testicular cancers, define the functions of known and novel germ cell–expressed genes, and identify unique germ cell targets for contraception. To understand the role of specific proteins in vivo, we have produced more than 70 transgenic mouse models, including mice lacking TGFβ superfamily ligands, binding proteins, and receptors; and downstream signaling proteins and their nuclear targets (eg, growth differentiation factor 9 [GDF9], bone morphogenic protein 15 [BMP15], inhibins [INHA], activins [INHBA], activin receptor type 2A [ACVR2A], SMADs, and pentraxin 3). Our studies identified inhibin as the first secreted tumor-suppressor factor that inhibits testicular and ovarian tumor development (Matzuk et al, 1992). Our research also showed that ACVR2A is a key regulator of follicle-stimulating hormone (FSH) synthesis and secretion from the anterior pituitary gland, and in the absence of ACVR2A, FSH levels are suppressed, resulting in a hypogonadal phenotype (Matzuk et al, 1995). Many mouse models have also been used to study female reproduction and/or gene function in both females and males (reviewed in Matzuk and Lamb, 2002, 2008).
Genes Important in the Male Germ Line
To study the genes and mechanisms that direct male reproduction, we have generated mutant mice that lack key proteins at almost every step of spermatogenesis (Table). To identify such critical proteins, we used functional genomic approaches, including analysis of evolutionary conservation, protein domain homology, and genomic synteny between mouse and human genomes. Our work effectively led to the discovery of essential genes at key stages of spermatogenesis. We identified several genes that show defects prior to as well as after meiotic division. Our studies with Dr Debra Wolgemuth’s group demonstrated that mutant mice, homozygous for a deletion of the cyclin A1 (Ccna1) gene, show spermatogenesis arrest prior to the first meiotic division (Liu et al, 1998). Mutant Ccna1-null males present with increased germ cell apoptosis, desynapsis abnormalities, and reduction of CDC2 kinase activation at the end of meiotic prophase, leading to meiotic arrest. Therefore, we conclude that cyclin A1 is essential for the mitosis-meiosis transition during spermatogenesis.
Table 1.
Mouse models with single-gene mutations and reproductive defectsa
| Mouse Gene–Target | Sex Affected | Reproductive Defect | Mouse Phenotype | Reference | Chr | |
|---|---|---|---|---|---|---|
| 1. | Activin receptor-type IIA (Acvr2a) | Both | Antral follicle block (F); small testes, delayed initiation of copulation, reduced mount, and intromission frequencies, and increased mount, intromission, and ejaculation latencies (M) | Infertile (F) Subfertile (M) |
Matzuk et al, 1995; Ma et al, 2005 | 2 |
| 2. | Anti-Müllerian hormone receptor (Amhr2) | Male | Uteri development causes obstruction | Secondary infertility | Mishina et al, 1996 | 15 |
| 3. | Bone morphogenetic protein 15 (Bmp15) | Female | Defects in cumulus-oocyte complex formation and ovulation | Subfertile | Yan et al, 2001 | X |
| 4. | Cyclin A1 (Ccna1) | Male | Block in spermatogenesis before the first meiotic division; haploinsufficiency observed in 129S6/SvEv inbred background | Infertile | Liu et al, 1998 | 3 |
| 5. | Folliculogenesis-specific basic helix-loop-helix (Figla) | Female | No primordial follicles develop at birth, and oocytes die | Infertile | Soyal et al, 2000 | 6 |
| 6. | Follistatin (Fst) | Female | Presence of male-specific coelomic vessel in ovary and loss of oocytes | Postnatal lethal | Yao et al, 2004 | 13 |
| 7. | Follicle-stimulating hormone β (Fshb) | Both | Preantral block in folliculogenesis (F); decreased testis size (M) | Infertile (F) | Kumar et al, 1997 | 2 |
| 8. | Germ cell protein with ankyrin repeats, sterile α and leucine zipper motifs (Gasz) | Male | Disrupted structure of intermitochondrial cement, zygotene-pachytene spermatocytes block | Infertile | Ma et al, 2009 | 6 |
| 9. | Growth differentiation factor 9 (Gdf9) | Female | Folliculogenesis arrest at the 1-layer follicle stage | Infertile | Dong et al, 1996; Elvin et al, 1999 | 11 |
| 10. | Inhibin α (Inha) | Both | Granulosa/Sertoli cell tumors, gonadotropin hormone dependent | Infertile (F) Secondary infertility (M) |
Matzuk et al, 1992 | 1 |
| 11. | Inhibin/activin βB (Inhbb) | Female | Delivery and nursing defects | Subfertile | Vassalli et al, 1994 | 1 |
| 12. | Kelch-like 10 (Klhl10) | Male (hetero) | Dysmorphic spermatozoa and impaired motility | Infertile | Yan et al, 2004 | 11 |
| 13. | Luteinizing hormone β (Lhb) | Both | Failure of spermatogenesis (M) and folliculogenesis (F) | Infertile | Ma et al, 2004 | 7 |
| 14. | LIM homeobox protein 8 (Lhx8) | Female | Primordial to primary follicle block and oocyte loss | Infertile | Pangas et al, 2006 | 3 |
| 15. | NOBOX oogenesis homeobox (Nobox) | Female | Primordial to primary follicle block and oocyte loss | Infertile | Rajkovic et al, 2004 | 6 |
| 16. | Nucleophosmin/nucleoplasmin 2 (Npm2) | Female | Maternal effect gene; partial block at 1-cell to 2-cell embryo stage | Subfertile | Burns et al, 2003 | 14 |
| 17. | 2′–5′ Oligoadenylate synthetase 1D (Oas1d) | Female | Defects in folliculogenesis and ovulatory efficiency | Subfertile | Yan et al, 2005 | 5 |
