Abstract
Polymeric scaffolds formed from synthetic or natural materials have many applications in tissue engineering and medicine, and multiple material properties need to be optimized for specific applications. Recent studies have emphasized the importance of the scaffolds’ mechanical properties to support specific cellular responses in addition to considerations of biochemical interactions, material transport, immunogenicity, and other factors that determine biocompatibility. Fibrin gels formed from purified fibrinogen and thrombin, the final two reactants in the blood coagulation cascade, have long been shown to be effective in wound healing and supporting the growth of cells in vitro and in vivo. Fibrin, even without additional growth factors or other components has potential for use in neuronal wound healing in part because of its mechanical compliance that supports the growth of neurons without activation of glial proliferation. This review summarizes issues related to the use of fibrin gels in neuronal cell contexts, with an emphasis on issues of immunogenicity, and considers the potential advantages and disadvantages of fibrin prepared from non-mammalian sources.
Introduction
The elastic properties of tissues and biomaterials designed to promote wound healing or regeneration in specific settings has until recently not been considered as an essential design feature. Most studies have addressed the biochemical and structural properties of scaffolds and extracellular matrices that dictate the molecular specificity of cell adhesions and the transport of soluble factors into and away from the site of repair. A series of recent studies has rejuvenated interest in studying how tissue and biomaterial stiffness influences the structure and function of cells by showing that matrix stiffness, under conditions where other factors are held constant, has a large effect on the rate of cell proliferation, specific programs of gene expression, cell motility, and the developmental fate of stem cells [1–3]. In some cases, matrix stiffness can override chemical stimuli, as illustrated by the lack of response to osteogenic growth factors when mesenchymal stem cells are plated on soft (< 1000 Pa) surfaces [4], and in other cases the nature of the adhesive ligand works in concert with substrate mechanics to direct specific processes such as the interplay between the type of integrin ligand and the substrate stiffness on the formation of actin stress fibers or the modulation of motility[5–7].
Not all cells respond similarly to matrix stiffness, and some cell types such as neutrophils seem not to respond to stiffness differences in the range that strongly affect other cell types [5]. One setting in which the elasticity of the substrate appears to have a highly specific effect is in central nervous system. The brain is among the softest human tissues, with a time-dependent shear storage modulus (or, depending on the type of rheologic measurement, Young’s modulus) that varies from 1000 Pa at millisecond time scales appropriate for modeling effects of impact, to a relatively steady level near 200 Pa at time scales on the order of seconds [8, 9]. At sites of injury, where glial scarring occurs, the local stiffness can be palpably higher, but is not yet quantitatively determined, and the stiffness difference at the interface of the glial scar can act as a physical as well as a chemical barrier to neurite extension and neuronal repair in severe injuries [9, 10].
The possibility that soft materials might be partially useful in restoration of diseased CNS tissue is related to the finding that two main cell types of the CNS, neurons and astrocytes, respond in very different ways to matrix stiffness [10], and that gels of low elastic modulus differentially support the neuronal development of precursor cells [9]. Spinal cord and cortical brain neurons extend neurites and form branches more avidly on soft materials, and are the only cell type thus far documented to be inhibited from extending as the matrix becomes stiffer than the stiffness of a normal brain (<1000 Pa) [9, 11–15]. In contrast, astrocytes, like numerous other cell types, develop stress fibers, a larger spread area, and become activated on stiff surfaces [10]. This article will focus on evidence of the effects of manipulating substrate stiffness that may have utility in central nervous system and other injury settings and on the specific properties of matrices derived from non-mammalian clotting factors such as salmon fibrinogen and thrombin that have potential advantages or complementary properties compared to synthetic or human-derived materials.
Advantages of fibrin from non-mammalian sources
Fibrin has a long and extensive record of use in wound healing including treatment of trauma to the brain and spinal cord [16, 17]. Fibrin is the normal scaffold that first forms at sites where trauma to cells initiates the cascade of reactions leading to blood clotting. Purification of the two final reactants, fibrinogen and thrombin, and administration in controlled amounts at defined locations has many clinical applications [18]. The fibrin scaffold can be supplemented with growth factors and other agents for specific settings and is simple to administer, with a straightforward injection into the affected region [19]. The reaction occurs at physiological temperature and pH, and both the rates of gelation and the mechanical properties of the polymerized gel can be controlled easily by adjusting the injection mix [20, 21].
Limitations of the use of fibrin in CNS or other injuries include the fact that fibrin is designed to degrade at a rate that depends on the production of plasmin and other proteases generated at the injured site, and in some settings such as neural regeneration, fibrin degradation proceeds too rapidly to allow neurite infiltration without the use of protease inhibitors that can have known or unanticipated negative effects. Cells of the CNS, including neurons, astrocytes, and microglia have receptors for thrombin, fibrinogen or fibrin that also have potentially negative effects on neural wound healing. Human fibrin is also optimized to polymerize at slightly below 37°C, and fails to clot at lowered temperatures that are required in some surgical settings, including CNS trauma. Additional concerns involve the potentials for infectious agents introduced by using proteins derived from pooled human or other mammalian sources. Some of these limitations can be overcome by non-mammalian coagulation proteins, such as have been purified from salmon blood [22–26]. Worldwide production of farmed salmon now exceeds a million metric tons annually. Therefore, millions of liters of blood with consistent quality are available from animals where genetics, nutrition, and environment are controlled or closely monitored.
