Abstract
Microtubule-associated proteins (MAPs) are involved in microtubule (MT) bundling and in crossbridges between MTs and other organelles. Previous studies have assigned the MT bundling function of MAPs to their MT-binding domain and its modulation by the projection domain. In the present work, we analyse the viscoelastic properties of MT suspensions in the presence or the absence of cAMP. The experimental data reveal the occurrence of interactions between MT polymers involving MAP2 and modulated by cAMP. Two distinct mechanisms of action of cAMP are identified, which involve on one hand the phosphorylation of MT proteins by the cAMP-dependent protein kinase A (PKA) bound to the end of the N-terminal projection of MAP2, and on the other hand the binding of cAMP to the RII subunit of the PKA affecting interactions between MTs in a phosphorylation-independent manner. These findings imply a role for the complex of PKA with the projection domain of MAP2 in MT-MT interactions and suggest that cAMP may influence directly the density and bundling of MT arrays in dendrites of neurons.
Keywords: microtubules, microtubule-associated protein 2, protein kinase A, cyclic AMP, interactions
INTRODUCTION
The structural Microtubule-Associated Proteins (MAPs) of neurons consist of two classes,34the HMW-MAPs (MAP1a,b and MAP2a,b,c,d) and the Tau family. In contrast with MAP1a,b in which the MT-binding domains are located in the N-terminal domain (Halpain and Dehmelt, 2006), MAP2 and Tau isoforms contain tubulin-binding motives in the C-terminal domain while the N-terminal domain of the molecules form a projection extending away from the MT wall (Gustke et al, 1994; Sanchez et al, 2000; Dehmelt and Halpain, 2004). Both domains of structural MAPs contain numerous phosphorylation sites and are substrates for a large number of protein kinases (Schneider et al, 1999; Chang et al, 2003; Sanchez et al, 2004; Trivedi et al, 2005), including the cAMP-dependent protein kinase PKA bound to the N-terminal domain of MAP2 (Vallee et al, 1981; Rubino et al, 1989; Obar et al, 1989), which was the first described member of the AKAP (A-kinase anchoring protein) family (Diviani D. and Scott J.D. 2001). MAPs accelerate the polymerization of tubulin dimers and induce the stabilization of the MT polymers (Hirokawa et al, 1988; Wiche, 1989; Wallis et al, 1993: Gustke et al, 1994). These functions are regulated by the phosphorylation level of MAPs (Murthy and Flavin, 1983; Burns et al, 1984; Itoh et al, 1997; Schneider et al, 1999). MAPs also determine the spacing between MTs and other cytoplasmic organelles in situ, and the MAP specific distance between sedimented MTs in vitro (Brown and Berlin, 1985; Black, 1987), supporting the concept that MAPs are sterically repulsive to other structures. However, MAPs mediate crossbridges between MTs and other subcellular structures in vitro (Leterrier et al, 1982; Linden et al, 1989; Leterrier et al, 1990; Severin et al, 1991) and in situ (Hirokawa et al, 1988; Cunningham et al, 1997; Farah et al, 2005). Since MAP2 and Tau molecules are bound to the MT wall through their C-terminal domain (Gustke et al, 1994; Sanchez et al, 2000; Al-Bassam et al, 2002; Dehmelt and Halpain, 2004), it is likely that the N-terminal domain of these proteins is responsible for spacing and interactions with other organelles. The tubulin-binding properties of MAPs interacting with MTs have been well characterized (Tokuraku et al, 1999; Trinczek et al, 1995; Sanchez et al, 2000). In contrast, little is known of the putative binding sites for other proteins present in the N-terminal domain of MAPs, beside the binding sequence for the regulatory subunit of PKA on MAP2 (Vallee et al, 1981; Rubino et al, 1989; Obar et al, 1989).
In the present work, we present evidence for MAPs-mediated weak interactions in vitro between MTs, resulting in a fragile gel. Reconstitution experiments demonstrate that MAP2 and not Tau mediates these interactions. The formation of a MT-MT network is regulated by cAMP in a conventional manner involving the phosphorylating activity of the PKA, but also by cAMP alone in the absence of ATP. These findings suggest that the projection domain of MAP2 is directly involved in interactions between MTs, and that this activity is regulated by the MAP2-bound PKA.
MATERIALS AND METHODS
Chemicals
PIPES (piperazine-N,N'-bis(2-ethanesulfonic acid)), MgCl2, ethylene glycol-bis (β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), ATP (vanadate free, Mg salt, from equine muscle), dithiotreitol, cyclic AMP, colchicine, paclitaxel from Taxus brevifolia (taxol), GTP (lithium salt), the protein kinase A (PKA) thermostable inhibitor (Walsh preparation) and protease inhibitors (N-p-Tosyl-L-Arginine methyl ester, aprotinin, pepstatin, leupeptin, phenylmethane sulfonylfluoride (PMSF), chloroquinine and soybean trypsin inhibitor) were from Sigma. All other compounds were from Merck. The antibody against the exchange protein directly activated by cAMP (EPAC) EPAC2 (Pab ab21237) was from Abcam (UK). Antibodies against RIIα,β PKA subunits (Mab PKA RIIa clone 40; Mab PKAβ RII clone 45) were from Becton Dickinson (UK). The polyclonal antibody against catalytic PKA β subunits (PKAβ cat (C-20)Sc-904) was obtained from Santa Cruz (Tebu SA France). Polyclonal antibodies against MAP2 and Tau were made by multiple subcutaneous immunisation of rabbits with 100 μg of the pure proteins in complete Freund adjuvant, followed by the same amount of proteins in incomplete adjuvant 4 weeks later. The specificity of the antibodies is shown in figure 3A. Secondary antibodies coupled to peroxidase were from Tebu S.A. (France). The chemiluminescent reagent ECL was from Amersham.