| 18. | Oxytocin (Oxt) | Both | Maternal nursing defects (F); social memory defect and increased aggression (M) | Fertile | Nishimori et al, 1996 | 19 |
| 19. | Oxytocin receptor (Oxtr) | Both | Maternal nursing defects (F); social memory defect and increased aggression (M) | Fertile | Takayanagi et al, 2005 | 6 |
| 20. | Platelet-activating factor acetylhydrolase, isoform 1b, beta1 subunit (Pafah1b1; Lis1) | Male | Gene trap insertion; spermiogenic block, spermatids fail to form correct acrosomes, and nuclei are distorted in size and shape | Infertile | Yan et al, 2003 | 11 |
| 21. | Sma/MAD homolog 5 (Smad5) | Both | Developing embryos lose primordial germ cells | Lethal | Chang and Matzuk, 2001 | 13 |
| 22. | Superoxide dismutase 1 (Sod1) | Female | Folliculogenesis defect, failure to maintain pregnancy | Subfertile | Ho et al, 1998; Matzuk et al, 1998 | 16 |
| 23. | Spermatogenesis and oogenesis-specific basic helix-loop-helix 1 (Sohlh1) | Both | Spermatogenesis block at meiotic entry (M); primordial to primary follicle block and oocyte loss (F) | Infertile | Pangas et al, 2006 | 2 |
| 24. | Tektin 3 (Tekt3) | Male | Sperm motility defects | Fertile | Roy et al, 2009 | 11 |
| 25. | Tektin 4 (Tekt4) | Male | Sperm motility defects and ultrastructural defects in flagellum | Subfertile | Roy et al, 2007 | 17 |
| 26. | Testis-expressed gene 14 (Tex14) | Male | Absence of germ cell intercellular bridges; meiotic defects and block at pachytene stage | Infertile | Greenbaum et al, 2006 | 11 |
| 27. | Y-box protein 2 (Ybx2; Msy2) | Both | Elongating spermatid arrest (M); follicular atresia, oocyte loss, anovulation (F) | Infertile | Yang et al, 2005 | 11 |
| 28. | Zygote arrest 1 (Zar1) | Female | Maternal effect gene, block at 1-cell to 2-cell embryo stage | Infertile | Wu et al, 2003b | 5 |
| 29. | Zona pellucida–binding protein (Zpbp) | Male | Abnormal acrosome and globozoospermia | Infertile | Lin et al, 2007 | 11 |
| 30. | Zona pellucida–binding protein 2 (Zpbp2) | Male | Abnormal acrosome formation and sperm head defects | Subfertile | Lin et al, 2007 | 11 |
Abbreviations: Chr, mouse chromosome location of the gene; hetero, haploinsufficiency phenotype observed in heterozygous mice; F, female; M, male.
Mutations were generated by using embryonic stem cell knockout technology and were created and/or phenotypically characterized by the Matzuk laboratory. Only the first reference is cited. Definitions: asthenozoospermia, reduced sperm motility; azoospermia, no sperm; globozoospermia, rounded sperm head; oligozoospermia, low sperm counts; teratozoospermia, abnormal sperm head morphology.
Other collaborative studies with Drs Norman Hecht and Richard Schultz led to the discovery of a key role for the germ cell–specific protein from the Y-box family of DNA/RNA-binding proteins; namely, male-specific, Y-box protein 2 (MSY2), also known as YBX2 (Yang et al, 2005). MSY2 was proposed to be a coactivator of transcription in postmeiotic spermatids by stabilizing the storage of parental mRNAs in the cytoplasm. Mice lacking MSY2 demonstrate disrupted spermatogenesis in postmeiotic germ cells, with many misshapen and multinucleated spermatids, resulting in azoospermia. Although increased apoptosis was observed, the major defect was due to large reductions of the mRNA levels in postmeiotic male germ cell transcripts. Interestingly, defects in MSY2-null female mice were observed at later stages of oogenesis in adults and include increased oocyte loss, anovulation, and multiple morphologic defects of oocytes and follicles. Thus, MSY2 represents one of a small number of germ cell–specific genes whose deletion leads to the disruption of both spermatogenesis and oogenesis.
Considerable progress has been made in the investigation of several genes involved in the later stages of spermatogenesis. To study proteins responsible for normal morphology of the flagella and acrosome in mature spermatozoa, we have created a number of mutant mice, including knockout models of tektin 3 (TEKT3), tektin 4 (TEKT4), and zona pellucida–binding proteins 1 (ZPBP1) and 2 (ZPBP2; Table). We demonstrated that homozygous deletions of these genes cause ultrastructural defects in the spermatozoal flagella and sperm motility defects that, in turn, lead to subfertility or infertility (Roy et al, 2007, 2009). Sperm that are null for Tekt4 exhibit severely reduced forward progression and uncoordinated movement of the flagellum. Transmission electron microscopy revealed that spermatozoal flagellar ultrastructure is mainly unaltered. However, the ineffective flagellar strokes in Tekt4-null sperm lead to nearly 10-fold higher consumption of intracellular adenosine triphosphate, resulting in rapid loss of progressive motility. Although male mice lacking TEKT3 are fertile, they similarly produce sperm with reduced motility and forward progression and with increased flagellar structural bending defects. Furthermore, male mice null for both TEKT3 and TEKT4 show subfertility, suggesting partial nonredundant roles for these 2 proteins in sperm physiology. Thus, tektins are necessary for the proper coordinated beating of the sperm flagellum and forward movement and are potential candidate genes for nonsyndromic asthenozoospermia in humans.
