Abstract
With the recent revolution in Molecular Biology and the deciphering of the Human Genome, our understanding of the building blocks that comprise living systems has advanced rapidly. We have yet to understand, however, how the physical forces that animate life affect the synthesis, folding, assembly, and function of these molecular building blocks. We are equally uncertain as to how these building blocks interact dynamically to create coupled regulatory networks from which integrative biological behaviors emerge. Here we review several recent advances in the field of biomechanics at the cellular and molecular levels, and we set forth some of the challenges confronting the field. Living systems work and move as multi-molecular collectives, and in order to understand key aspects of health and disease we must first be able to explain how physical forces and mechanical structures contribute to the ‘active’ material properties of living cells and tissues, as well as how these forces impact information processing and cellular decision making. Such insights will no doubt inform basic biology and rational engineering of effective new approaches to clinical therapy.
INTRODUCTION
In this post-genomic era, the challenge that all areas of biomedical research now face is to understand how the many expressed and biosynthesized molecules fold, assemble and function within the context of living cells, tissues, and organs. Just as challenging is how complex biological characteristics subsequently emerge through collective interactions and within dynamically coupled regulatory networks. Systems Biology presently emphasizes information transfer14, but three-dimensional geometry and physical forces have yet to be taken into account despite the central roles that these biomechanical factors play in biological structure and function. But without ‘Biomechanics Inside’ there would be no form, no function, no life.
The field of Mechanics, and the shapes and forces that it describes and predicts, are the basis of modern developments from the smallest level of single atoms to the largest scale applications in space flight. Nonetheless, we still have no comparable conceptual framework that embraces basic paradigms of biology together with physical principles such as conservation of mass, momentum and energy. We lack a comprehensive theory that permits prediction of the many shapes, material properties, motions, and fluxes that are encountered in the living world.45, 64
Most diseases present as a complex genetic profile, with multiple changes in molecular expression.75, 117 Nonetheless, a patient goes to the doctor’s office often because of a mechanical defect in a tissue or organ: a new swelling or lump, pain due to nerve compression, stiffness that limits movement, edema caused by a leak of tissue bodily fluids, constricted blood flow or lymph flow, or obstructed airflow that restricts breathing. Cures and remedies are judged successful by the patient only when such mechanical defects are remedied. In order to understand health-related and disease-related aspects of living systems – all of which work and move as multi-molecular collectives – we must first be able to explain how physical forces and mechanical structures contribute to the ‘active’ material properties of living cells and tissues, as well as how these forces impact information processing and cellular decision making. Such insights will inform not only basic biology but also rational engineering of effective new approaches to clinical therapy. This challenge has led to the emergence of modern Biomechanics - a field that is evolving rapidly and bringing together approaches from engineering, the physical sciences, and virtually all biological disciplines. Here we discuss key obstacles and major opportunities confronting the field of Biomechanics, as well as implications for the future of science, engineering and healthcare.
HISTORICAL BACKGROUND
In the first half of the 20th century, a dominant doctrine in biology held that mechanical forces act as key causative agents both during tissue morphogenesis and throughout adult life. Biological materials ranging from pollen grains studied by Robert Brown of ‘Brownian motion’ to proteins, lipids, and DNA were all subjects of seminal studies of complex fluids. Physical forces were viewed then as the source of pattern formation in various life forms.104 At a time when the molecular basis of viscosity was being developed by Einstein24, some of the earliest quantitative evidence of non-Newtonian viscosity also emerged from studies of biological fluids. Even though the reality of molecules, as distinct from colloidal particles, was still being contested, the unusual mechanical properties of cytoplasm led to visionary proposals that the cell contained a system of molecular filaments.
Clinicians have always recognized the central importance of physical forces in physiological control. The effects of inspiratory pressure on lung function, hemodynamic shear stress on vascular remodeling, compression on bone generation, and tension on skin aging are well-known examples. The richness of chemical and molecular control had often taken center stage in biological analyses without recognition of the ‘trees’ in the ‘forest’, until approximately twenty years ago with the realization of the pivotal role of physical forces in genetic and cellular regulation, as well as developmental control. Interest in biomechanics has since grown exponentially and now includes researchers in a wide range of biological disciplines including molecular biophysics, cell biology, developmental biology, genetics and physiology, as well as mechanical engineering, materials science and nanotechnology.
CURRENT STATUS OF THE FIELD
Many clinical therapies already rely on biomechanical cues to treat disease51, but they are typically not presented in the appropriate context of “Biomechanics Inside”. Some of these treatments are ancient, such as a mechanical support of the skin with a bandage in venous ulcers. Modern examples are many and include stents, ventilators, and vasodilators/constrictors. A recent, intriguing example is the vacuum-assisted closure sponge 88, in which plastic surgeons have found that application of cyclic suction to non-healing wounds is more effective at healing than two other FDA-approved therapeutics: platelet derived growth factor (PDGF) or a tissue engineered implant with stem cells. Foams are used to close blood vessels during uncontrolled angiogenesis and venous ulcerations. Better understanding of the molecular biophysical basis of cellular mechanotransduction seems likely lead to new drug-based and nanotechnology-based therapeutics across a broad spectrum of disorders. Some heart arrhythmias will almost certainly come to be treated with inhibitors of stress-sensitive ion channel, for example. Moreover, medical diagnostics based on mechanics are ubiquitous and include palpation and ultrasound detection of variations in tissue mechanics, suggesting that further opportunities are likely to have a basis in biomechanics.
Microscopic changes in cell mechanics and extracellular matrix structure are expected to dysregulate molecular mechanisms of mechanotransduction – which is the process by which cells sense mechanical signals and convert them into a chemical response. Examples include numerous developmental abnormalities (e.g., osteogenesis imperfecta) that result from altered matrix mechanics; enhanced cancer cell metastasis within the microvasculature that result from hydrodynamic flow-mediated tumor cell adhesion60, 99, loss of lung elasticity in emphysema69, 87, 101, excessive narrowing of airways in asthma34, increased wall stiffness in hypertension, enhanced rigidity and adhesion of red cells to the endothelium in malaria and sickle cell disease, and abnormal cellular mechanotransduction in deafness as well as polycystic kidney disease; but there are numerous other examples in virtually all areas of medicine and surgery51. Even in embryonic development and cancer, it is physical forces, material flows, and differences in cellular mechanics that provide essential inputs in the program that drives cell sorting, differentiation, growth and angiogenesis.53, 79 In these cases, biomechanics underlies the abilities of the cell, tissue or whole organ to carry out normal functions in health or to malfunction in disease.