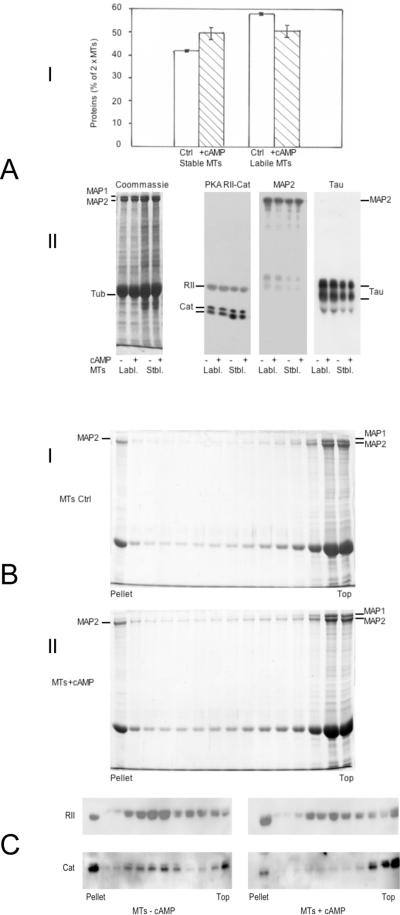
Figure 3.
A: Effect of 5 μM cAMP on the repartition of MT proteins during a polymerization cycle
1× MTs (6 mg/ml) in buffer B were repolymerized for 45 minutes at 35 °C in the absence or the presence of 5 μM cAMP (no ATP). MT pellets recovered by centrifugation 1 h at 48 000 × g at 35 °C were resuspended at 4 °C in 1/5th of initial volume of buffer B +/− 5 μM cAMP for depolymerization. After centrifugation at 100 000 × g for 40 minutes, the total amount of proteins in supernatants (2× cold-labile MTs) and pellets (2× cold-stable MTs) were measured.
I: The repartition of MT proteins into cold-stable and cold-labile fractions after a second polymerization cycle is expressed as the % of total initial amount of MTs 1×. The mean values +/− s.d. of two separate experiments are shown.
II: 7.5% acrylamide SDS-PAGE and immunoblotting of identical amounts of cold-labile (7.5 μg) and cold-stable (12 μg) MTs obtained from the second polymerization cycle +/− cAMP. The samples were probed successively with antibodies against the RII and catalytic subunits of MAP2-bound PKA, and antibodies against MAP2, and Tau. Left panel: Coommassie blue staining of the same MT proteins (40 μg).
B: Selective aggregation of MAP2-containing MTs during MT gelation
2× MTs (3 mg/ml) in buffer B containing 7.5% sucrose were mixed at 4 °C +/− 5 μM cAMP and incubated at 35 °C on top of a linear 15%–60% sucrose gradient in the same buffer. After 2 h, the gradients tubes were centrifuged for 30 minutes at 35 000 × g and 35 °C, 0.8 ml fractions were collected and 25 μl aliquots were analyzed on 7.5% acrylamide SDS-PAGE, stained with Coomassie blue. I: Control MTs: 36.6% of total MT proteins enter the gradient (pellet + fractions 1–9 / total MTs loaded). II: MTs + 5 μM cAMP: 44.2% of total MT proteins enter the gradient (pellet + fractions 1–9 / total MTs loaded).
C: cAMP induces the segregation of RII and Catalytic subunits of the PKA on distinct MT species during MT gelation
2× MTs (4 mg/ml) in buffer B containing 7.5% sucrose were incubated +/− cAMP at 35°C and centrifuged on top of a linear 15%–60% sucrose gradient in the same conditions as in figure 3B. 25 μl aliquots of all fractions were analyzed by SDS-PAGE (7.5% acrylamide) and immunoblotting for the detection of the PKA subunits by specific antibodies.
Methods
MT were obtained from rat forebrain by the procedure of Shelanski et al (1973). Tissues were homogenized in buffer A: PIPES 0.08 M, MgCl2 1 mM, EGTA 1 mM, dithiotreitol 2 mM, pH 6.8, containing 1 mM GTP and protease inhibitors (0.1 mg / ml N-p-Tosyl-L-Arginine methyl ester, 0.05 U / ml aprotinin, 1 μM pepstatin, 1 μM leupeptin, 1 mM phenylmethane sulfonylfluoride, 0.1 mM chloroquine, 10 nM soybean trypsin inhibitor and 0.1 mM Nα-p-Tosyl-L-Lysine chloro-methyl ketone). MT pellets were dissociated in buffer A, frozen in liquid N2 and stored at −80 °C.
Tubulin and MAPs were separated from each other by chromatography on phosphocellulose (PC) of 3× polymerized MTs in buffer A (Weingarten et al, 1975). Pure tubulin samples were immediately frozen for storage in liquid nitrogen. An alternative tubulin purification procedure was made according to Castoldi and Popov (2003), which yields highly concentrated protein of much higher polymerization efficiency than PC tubulin (Castoldi and Popov, 2003).
MAPs were recovered from the PC by successive elution with 0.35 M NaCl (MAP2 + Tau) and 1 M NaCl (MAP1 + MAP2) in buffer A. MAP fractions were concentrated by ammonium sulfate precipitation and dialysis against buffer A. Heat-stable MAP2 and Tau were obtained according to Fellous et al (1977) by heat-denaturation for 10 minutes at 100 °C in buffer A + 10mM dithiotreitol of either 2× MTs or the purified (native) MAP2 and Tau, followed by centrifugation for 30 min at 100 000 × g, concentration of the heat-soluble proteins by ammonium sulfate precipitation and dialysis against buffer A. Both native and thermostable MAP2 and Tau were further separated by chromatography on sepharose (ultrogel ACA34, LKB Pharmacia) in buffer A, concentrated again by ammonium sulfate precipitation and dialysis against buffer A + 3% glycerol for storage in liquid N2.