Proteins derived from salmon or other non-mammalian tissues are likely to be safe from mammalian infectious agents due to the wide evolutionary distance between fish and humans. The low body temperature of cold water fish serves as another barrier to cross-species survival of bacteria or viruses. Prion infection is also most probable in species with similar prion proteins. Although salmon do have a normal isoform of prion protein, its structure is quite different from the mammalian protein [27] and therefore presents little risk. Also, there is no evidence of prion disease in fish, and even if farmed fish ingest mammalian prions, the infectivity is quickly cleared [28]. Prion contamination is a special concern in fibrinogen products, because mammalian fibrinogen can selectively bind the infective part of the prion protein [29].
Salmon fibrinogen and thrombin are sufficiently similar to human fibrinogen and thrombin to be interchangeable in terms of fibrin polymerization, but they differ subtly from those of the human proteins both in amino acid sequence and the nature of glycosylation [23, 24, 30]. For example, whereas salmon thrombin activates human platelets (a cell type absent in fish) the time course of platelet aggregation in vitro is slightly different, suggesting that salmon thrombin activates the major human platelet receptor, but might not fully activate other cellular targets [23]. The A-alpha chain of salmon fibrinogen is significant lower molecular weight compared to human A-alpha, and the gamma chain of zebrafish fibrinogen, the closest species to salmon that has been sequenced, lacks homology in a region of human fibrinogen that activates microglia [31] and is thought to contribute to inflammation in the CNS.
Differences between salmon and mammalian fibrin are apparent in several studies in which these scaffolds have been compared in cell culture and animal models. Mammalian neurite outgrowth in vitro is significantly less in human or bovine fibrin compared to salmon fibrin in three dimensional fibrin gels [33]. Human fibrin did not improve functional recovery in a rat model of spinal cord injury [33]. Human fibrinogen inhibits neurite outgrowth while salmon fibrin does not, possibly via outgrowth by triggering an inhibitory signal transduction pathway in neurons [34]. Human fibrinogen polymerizes slowly below 37°C but salmon fibrinogen clots normally down to 0°C [30]. Since outcomes can be improved by hypothermia after traumatic brain injury [35] and possibly spinal cord injury [36], salmon fibrin could be effective for the coagulopathy seen at low body temperature.
Issues of immune response to foreign proteins and biomaterial scaffolds
The same structural differences between salmon and mammalian fibrinogen and thrombin that in some context confers possible advantages to use of non-mammalian fibrin is potentially countered by the presumed higher antigenicity of salmon proteins in a mammalian host. The inflammatory response to synthetic and natural tissue adhesives is variable, depending primarily on the contact of tissue fluids around the biomaterial and the access of host blood cells. In general, host reactions following administration of biomaterials include stages of blood-material interaction, provisional matrix formation, acute inflammation, chronic inflammation, granulation tissue development, foreign body reaction, and fibrosis (fibrous capsule) development [37]. In the cascade of these events the role of the immune system is significant, but immune system activation largely depends on the immunogenicity of tissue adhesives.
Synthetic tissue adhesives, as most widely used and continuously developed, are relatively inert for the host immune system. For example, if administered intravascularly to the rats, N-butyl-2-cyanoacrylate induces only mild eosinophilic inflammation during the first day and after 7 days the tissue reaction is minimal [38]. However, small particles of cyanoacrylates can modulate immune response to external antigens as demonstrated by Simeonova et al. [39]. Currently it is believed that the adjuvant effect of synthetic materials in stimulating dendritic cells and the adaptive immune response to co-expressed (auto)antigens may be more important than was previously thought [40]. In addition, systemic inflammatory reactions and septic complications can develop, but the conditions that are needed for their development are not well known. Some of these rare reactions can be life threatening due to fulminant inflammatory reactions [41]. Delayed and recurrent chronic inflammatory and granulomatous reactions could be seen in response to some synthetic gels [42]. However, polyethylene glycol based biodegradable hydrogels used as tissue sealants do not appear to induce immediate humoral or cellular immune reactions [43, 44].
Thus in general, synthetic tissue adhesives do not necessarily strongly initiate cross-talk with hosts tissues and cells due to their relatively passive role in tissue repair. However, the immune reactions largely depend on the chemistry and physics of synthetic biomaterials surfaces which contact the host tissue. Biomaterials’ surface characteristics have a significant impact on antigen presenting cells, including macrophage and dendritic cells’ responses such as adhesion, apoptosis and cytokine secretion. These cells continuously sense and internalize their surroundings by pattern recognizing receptors orchestrating the immune system and inflammatory reactions towards the target tissue. As an example, alginate, which is composed of alternating mannuronic and guluronic acid residues has been shown to induce inflammatory response depending on the amount of mannuronic acid residues in the preparation [45]. Thus by controlling the alginate composition it is possible to modulate inflammatory reactions induced by sealants containing alginate. Synthetic materials like polyglycolic acid are also able to enhance immune response [40]. Unfortunately, thus far the interactions between synthetic tissue adhesives and the immune system are only superficially studied in spite of the fact that specially designed materials could stimulate the immune system to improve tissue healing [46].