PAST ACCOMPLISHMENTS IN CELL MECHANICS
Our understanding of how cells generate and respond to mechanical forces has advanced significantly as have our insights into how biomechanical factors contribute to extracellular matrix assembly and remodeling during embryological development and throughout adult life. Indeed, biomechanical approaches have led to entirely new insights into biological control. An essential aspect of biomechanics emerging from many lines of evidence is that cells are not only exposed to forces, stresses, and tensions, but that they also actively generate their own. This and additional determinants are summarized in a few pertinent examples:
Cardiovascular cell mechanics and microcirculation
One of the most thorough analyses of the mechanical properties of living cells has been carried out on the mammalian red blood cell, which is a uniquely simple structure with predominantly two components – a membrane with bending and shearing properties that are dependent upon strain, strain rate, and strain history, and a cytoplasm that in the normal red cell is predominantly a Newtonian viscous fluid.16 Quantitative passive biomechanical models were developed that serve to predict red cell motion and deformation in a large number of in vivo situations. 39, 40, 89, 98 A key element of these models was the recognition that under the influence of membrane tension the lipid bilayer preserves membrane area within narrow limits. Discrete network models of the red blood cell membrane are increasingly taking into account the particular load-bearing functions of specific proteins (e.g., flexible spectrin springs, and actin protofilament nodes) as well as the key role of prestress for shape stability.107 Newly developed constitutive models for the red cell membrane show the full power of biomechanical analysis not only as a starting point for prediction of whole cell and cell suspension behavior but also as a reference for molecular models of cell membranes derived from the crystal structure of its constituents.
As another component of whole blood, several generations of biomechanical models have been developed for white blood cells 21, 27 97, which are the basis of immune surveillance and inflammation (Fig. 1). These models have found use in prediction of cell-cell interactions in the microcirculation 19, and similar models were developed for endothelium, platelets and metastatic tumor cells. These passive mechanical models were developed with a view towards the microcirculation, the cardiovascular system, and their modeling was based on fundamental principles of mechanics.
Fig. 1.
Distribution of normalized fluid stresses acting on the membrane of a migrating leukocyte with active cytoplasmic projections (pseudopods). The cell is attached to a flat glass surface and exposed to a constant shear stress. The fluid shear stress was determined by solution of the equation of motion for a Newtonian fluid (plasma) with non-slip condition on the cell membrane and on the substrate. All stress values are normalized by the applied shear stress (2.2 dyn/cm2) on the cell substrate away from the cell. The cell shape was reconstructed from a three-dimensional stack of confocal images with a fluorescent membrane label. Su, SS: Fluid Stress on the Surface of a Migrating Leukocyte in a Flow Field and the Involvement of Formyl Peptide Receptor in Its Mechanotransduction. Ph.D. Thesis. Department of Bioengineering, University of California San Diego, 2007.
By integration of whole-cell viscoelastic mechanical models with traditional biofluid mechanics it has been possible to predict a considerable number of microvascular events.89 Biomechanical analyses of different cell types in the circulation has yielded a new level of understanding of cell interactions in the circulation, and it is now possible to predict cell behavior in narrow vessels, such as capillaries. This has served to place a number of issues in blood diseases, inflammation, and cardiovascular disease on firm biomechanical footing. Models have been developed in several organs (e.g. lung, heart, skeletal muscle, connective tissue) that quantitatively predict basic aspects of organ perfusion. 35 100 In addition, there is increasing evidence to suggest that key steps in vascular development are under the control of biomechanical forces. Fluid shear stress may play a central role in the development of the first blood vessels in the yolk sac and the heart, and influences remodeling of the embryonic primary capillary plexus.4, 49 Blood pressure controls the development of arterioles106 and cytoskeletal forces that capillary cells exert on extracellular matrix control angiogenesis during embryonic lung development.73
A major new area of research in endothelial cell mechanics is the role of the thin 150–500 µm thick layer of membrane bound macromolcules that ubiquitously coats the inner surface of all blood vessels.108 Despite the striking fragility of this layer in response to light or chemical enzymes, it provides several vital functions necessary for life. It provides the molecular sieve that determines the oncotic forces necessary for the movement of water into and out of our microvessels 70, 112, provides a protective barrier that prevents adhesive molecular interaction between red and endothelial cell membranes 112, allows white cells to roll freely through our circulation preventing the penetration of leukocyte microvilli except in areas of inflammation91, and appears to be the critical layer in the transmission of fluid shear stress into the cytoskeleton.91, 115 Recent experiment have shown that the integrity of the layer is necessary for flow alignment under fluid shear 103, 118 and the regulation of eNOS release.30 As summarized by Weinbaum et al.114, all of these phenomena have been described using cellular level biomechanical models.
Effects of fluid shear stress on vascular and epithelial cell function and dysfunction
Disturbed or turbulent flow is widely recognized as being a leading cause of atherosclerosis. By manipulating fluid flow above them, underlying vascular cells can be transformed from normal to abnormal phenotypes. Ever since the earliest observations by Patel that endothelial cells orient themselves in the direction of fluid shear, a large body of evidence has come forward to show that in endothelial cells just about all cell functions - from morphology, to signal transduction and gene expression - can be influenced by the relatively modest forces generated by fluid shear stress. Today we recognize that just about all cell types inside and outside the cardiovascular system respond to fluid shear stress. 74 Even cells that are much more primitive than mammalian cells, like dinoflaggelates (red tide) in the ocean, respond to modest fluid shear stress.11 Responses to similar shear stresses are cell-type specific, however. For example, endothelial cells elongate in the direction of flow but vascular smooth muscle cells elongate in a direction perpendicular to the direction of fluid shear, as do endothelial cells derived from heart valves. Cardiac myocytes, by contrast, respond by changing the beating rate of their sarcomeres without significant change in cell shape; migrating leukocytes show a dramatic changes in cell shape. There are also responses of cells to normal stress, but tend to require higher stress levels and produce a more selective response. As described below, mechanisms by which cells sense fluid shear stress is a very active area of study. The fact that the stimulus is the shear stress itself, rather than a chemical whose local concentration is affected by fluid flow, is now well established. Recent evidence suggest that cells may utilize existing receptors (e.g., G-protein coupled receptors, integrin membrane adhesion receptors) 65, a feature that may underlie the cell-specific response encountered in mechanotransduction and may suggest that biochemical pathways (e.g., via agonists and antagonists) may also be responsive to fluid shear stress. The interaction of membrane receptors with the glycocalyx and cytoskeletal structures may contribute to amplification mechanisms.