Viscosity measurements of MTs in buffer B (buffer A + 3 mM MgCl2, 1 mM GTP, 0.4 M sucrose, protease inhibitors) were performed according to McLean-Fletcher and Pollard (1980) as previously reported (Eyer et al, 1989) using 75 μl capillaries (Drummond) and 0.7 mm diameter stainless steel ball (Marteau et Lemarié, Paris, Fr.). Samples (100 μl) were prepared at 4 °C. The velocity of the falling ball was measured at 35 °C in a water- jacketed chamber over a distance of 5 cm at an angle of 45 ° or 80 ° to the horizontal. The viscosity of MT suspensions was expressed in Pascal.seconds by comparison with the velocity of the ball in glycerol solutions of known concentrations. Elasticity measurements were made by small strain (<2%) oscillatory deformation of MT samples (1ml) at 10 rads/s during the course of the gelation using a Rheometrics (Piscataway NJ) RFSII instrument (Janmey et al, 1994). The shear storage modulus, G', a measure of the elastic resistance of the material, was derived from these measurements by a method reported elsewhere (Leterrier et al, 1996).
Video-enhanced microscopy was performed according to Kurachi et al. (1999) using a DIC microscope (Carl Zeiss, Axiovert 135 TV, Germany) equipped with a Plan Apochromat 63× immersion objective lens (numerical aperture = 1.4) and 4× zoom lens, an oil-immersion condenser lens for high-magnification objectives. MT samples were introduced in slide-coverslip chambers made by double side tape, further sealed by vaselin-lanolin-paraffin in the ratio 1/1/1. Samples were incubated at 36 °C for several hours in a temperature-controlled incubation box surrounding the microscope stage. The organisation of MT suspensions at increasing incubation times was recorded with a Newvicon camera (Hamamatsu, C2400–07, Hamamatsu, Japan) and the contrast of images was enhanced and averaged over several (4 or 8) frames in real time by an image processor (Hamamatsu, ARGUS-10, Hamamatsu, Japan).
Protein measurements were conducted according to Lowry et al (1951), using bovine serum albumin as a standard. Proteins were resolved by 7.5% acrylamide SDS-PAGE according to Laemmli (1970). Immunoblotting were made according to Towbin et al (1979), and the detection of peroxidase-coupled secondary antibodies was done by chemiluminescence with ECL (Amersham).
RESULTS
I-Evidence for weak interactions between MTs
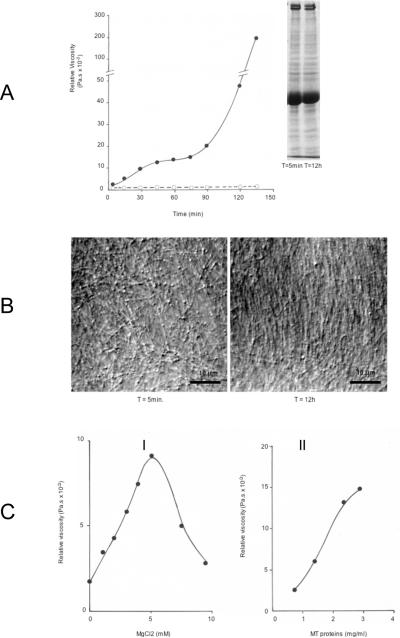
The polymerization of MAPs-saturated MTs at concentrations above 1mg/ml occurs routinely within 20–30 minutes at 37 °C in the presence of 1 mM GTP and is completed within 1 h. The measurement of viscosity in polymerizing MTs (4 mg/ml in figure 1A) shows a mild initial increase with no major change for the first 60 minutes followed by a rapid increase toward a gel state which is reached after several hours (not shown). The initial phase of mild viscosity change likely corresponds to the polymerization of MT proteins into polymers. The delayed increase in viscosity, unrelated to the MT polymerization process already terminated, suggests instead distinct biophysical events involving polymers in suspension.
Figure 1.
A: Time-dependent changes in the viscosity of MT suspensions
The viscosity of 4 mg/ml MTs incubated at 35 °C (closed symbols) rose during the polymerization step and remained at a low constant level during the first hour, before increasing again, reaching a gel state after several hours with protein concentrations higher than 2mg/ml. Gels remained stable until broken by shearing at 35 °C or depolymerization by cold. No viscosity change occurred in samples containing 5 μM colchicin (open symbols). Values are the mean of duplicate assays.
Inset: 7.5% acrylamide SDS-PAGE analysis of 50 μg MT proteins taken after 5 minutes and 12h incubation at 35 °C (Coomassie blue staining), showing no alteration of the pattern of MT proteins during long incubation time in the presence of protease inhibitors.
B: Progressive formation of MT bundles with incubation time
AVEC-DIC videomicroscopy of 3mg/ml MT suspensions in buffer B, sealed in slide-coverslip incubation chambers. Images were taken within the first 5–10 minutes and after 12 h incubation at 35 °C in the humidity- and temperature-controlled microscope chamber. MTs observed after 5 min. at 10 μm from the glass surface are independent from each other and freely moving with Brownian motion. The same sample after 12 h contained large arrays of immobilized parallel MTs in bundles throughout the whole sample space (image taken at 20 μm from the glass surface).
C: Dependance of MT viscosity on Mg ions and protein concentration
I: Viscosity changes in 3 mg/ml MT suspensions after 2 h at 35 °C in the presence of increasing concentrations of MgCl2. The sample with no MgCl2 was treated with 4 mM EDTA to quench the 3 mM MgCl2 present in buffer B. Values are the mean of duplicate assays.
II: Viscosity change after 2 h at 35 °C in suspensions of increasing MT concentrations in buffer B. Values are the mean of duplicate assays.
The observation of the MT samples by DIC videomicroscopy shows that MTs are independent from each other and freely moving with Brownian motion during the initial phase of polymerization, which results in the random orientation of the polymers (T = 5min, figure 1B). The observation of the same sample after several hours incubation at 37 °C reveals that MTs are organized into large bundles of semi-parallel polymers and do not move observably with Brownian motion (T = 12h, figure 1B). This bundle organization of immobilized MTs after long incubation time is found throughout the whole sample (not shown). This structural change of MT suspensions could result from liquid crystalline ordering of the rigid MTs which can occur at such a high concentration. Alternatively, the possibility that a MT network is formed through interactions between MT polymers of the suspension, favoring their alignment in semi-parallel arrays, is also suggested from the immobilization of MTs within bundles.