Native tissue adhesives represent natural bio-compounds of the host to mimic the physiological situation and therefore their interaction with cellular and humoral factors of the immune system is more immediate. Here the immune system gets stronger direct signals for its activation. The extent of activation depends on genetic differences between donor (from where biomaterial is prepared) and host tissues. Tissue sealants prepared from the individual’s tissues to be treated (autologous adhesives) are not immunogenic if their composition is not physically or chemically altered i.e. there are no neo-antigens recognized by the immune system as non-self. In a study by van Nooten et al. [47] a 2-component glue with sufficient elasticity and tensile strength was made by mixing canine autologous plasma concentrate with 7.5 % glutaraldehyde and applied to dogs. As a result, only mild inflammation with few lymphocytes and plasma cells around the glue was formed. The absence of CD4+ T lymphocytes in the infiltration was taken as evidence for the absence of antigen presentation in the tissue treated with glue [47]. Protein preparations from other individuals of the same species can acquire immunogenicity (for example due to blood group antigens contamination) whereas xenogenic biomaterials from tissues of other species are always immunogenic. The combination of allogenous and heterologous proteins in fibrinogen-coated collagen patches seems to diminish immunological reactions against the patches’ components [48], but comprehensive immunological studies on these tissue adhesives are still not available.
The use of recombinant human coagulation proteins, instead of those prepared from blood with a possibility of contamination with infectious agents, seems to offer a treatment device that has no problems. However, an expectation that human recombinant protein preparations would be non-immunogenic in patients capable of synthesizing these proteins by themselves, is somewhat premature, if we take into account the possibility that these preparations may include trace amounts of ingredients originating from the expression systems used for their preparation. Also incomplete or abnormal post-translational modification or other, even minimal changes in the composition can render these preparations immunogenic [49]. Still, recombinant human thrombin is less immunogenic for clinical use than heterologous thrombin as has also been demonstrated by the comparative clinical analysis of Weaver et al. [50]. In this study no patients who underwent either a peripheral arterial bypass or arteriovenous graft procedure and received recombinant human thrombin seroconverted or had an increase in anti-thrombin antibody titer. In another study, the development of non-neutralized antibodies to recombinant human thrombin was detected in 3 out of 198 patients [32].
The most commonly used native tissue sealants are thrombin- and fibrinogen-based. Thrombin-based products are often produced from bovine plasma and fibrinogen-based hemostatic agents derived from human plasma [51]. Accordingly, the most serious immunological problems could rise if bovine thrombin based fibrin glue is used. Although tissue glues of such types have been safely and effectively used for many years in thousands of patients, a number of immunological complications have been reported. In most cases, these complications are related to the development of immune reactions against bovine thrombin preparations. Even a single administration of bovine thrombin can mount a significant immune response, as has been shown by the development of autoantibodies characteristic for the systemic autoimmunity in non-autoimmune-prone mice [52]. If the patient has a propensity to develop such antibodies or has pre-existing autoantibodies to thrombin, the administration of bovine thrombin containing glue could induce or potentiate immune reactions against autologous thrombin. In such cases serious clinical consequences from the antithrombin autoantibodies may appear. It is possible that these autoantibodies cause either thrombosis [53] or hemorrhage [54]. The pathological outcome of antithrombin antibodies likely depends on the antigenic epitope to which they react. According to recent studies, thrombin autoantibodies can be found in up to 40.9 % of patients with systemic lupus erythematosus and antiphospholipid syndrome [55]. Somewhat lower frequencies have been reported earlier [56], however thrombin may share common epitopes with prothrombin and beta2- glycoprotein I, well known autoantigens in antiphospholipid syndrome [57]. Similarly, antibodies to other bovine fibrin glue components like factor Va could arise [53], leading to the development of autoantibodies against human factor Va which would impair the systemic coagulation with serious clinical complications similar to those seen in cases of spontaneously developed autoantibodies [58].
Administration of bovine fibrin glue can also induce immediate reactions in the form of Immunoglobulin E (IgE)-mediated anaphylaxis against thrombin [59, 60]. If aprotinin of bovine origin is used in fibrin sealant to prevent lysis of the clot, anaphylactic reactions can develop [61, 62]. A specific immune response induced by local aprotinin administration does not differ from that seen in intravenous exposure [61].
The development of immune reactions to bovine fibrin glue and other glue materials of bovine origin is mostly due to the presence of xenogenic carbohydrates such as Galα1-3Gal in bovine products [63]. These carbohydrates, not present in human fibrinogen or thrombin, are strong stimulators of immune response among humans. In bovine thrombin preparations the source of Galα1-3Gal may not be thrombin itself but rather this xenoantigen can be expressed on contaminating proteins. Whatever the cause, 90 % of humans exposed to a bovine thrombin preparation develop IgG antibodies against one or more of the proteins in the preparation [53].
In contrast to tissue sealants containing constituents of bovine origin, coagulation proteins of salmon used for fibrin glue development do not express highly antigenic carbohydrate Galα1-3Gal. However IgE and IgG antibodies can be developed to fish proteins, probably induced by proteins rather than by carbohydrate residues. Among fish proteins with potency to activate IgE (i.e. immediate allergen) reactions, parvalbumin, a muscle protein, holds a special position since parvalbumins from fish are considered to be the major or sole allergens for 95 % of the patients suffering from IgE-mediated fish allergy [64]. So far parvalbumins of about 30 fish species have been characterized [65] including salmon allergen Sal s1 [66, 67]. Parvalbumins of different fish species show considerable cross-reactivity and therefore are dangerous for individuals with known fish allergy that account for about 0.1–0.3 % of the general population [65]. This salmon protein was also demonstrated to be antigenic by the use of salmon antibodies in IgG ELISA and immunoelectrophoretic methods [66]. However, since salmon thrombin and fibrinogen are prepared from salmon blood the possibility that allergen Sal s1 situating in the fish muscle would contaminate salmon fibrin glue should be minimal.