Development and verification of effective surface tension as a mechanism for cell sorting
The active segregation of mixed cell types into multiple homogenous structures that lead to specific organs is a hallmark of early development. As early as the 1960's embryologists such as M. Steinberg proposed that cells spread and sort according to adhesion and surface tension, with multiple demonstrations of how organizational sorting could occur through a hierarchy of physical forces.32 111 Modern molecular biology has identified at least some of the proteins and signaling pathways that are used for sorting, but the physical principles remain the underlying predictive mechanisms by which cell sorting occurs. 32
Shape-dependent control of cell fate switching
The importance of cell shape and cell deformation for control of cell growth and function was first recognized almost thirty years ago.31 Since that time it has become clear that mechanical distortion of cells resulting from physical interactions between cells and their extracellular matrix adhesions can regulate their responsiveness to soluble cues, and thereby govern switching between different fates, including growth, differentiation and apoptosis, as well as control directional cell motility.12, 26, 78, 96 Lineage switching of the human mesenchymal stem cell can even be controlled by either physically distorting the cells67 or simply by varying the mechanical compliance of the extracellular matrix.26
Effect of extracellular matrix stiffness and tractions on cell structure and function
Studies from the 1980's and earlier 44 showed that cells generally apply traction forces to the networks or the surfaces to which they are bound (Fig. 2). The inability of many solid tissue cell types to grow on liquid or very soft surfaces has long been used as a diagnostic to detect transformed cells. The importance of matrix stiffness for control of cell shape and function had thus been hinted at for some time, but the effects were most definitively demonstrated by Y-L. Wang in the 1990’s with studies of fibroblasts and epithelial cells on collagen-coated polyacrylamide gels of varied flexibility.3, 44 Traction force microscopy methods were subsequently developed to quantify this response.16 Cell tension generated by nonmuscle myosins described first in the 1960/70’s and the level of prestress (isometric tension) in the cytoskeleton have consistently proven key to the sensitivity of cells to matrix elasticity; but the myosins only ‘pull the trigger’ that initiates downstream signal trajectories that continue to be obscure. It is nonetheless clear that in determining cell morphology, transcriptional programs, and cell fate, the stiffness of a cell's substrate and cell traction forces provide as much input as do chemical messengers.26, 50
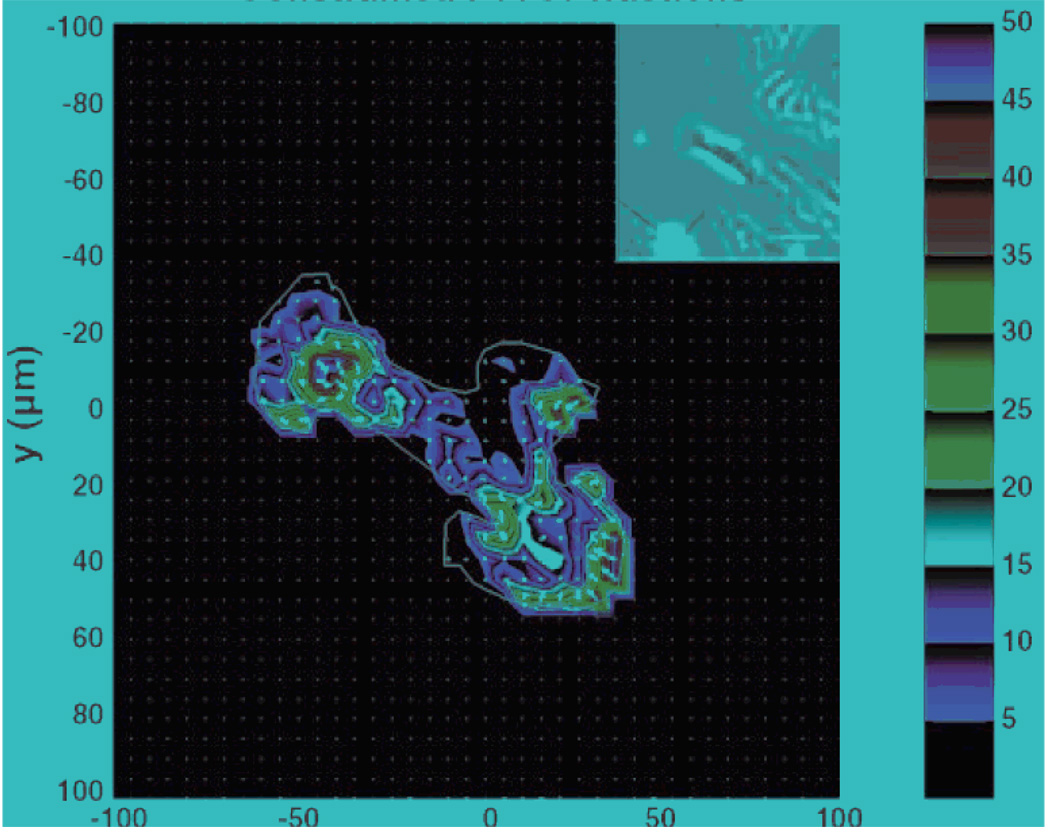
Fig. 2.
Traction microscopy image: Traction stress field exerted by a rat pulmonary microvascular endothelial cell upon its substrate. Inset: Phase contrast image at reduced magnification. Scale: Shear stress in Pascals. Adapted from 1
Mechanotransduction by primary cilia and other elongated structures
In the ear and kidney there are specialized cellular projections that serve as sensors of mechanical force. Auditory sensation is mediated by acoustic forces applied to the tips of stereocilia which are communicated to the transduction channels regulating the transport of Ca++ by gating springs that are stretched when the hair bundle is displaced towards its tall edge.54 More recent studies have shown that there are two distinct mechanisms for transducer adaptation, one acting on a time scale of 1 ms or less in addition to the slower mode of 10 ms or more first identified in the pioneering work of Hudspeth and co-workers.54 As a result, two models of hair cell adaptation have been proposed, an active motor associated with a tension generating system that slowly slips down an actin-myosin network and a calcium closure mechanism that acts on a shorter time scale. 48
In the kidney, two possible mechanical sensors of flow have been proposed. In the proximal tubule the fundamental mystery, since it was first observed four decades ago 90, is glomerular tubular balance, which is the ability of the brush border epithelium to sense the filtered load that enters the tubule and reliably reabsorb 2/3 of this flow independent of the glomerular filtration rate. Guo et al 42 proposed that the 4000 microvilli per cell act as a flow sensor that responds collectively to the bending moment produced by the fluid shear stress acting on microvilli tips. This hypothesis was quantitatively confirmed by the experiments of Du et al. 23 using a mathematical model for the hydrodynamic forces acting on brush border microvilli. In the cortical collecting duct there are two cell types, principal and intercalated cells, with the former secreting K+ and the latter regulating pH. Praetorius and Spring 83 have proposed that principal cells have primary cilia which regulate intracellular calcium. In both cell types it has been difficult to determine whether it is flow or stretch that leads to the abrupt increase in intracellular calcium 62 and, in principal cells, whether the flow sensor is the primary cilia. In addition, defects in the structure/function of the primary cilia in polycystic kidney disease appear to be due to the stunting of these cellular projections.61 Biomechanical models have contributed greatly to the interpretation of these experiments.