To understand the final stages of spermatogenesis and fertilization, we constructed mouse models with a knockout of 2 novel proteins expressed in the acrosome of mature spermatozoa, ZPBP1 and ZPBP2 (Lin et al, 2007). Mutant male mice lacking ZPBP1, ZPBP2, or both demonstrated abnormal acrosome formation due to various morphologic defects of the sperm head. Male mice null for Zpbp1 were sterile and presented with characteristic abnormal round-headed sperm morphology (similar to globozoospermia) and no forward sperm motility. The abnormal phenotype of null Zpbp1 is probably caused by improper acrosome compaction, ultrastructural acrosome fragmentation, and disruption of the Sertoli cell-spermatid junctions, resulting in the inability of oocyte fertilization. Males missing ZPBP2 were subfertile and produced dysmorphic sperm with a reduced ability to penetrate the zona pellucida. Further studies of mutant Zpbp2 mice demonstrated that aberrant acrosomal membrane invaginations resulted in abnormal morphology. Although double-mutant mice heterozygous for the Zpbp1 mutation and homozygous for the mutant Zpbp2 allele had more severe morphologic defects and reduced fecundity, double homozygous mutant mice were sterile, similar to the Zpbp1-null mice. These results suggested that the 2 paralogous genes play important structural roles during spermiogenesis and likely cooperate in sperm-egg interaction.
The Immortal Male Germ Line
Spermatogenesis is a continuous process originating from spermatogonial stem cells (SSCs). Because SSCs can self-renew and maintain in an undifferentiated state, sperm are produced for the lifetime of an adult male. Self-renewal of SSCs is strictly regulated by extrinsic stimuli as well as intrinsic signal pathways and transcriptional mechanisms. Extrinsic factors that regulate self-renewal and differentiation of SSCs have been analyzed. Glial cell line–derived neutrophic factor (GDNF) is an essential growth factor for SSCs to self-renew and is produced by Sertoli cells. GDNF stimulates SSCs to self-renew by signaling through a heterodimer of the tyrosine kinase, protooncogene rearranged during transfection (RET), and GDNF receptor family alpha 1 (GFRα1; Figure 1) (Jing et al, 1996; Treanor et al, 1996). In testes heterozygous for Gdnf null mutation, spermatogenesis is impaired by defects in self-renewal of SSCs. In contrast, there is an abnormal increase of SSCs in testes with overexpression of GDNF (Meng et al, 2000). When normal SSCs are transplanted into GDNF-deficient testes, SSCs cannot be maintained in an undifferentiated state and disappear from the transplanted testes (Naughton et al, 2006). Basic fibroblast growth factor (bFGF) can enhance self-renewal of SSCs together with GDNF in vitro, although SSCs cannot self-renew with bFGF alone (Kubota et al, 2004). Recently, colony-stimulating factor 1 (CSF1) was shown to enhance self-renewal activity of SSCs in the presence of GDNF and bFGF in vitro (Oatley et al, 2009). On the other hand, Kit ligand (KITL) is an essential cytokine for spermatogonia to differentiate. In testes of mice mutant for KITL, undifferentiated spermatogonia accumulate because of a failure in differentiation (Ohta et al, 2000). Transplantation experiments reveal that the KIT tyrosine kinase receptor, the receptor for KITL, is absent in SSCs and present in differentiated spermatogonia (Shinohara et al, 2000).
Figure 1.
Essential molecules regulating self-renewal of spermatogonial stem cell (SSC). Glial cell line–derived neutrophic factor (GDNF) stimulates SSC self-renewal, and basic fibroblast growth factor (bFGF) and colony-stimulating factor 1 (CSF1) enhance this activity. Transcriptional repressor, encoded by B-cell chronic lymphocytic leukemia/lymphoma 6, member b (Bcl6b) and the transcription factors Ets variant 5 (Etv5) and LIM homeobox 1 (Lhx1), are activated by the GDNF stimuli. Independently from GDNF signaling, the transcriptional repressor promyelocytic leukemia zinc finger; (PLZF; also known as ZBTB16 and ZFP145) and transcription factor TATA box-binding protein–associated factor (TAF4b) regulate SSC self-renewal.