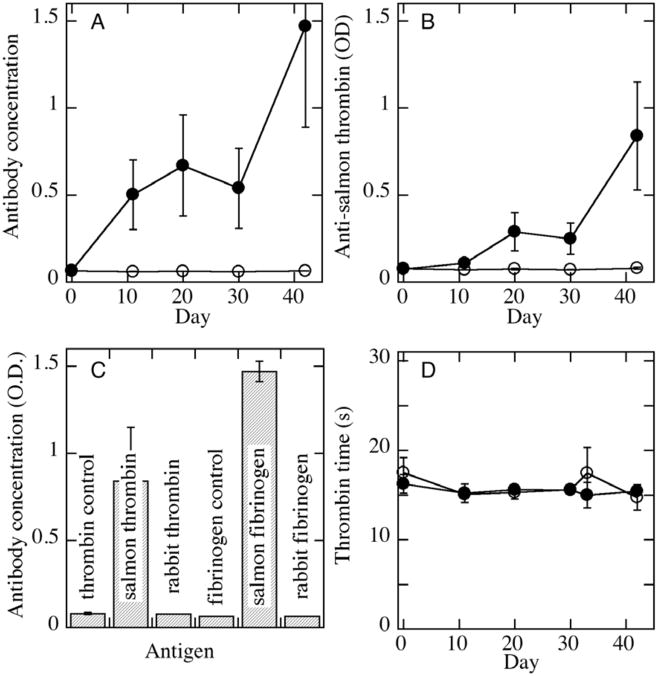
Some reactions to fish proteins may also develop through cross-reactions. Thus, pre-existing autoantibodies can react with fish thrombin and fibrinogen preparations as has been shown in previous studies [23, 24]. In these studies, as well as a later one by Laidmäe et al. [30], no assays of immune response showed any unusual antigenicity of the salmon fibrin sealant (Figure 1). Humoral immune response to salmon thrombin and fibrinogen was studied after intraperitoneal administration of salmon fibrin glue to rabbits and rats. Using an enzyme-linked immunosorbent assay and salmon thrombin and fibrinogen as antigens, only a mild increase of IgG binding to antigens was demonstrated 1–3 weeks after the first fibrin glue administration. As expected, after a second dose of salmon fibrin glue, antibodies levels were higher (Fig 1A,B). In no case was an increase in clotting times observed, demonstrating that the antibodies developed did not affect the normal coagulation process (Fig 1D). Notably, when rabbit blood samples were tested with purified rabbit fibrinogen and thrombin, no antibodies were detected showing that autoantibodies to host proteins have not been developed (Fig 1C). These results were further confirmed in immunoblot studies on 23 control and 23 experimental rats where low intensity antibody reactions to other polypeptides in thrombin and fibrinogen preparations were found [30]. As also demonstrated by other authors in association with human proteins [68], salmon fibrinogen is more immunogenic than salmon thrombin. Most importantly, however, no cross- reactivity to human factor Va was demonstrated in animals immunized with salmon thrombin. This result shows that salmon fibrin glue does not induce immune reactions that might be cross-reactive with human factor Va, which is the most problematic immunological cross-reactivity associated with the use of bovine fibrin glue [69]. Also, peripheral blood C-reactive protein levels were similar in experimental and control rats at the next day of second salmon fibrin glue challenge (Laidmäe et al., unpublished). These results show that there is no immediate inflammatory reaction in response to salmon fibrin glue application. The absence of antibody cross-reactivity between salmon and mammalian proteins is likely due to differences in glycosylation and other posttranslational modifications which are substantially different between fish and mammals.
Figure 1.
Development of antibodies to salmon, but not endogenous, proteins after administration of salmon fibrin. Antibodies to salmon fibrinogen (A) or thrombin (B) were measured in rabbit plasma samples taken at various times before and after intraperitoneal administration of salmon fibrin on days 2 and 32. Open symbols are data for samples from control animals treated with saline and solid symbols are from animals treated with salmon fibrin. (C) Comparison of antibody titres on day 42 for rabbits treated with salmon fibrin on days 2 and 32 reacting against salmon or rabbit fibrinogen or thrombin. Controls are for ELISA assays done on the same strips using plasma from control animals treated with 0.9% saline in place of salmon fibrin. D. Thrombin times performed on the same samples as used for antibody analyses. Adapted from [30].
Performance of salmon fibrin in hemostasis and neuronal wound healing
Salmon fibrin has been tested in animal models of both hemostasis and neuronal wound healing. In two models of bleeding, purified salmon fibrinogen and thrombin preparations performed as well as commercial preparations of human proteins. Salmon fibrin dressing efficiently stopped bleeding in a rat hip penetrating injury model [70] and bandages lined with lyophilized salmon fibrinogen and thrombin controls arterial bleeding in a swine model where a 4.4 mm hole was surgically formed in the aorta [71]. Gels made from salmon fibrin were superior to human fibrin gels in supporting the growth and tubulogenesis of human umbilical vein endothelial cells in vitro [72].