The Musculoskeletal System
Bone cells (osteocytes) live in a rigid mineralized tissue. Accordingly, one of the most intriguing problems in mechanotransduction is how such cells are able to sense mechanical loading associated with locomotion. Bone is widely recognized to atrophy in the weightlessness of space or prolonged bed rest, for example, and fractures resulting from osteoporosis are one of the most costly health care risks in an aging population. Wolff recognized more than a century ago that trabecular bone adapts to loading by developing an architecture that closely paralleled the principal stresses in bone tissue.116 Although Piekarski and Munro81 postulated that bone cells receive their nutrition though load-induced fluid flow in the lacunar-canalicular system, there is little connection between this hypothesis and the remarkable observation by Rubin and Lanyon86 that bone maintenance requires nothing more than a few cycles per day of mechanical loading that produce in excess of 1000 µstrain. The critical connection was made in Weinbaum et al 113 who hypothesized that the fluid shear stress acting on cell processes was the activating signal. If the pericellular space contains a matrix with sieving properties that exclude albumin, their theoretical model then predicts that fluid shear stress would be comparable in magnitude to that acting on vascular endothelial cells even though dimensions of these pores are two orders of magnitude smaller than that of capillaries. This highly non-intuitive prediction conforms flow culture experiments of Reich and Frangos 85 and numerous subsequent investigators.
Although this flow model has proven to be basically correct, subsequent analysis suggested that fluid drag on the pericellular matrix far exceeded the shear stress on the process membrane. Moreover, the actin filament bundle in dendritic processes led to a highly polarized cell whose processes were several hundred times stiffer than the osteocyte cell body. Accordingly, a new cellular excitation hypothesis proposed that the processes were attached along their length by tethering filaments, and that flow-induced drag on these filaments would produce a tension that could greatly amplify the very small whole tissue strains at the cellular level.119 Using new electron microscopic fixation techniques, tethering filament were seen for the first time and a greatly refined model was developed to explain in detail the strain amplification hypothesis.43 This model has since been extended to examine the role of focal integrin attachments along the process and their role in the initiation of intracellular signaling.110
More broadly, tissues of the musculoskeletal system show exquisite sensitivity to their biomechanical environment, and it is now clear that mechanical forces serve as a regulatory factor not only in remodeling of bone, but also muscle and cartilage. Disuse of joints leads to cartilage atrophy, whereas overuse and abnormal loading are associated with degenerative joint diseases such as osteoarthritis.41 These processes appear to be mediated at the cellular level through complex interactions between biomechanical factors, soluble mediators, and genetic programming. Thus a further understanding of such mechanisms regulating cell behavior under physiologic or pathologic conditions could provide new insight into the development of physical or pharmacologic therapies for mechanically-based musculoskeletal diseases such as osteoporosis, osteoarthritis, and muscular dystrophy/atrophy.
Cell stretch, bronchospasm, and asthma
Of all known bronchodilatory agents or drugs, the single most potent is a simple deep inspiration. In asthma, however, this innate protection agency fails, and this failure has been implicated as a proximal cause of excessive airway narrowing. 34 We now have insights into this fundamental bronchodilatory mechanism: a deep inspiration stretches the airway smooth muscle cell and causes its contractile machinery and cytoskeletal scaffolding to fluidize.76, 105 We understand less well the failure of the airway smooth muscle cell to fluidize in the spontaneous asthmatic attack, but this failure is now known to involve transition of the airway smooth muscle cell to a state in which its cytoskeletal network becomes frozen in a stiff glassy phase, and involves the modulation of that transition within the airway smooth muscle (ASM) cell by MAP kinase pathways 22, remodeling of the ASM contractile apparatus and its cytoskeletal scaffolding47, 93, increase of ASM mass 56, and by remodeling of connective tissues within the surrounding airway wall.76
Stretch and mechanoprotection
Cells exhibit a repertoire of strategies to protect themselves against damage caused by imposed mechanical stretch – mechanoprotection. The best known strategy is reinforcement and resulting cytoskeletal stiffening that results from activation of stress-activated ion channels, the small GTPase Rho, and myosin-dependent focal adhesion assembly, as well as tensegrity-based interactions within the microfilament-intermediate filament-microtubule lattice of the deep cytoskeleton.66, 109 Recent studies suggest that the cell can deploy another strategy for mechanoprotection that is based on cytoskeletal fluidization.7, 76, 105 In cells resident in organs that routinely undergo large stretch (heart, gut, lung), fluidization implies dramatically augmented mobility of macromolecules which, ordinarily, is thought to be markedly retarded due to molecular crowding, caging, and assembly. 18, 38, 63, 71 Hence, molecular crowding and cell stretch are now understood to have potent but opposite compensatory effects. Finally, prompt fluidization followed by slow resolidification provides the freedom for the cell to move and reorganize its contractile units, stress fibers, and adhesion processes in response to mechanical stress or other stimuli.15
Mechanotransduction
Cells are constantly pulling on their surroundings in order to probe and adjust to their mechanical microenvironment.84 Stem cell differentiation was already mentioned as strongly influenced by the substrate stiffness.26 Recent evidence suggests that a number of molecules and cellular structures are involved: stress-activated ion channels, transmembrane proteins that mediate cell-matrix or cell-cell contacts, focal adhesion complexes, membrane lipids, glycocalyx proteins, and also G-protein coupled receptors can all serve as mechanosensors and transducers.52 Thus, receptors that traditionally have been thought of as being under the control of biochemical agonist and antagonist molecules, might also respond to fluid shear or substrate strain by a conformational change to initiate intracellular signaling events. 6 However, activation of these receptor signaling cascades by mechanical cues may produce entirely different physiological responses depending on the overall deformation state of the cell and cytoskeleton, and the physical context of the tissue and organ in which the cell normally experiences mechanical stresses.52
CHALLENGES FOR THE FUTURE
What determines stiffness and other constitutive physical properties of the cell?