Extrinsic signals stimulate downstream signal transduction pathways as well as transcription machineries. The most important transcriptional regulator of SSCs is promyelocytic leukemia zinc finger (PLZF; also known as ZFP145 and ZBTB16; Figure 1) (Buaas et al, 2004; Costoya et al, 2004). PLZF is a POK (POZ/BTB and Kruppel)–type transcription suppressor and regulates transcription by negative cooperation with the core-pressors and histone deacetylase (HDAC) (Barna et al, 2002). The expression of PLZF is restricted to SSCs and early spermatogonia. Mutations in the Plzf locus lead to male infertility by progressive loss of germ cells. In PLZF-mutant testes, spermatogonia exist at birth, and spermatozoa are produced in the first few rounds of spermatogenesis, but ultimately all germ cells disappear. PLZF-mutant SSCs fail to colonize when transplanted in germ cell–depleted testes. However, environmental somatic cells in PLZF-null testes can support self-renewal of wild-type SSCs. PLZF may regulate genes related to differentiation through epigenetic regulations, because PLZF interacts with polycomb group proteins and HDAC (Barna et al, 2002).
SSCs express Octomer-4 (OCT4; also known as POU domain class 5 transcription factor 1 [POU5F1]), which is essential for cell pluripotency and cell survival of embryonic stem (ES) cells and primordial germ cells (PGCs). OCT4 is a transcription factor specifically expressed in ES cells, preimplantation embryos, and germ cells (Pesce et al, 1998). In testes, OCT4 localizes to spermatogonia and is used as a marker of SSCs. Recently, OCT4 was shown to be important for SSC maintenance by knockdown experiments (Dann et al, 2008). OCT4 knockdown SSCs cannot colonize in culture and transplanted testes. Because PLZF is not affected by knockdown of OCT4, OCT4 and PLZF function in different pathways to maintain the SSCs in an undifferentiated state.
A basic helix-loop-helix (bHLH) transcription factor, neurogenin 3 (NGN3), localizes to only early spermatogonia (between As and Aal) in testes (Yoshida et al., 2004). NGN3 is a useful marker of SSCs because NGN3 starts to express in spermatogonia after birth but is not expressed in PGCs. At present, the role of NGN3 in SSCs remains unclear. Germ cell–specific bHLH transcription factor testis- and ovary-specific bHLH transcriptional factor 1 (SOHLH1) and SOHLH2 are expressed in type A spermatogonia (Ballow et al, 2006a, 2006b). In testes lacking SOHLH1 or SOHLH2, differentiation of spermatogonia is inhibited, and only type A spermatogonia remain (Ballow et al, 2006a; Hao et al, 2008; Toyoda et al, 2009). SOHLH1 and SOHLH2 can form heterodimers and control transcription in spermatogonia. Interestingly, expression of NGN3 is decreased in SOHLH1-null testes, which are enriched in undifferentiated spermatogonia, suggesting that SOHLH1 might regulate transcription of NGN3 (Ballow et al, 2006a).
Oatley et al (2006) identified genes regulated by GDNF by using an in vitro culture system. Among these genes, the gene that showed the most dramatic change in expression is B-cell chronic lymphocytic leukemia/lymphoma 6, member B (BCL6B; also known as BAZF). BCL6B, like PLZF, is a POK family transcriptional repressor. SSCs, in which BCL6B was down-regulated by small interfering RNA, were decreased in both number and size of colonies. Furthermore, colony formation was decreased when BCL6B knockdown SSCs were transplanted into recipient testes. Although BCL6B-null mice are fertile, about 24% of seminiferous tubules degenerated and/or showed defects in spermatogenesis. BCL6B is suggested to be an important molecule in self-renewal of SSCs and is a gene that connects the GDNF pathway and SSC self-renewal. Ets variant 5 (ETV5; also known as ERM) and Lim homeobox 1 (LHX1) were other transcription factors regulated by GDNF and shown to be important for SSC maintenance in vitro (Oatley et al, 2007).
Whereas PLZF and BCL6B are essential for SSC self-renewal and regulate transcription negatively, TATA box-binding protein (TBP)–associated factor 4b (TAF4B) was shown to be an important protein in SSC self-renewal that regulates transcription positively (Figure 1) (Falender et al, 2005). TAF4B is a germ cell–specific component of the transcription factor IID (TFIID) transcription complex. TAF4B-null mice are fertile at a young age but become sterile after 11 weeks of age (Freiman et al, 2001). TAF4B localizes to spermatogonia and spermatids but is not expressed in meiotic cells or somatic cells in testis. Although there were no defects in neonatal TAF4B-null testes, proliferation defects and decreased expression of SSC marker genes began to appear at 3 days of age. Degeneration of spermatogenesis became more severe by 8 weeks of age, and almost all tubules lacked germ cells by 8 months of age. Because spermatogenesis was recovered by transplantation of normal SSCs into TAF4B-null testes, the surrounding environment could support spermatogenesis, and mutant SSCs lacked self-renewal activity. Thus, TAF4B likely activates SSC genes related to self-renewal, proliferation, and differentiation.
Recently, somatic cells were shown to be converted into a pluripotent state by induction of 4 transcription factors only (Takahashi and Yamanaka, 2006; Takahashi et al, 2007; Wernig et al, 2007; Yu et al, 2007; Park et al, 2008b). Progress with induced pluripotent stem (iPS) cells is a major topic in regenerative medicine because iPS cells allow us to exclude various conflicts about moral issues and immunotolerance (Park et al, 2008a). On the other hand, SSCs can be converted into a pluripotent state in culture in vitro (Kanatsu-Shinohara et al, 2004; Guan et al, 2006; Conrad et al, 2008). Interestingly, SSCs express all of the genes that are required for induction of pluripotency (ie, Oct4, Sox2, Klf4, Myc, and Lin28) except for Nanog (Kanatsu-Shinohara et al, 2005; Oatley et al, 2006). Constitutive expression of NANOG enables autonomous self-renewal of ES cells, whereas lack of NANOG leads to early embryonic lethality (Chambers et al, 2003; Mitsui et al, 2003). In addition, NANOG is important for PGC maturation during embryonic development (Chambers et al, 2007).