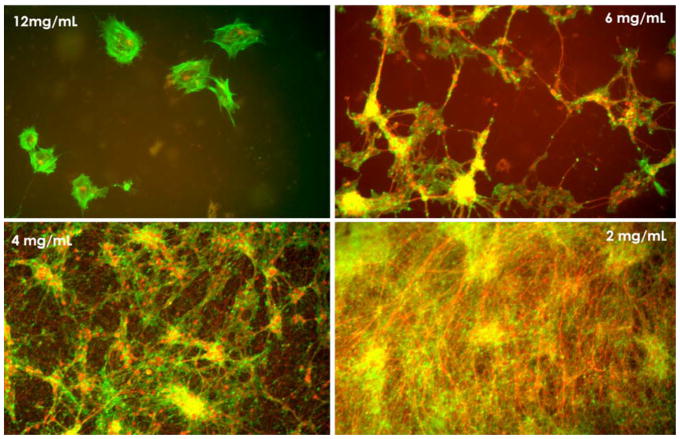
In addition to its utility in hemostasis and formation of a matrix in which angiogenesis can initiate, fibrin also has advantages for repair of central and peripheral nervous system injuries in part because of it mechanical properties, which can be tuned by varying fibrinogen and thrombin concentrations. Neurons, unlike other cell types, appear to grow best on very soft materials [11, 12] and fibrin fibers are among the softest biopolymers [73–75]. One consequence of the mechanical preference of neurons is evident in Figure 2, which shows how the relative amounts of neurons and astrocytes grown from a whole cortical mouse brain preparation are altered as the elastic modulus of the salmon fibrin gel is changed from 1800 Pa to 74 Pa. The softest gels, which simulate the softness of normal brain [1,3] strongly promote growth of neurons and astrocytes, whereas stiffer gels promote astrocyte adhesion and spreading but not neuronal extension.
Figure 2.
Cultures of dissociated embryonic mouse brain cortices at 1 week in salmon fibrin gels of different concentrations correponding to elastic modul of 1800 Pa, 575 Pa, 300 Pa and 75 Pa with decreasing concentration. Neurons are labeled for bIII-tubulin (red) and astrocytes for GFAP (green). Adapted from [10].
Formation of a soft-cell-compatible matrix is accomplished by fibrin from any source, but the suitability of this matrix for specific applications depends on many factors such as the stability of the matrix to degradative enzymes released at the site of injury and the multiple signals than can be elicited by sites within the protein filaments. For the long-term cultures needed to produce robust neurite outgrowth with minimal activation of inflammation, salmon fibrin is more effective than human or bovine thrombin to support neuronal growth in vitro [33]. In part this is because salmon fibrin is more resistant to rapid degradation in a neuronal setting, and in part perhaps because of beneficial differences in post-translational modifications or primary structure. Figure 3 shows the greater extension and more robust growth of neurites from rat brain cortices in salmon fibrin compared to human or bovine fibrin. Whereas the mechanisms that account for the greater growth of mammalian neurons in salmon fibrin are not known, preliminary studies in a rat spinal cord injury model are consistent with these in vitro findings.
Figure 3.
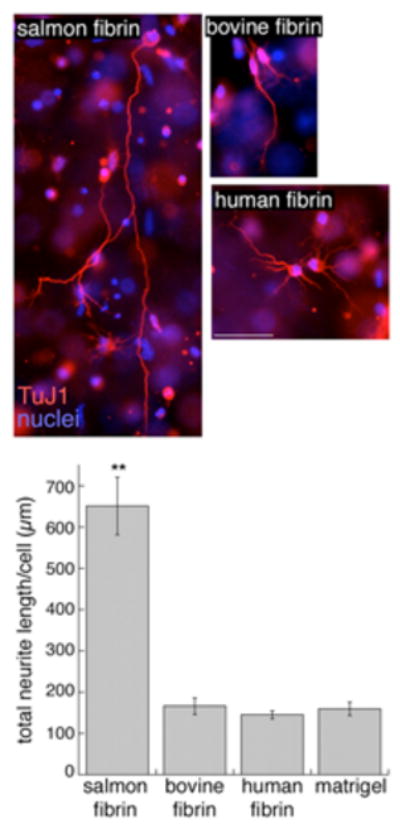
Rat cortical neurons embedded in salmon, bovine or human fibrin for 3 days were immunostained with TuJ1 or Map5/Map1b antibodies to visualize neurites. . Scale bar is 50 μm. The effect of the different matrices on neurite growth was quantified by measuring the total neurite length per cell (**p<0.001 salmon fibrin vs. bovine or human fibrin). Adapted from [33].
Conclusion
The biochemical and mechanical properties of fibrin gels, characterized by a large mesh size, a low elastic modulus, and binding sites for integrins and other cellular targets are well suited to use in neuronal and other tissue engineering contexts. The effectiveness of fibrin gels in vivo cannot be inferred from its efficacy in vitro without consideration of its stability at wound sites and the immunologic response of the host to this foreign material. For proteins derived from blood or even from recombinant sources possible adverse effects from infectious agents or bacterial endotoxins are also an important consideration. Such issues have motivated studies of fibrin derived from non-mammalian source such as farmed salmon, and structural differences between salmon and mammalian fibrin might also contribute to its advantageous use in neural cell growth and repair.