To answer this question, top-down reductionist versus bottom-up integrative approaches presently define a divide. Bottom-up reductionist approaches 2, 82 are sometimes rooted in the traditional viscoelastic paradigm. At the level of fundamental constituents and their use in reconstituted systems in vitro, cell-derived materials and molecules are terrifically rich because, among other things, they are well-defined, they can have motors and crosslinkers that provide contraction and change on/off rates, and they can be manipulated precisely. 10, 13, 94 Additionally, like living cells these materials can get around limits set by thermal forces and the fluctuation-dissipation theorem.72 Nonetheless, current understanding of cell-derived materials and their major molecular components have thus far failed to account for integrated cell physical properties, such as stiffness, in most cases even to within orders of magnitude. 37 In certain limiting cases 17, or if the networks are prestressed37, physical properties comparable to those observed in cells can be approximated, but mechanism remains unclear. As such, constitutive models in cell mechanics are available today, but with limited ability to predict intracellular biological events. From a top-down perspective, by contrast, there has emerged a striking analogy between the dynamics of the intact living cell and that of the universal but relatively featureless pattern of inert soft glassy materials such as foams, pastes and colloids.28, 55, 105 Glassy dynamics cannot predict what will happen to matrix dynamics if a particular protein is mutated, but suggest that dynamics of cytoskeletal proteins generically, and transitions between fluid-like versus solid-like behavior in particular, are governed by free energy barriers incorporated into a rough energy landscape.33 Integration of the competing paradigms of viscoelasticity versus glassy dynamics, along with the central role of cytoskeletal prestress102, into a single unified framework has become a major challenge in cell biophysics, but promising inroads have been suggested in a recently-proposed model of a ‘glassy wormlike chain’.55, 92 Whether similar concepts apply to the observed power law rheology of the chromatin-packed nucleus remains unclear, but there are intriguing differences in nuclear mechanics between embryonic stem cells and committed cells.77
How to measure stiffness and forces more accurately and non-invasively
Developments in ultrasound, MRI, and other methods might make it possible to determine frequency and/or strain rate dependent viscoelastic properties in whole tissues without disrupting resident cells. Spatial resolution at the cellular scale within intact tissues is not so clearly on the horizon, but concepts of super-resolution in optics and other imaging fields could provide opportunities. Effective methods exist to measure details of mechanical strain and strain-rates for localized cytoplasmic regions.68, 95
Impacts on new biomaterials and artificial organs
Chemical synthesis and degradation as well as issues such as cell attachment and solute diffusion have been principal design criteria for tissue replacements and bioreactors to date. A growing field, termed “Functional Tissue Engineering”, has sought to further emphasized the role of biomechanical factors in the repair and regeneration of tissue.8 In particular, a further understanding of the ability of cells to perceive and respond to their mechanical environment is necessary within the context of engineered tissue replacements. At the cellular and subcellular level, compliance and such features as cytoskeletal rheology, strain-stiffening, and fluidization, and their interaction with scaffolds and other artificial extracellular matrices will likely need to be worked into the operating principles of tissue engineers.
Biomechanical therapeutics and diagnostics
Investigation of the role of cell mechanics in inflammatory cardiovascular cell activation is still in an early state. This is a field with considerable opportunities, since inflammation is now being recognized as common to many human diseases, including aging, cancer41, 42, and a large number of chronic and acute diseases. One of the hallmarks of inflammation is the activation of endothelial cells, leukocytes and platelets with local actin polymerization in form of pseudopod formation. These events serve as ready diagnostic targets, as signposts to trace the origins of inflammation, and as therapeutic targets. Inflammation is associated with cell interactions that include leukocyte attachment to endothelium, homotypic cell interactions, and highly regulated cell interactions to specific tissue structures (e.g. lymphocytes to high endothelium in lymph nodes). Increasing evidence shows that pseudopod formation is controlled by a group of proteins that controls not only the shape of the actin polymers to be formed, but also the location and rate of the polymerization, its attachment to integrins, and specific actions by myosin-like motions with actin polymers.
Leukocyte rolling and attachment to the endothelium 20, 57 have become the subjects of extensive analysis, but less studied are mechanisms for migration across the endothelium, mechanisms for endothelial pore formation, and mechanics of extracellular matrix proteins such as the basement membrane, which appears to a be relatively strong mechanical structure that requires proteolytic breakdown in inflammation. Migration of cells in the interstitial space, as a coordinated event between pseudopod formation/retraction and controlled membrane attachment/detachment, is largely unexplored at the whole cell level; few predictive theories have been advanced and tested. The role of the extracellular matrix and basement membrane in tumors is also beginning to attract increased attention from a biomechanics point view because changes in matrix structure and mechanics can promote tumor formation. 79 Other important biomechanical events in inflammation are mechanisms that control mechanical attachment of adhesion molecules in cell layers, such as the endothelium or the epithelium in the skin or in the intestine.
Preservation of barrier properties is one of the key mechanisms to minimize the inflammatory cascade as well as cancer metastasis 59, 80. Separation of cell sheets (e.g. interendothelial pores) or pore formation inside a cell cytoplasm (e.g. transendothelial pores) are controlled by biomechanical events including the effects of fluid shear stress and anchoring by inter-endothelial adhesion molecules, like VE-cadherin.
Another key event to minimize the inflammatory cascade is the transport in the lymphatic system. Although mechanical contributions to lymphatic function have been long recognized, these responses have never been quantified or defined at the molecular biophysical level. The fundamental function of the lymphatic system is still open to speculation and disagreement, and therefore biomechanics needs to play a central role to identify its modus operandi, including transport of antigens, viruses, cellular reactions to antigen presenting cells, cell contact, and apoptosis.
From genomics to biomechanics
There is ample evidence that many biological processes surrounding growth and disease are governed by mechanics. Mechanics, in turn, is concerned with three-dimensional shapes, dynamics of deforming bodies, forces, and energies. Mechanics is based on fundamental physical principles that give it the unique ability to predict and anticipate events on many different scales. While modern mechanics in its different forms is able to make remarkable predictions from the largest scales in the Universe to the smallest scales inside an atom, we cannot yet predict the rich world of shapes in the living world. Given a full genomic blueprint today and given a good deal of homology between proteins among many cell and animal types, we have no formulation of physical laws from which we might predict stem cell fate in a given niche or microenvironment, let alone the shape of the encoded organism or its motions. 45 Progress is being made on mechanics of chromatin, tension-dependent controls of chromosome movements, and biomechanics of RNA polymerases, but our limited understanding of protein folding and unfolding46 5, 6, molecular crowding 18, 25, 63, and the thermodynamics of small open systems that reside far from thermodynamic equilibrium 45 64 remain important barriers.
Three-dimensional matrices
Cell biology is in the early phase of moving from cells cultured on flat rigid 2D substrates to cells grown within 3D compliant substrates, a state far more physiologic for most cells. Associated biological knowledge and methodological approaches require much further development. Transport issues need to become an integral part of compliant 3D tissue matrices, as layers of metabolically active cells will consume and create gradients of oxygen as well as other metabolites. Interstitial velocities that arise from cell motion might be small, but shear stresses on interstitial cells might well reach physiologically relevant levels and shape interstitial structures.
Many soluble factors such as growth factors interact with matrix and their homogeneity seems unlikely; growth factor expression even appears to depend on matrix elasticity.54, Heterogeneity in 3D cultures could couple to stress generation of cells and seems likely to have biologically significant effects on key pathways that include oxygen sensitive transcription factors. Cells in interstitial spaces are not only subject to such soluble factors (e.g. cytokines) but also are simultaneously subject to solid molecular attachments (e.g. via integrins) as well as to fluid shear stresses, with sensors that include G-protein coupled receptors and stretch-sensitive ion channels. How these multiple cues are integrated by cells is a challenging facet of “Biomechanics Inside”.