The gelation of MT samples requires higher Mg++ concentrations than that needed for their polymerization (1 mM MgCl2), at which little viscosity change is recorded during the gelation kinetic, but a linear increase in viscosity of the sample, measured after 2h, is obtained between 1 and 5 mM MgCl2 (figure 1C-I). Higher MgCl2 concentrations inhibit the MT gelation process (figure 1C-I) by a polymerization-independent mechanism since high Mg++ stimulates efficiently tubulin polymerization (Lee and Timasheff, 1975). Accordingly, all further studies were performed using 3 mM MgCl2 which allows a linear increase of MT gelation with time. Under these conditions, the viscosity change with time in MT suspensions is directly proportional to the concentration of proteins, with a critical concentration close to 1 mg/ml MT proteins (figure 1C-II).
The requirement of Mg ions at a concentration range higher than that necessary for tubulin polymerization suggests that other biochemical / biophysical mechanisms are responsible for the gelation process.
2-Rheological properties of MT suspensions. Effect of activation of the MAP2-bound PKA by cAMP + ATP
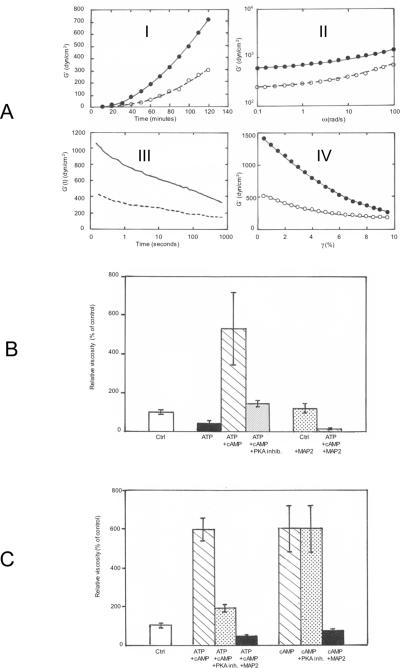
To characterize the physical properties of MT gels, 2× MT (6 mg/ml) in buffer B (containing 3 mM MgCl2) were incubated in a strain-controlled rheometer at 35 °C and several conditions were applied to the MT suspensions for the study of their mechanical resistance (figure 2A). Since the phosphorylation of MAPs by the MAP2-bound PKA affects their binding to tubulin and their acceleration of tubulin polymerization (Murphy and Flavin, 1983; Burns et al, 1984; Itoh et al, 1997), rheological studies were performed in the absence or the presence of 0.1 mM ATP 5 +/− μM cAMP to induce the complete activation of the enzyme (figure 2A). The recording of the shear storage (elastic) modulus (G') with incubation time shows that significant values appear only after 30 minutes when MT polymerization, as judged e.g. by light scattering, is already complete (figure 2A-I). Full activation of PKA stimulates the gelation kinetic by ≈ 2.5 (figure 2A). The shear storage modulus of the MT gels already formed after 2 h, measured by small strain oscillatory deformations, is only weakly sensitive to oscillation frequency and is significantly larger than the loss modulus (G"), characterizing a true viscoelastic gel (figure 2A-II). The gel is weak, since a constant strain applied to the sample is followed by the progressive decrease in shear stress, quantified by the elastic modulus G during the time after the strain, indicative of the relaxation of the gel in response to its deformation (figure 2A-III). Similarly, the application of increasing strains to the MT gel does not provoke the strain-hardening observed for gels made of semiflexible polymers (Leterrier et al, 1996; Storm et al, 2005), but results instead in its rupture, illustrative of a highly fragile structure of the MT network (figure 2A-IV).
Figure 2.
A: Rheological study of MT gelation. Stimulation by cAMP + ATP
2× MTs (6 mg/ml) in buffer B, were incubated in a strain-controlled rheometer at 35 °C in the presence (closed symbols and plain line) or the absence (open symbols and dotted line) of 5 μM cAMP and 100 μM ATP.
I: Shear storage modulus (G') of MT suspensions during the polymerization initiated by bringing the sample from 4 °C in ice to 35 °C in the rheometer. The values of G' increases progressively after 30 minutes in both samples.
II: Mild increase in G' measured in the fully polymerized samples (after 2 h incubation at 35 °C for samples in I) at increasing oscillation frequencies of the rheometer, indicative of the gel state of the suspension.
III: MT gels were submitted to a small strain (2%) at T = 0 (same samples as in II). The static shear modulus G' was recorded during the relaxation of samples following the constant strain. The time dependent linear decrease in G shows that MT gels are fragile and reorganize after the application of a mild strain.
IV: G' measured in MT gels submitted to increasing strain does not exhibit strain-hardening. The strain-dependent lower elasticity suggests instead that the gel breaks easily.
B: Regulation of MT viscosity by the phosphorylation of MAPs via the cAMP-dependent MAP2-bound PKA
Samples of 2× MTs (6 mg/ml) in buffer B were mixed at 4 °C with or without 100 μM ATP +/− 5 μM cAMP, in combination or not with 0.25 μM PKA thermostable inhibitor or 50 μg thermostable MAP2, as indicated. The viscosity of samples was measured on triplicate samples after 12 h incubation at 35 °C in sealed capillaries. Average values +/− s.d. are expressed as the percentage of control MTs (no addition).
C: Comparison of the effects on MT gelation of the activation of the MAP2-bound PKA activity by cAMP with that of cAMP alone in the absence of ATP
Samples of 2× MTs (6 mg/ml) in buffer B were mixed at 4 °C with 100 μM ATP + 5 μM cAMP, in combination or not with 0.25 μM PKA thermostable inhibitor or 50 μg thermostable MAP2, as indicated. In a parallel set of assays, the same conditions were adopted but without adding ATP. The viscosity of samples was measured on triplicate samples after 12 h incubation at 35 °C in sealed capillaries. Average values +/− s.d. are expressed as percentage of control MTs (no addition).