In summary, maturation of SSCs from PGCs is associated with inactivation of Nanog, and induction of pluripotency in SSCs requires reactivation of Nanog. There are many similarities between SSCs and pluripotent stem cells. However, despite accumulating information about the transcriptional network in SSC self-renewal after development of transplantation technology and generation of germ cell–specific knockout mice, complete knowledge is still limited. The understanding of regulatory mechanisms of SSC self-renewal may provide unique insights into not only self-renewal of other stem cells, but also nuclear reprogramming, because SSCs share many transcription factors with ES cells and are the only stem cells that can transfer genetic information to the next generation.
Discovery of the First Germ Cell Intercellular Bridge Protein (TEX14)
Cytokinesis, which separates a mother cell into two daughter cells, occurs in all multicellular organisms. In somatic cells, abscission of the midbody is the last step of cytokinesis and separates completely the two daughter cells. In contrast to somatic cells, abscission is blocked in differentiating germ cells and results in germ cells interconnected by a stable structure called the intercellular bridge (Dym and Fawcett, 1971; Huckins, 1978). In 1955, Fawcett used electron microscopy of cat spermatids to convincingly define the intercellular bridge as a stable cytoplasmic channel connecting the germ cells, and within a short period, intercellular bridges were identified to be conserved in a wide variety of species, including human (Fawcett et al, 1959). The conservation among various species and the interconnection of as many as 650 germ cells in a syncytium suggest that intercellular bridges have key roles in multiple stages of spermatogenesis in mammals. The reason why intercellular bridges connect mammalian germ cells is still unknown, although intercellular bridges are believed to function in synchronization of germ cells as well as permitting the passage of organelles and molecules between germ cells (especially important postmeiotically in haploid spermatids) (Braun et al, 1989; Ventela et al, 2003).
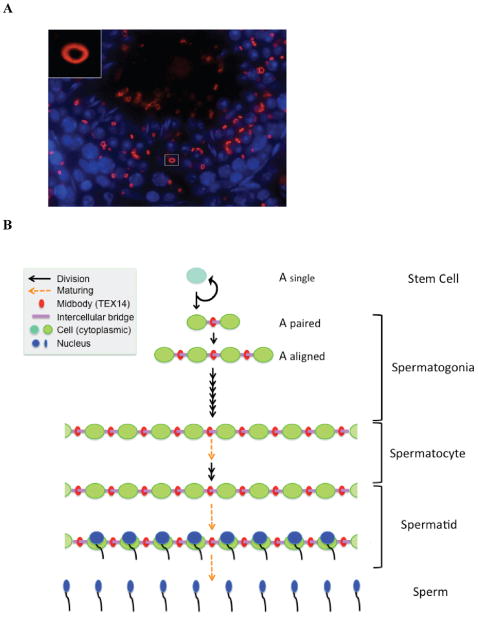
We identified testis-expressed gene 14 (Tex14) as a germ cell–specific gene by using in silico subtraction (Wu et al, 2003a). In parallel, Wang et al (2001) identified a portion of TEX14 as a spermatogonial-enriched gene protein. We showed that full-length TEX14 is a 162.5-kDa protein with 3 ankyrin repeats, a protein kinase–like domain, and a leucine zipper dimerization motif (Wu et al, 2003a). TEX14 localizes to male and female germ cell intercellular bridges (Figure 2A) (Greenbaum et al, 2007). Knockout of Tex14 disrupts intercellular bridges in male and female mice but only arrests meiosis in spermatocytes. As a result, Tex14 null mice present with disrupted spermatogenesis and sterility only in males (Greenbaum et al, 2006). Our studies indicate that TEX14 is the first essential protein of the germ cell intercellular bridge and demonstrate that intercellular bridges are essential for fertility and spermatogenesis.
Figure 2.
(A) The localization of TEX14 in the testis. Immunofluorescence using anti–testis-expressed gene 14 (TEX14) antibody shows that TEX14 localizes to intercellular bridge. Red, TEX14; blue, 4′,6-diamidino-2-phenylindole. The tissue is from a 6-week-old male mouse testis. Inset shows a representative intercellular bridge at a high magnification. (B) A summary of intercellular bridges. In testes, the A-type single (As) spermatogonium is the stem cell. The daughter cells of As spermatogonia are self-renewed As spermatogonia without intercellular bridges and A-paired (Apr) spermatogonia interconnecting by intercellular bridges. All differentiating germ cells, which are present after the As cell differentiates to form an Apr spermatogonia and through the haploid germ cell stages, are connected by intercellular bridges. TEX14 localizes initially to the midbody during cytokinesis, and its presence results in stable intercellular bridges in differentiating germ cells. TEX14 is a specific marker for intercellular bridges, the differentiation process, and the initiation of the spermatogenesis pathway. Details of the model are as follows: black arrow, dividing cell; orange arrow, maturing cell; red ring, midbody and TEX14; purple bar, intercellular bridge; turquoise blue and bright green circles, cytoplasm of stem cell and differentiating germ cell; blue, maturing nucleus during spermiogenesis.