Funding: obstacles and opportunities
Paradoxically, a major obstacle surrounding funding in biomechanics may have stemmed from the central dogma of molecular biology, in which the gene is held to be at the apex of a causal cascade of highly specific signaling pathways, and a physical picture in which this cascade of events plays out according to the rules of dilute solution biochemistry. Within such a conceptual framework, physical forces are easily dismissed as being merely end-effects that reside far downstream of initiating causal events. Related funding obstacles have been concerns surrounding causality and specificity; physical force, after all, is innately nonspecific in both its causes and its effects. Specific genes and signals do indeed control cell-based generation of physical forces, but, as described above, there is now a growing recognition that physical forces, cell shape, cytoskeletal tension, and cell deformation – all of which are nonspecific – act to control signal transduction, gene expression, and stem cell fate.12, 78, 96 36 Therefore, the framework of linear and specific causal cascades playing out according to the rules of dilute solution chemistry, with genes at the top of the cascade and physical forces near the bottom, is now being replaced by the notions that the cell interior is a crowded chemical space 18, 63 that is much closer to the solid state than the fluid state 29, 105, and a framework in which there plays out a web of causality in which cell structure, physical forces, and epigenetic factors are seen to play indispensable roles.
Every obstacle is an opportunity. Taken together, these obstacles comprise a creative tension that highlights the need for fuller integration of quantitative cell mechanics into normative biology. Because cell mechanics has application to all human diseases, its inclusion into health related research efforts represents a major opportunity.
CONCLUSION
Despite remarkable progress to date, the challenges outlined above suggest that the greatest discoveries in cell mechanics are yet to come. It remains unclear how to augment basic principles of mechanics (conservation of mass, momentum, and energy) with a set of principles embracing basic biological paradigms so as to unify mechanics with cell biology, development, and molecular biology. With suitable resources and genomic data, we should one day be able to predict quantitatively and from first principles the way a cell divides, differentiates, matures and migrates. We should be able to predict how a cell generates and incorporates itself into an extracellular matrix, as well as the shapes it assumes. Biology could thus become a truly predictive and mechanistic science in which forecasts of multifactorial processes impacting living systems – in groups as well as at the individual level – could be used not only to make informed decisions but also to improve health by creating new effective drugs and inexpensive replacement tissues.
Nowhere is our current limited ability to predict biological processes better visible than at the level of individual cells, i.e. in cell biomechanics. Few cell shapes or material properties have ever been predicted based on fundamental principles of mechanics. We will have to learn how to understand the mechanics of single proteins and lipid membranes, groups or modules of proteins and lipid structures, and integrate these into predictions for whole cell behavior.14 These opportunities are rich and enduring. There are many immediate opportunities to study the role of cell biomechanics in reproduction, growth and tissue repair, in numerous organ systems such as orthopedic and cardiovascular mechanics, as well as in a long list of diseases, from the malformations of primary genetic defects to inflammation and eventual cell death.
Fig. 3.
In vitro flow assays yield images obtained from both top-view and side-view of an adherent cell under flow conditions compared with in vivo images. 9, 58
Contributor Information
Jeffrey J. Fredberg, Email: jeffrey_fredberg@harvard.edu, Harvard University.
Dennis Discher, Email: discher@seas.upenn.edu, University of Pennsylvania.
Cheng Dong, Email: cxd23@psu.edu, Pennsylvania State University.
Farshid Guilak, Email: guilak@duke.edu, Duke University.
Donald Ingber, Email: donald.ingber@childrens.harvard.edu, Harvard Medical School.
Paul Janmey, Email: janmey@mail.med.upenn.edu, University of Pennsylvania.
Roger D. Kamm, Email: rdk@MIT.edu, MIT.
Geert W. Schmid-Schönbein, Email: gwss@bioeng.ucsd.edu, Univ. of California, San Diego.
Sheldon Weinbaum, Email: weinbaum@ccny.cuny.edu, City College of New York.
References
- 1.An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK-and Rho kinase-dependent pathways. Am J Physiol Cell Physiol. 2005;289(3):C521–C530. doi: 10.1152/ajpcell.00429.2004. [DOI] [PubMed] [Google Scholar]
- 2.Bausch AR, Kroy K. A bottom-up approach to cell mechanics. Nature Physics. 2006 [Google Scholar]
- 3.Ben-Ze'ev A, Robinson GS, Bucher NL, Farmer SR. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988;85(7):2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatnik JS, Schmid-Schonbein GW, Sung LA. The influence of fluid shear stress on the remodeling of the embryonic primary capillary plexus. Biomech Model Mechanobiol. 2005;4(4):211–220. doi: 10.1007/s10237-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 5.Brujic J, Hermans R, Walther K, Fernandez JM. Single-molecule force spectroscopy reveals signatures of glassy dynamics of the energy landscape of ubiquitin. Nature Physics. 2006;2:282–286. [Google Scholar]
- 6.Brujic J, Hermans RI, Garcia-Manyes S, Walther KA, Fernandez JM. Dwell-time distribution analysis of polyprotein unfolding using force-clamp spectroscopy. Biophys J. 2007;92(8):2896–2903. doi: 10.1529/biophysj.106.099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4:557–571. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 8.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122(6):570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Donell B, Deaver DR, Lawrence MB, Dong C. In vitro side-view imaging technique and analysis of human T-leukemic cell adhesion to ICAM-1 in shear flow. Microvasc Res. 1998;55(2):124–137. doi: 10.1006/mvre.1997.2064. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445(7125):295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen AK, Latz MI, Sobolewski P, Frangos JA. Evidence for the role of G-proteins in flow stimulation of dinoflagellate bioluminescence. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R2020–R2027. doi: 10.1152/ajpregu.00649.2006. [DOI] [PubMed] [Google Scholar]
- 12.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 13.Choy JL, Parekh SH, Chaudhuri O, Liu AP, Bustamante C, Footer MJ, Theriot JA, Fletcher DA. Differential force microscope for long time-scale biophysical measurements. Rev Sci Instrum. 2007;78(4):043711. doi: 10.1063/1.2727478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusick ME, Klitgord N, Vidal M, Hill DE. Interactome: gateway into systems biology. Hum Mol Genet. 2005;14(Spec No. 2):R171–R181. doi: 10.1093/hmg/ddi335. [DOI] [PubMed] [Google Scholar]
- 15.De R, Zemel A, Safran S. Dynamics of cell orientation. Nature Physics. 2007;3:655–659. [Google Scholar]
- 16.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76(4):2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng L, Trepat X, Butler JP, Millet E, Morgan KG, Weitz DA, Fredberg JJ. Fast and slow dynamics of the cytoskeleton. Nat Mater. 2006:636–640. doi: 10.1038/nmat1685. [DOI] [PubMed] [Google Scholar]