3-MT gelation is activated by cAMP-dependent phosphorylation of MT proteins and by a cAMP-dependent, phosphorylation-independent mechanism
The findings of figure 2A suggest that the phosphorylation of MAP2 and Tau by the MAP2-bound PKA affects directly MT-MT interactions. Further evidence is obtained by measuring the viscosity of MTs after several hours at 35 °C (when gelation is complete) in the presence of ATP +/– cAMP and the presence or the absence of a specific inhibitor of the catalytic subunit of the PKA (figure 2B). ATP alone induces a significant reduction of MT viscosity which is instead strongly activated by cAMP + ATP (figure 2B). This later effect is markedly reduced when a saturating amount of the heat stable PKA inhibitor (Walsh inhibitor) is present (figure 2B). These results suggest that the phosphorylation of the main substrates of PKA (MAP2 and Tau) activates MT gelation, while their phosphorylation by MT-associated kinases other than the PKA (the only active kinases in the absence of cAMP) (Sanchez et al, 2000) induces an opposit effect (inhibition) on the basal level of MT gelation.
The phosphorylation of MAPs by the cAMP-dependent MAP2-bound PKA is not the only pathway by which cAMP affects MT gelation. Comparison of MT gels formed after several hours at 35°C (same conditions as for figure 2B) in the presence of either cAMP+ATP or cAMP alone (no ATP added) reveals that cAMP alone induces a strong activation of gelation unaffected by the inhibitor of the PKA catalytic subunit in the absence of ATP (GTP is not a substrate for the enzyme and the Walsh inhibitor binds exclusively to the complex ATP-catalytic subunit (Whitehouse and Walsh, 1983)) (figure 2C). Consequently, the activation of MT gelation by cAMP alone likely involves other mechanisms than the phosphorylation of MAPs. Thermostable MAPs (devoid of bound PKA) inhibit similarly the stimulation of MT gelation by cAMP in the presence and the absence of ATP (figures 2B and 2C).
The presence of cAMP (alone) during a temperature-dependent polymerization cycle of MTs increases by ≈ 10% the relative amount of MT proteins that are not dissociated by incubation at 4 °C after polymerization at 35 °C (figure 3A-I). This observation suggests that the binding of cAMP to the MAP2-bound PKA enhances conformational changes of MAPs resulting in their lower solubility. When these fractions were analyzed by SDS-PAGE and immunoblotting for the presence of the PKA subunits, no cAMP-dependent change was found in the relative amount of the regulatory subunit RII or the catalytic subunit C in either cold-stable or cold-labile MT fractions (figure 3A-II). This demonstrates that the binding of cAMP to the MAP2-bound PKA does not induce the solubilization of either the C or the RII subunits, both subunits remaining bound to the MT structure after dissociation of the kinase by cAMP.
The preferential participation of MAP2 in MT-MT complexes is reported in figure 3B. 2× MTs were layered on top of dense (15–60%) sucrose gradients and further incubated under conditions favoring their polymerization and gelation in the presence or the absence of cAMP (no ATP), before sedimentation for the separation of MT complexes from single MTs (figure 3B). Fractionation of the gradients and analysis by SDS-PAGE of the fractions reveals that MTs containing only MAP2 and not MAP1 were found along the gradient and as a pellet. The MTs on top of the gradient contained all MAP1 and the remaining MAP2. The presence of cAMP enhances mildly the relative amount of dense MAP2-MTs complexes: the quantification of MT proteins shows that 36.6% (−cAMP) and 44.2% (+cAMP) of total MT proteins sediment in the sucrose gradient (figure 3B-II). These findings show that MAP2 is enriched in a subpopulation of polymerized MTs sedimenting at high sucrose densities, i.e. which are associated together as multi-polymer complexes. The analysis of the behaviour of the PKA subunits in the fractions of sucrose gradients of MTs +/− cAMP (figure 3C) reveals that the RII subunits are concentrated in heavy MT fractions penetrating the gradient. Adding cAMP during preincubation of MTs before centrifugation increases slightly the amount of RII associated to the pellet (figure 3C). In contrast, the catalytic subunits of the PKA, which are preferentially associated to heavy MT fractions in the control sample (no cAMP added) are mainly found in the light MT fractions of the gradient when cAMP is present (figure 3C). These data show that the dissociation of the PKA by cAMP results also in the partial segregation of both subunits between single MTs and MT complexes separated by sedimentation on sucrose gradient.
4-Direct contribution of MAP2 and not of Tau to interactions between MTs
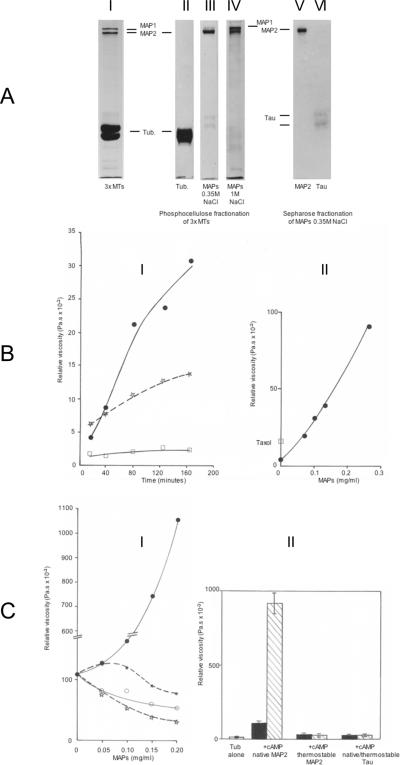
The requirement of MAP2 for MT gelation was further explored in reconstitution experiments, using pure tubulin and various MAPs fractions shown in figure 4A.
Figure 4.
A: SDS-PAGE of MT proteins
I: 3 × MTs (30 μg). II–IV: Separation of MT proteins by chromatography on phosphocellulose. II: pure tubulin (30 μg) eluted in the void volume. III: MAP2 + Tau (15 μg) eluted by 0.35M NaCl in buffer A. IV: MAP1 and MAP2 + proteins of low MW (20 μg) eluted by 1M NaCl in buffer A. V,VI: MAP2 and Tau separated by chromatography on ultrogel ACA 34 in buffer A. V: MAP2 (10 μg). VI: Tau (2.5 μg). Identical patterns in SDS-PAGE of heat-stable MAP2 and Tau were obtained (not shown).