In testes, the A-type single (As) spermatogonium is the stem cell for spermatogenesis and is the only postnatal male germ cell without intercellular bridges (Figure 2B). Intercellular bridges are typically observed in all differentiating spermatogonia after the As cell differentiates to form A-paired (Apr) spermatogonia and through the haploid germ cell stage. TEX14-positive intercellular bridges interconnect Apr spermatogonia cells and continue to interconnect male germ cells up through formation of mature spermatozoa (Greenbaum et al, 2006). Thus, the intercellular bridges and TEX14 are specific markers for the differentiation process and initiation of the spermatogenesis pathway.
We developed a biochemical method to enrich intercellular bridges from mouse testes, using TEX14 as a marker protein (Greenbaum et al, 2007), and performed proteomic analysis to identify 19 cytokinesis proteins, including Mitotic Kinesin-Like Protein 1 (MKLP1) and Male germ cell Rac guanosine triphosphatase–activating protein 1 (MgcRacGap1), that are necessary for cytokinesis and also are components of the intercellular bridge (Van de Putte et al, 2001; Matuliene and Kuriyama, 2004). TEX14 converts these midbody proteins into stable intercellular bridge components (Greenbaum et al, 2007). The mechanisms by which these proteins come together to prevent the completion of cytokinesis and form a stable intercellular bridge and the precise functions of the intercellular bridge throughout spermatogenesis remain elusive. We are studying the interactions among these bridges and cytokinesis proteins, and how a stable intercellular bridge forms. We believe that TEX14 and other intercellular bridge proteins are unique targets for male contraception, and small-molecule contraceptives that disrupt the intercellular bridge have important clinical applications. Furthermore, defining how intercellular bridges function in germ cell biology and spermatogenesis is the long-range objective of our basic research program.
Translation to Clinical Practice
Although findings in knockout mouse models are very important for the dissection of mammalian reproductive pathways, the discovery of genetic defects in humans, translation of these findings to clinical diagnoses, and providing potential targets for therapeutics are the ultimate goals. Although the investigation of mutant animal models has been extremely useful in studying simple diseases, study of genetic defects causing human infertility is complicated by the enormous number of genes involved and the phenotypic heterogeneity of infertility (Matzuk and Lamb, 2008).
One noteworthy example of the translational research in the Matzuk laboratory is the study of human kelch-like 10 (KLHL10) in infertile oligozoospermic patients. Our earlier mouse knockout studies have showed that the germ cell–specific KLHL10 protein is involved in protein ubiquitination, is critical for the maturation process of mouse spermatozoa, and is one of the essential proteins for survival of postmeiotic spermatozoa (Yan et al, 2004; Wang et al, 2006). Male mice heterozygous for the Klhl10 gene deletion demonstrate a failure of spermatozoal maturation, resulting in oligozoospermia. These results suggested to us that a mutated KLHL10 gene in human males might have dominant effect as well. Consequently, we devised a strategy to determine whether mutations in KLHL10 might be associated with human oligozoospermia and male infertility.
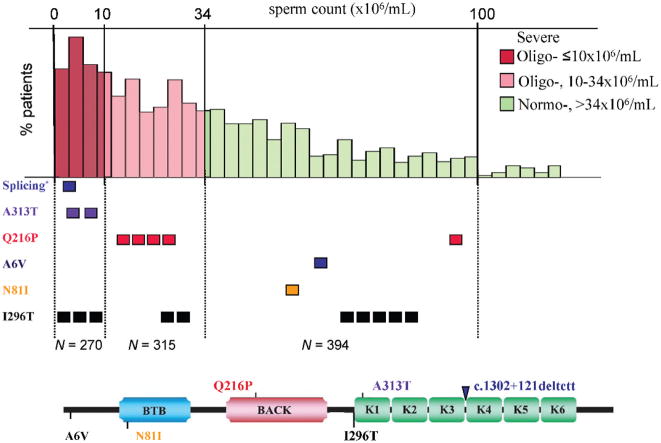
Although transcription is repressed in mature sperm, many postmeiotically expressed mRNAs and micro-RNAs persist in human ejaculate spermatozoa and are even delivered to the oocyte (reviewed by Krawetz, 2005). Accordingly, detection of specific RNAs would provide an imprint of testicular gene expression. Therefore, we developed a noninvasive, high-throughput screening technique that effectively uses reverse transcription–polymerase chain reaction (RT–PCR) of germ line mRNAs obtained from human ejaculate samples of oligozoospermic and normozoospermic infertile men (Yatsenko et al, 2006). We employed this approach to study KLHL10 mutations in oligozoospermic, infertile patients and identified 7 of 550 patients (1.2%) with missense and/or splicing mutations in KLHL10 (Figure 3) (Yatsenko et al, 2006). Importantly, we confirmed the pathogenic effect of identified KLHL10 mutations. We demonstrated abnormal gene splicing via RT-PCR, and using an in vitro yeast 2-hybrid functional assay, showed abnormal protein dimerization of mutant proteins with the various missense mutations. These results support our initial hypothesis that mutations in human KLHL10 have a dominant effect on human spermatogenesis.
Figure 3.