- 18.Dobson CM. Chemical space and biology. Nature. 2004;432(7019):824–828. doi: 10.1038/nature03192. [DOI] [PubMed] [Google Scholar]
- 19.Dong C, Cao J, Struble EJ, Lipowsky HH. Mechanics of leukocyte deformation and adhesion to endothelium in shear flow. Ann Biomed Eng. 1999;27(3):298–312. doi: 10.1114/1.143. [DOI] [PubMed] [Google Scholar]
- 20.Dong C, Lei XX. Biomechanics of cell rolling: shear flow, cell-surface adhesion, and cell deformability. J Biomech. 2000;33(1):35–43. doi: 10.1016/s0021-9290(99)00174-8. [DOI] [PubMed] [Google Scholar]
- 21.Dong C, Skalak R, Sung KL, Schmid-Schonbein GW, Chien S. Passive deformation analysis of human leukocytes. J Biomech Eng. 1988;110(1):27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- 22.Dowell ML, Lakser OJ, Gerthoffer WT, Fredberg JJ, Stelmack GL, Halayko AJ, Solway J, Mitchell RW. Latrunculin B increases force fluctuation-induced relengthening of ACh-contracted, isotonically shortened canine tracheal smooth muscle. J Appl Physiol. 2005;98(2):489–497. doi: 10.1152/japplphysiol.01378.2003. [DOI] [PubMed] [Google Scholar]
- 23.Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci U S A. 2004;101(35):13068–13073. doi: 10.1073/pnas.0405179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einstein A. Uber die von der molekularkinetischen Theorie der Warme geforderte Bewegung von in ruhenden Flussigkeiten suspendierten Teilchen. Ann. Physik. 1905;19:371–381. [Google Scholar]
- 25.Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci. 2001;26(10):597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87(14):148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 29.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, Fredberg JJ. Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68(4 Pt 1):041914. doi: 10.1103/PhysRevE.68.041914. [DOI] [PubMed] [Google Scholar]
- 30.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93(10):e136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 32.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278(1):255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes and motions of proteins. Science. 1991;254(5038):1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- 34.Fredberg JJ. Frozen objects: small airways, big breaths, and asthma. J Allergy Clin Immunol. 2000;106(4):615–624. doi: 10.1067/mai.2000.109429. [DOI] [PubMed] [Google Scholar]
- 35.Fung Y. Biodynamics: Circulation. New York: Sringer-Verlag; 1984. [Google Scholar]
- 36.Garcia HG, Grayson P, Han L, Inamdar M, Kondev J, Nelson PC, Phillips R, Widom J, Wiggins PA. Biological consequences of tightly bent DNA: the other life of a macromolecular celebrity. Biopolymers. 2007;85(2):115–130. doi: 10.1002/bip.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci U S A. 2006;103(6):1762–1767. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodsell D. Biomolecules and nanotechnology. Am Sci. 2000;88:230–237. [Google Scholar]
- 39.Gov NS. Active elastic network: cytoskeleton of the red blood cell. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;75(1 Pt 1):011921. doi: 10.1103/PhysRevE.75.011921. [DOI] [PubMed] [Google Scholar]
- 40.Gov NS, Safran SA. Red blood cell membrane fluctuations and shape controlled by ATP-induced cytoskeletal defects. Biophys J. 2005;88(3):1859–1874. doi: 10.1529/biophysj.104.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33(4):195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol. 2000;279(4):F698–F712. doi: 10.1152/ajprenal.2000.279.4.F698. [DOI] [PubMed] [Google Scholar]
- 43.Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004;101(47):16689–16694. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 45.Haw M. From steam engines to life. American Scientist. 2007;95:472–473. [Google Scholar]
- 46.Henzler-Wildman K, Kern D. Dynamic personalities of proteins. Nature. 2007;450(7172):964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 47.Herrera AM, Martinez EC, Seow CY. Electron microscopic study of actin polymerization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1161–L1168. doi: 10.1152/ajplung.00298.2003. [DOI] [PubMed] [Google Scholar]
- 48.Holt JR, Corey DP. Two mechanisms for transducer adaptation in vertebrate hair cells. Proc Natl Acad Sci U S A. 2000;97(22):11730–11735. doi: 10.1073/pnas.97.22.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 50.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1(5):E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 51.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 52.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20(7):811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 53.Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50(2–3):255–266. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- 54.Jaramillo F, Hudspeth AJ. Displacement-clamp measurement of the forces exerted by gating springs in the hair bundle. Proc Natl Acad Sci U S A. 1993;90(4):1330–1334. doi: 10.1073/pnas.90.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroy K, Glaser J. The Glassy Wormlike Chain. New Journal of Physics. 2007;9:416. [Google Scholar]
- 56.Lazaar AL, Panettieri RA., Jr Is airway remodeling clinically relevant in asthma? Am J Med. 2003;115(8):652–659. doi: 10.1016/j.amjmed.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Lei X, Lawrence MB, Dong C. Influence of cell deformation on leukocyte rolling adhesion in shear flow. J Biomech Eng. 1999;121(6):636–643. doi: 10.1115/1.2800866. [DOI] [PubMed] [Google Scholar]
- 58.Leyton-Mange J, Yang S, Hoskins MH, Kunz RF, Zahn JD, Dong C. Design of a side-view particle imaging velocimetry flow system for cell-substrate adhesion studies. J Biomech Eng. 2006;128(2):271–278. doi: 10.1115/1.2165689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang S, Sharma A, Peng HH, Robertson G, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res. 2007;67(12):5814–5820. doi: 10.1158/0008-5472.CAN-06-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang S, Slattery MJ, Dong C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Exp Cell Res. 2005;310(2):282–292. doi: 10.1016/j.yexcr.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289(5):F978–F988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 62.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285(5):F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 63.Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int Rev Cytol. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. [DOI] [PubMed] [Google Scholar]
- 64.Macklem PT. Viewpoint: Emergent phenomena and the secrets of life. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.00942.2007. [DOI] [PubMed] [Google Scholar]
- 65.Makino A, Shin H, Komai Y, Fukuda S, Coughlin MF, Sugihara-Seki M, Schmid-Schonbein G. Mechaotransduction in leukocyte activation. Biorheology. 2007;44:221–249. [PubMed] [Google Scholar]
- 66.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119(Pt 3):508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 67.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 68.McCulloch AD, Omens JH. Non-homogeneous analysis of three-dimensional transmural finite deformation in canine ventricular myocardium. J Biomech. 1991;24(7):539–548. doi: 10.1016/0021-9290(91)90287-w. [DOI] [PubMed] [Google Scholar]
- 69.Mead J, Lindgren I, Gaensler EA. The mechanical properties of the lungs in emphysema. J Clin Invest. 1955;34(7, Part 1):1005–1016. doi: 10.1172/JCI103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michel CC. Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol. 1997;82(1):1–30. doi: 10.1113/expphysiol.1997.sp004000. [DOI] [PubMed] [Google Scholar]