B: MT gelation requires MAPs
I: Time-dependent viscosity changes at 35 °C of pure PC tubulin (6.4 mg/ml) in buffer B, incubated at 35 °C alone (open squares) or in the presence of either 0.14 mg/ml of the MAP fraction eluted from the PC column by 0.35M NaCl (MAP2 + Tau) (closed circles) or 15 μM taxol (dotted line, open stars). Values are the mean of duplicate assays.
II: Viscosity measured after 2 h at 35 °C of pure PC tubulin (6.4 mg/ml) incubated in the presence of increasing concentrations of the MAP fraction eluted from the PC column by 1M NaCl (MAP1 + MAP2). The value obtained for the same tubulin sample incubated for 2 h in the presence of 15 μM taxol is shown (open square). Values are the mean of duplicate assays.
C: Pure tubulin requires native MAP2 for the induction of a cAMP-dependent stimulated gelation
I: The viscosity of pure tubulin (4.9 mg/ml) purified according to (Castoldi and Popov, 2003), was measured after incubation for 30 min at 35 °C in buffer B, in the presence of increasing concentrations of native MAP2 (closed circles), thermostable MAP2 (dotted line, closed stars), native Tau (open circles), or thermostable Tau (dotted line, open stars). Values are the mean of duplicate assays.
II: Viscosity of pure tubulin (3.7 mg/ml) incubated for 30 min at 35 °C alone or in the presence of native MAP2 (0.15 mg/ml), thermostable MAP2 (0.15 mg/ml), or native or thermostable Tau (0.15 mg/ml). Samples contained 5 μM cAMP (hatched columns) or not (black columns). Assays were done in triplicate and values are shown +/− s.d.
The viscosity of PC tubulin samples in buffer B was measured during incubation time alone, with taxol or in the presence of the MAP fraction eluted form PC by 0.35 M NaCl (figure 4B-I). A weak increase in viscosity is obtained when the polymerization of tubulin is enhanced by taxol, reaching a plateau with longer incubation times (not shown), while the sample of tubulin alone remains fluid (figure 4B-I). The presence of MAPs induces a net initial rise in viscosity which keeps increasing for hours (figure 4B-I). This effect is linearly proportional to the concentration of MAPs (figure 4B-II).
Since the 0.35 M NaCl MAPs purified from the PC column contain MAP2 and Tau (figure 4A-III), the respective contribution of each protein to the gelation of tubulin was investigated, using either native or heat-treated MAP2 and Tau (figure 4A-V,VI). The pure tubulin used in this experiment was obtained according to a procedure selected for its high polymerization capacity as compared to PC tubulin (Castoldi and Popov, 2003). The addition of increasing amounts of native MAP2 (unheated) produces a strong increase in the viscosity of the sample measured after 30 minutes (figure 4C-I, plain line and filled circles), in contrast with the lack of viscosity change observed in the presence of heat-treated MAP2 (figure 4C-I, plain line and open circles). Adding either native Tau (figure 4C-I, filled stars and dotted line) or heat-treated Tau (figure 4C-I, open stars and dotted line) did not result in any significant increase in the viscosity of the suspension but induced instead, like heat-treated MAP2, a concentration-dependent inhibition of the basal viscosity of the tubulin sample. Furthermore, the stimulation of the MT gelation kinetic by native MAP2 is enhanced strongly by the addition of cAMP alone (no ATP) (figure 4C-II).
DISCUSSION
The present results bring new insights into the mechanisms of MAPs-mediated MT bundling. The process of MT bundling in cells has been analyzed in studies showing the direct contribution of both the HMW-MAP2 (MAP2a,b) and the LMW MAP2 (MAP2c,d) species (Umeyama et al, 1993; Leclerc et al, 1996), as well as Tau (Kanai et al, 1992; Brandt and Lee, 1993) to the induction of cytoplasmic processes filled with thick MT bundles. Most studies revealed an opposite contribution of the MT-binding domains and the projection domains of MAPs to the formation of MT bundles: from constructs including various parts of the molecules, it appears that the strongest MT bundling activity resides in the MT-binding domains of MAPs, which is either modulated or inhibited by the projection domain of MAP2 (Belanger et al, 2002),Tau (Gustke et al, 1994) or MAP4 (Iida et al, 2002). Alternatively, the projection domain of MAPs is thought to be responsible for a repulsion between adjacent MTs, as it has been established that the spacing between MTs within bundles depends on the type of MAPs involved in vivo (Chen et al, 1992) and in vitro (Brown and Berlin, 1985; Black, 1987). Studies in vitro failed to demonstrate that intact MAPs mediate strong interactions between MTs (Brandt and Lee, 1993; Iida et al, 2002), in contrast with truncated MAPs constructs restricted to the MT-binding domain which induce MT bundling (Brandt and Lee, 2003; Gustke et al, 1994).
Only few studies exist of the rheology of MT suspensions (Buxbaum et al, 1987; Friden et al, 1988; Sato et al, 1988), and these point to a lack of strong interactions between MTs containing native MAPs. However, these studies revealed that unexpected behavior of MTs suspensions could be explained by weak interactions between polymers (Sato et al, 1988), and that the viscosity of MT samples is significantly lowered by tryptic digestion of the MAPs projections (Friden et al, 1988). Our data confirm and expand these findings by the systematic analysis of the viscosity and viscoelasticity of MT suspensions: slowly-forming elastic gels of MTs at high protein concentrations follow a kinetic initiated after the polymerization of the MT proteins. No or very little gelation occurs at the concentration of MgCl2 required for the polymerization of tubulin subunits. Instead, MT gelation requires a significantly higher amount of Mg ions between 2 and 5 mM, which is reversed at concentrations above 5 mM (figure 1C). This effect of Mg ions on MT gelation could be related to the Mg-dependent conformational changes of MAP2 antiparallel dimers previously described (Wille et al, 1992a), or to the effects of divalent cations on anionic polyelectrolytes, as has been seen for actin and neurofilament suspensions (Tang et al, 1997: Rammensee et al, 2007). Rheological analysis revealed that MT gels break easily under strain (figure 2A), suggesting that the interactions between MTs in vitro are much weaker than those found with other types of gels such as actin or neurofilament gels (Storm et al, 2005; Janmey et al, 2007; Wagner et al, 2007). The direct participation of MAPs in the MT gelation process has been established by several approaches: No gel is ever formed in highly concentrated tubulin solutions in the presence of taxol (fig 4B-I) but the gelation of tubulin solutions depends upon the addition of either a mixture of MAPs or of purified native MAP2 (figs 4B,C). Further evidence for the direct involvement of MAP2 in MT gelation is obtained by the finding that interactions between MTs are stimulated by cAMP (figures 2, 4C-II), since MAP2 is the only MAP that is regularly associated with the cAMP receptor of the PKA through a specific binding site located at the end of the N-terminal projection (Vallee et al, 1981; Rubino et al, 1989; Obar et al, 1989).