Acknowledgments
This work was supported by the US National Institutes of Health through NIH-SBIR (2-R44 NS048734-03)
References
- 1.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Georges PC, Rojas WDJ, Levental Ilya, Solon J, Byfield J, Janmey PA. Effect of substrate stiffness on the structure and function of cells. Biophys Rev and Lett. 2006;1:401–410. [Google Scholar]
- 3.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 6.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 7.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 8.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;1:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 9.Saha K, Keung A, Irwin E, Li Y, Little L, Schaffer D, Healy KE. Substrate Modulus Directs Neural Stem Cell Behavior. Biophys J. 2008 doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–8. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–84. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed. 2004;15:1521–31. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 14.Jiang FX, Yurke B, Firestein BL, Langrana NA. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann Biomed Eng. 2008;36:1565–79. doi: 10.1007/s10439-008-9530-z. [DOI] [PubMed] [Google Scholar]
- 15.Kostic A, Sap J, Sheetz MP. RPTP{alpha} is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. J Cell Sci. 2007;120:3895–904. doi: 10.1242/jcs.009852. [DOI] [PubMed] [Google Scholar]
- 16.Fawcett JW. Spinal cord repair: from experimental models to human application. Spinal Cord. 1998;36:811–7. doi: 10.1038/sj.sc.3100769. [DOI] [PubMed] [Google Scholar]
- 17.Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–31. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 18.Janmey P, Winer J, Weisel J. Fibrin Gels. J Royal Soc Interface. 2008 doi: 10.1098/rsif.2008.0327. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 20.Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14:236–41. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- 21.Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007;5(Suppl 1):116–24. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- 22.Murtaugh PA, Halver JE, Gladner JA. Cross-linking of salmon fibrinogen and fibrin by factor XIII and transglutaminase. Biochem Biophys Res Commun. 1973;54:849–55. doi: 10.1016/0006-291x(73)90771-7. [DOI] [PubMed] [Google Scholar]
- 23.Michaud SE, Wang LZ, Korde N, Bucki R, Randhawa PK, Pastore JJ, Falet H, Hoffmeister K, Kuuse R, Uibo R, Herod J, Sawyer E, Janmey PA. Purification of salmon thrombin and its potential as an alternative to mammalian thrombins in fibrin sealants. Thromb Res. 2002;107:245–54. doi: 10.1016/s0049-3848(02)00333-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang LZ, Gorlin J, Michaud SE, Janmey PA, Goddeau RP, Kuuse R, Uibo R, Adams D, Sawyer ES. Purification of salmon clotting factors and their use as tissue sealants. Thromb Res. 2000;100:537–48. doi: 10.1016/s0049-3848(00)00362-5. [DOI] [PubMed] [Google Scholar]
- 25.Manseth E, Skjervold PO, Flengsrud R. Sample displacement chromatography of Atlantic Salmon (Salmo salar) thrombin. J Biochem Biophys Methods. 2004;60:39–47. doi: 10.1016/j.jbbm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Manseth E, Skjervold PO, Fjaera SO, Brosstad FR, Bjornson S, Flengsrud R. Purification and characterization of Atlantic salmon (Salmo salar) fibrinogen. Comp Biochem Physiol B Biochem Mol Biol. 2004;138:169–74. doi: 10.1016/j.cbpc.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs CJ, Jr, Bolis CL. Normal isoform of amyloid protein (PrP) in brains of spawning salmon. Mol Psychiatry. 1997;2:146–7. doi: 10.1038/sj.mp.4000250. [DOI] [PubMed] [Google Scholar]
- 28.Ingrosso L, Novoa B, Valle AZ, Cardone F, Aranguren R, Sbriccoli M, Bevivino S, Iriti M, Liu Q, Vetrugno V, Lu M, Faoro F, Ciappellano S, Figueras A, Pocchiari M. Scrapie infectivity is quickly cleared in tissues of orally-infected farmed fish. BMC Vet Res. 2006;2:21. doi: 10.1186/1746-6148-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer MB, Roeckl C, Parizek P, Schwarz HP, Aguzzi A. Binding of disease-associated prion protein to plasminogen. Nature. 2000;408:479–83. doi: 10.1038/35044100. [DOI] [PubMed] [Google Scholar]
- 30.Laidmae I, McCormick ME, Herod JL, Pastore JJ, Salum T, Sawyer ES, Janmey PA, Uibo R. Stability, sterility, coagulation, and immunologic studies of salmon coagulation proteins with potential use for mammalian wound healing and cell engineering. Biomaterials. 2006;27:5771–9. doi: 10.1016/j.biomaterials.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K. The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–82. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman WC, Singla N, Genyk Y, McNeil JW, Renkens KL, Jr, Reynolds TC, Murphy A, Weaver FA. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surg. 2007;205:256–65. doi: 10.1016/j.jamcollsurg.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Ju YE, Janmey PA, McCormick M, Sawyer ES, Flanagan L. Enhanced neurite growth from mammalian neurons in threedimensional salmon fibrin gels. Biomaterials. 2007;28:2097–108. doi: 10.1016/j.biomaterials.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, Zheng B, Akassoglou K. Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci U S A. 2007;104:11814–9. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies AR. Hypothermia improves outcome from traumatic brain injury. Crit Care Resusc. 2005;7:238–43. [PubMed] [Google Scholar]