- 71.Minton A. How can biochemical reactions within cells differ from those in test tubes? Journal of Cell Science. 2006;119:2863–2869. doi: 10.1242/jcs.03063. [DOI] [PubMed] [Google Scholar]
- 72.Mizuno D, Tardin C, Schmidt CF, Mackintosh FC. Nonequilibrium mechanics of active cytoskeletal networks. Science. 2007;315(5810):370–373. doi: 10.1126/science.1134404. [DOI] [PubMed] [Google Scholar]
- 73.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232(2):268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 74.Murray C. The physiological principle of minimum work. I. The vascular system and the cost of blood volume. Proceedings of the National Academy of Sciences USA. 1926;12:207–214. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7(2):95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 76.Oliver MN, Fabry B, Marinkovic A, Mijailovich SM, Butler JP, Fredberg JJ. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am J Respir Cell Mol Biol. 2007;37(3):264–272. doi: 10.1165/rcmb.2006-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pajerowski J, Dahl K, Zhong F, Sammak P, Discher D. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J. 2002;16(10):1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 79.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 80.Peng HH, Liang S, Henderson AJ, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp Cell Res. 2007;313(3):551–559. doi: 10.1016/j.yexcr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269(5623):80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- 82.Pollard TD. The cytoskeleton, cellular motility and the reductionist agenda. Nature. 2003;422(6933):741–745. doi: 10.1038/nature01598. [DOI] [PubMed] [Google Scholar]
- 83.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184(1):71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 84.Raupach C, Zitterbart DP, Mierke CT, Metzner C, Muller FA, Fabry B. Stress fluctuations and motion of cytoskeletal-bound markers. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;76(1 Pt 1):011918. doi: 10.1103/PhysRevE.76.011918. [DOI] [PubMed] [Google Scholar]
- 85.Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol. 1991;261(3 Pt 1):C428–C432. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- 86.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. [PubMed] [Google Scholar]
- 87.Sato A, Hirai T, Imura A, Kita N, Iwano A, Muro S, Nabeshima Y, Suki B, Mishima M. Morphological mechanism of the development of pulmonary emphysema in klotho mice. Proc Natl Acad Sci U S A. 2007;104(7):2361–2365. doi: 10.1073/pnas.0607882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114(5):1086–1096. doi: 10.1097/01.prs.0000135330.51408.97. discussion 1097–1088. [DOI] [PubMed] [Google Scholar]
- 89.Schmid-Schonbein GW. Biomechanics of microcirculatory blood perfusion. Annu Rev Biomed Eng. 1999;1:73–102. doi: 10.1146/annurev.bioeng.1.1.73. [DOI] [PubMed] [Google Scholar]
- 90.Schnermann J, Wahl M, Liebau G, Fischbach H. Balance between tubular flow rate and net fluid reabsorption in the proximal convolution of the rat kidney. I. Dependency of reabsorptive net fluid flux upon proximal tubular surface area at spontaneous variations of filtration rate. Pflugers Arch. 1968;304(1):90–103. doi: 10.1007/BF00586722. [DOI] [PubMed] [Google Scholar]
- 91.Secomb TW, Hsu R, Pries AR. Effect of the endothelial surface layer on transmission of fluid shear stress to endothelial cells. Biorheology. 2001;38(2–3):143–150. [PubMed] [Google Scholar]
- 92.Semmrich C, Storz T, Glaser J, Merkel R, Bausch AR, Kroy K. Glass transition and rheological redundancy in F-actin solutions. Proc Natl Acad Sci U S A. 2007;104(51):20199–20203. doi: 10.1073/pnas.0705513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seow CY. Myosin filament assembly in an ever-changing myofilament lattice of smooth muscle. Am J Physiol Cell Physiol. 2005;289(6):C1363–C1368. doi: 10.1152/ajpcell.00329.2005. [DOI] [PubMed] [Google Scholar]
- 94.Shaevitz JW, Fletcher DA. Load fluctuations drive actin network growth. Proc Natl Acad Sci U S A. 2007;104(40):15688–15692. doi: 10.1073/pnas.0702601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simon SI, Schmid-Schonbein GW. Cytoplasmic strains and strain rates in motile polymorphonuclear leukocytes. Biophys J. 1990;58(2):319–332. doi: 10.1016/S0006-3495(90)82379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264(5159):696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 97.Skalak R, Dong C, Zhu C. Passive deformations and active motions of leukocytes. J Biomech Eng. 1990;112(3):295–302. doi: 10.1115/1.2891187. [DOI] [PubMed] [Google Scholar]
- 98.Skalak R, Ozkaya N, Skalak T. Biofluid Mecahnics. Annual Review of Fluuid Mechanics. 1989;21:167–204. [Google Scholar]
- 99.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. 2005;288(4):C831–C839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith NP, Kassab G. Analysis of coronary blood flow interaction with myocardial mechanics based on anatomical models. Phil. Trans. R. Soc. Lond. 2001;A 359:251–1263. [Google Scholar]
- 101.Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. 2003;168(5):516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- 102.Sultan C, Stamenovic D, Ingber DE. A computational tensegrity model predicts dynamic rheological behaviors in living cells. Ann Biomed Eng. 2004;32(4):520–530. doi: 10.1023/b:abme.0000019171.26711.37. [DOI] [PubMed] [Google Scholar]
- 103.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a "bumper-car" model. Proc Natl Acad Sci U S A. 2004;101(47):16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thompson DAW. On Growth and Form. Cambridge University Press; 1942. [Google Scholar]
- 105.Trepat X, Deng L, An S, Navajas D, Tschumperlin D, Gerthoffer W, Butler J, Fredberg J. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res. 2003;92(8):929–936. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- 107.Vera C, Skelton R, Bossens F, Sung LA. 3-D nanomechanics of an erythrocyte junctional complex in equibiaxial and anisotropic deformations. Ann Biomed Eng. 2005;33(10):1387–1404. doi: 10.1007/s10439-005-4698-y. [DOI] [PubMed] [Google Scholar]
- 108.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79(3):581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 109.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104(40):15941–15946. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wayne Brodland G, Chen HH. The mechanics of cell sorting and envelopment. J Biomech. 2000;33(7):845–851. doi: 10.1016/s0021-9290(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 112.Weinbaum S. Whitaker Distinguished Lecture: Models to solve mysteries in biomechanics at the cellular level; a new view of fiber matrix layers. Ann Biomed Eng. 1998;26(4):627–643. doi: 10.1114/1.134. 1997. [DOI] [PubMed] [Google Scholar]
- 113.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 114.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 115.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100(13):7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolff J. Das gesetz der transformation der knochen. Berlin: A Hirschwald; 1891. [Google Scholar]
- 117.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318(5853):1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 118.Yao Y, Rabodzey A, Forbes Dewey C., Jr Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007;293(2):H1023–H1030. doi: 10.1152/ajpheart.00162.2007. [DOI] [PubMed] [Google Scholar]
- 119.You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–1386. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]