These data raise however several unsolved questions:
The effect of cAMP on MT gelation is a new and intriguing finding, because two distinct situations exist:
1-If ATP is present, the addition of cAMP induces the phosphorylation of MT proteins (mainly Tau and MAP2) by the MAP2-bound PKA (Jameson et al, 1980; Gustke et al, 1994; Litersky et al, 1996; Schneider et al, 1999; Sanchez et al, 2000). The possibility of a direct involvement of the phosphorylating activity of the PKA catalytic subunit in modulating MT gelation is supported by the fact that the stimulation by cAMP+ATP of gelation is strongly reduced by the thermostable PKA inhibitor (fig 2B,C). This could suggest that the function of the MT proteins (likely MAPs) involved in the mechanism of interactions between MTs is regulated by their cAMP-dependent phosphorylation.
2-However, we observed simultaneously that cAMP can be as effective alone, when no phosphorylation of MAPs takes place (GTP does not substitute for ATP as a substrate for PKA) (fig 2C). The PKA inhibitor has no effect under these conditions (fig 2C), as expected from the lack of binding of the inhibitor peptide to the catalytic subunit unbound to ATP (Withehouse and Walsh, 1983). This later situation raises the intriguing question of how cAMP could stimulate MT gelation without involving the phosphorylating activity of the catalytic subunit of the PKA.
A first possibility was that cAMP could affect the MT network through another class of cAMP-binding proteins than the PKA R subunits, the exchange proteins directly activated by cAMP (EPACs) (Kawasaki et al, 1998; Boos, 2003). EPACs have been identified recently and contain both cAMP-binding and guanine nucleotide exchange factor domains (Bos, 2003). Direct interactions between MT and EPAC1 involve the light chains of MAP1a (Magiera et al, 2004; Gupta et al, 2005; Borland et al, 2006), and lead to activation of Rap1 GTPase activity by EPAC (Kawasaki et al, 1998). Immunoblotting attempts to identify EPAC proteins failed to reveal these molecules in 2× polymerization cycles purified MTs (not shown). Thus, the effect of cAMP in the absence and the presence of ATP is likely mediated by the RII subunits of the MAP2-bound PKA.
In light of the stimulation of MT gelation by cAMP alone, it is not clear why the strong stimulation of MT gelation by cAMP should not occur also in the presence of both ATP and the PKA inhibitor (figure 2B,C), since it does not require the phosphorylating activity of the catalytic subunit of the PKA (figure 2C). The main difference between both situations is the presence of ATP: when adding ATP alone to MTs, the catalytic subunit of PKA is inactive in the absence of cAMP. Instead, the only active kinases are MT-bound kinases other than the PKA (Sanchez et al, 2000) which phosphorylate different sites of MAP2 than the PKA of which the targets are majoritarly sites of the projection domain (Itoh et al, 1997). This results in a significant inhibition of MT gelation (figure 2B). When both cAMP, ATP and the PKA inhibitor are present, the inhibition of MT gelation activity in the presence of ATP alone due to other MT-bound kinases oppose the stimulatory effect of cAMP on the PKA of which the catalytic subunit is inactive (bound to the inhibitor), resulting in a lower gelation than with cAMP alone (no phosphorylation at all) or cAMP+ATP in which the PKA is fully active.
The ratio of MAP2-bound PKA molecules to MAP2 in MTs purified by cycles of polymerization-depolymerization is close to 1/40 (Theurkauf and Vallee, 1982). This projection of the molecule (Luo et al, 1990; Newlon et al, 2001). The binding site for all AKAPs (including MAP2) on PKA is formed by the dimerization of RII subunits through their N-terminal domain, generating the AKAP-binding site in an X-type four helix bundle which binds only one AKAP per RII dimer (Newlon et al, 2001). A first possible interpretation of the cAMP-stimulated gelation of MT suspensions in the absence of ATPwas that the relatively rare PKA molecules bound to MAP2 could become able, under cAMP binding, of linking an additional MAP2 free of bound PKA and belonging to another MT, thus mediating MT-MT interactions. This hypothesis seems unlikely since only one binding site for MAP2 exists on the X-type four helix bundle domain of the RII dimer (Newlon et al, 2001). Also, the occurence of interactions between MTs independent of cAMP (figs 1, 2A, 4BC), suggest that they cannot be attributed exclusively to the MAP2-bound PKA.