- 36.Shibuya S, Miyamoto O, Janjua NA, Itano T, Mori S, Norimatsu H. Post-traumatic moderate systemic hypothermia reduces TUNEL positive cells following spinal cord injury in rat. Spinal Cord. 2004;42:29–34. doi: 10.1038/sj.sc.3101516. [DOI] [PubMed] [Google Scholar]
- 37.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suga T, Akamatsu T, Kawamura Y, Saegusa H, Kajiyama M, Nakamura N, Takei M, Matsumoto A. Actual behaviour of N-butyl-2-cyanoacrylate (histoacryl) in a blood vessel: a model of the varix. Endoscopy. 2002;34:73–7. doi: 10.1055/s-2002-19384. [DOI] [PubMed] [Google Scholar]
- 39.Simeonova M, Chorbadjiev K, Antcheva M. Study of the effect of polybutylcyanoacrylate nanoparticles and their metabolites on the primary immune response in mice to sheep red blood cells. Biomaterials. 1998;19:2187–93. doi: 10.1016/s0142-9612(98)00126-4. [DOI] [PubMed] [Google Scholar]
- 40.Babensee JE. Interaction of dendritic cells with biomaterials. Semin Immunol. 2008;20:101–8. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Aoki N, Sakai T, Oikawa A. Postoperative inflammatory reaction developing focal but severe brain edema. A possible complication of topical application of Biobond-soaked oxycellulose. Acta Neurol Scand. 1998;98:288–91. doi: 10.1111/j.1600-0404.1998.tb07311.x. [DOI] [PubMed] [Google Scholar]
- 42.Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008;22:150–61. doi: 10.1111/j.1468-3083.2007.02354.x. [DOI] [PubMed] [Google Scholar]
- 43.Allen MS, Wood DE, Hawkinson RW, Harpole DH, McKenna RJ, Walsh GL, Vallieres E, Miller DL, Nichols FC, 3rd, Smythe WR, Davis RD. Prospective randomized study evaluating a biodegradable polymeric sealant for sealing intraoperative air leaks that occur during pulmonary resection. Ann Thorac Surg. 2004;77:1792–801. doi: 10.1016/j.athoracsur.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 44.Park EL, Ulreich JB, Scott KM, Ullrich NP, Linehan JA, French MH, Ho WY, White MJ, Talley JR, Fellah AM, Ramakumar S. Evaluation of polyethylene glycol based hydrogel for tissue sealing after laparoscopic partial nephrectomy in a porcine model. J Urol. 2004;172:2446–550. doi: 10.1097/01.ju.0000138159.69642.d9. [DOI] [PubMed] [Google Scholar]
- 45.Flo TH, Ryan L, Latz E, Takeuchi O, Monks BG, Lien E, Halaas O, Akira S, Skjak-Braek G, Golenbock DT, Espevik T. Involvement of toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J Biol Chem. 2002;277:35489–95. doi: 10.1074/jbc.M201366200. [DOI] [PubMed] [Google Scholar]
- 46.Chan G, Mooney DJ. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26:382–92. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Van Nooten GJ, Somers P, Forsyth R, Narine K, Van Belleghem Y, Jacobs S, De Somer F. Autologous glue: part of the sticky mystery unraveled. J Thorac Cardiovasc Surg. 2007;134:415–23. doi: 10.1016/j.jtcvs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Erdogan D, van Gulik TM. Evolution of fibrinogen-coated collagen patch for use as a topical hemostatic agent. J Biomed Mater Res B Appl Biomater. 2008;85:272–8. doi: 10.1002/jbm.b.30916. [DOI] [PubMed] [Google Scholar]
- 49.Mukovozov I, Sabljic T, Hortelano G, Ofosu FA. Factors that contribute to the immmunogenicity of therapeutic recombinant human proteins. Thromb Haemost. 2008;99:874–82. doi: 10.1160/TH07-11-0654. [DOI] [PubMed] [Google Scholar]
- 50.Weaver FA, Lew W, Granke K, Yonehiro L, Delange B, Alexander WA. A comparison of recombinant thrombin to bovine thrombin as a hemostatic ancillary in patients undergoing peripheral arterial bypass and arteriovenous graft procedures. J Vasc Surg. 2008;47:1266–73. doi: 10.1016/j.jvs.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 51.Schoenecker JG, Johnson RK, Fields RC, Lesher AP, Domzalski T, Baig K, Lawson JH, Parker W. Relative purity of thrombin-based hemostatic agents used in surgery. J Am Coll Surg. 2003;197:580–90. doi: 10.1016/S1072-7515(03)00670-7. [DOI] [PubMed] [Google Scholar]
- 52.Schoenecker JG, Johnson RK, Lesher AP, Day JD, Love SD, Hoffman MR, Ortel TL, Parker W, Lawson JH. Exposure of mice to topical bovine thrombin induces systemic autoimmunity. Am J Pathol. 2001;159:1957–69. doi: 10.1016/S0002-9440(10)63043-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortel TL, Mercer MC, Thames EH, Moore KD, Lawson JH. Immunologic impact and clinical outcomes after surgical exposure to bovine thrombin. Ann Surg. 2001;233:88–96. doi: 10.1097/00000658-200101000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costa JM, Fiessinger JN, Capron L, Aiach M. Partial characterization of an autoantibody recognizing the secondary binding site(s) of thrombin in a patient with recurrent spontaneous arterial thrombosis. Thromb Haemost. 1992;67:193–9. [PubMed] [Google Scholar]
- 55.Matsuda J, Matsuyama A, Atsumi G, Ohkura N. Sole existence of antithrombin antibody in patients with systemic lupus erythematosus showing tendency of its antigenic determinants directing against exosite II (antithrombin/heparin binding site) of thrombin. Blood Coagul Fibrinolysis. 2008;19:66–9. doi: 10.1097/MBC.0b013e3282f2b5a9. [DOI] [PubMed] [Google Scholar]