Distribution of identified KLHL10 mutations among categories of sperm concentration in infertile men. Three major categories are shown in the upper part of the figure: severe (red) and moderate (pink) oligozoospermia, and normozoospermia (green). The lower part shows all of the KLHL10-identified mutations with respect to their corresponding position in the KLHL10 protein. Mutations present above the protein are only present in oligozoospermic patients, whereas those depicted below the protein are also observed in normozoospermic patients (believed to be rare polymorphisms). Splicing* denotes splicing KLHL10 mutation, c.1302+121_124del4bp. Known functional domains of the KLHL10 protein are shown by different colors and named after their respective domains, as BTB, BACK, and K1–K6 (Kelch motifs 1–6).
Recently, more encouraging discoveries toward non-syndromic male infertility have been reported. Dr Ray’s group reported that a novel testis-expressed gene, aurora kinase C (AURKC), previously shown to play a role in meiotic division in mice (Tang et al, 2006), is responsible for male infertility (Dieterich et al, 2007). Male patients harboring homozygous single-nucleotide deletion in the AURKC gene presented with large-headed, multiflagellar polyploid spermatozoa that showed nearly 100% morphologically abnormal spermatozoa with oversized irregular heads, abnormal midpiece and acrosome, and up to 6 flagella. In another report, the spermatogenesis-specific gene SPATA16 was shown to be responsible for globozoospermia in 3 related male infertile patients (Dam et al, 2007). This study described 3 affected brothers from a consanguineous family, in which each brother is homozygous for mutation in the SPATA16. However, a follow-up study of unrelated patients with complete or partial globozoospermia failed to identify SPATA16 mutations, highlighting the common difficulty in identifying genetic mutations in such a complex etiology as male infertility.
Implications for the Future
Current discoveries of crucial genetic and molecular aspects of mammalian reproductive pathways are a fundamental step toward clinical management as well as effective therapy. Therefore, more human genetic studies of male infertility and novel effective, noninvasive screening detection methods in the germ line are needed for future progress. Ultimately, recognized genetic alterations associated with human spermatogenesis defects should be applied for various diagnostic applications in the fertility clinic.
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants U01HD60496, P01HD36289, and R01HD57880 and NCI grant R01CA60651 to M.M.M.
References
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006a;294(1):161–167. doi: 10.1016/j.ydbio.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A. Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns. 2006b;6(8):1014–1018. doi: 10.1016/j.modgep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Barna M, Merghoub T, Costoya JA, Ruggero D, Branford M, Bergia A, Samori B, Pandolfi PP. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3(4):499–510. doi: 10.1016/s1534-5807(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337(6205):373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36(6):647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Burns KH, Viveiros MM, Ren Y, Wang P, DeMayo FJ, Frail DE, Eppig JJ, Matzuk MM. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science. 2003;300(5619):633–636. doi: 10.1126/science.1081813. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450(7173):1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- Chang H, Matzuk MM. Smad5 is required for mouse primordial germ cell development. Mech Dev. 2001;104(1–2):61–67. doi: 10.1016/s0925-4773(01)00367-7. [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Bühring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456(7220):344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36(6):653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Dam AH, Koscinski I, Kremer JA, Moutou C, Jaeger AS, Oudakker AR, Tournaye H, Charlet N, Lagier-Tourenne C, van Bokhoven H, Viville S. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81:813–820. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26(11):2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- Dieterich K, Soto Rifo R, Faure AK, Hennebicq S, Ben Amar B, Zahi M, Perrin J, Martinez D, Sele B, Jouk PS, et al. Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nat Genet. 2007;39(5):661–665. doi: 10.1038/ng2027. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4(2):195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor-9-deficient ovary. Mol Endocrinol. 1999;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- Falender AE, Freiman RN, Geles KG, Lo KC, Hwang K, Lamb DJ, Morris PL, Tjian R, Richards JS. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. 2005;19(7):794–803. doi: 10.1101/gad.1290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Biochem Cytol. 1959;5(3):453–460. doi: 10.1083/jcb.5.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman RN, Albright SR, Zheng S, Sha WC, Hammer RE, Tjian R. Requirement of tissue-selective TBP-associated factor TAFII105 in ovarian development. Science. 2001;293(5537):2084–2087. doi: 10.1126/science.1061935. [DOI] [PubMed] [Google Scholar]
- Greenbaum MP, Ma L, Matzuk MM. Conversion of midbodies into germ cell intercellular bridges. Dev Biol. 2007;305:389–396. doi: 10.1016/j.ydbio.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A. 2006;103(13):4982–4987. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440(7088):1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Hao J, Yamamoto M, Richardson TE, Chapman KM, Denard BS, Hammer RE, Zhao GQ, Hamra FK. Sohlh2 knockout mice are male-sterile because of degeneration of differentiating type A spermatogonia. Stem Cells. 2008;26(6):1587–1597. doi: 10.1634/stemcells.2007-0502. [DOI] [PubMed] [Google Scholar]
- Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998;273(13):7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- Huckins C. Spermatogonial intercellular bridges in whole-mounted seminiferous tubules from normal and irradiated rodent testes. Am J Anat. 1978;153(1):97–121. doi: 10.1002/aja.1001530107. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85(7):1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119(7):1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72(4):985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- Krawetz SA. Paternal contribution: new insights and future challenges. Nat Rev Genet. 2005;6(8):633–642. doi: 10.1038/nrg1654. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101(47):16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Lin YN, Roy A, Yan W, Burns KH, Matzuk MM. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol. 2007;27(19):6794–6805. doi: 10.1128/MCB.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20(4):377–388. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Ma L, Buchold GM, Greenbaum MP, Roy A, Burns KH, Zhu H, Han DY, Harris RA, Coarfa C, Gunaratne PH, Yan W, Matzuk MM. GASZ is essential for male meiosis and suppression of retro-transposon expression in the male germline. PLoS Genet. 2009;5(9):e1000635. doi: 10.1371/journal.pgen.1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone b subunit leads to hypogonadism, defects in gonadal steroidogenesis and infertility. Proc Natl Acad Sci U S A. 2004;101(49):17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Reyna A, Mani SK, Matzuk MM, Kumar TR. Impaired male sexual behavior in activin receptor type II knockout mice. Biol Reprod. 2005;73(6):1182–1190. doi: 10.1095/biolreprod.105.043794. [DOI] [PubMed] [Google Scholar]
- Matuliene J, Kuriyama R. Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol Biol Cell. 2004;15(7):3083–3094. doi: 10.1091/mbc.E03-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. a-Inhibin is a tumor-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Med. 2002;8(suppl 1):S41–S49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287(5457):1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74(2):314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282(35):25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103(25):9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136(7):1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yomogida K, Dohmae K, Nishimune Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127(10):2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A. 2006;103(21):8090–8095. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008a;134(5):877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008b;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Schöler H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71(1–2):89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM. Absence of tektin 4 causes asthenozoospermia and subfertility in male mice. FASEB J. 2007;21(4):1013–1025. doi: 10.1096/fj.06-7035com. [DOI] [PubMed] [Google Scholar]
- Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM. Tektin 3 is required for progressive sperm motility in mice. Mol Reprod Dev. 2009;76(5):453–459. doi: 10.1002/mrd.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97(15):8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J. FIGa, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127(21):4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102(44):16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CJ, Lin CY, Tang TK. Dynamic localization and functional implications of Aurora-C kinase during male mouse meiosis. Dev Biol. 2006;290(2):398–410. doi: 10.1016/j.ydbio.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Toyoda S, Miyazaki T, Miyazaki S, Yoshimura T, Yamamoto M, Tashiro F, Yamato E, Miyazaki J. Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev Biol. 2009;325(1):238–248. doi: 10.1016/j.ydbio.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382(6586):80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Van de Putte T, Zwijsen A, Lonnoy O, Rybin V, Cozijnsen M, Francis A, Baekelandt V, Kozak CA, Zerial M, Huylebroeck D. Mice with a homozygous gene trap vector insertion in mgcRacGAP die during pre-implantation development. Mech Dev. 2001;102(1–2):33–44. doi: 10.1016/s0925-4773(01)00279-9. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8(4):414–427. doi: 10.1101/gad.8.4.414. [DOI] [PubMed] [Google Scholar]
- Ventela S, Toppari J, Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing. Mol Biol Cell. 2003;14(7):2768–2780. doi: 10.1091/mbc.E02-10-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27(4):422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- Wang S, Zheng H, Esaki Y, Kelly F, Yan W. Cullin3 is a KLHL10-interacting protein preferentially expressed during late spermiogenesis. Biol Reprod. 2006;74(1):102–108. doi: 10.1095/biolreprod.105.045484. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Wu MH, Rajkovic A, Burns KH, Yan W, Lin YN, Matzuk MM. Sequence and expression of testis-expressed gene 14 (Tex14): a gene encoding a protein kinase preferentially expressed during spermatogenesis. Gene Expr Patterns. 2003a;3(2):231–236. doi: 10.1016/s1567-133x(03)00036-x. [DOI] [PubMed] [Google Scholar]
- Wu X, Viveiros M, Eppig J, Bai Y, Fitzpatrick S, Matzuk MM. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat Genet. 2003b;33(2):187–191. doi: 10.1038/ng1079. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo F, Elvin J, Carino C, Prasad S, Skinner S, Dunbar B, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yan W, Assadi AH, Wynshaw-Boris A, Eichele G, Matzuk MM, Clark GD. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2003;100(12):7189–7194. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Ma L, Burns KH, Matzuk MM. Haploinsufficiency of kelch-like protein homolog 10 causes infertility in male mice. Proc Natl Acad Sci U S A. 2004;101(20):7793–7798. doi: 10.1073/pnas.0308025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Ma L, Pangas SA, Burns KH, Bai Y, Schultz RM, Matzuk MM. Mice deficient in oocyte-specific oligoadenylate synthase-like protein, OAS1D display reduced fertility. Mol Cell Biol. 2005;25(11):4615–4624. doi: 10.1128/MCB.25.11.4615-4624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Medvedev S, Yu J, Tang LC, Agno JE, Matzuk MM, Schultz RM, Hecht NB. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc Natl Acad Sci U S A. 2005;102(16):5755–5760. doi: 10.1073/pnas.0408718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HHC, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsenko AN, Roy A, Chen R, Ma L, Murthy LJ, Yan W, Lamb DJ, Matzuk MM. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15(23):3411–3419. doi: 10.1093/hmg/ddl417. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269(2):447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]