- 56.Miesbach W, Matthias T, Scharrer I. Identification of thrombin antibodies in patients with antiphospholipid syndrome. Ann N Y Acad Sci. 2005;1050:250–6. doi: 10.1196/annals.1313.026. [DOI] [PubMed] [Google Scholar]
- 57.Hwang KK, Grossman JM, Visvanathan S, Chukwuocha RU, Woods VL, Jr, Le DT, Hahn BH, Chen PP. Identification of anti-thrombin antibodies in the antiphospholipid syndrome that interfere with the inactivation of thrombin by antithrombin. J Immunol. 2001;167:7192–8. doi: 10.4049/jimmunol.167.12.7192. [DOI] [PubMed] [Google Scholar]
- 58.Kalafatis M, Simioni P, Tormene D, Beck DO, Luni S, Girolami A. Isolation and characterization of an antifactor V antibody causing activated protein C resistance from a patient with severe thrombotic manifestations. Blood. 2002;99:3985–92. doi: 10.1182/blood.v99.11.3985. [DOI] [PubMed] [Google Scholar]
- 59.Tadokoro K, Ohtoshi T, Takafuji S, Nakajima K, Suzuki S, Yamamoto K, Ito K, Miyamoto T, Muranaka M. Topical thrombin-induced IgE-mediated anaphylaxis: RAST analysis and skin test studies. J Allergy Clin Immunol. 1991;88:620–9. doi: 10.1016/0091-6749(91)90156-i. [DOI] [PubMed] [Google Scholar]
- 60.Wuthrich B, Bianchi-Kusch E, Johansson SG. Allergic urticaria and angioedema caused by a hemostatic sponge of bovine fibrin used in tooth extraction. Allergy. 1996;51:49–51. doi: 10.1111/j.1398-9995.1996.tb04549.x. [DOI] [PubMed] [Google Scholar]
- 61.Scheule AM, Beierlein W, Wendel HP, Jurmann MJ, Eckstein FS, Ziemer G. Aprotinin in fibrin tissue adhesives induces specific antibody response and increases antibody response of high-dose intravenous application. J Thorac Cardiovasc Surg. 1999;118:348–53. doi: 10.1016/S0022-5223(99)70226-6. [DOI] [PubMed] [Google Scholar]
- 62.Shirai T, Shimota H, Chida K, Sano S, Takeuchi Y, Yasueda H. Anaphylaxis to aprotinin in fibrin sealant. Intern Med. 2005;44:1088–9. doi: 10.2169/internalmedicine.44.1088. [DOI] [PubMed] [Google Scholar]
- 63.Schoenecker JG, Hauck RK, Mercer MC, Parker W, Lawson JH. Exposure to topical bovine thrombin during surgery elicits a response against the xenogeneic carbohydrate galactose alpha1–3galactose. J Clin Immunol. 2000;20:434–44. doi: 10.1023/a:1026455631876. [DOI] [PubMed] [Google Scholar]
- 64.Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, Valenta R, Spitzauer S. Recombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergy. J Immunol. 2002;168:4576–84. doi: 10.4049/jimmunol.168.9.4576. [DOI] [PubMed] [Google Scholar]
- 65.Faeste CK, Plassen C. Quantitative sandwich ELISA for the determination of fish in foods. J Immunol Methods. 2008;329:45–55. doi: 10.1016/j.jim.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Lindstrom CD, van Do T, Hordvik I, Endresen C, Elsayed S. Cloning of two distinct cDNAs encoding parvalbumin, the major allergen of Atlantic salmon (Salmo salar) Scand J Immunol. 1996;44:335–44. doi: 10.1046/j.1365-3083.1996.d01-314.x. [DOI] [PubMed] [Google Scholar]
- 67.Van Do T, Hordvik I, Endresen C, Elsayed S. Expression and analysis of recombinant salmon parvalbumin, the major allergen in Atlantic salmon (Salmo salar) Scand J Immunol. 1999;50:619–25. doi: 10.1046/j.1365-3083.1999.00637.x. [DOI] [PubMed] [Google Scholar]
- 68.Kroez M, Lang W, Dickneite G. Wound healing and degradation of the fibrin sealant Beriplast P following partial liver resection in rabbits. Wound Repair Regen. 2005;13:318–23. doi: 10.1111/j.1067-1927.2005.130315.x. [DOI] [PubMed] [Google Scholar]
- 69.Lawson JH. The clinical use and immunologic impact of thrombin in surgery. Semin Thromb Hemost. 2006;32(Suppl 1):98–110. doi: 10.1055/s-2006-939559. [DOI] [PubMed] [Google Scholar]
- 70.Reid T, Fuller E, Janmey P, Sawyer E, Fudge J, Mochmer K, Peat R, Seelbaugh J. Efficacy of hemostatic dressings with salmon thrombin and fibrinogen in a rat hip penetrating injury model. Blood. 2001;98:76B–76B. [Google Scholar]
- 71.Rothwell SW, Reid TJ, Dorsey J, Flournoy WS, Bodo M, Janmey PA, Sawyer E. A salmon thrombin-fibrin bandage controls arterial bleeding in a swine aortotomy model. J Trauma. 2005;59:143–9. doi: 10.1097/01.ta.0000171528.43746.53. [DOI] [PubMed] [Google Scholar]
- 72.Sieminski AL, Gooch KJ. Salmon fibrin supports an increased number of sprouts and decreased degradation while maintaining sprout length relative to human fibrin in an in vitro angiogenesis model. J Biomater Sci Polym Ed. 2004;15:237–42. doi: 10.1163/156856204322793610. [DOI] [PubMed] [Google Scholar]
- 73.Guthold M, Liu W, Sparks EA, Jawerth LM, Peng L, Falvo M, Superfine R, Hantgan RR, Lord ST. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys. 2007;49:165–81. doi: 10.1007/s12013-007-9001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Jawerth LM, Sparks EA, Falvo MR, Hantgan RR, Superfine R, Lord ST, Guthold M. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006;313:634. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collet JP, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci U S A. 2005;102:9133–7. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]