The analysis of MT gelation with pure tubulin and purified MAPs demonstrated that MAP2 alone and not Tau is able to induce MT gelation, in a cAMP-activated manner (fig 4C). The large extent of stimulation of MT gelation by cAMP using pure native MAP2 and tubulin may result from the absence of the other MAP species (Tau, MAP1) in the assay. Tau was found not to stimulate and may even inhibit the MT gelation activity (figure 4C) and MAP1, which could also contribute to MT bundling (figure 4B-II) is not associated with PKA and thus may contribute to a higher basal gelation level of 2× MTs in the absence of cAMP. Our experiments demonstrate unambiguously that only the native form of MAP2 is able to mediate the formation of MT networks with pure tubulin (fig 4C). The thermostable MAP2 preparation obtained by thermal denaturation of MT proteins is inactive on the gelation of pure tubulin MTs (fig 4C) and inhibits efficiently the gelation of 2× MTs containing all MAPs (figs 2B,C). This opposite behaviour of native and thermostable MAP2 molecules indicates for the first time that the presumed unstructured MAP2 molecule (Hernandez et al, 1986; Mukhopadhyay and Hoh, 2001) may nevertheless contain folded domains that are lost after thermal denaturation and are required for efficient interactions between MTs, while both native and thermostable MAP2 are similarly efficient in promoting tubulin polymerization (Hernandez et al, 1986). Native and heat-treated MAP2 have been compared and shown very similar (Hernandez et al, 1986). However, examination of the data from this report shows that the fluorescence emission spectra of native MAP2, heat-treated MAP2 and MAP2 in 6M guanidine hydrochloride differ significantly (Hernandez et al, 1986), thus suggesting subtle structural differences between the MAP2 molecules before and after heating. In addition, the notion of a disorganized structure of MAP2 is inconsistent with the fact that MAP2 adopts a linear semi-rigid rod-like aspect in electron microscopy (Voter and Erikson, 1982; Wille et al, 1992a,b), which is instead in agreement with the existence of a repulsive force exerted by MAPs in packed MTs, resulting in MAP-specific distances between polymers (Brown and Berlin, 1985; Black, 1987; Kindler et al, 1990; Chen et al, 1992). Altogether, there are experimental indications pointing at the possibility of some degree of structure in the projection domain of MAP2.
A functional model of the MT gelation process supposes that MAP2 itself, and not the MAP2-bound PKA, mediates interactions between polymers. This hypothesis is consistent with the experimental data demonstrating that MAP2 molecules self-associate in vitro in an anti-parallel manner (Wille et al, 1992a,b). Antiparallel dimers are formed by the 36 kDa MT-binding fragment of MAP2 alone and by both the large (MAP2a,b) and the short (MAP2c) MAP2 molecules (Wille et al, 1992a,b). The evidence that antiparallel dimers of all MAP2(a,b,c) isoforms consist of molecules tightly associated along their entire length, in register, staggered or associated into multimolecular fibers (Wille et al, 1992a,b) suggest that domains of MAP2 other than the MT-binding segment contain also binding sites for another MAP2 in antiparallel fashion.
Based on these data and the present observations, we propose that (weak) antiparallel interactions between MAP2 molecules borne by separate MTs are the effectors of MT gelation. The present findings raise further the hypothesis of Mg ions- and temperature-dependent conformational states of the projection involved in mediating anti-parallel interactions between MAP2 molecules. Our results suggest further that such putative conformational states of MAP2 could be enhanced by the binding of cAMP to the RII dimer of the MAP2-bound PKA in the absence of ATP, while in the presence of ATP the phosphorylation of the MAP2 projection domain by the bound PKA activated by cAMP may result in a similar conformational change favoring inter-MTs interactions. The observed effects of cAMP alone on MT gelation implies that a stable complex exists between the RII dimer and MAP2, independently of the binding of cAMP to RII. This was shown earlier by Theurkauf and Vallee (1982) who found that the RII subunit of the MAP2-bound PKA remains strongly associated with MAP2 after their binding of cAMP. Our study indicates further that both the RII and the catalytic subunits of the MAP2-bound PKA remain associated with the MT proteins during a full cycle of polymerization-depolymerization in the presence of cAMP (figure 3A-II), which is suggestive of MAP2-specific substrate-PKA interactions similar to those analyzed by Vigil et al (2004). However, the PKA subunits do not distribute systematically together within the same MAP2 molecules in MTs during gelation in the presence of cAMP. The figure 3C show that although RII subunits are found mainly associated with MAP2-enriched MT complexes sedimenting in the sucrose gradient in a fashion that is mildly affected by the presence of cAMP, the catalytic subunits exhibit a significant shift between heavy MT complexes and the single MTs remaining on top of the gradient consecutively to the binding of cAMP to the PKA (figure 3C). The preferential segregation of RII subunits in heavy MT complexes suggests that the RII-bound MAP2 molecules are directly involved in the cAMP-stimulated MT gelation. On the contrary, the catalytic subunits, which exhibit a distribution pattern in MTs along the sucrose gradient similar to that of the RII subunits in the absence of cAMP (a consequence of their tight binding to the RII subunits in the absence of cAMP), are excluded in the presence of cAMP from the heavy MT complexes and thus may preferentially bind to single MTs sedimenting on the top of gradients (figure 3C). Such a segregation may involve the preferential binding of the free catalytic subunit to its protein substrates (MAP2 and Tau) characterized by specific structural properties such as their phosphorylation level. In contrast with the binding site of the RII subunits to the N-terminal domain of MAP2 described since several years (Vallee et al, 1981; Rubino et al, 1989; Obar et al, 1989), the putative binding sites of the free catalytic subunits to MT proteins have not been investigated previously. In addition, our data suggest also that the stochiometry between the RII and the catalytic subunits may not be systematically the presumed ratio RII2-Cat2 on MAP2 molecules of MT preparations (Luo et al, 1990; Newlon et al, 2001), a possibility that is suggested by the comparison between the staining patterns of both subunits of the PKA in different MT preparations (figure 3A-II and figure 3C). These questions raised by the present work remain to be explored in further investigations.
If the cAMP-stimulated MT-MT interaction process in vitro described in this report reflects molecular events occurring in the dendritic tree of neurons, the preferential localization of the PKA on MAP2-containing MTs of this cellular domains (Harada et al, 2002) may affect the plasticity of neurons following the activation of the synaptic adenylate cyclase. An increased density of MT bundles should result from the activation of local cAMP production by the adenylate cyclase, with consecutive alterations of the morphological structure and possibly the physiological activity of the dendritic tree.
A possible extension of the present work could be the further exploration of the structural properties of native versus thermostable MAP2 and the effect of cAMP on the secondary structure of the complex MAP2-PKA, using methods such as those developed by Wille et al (1992a,b). However, since MAP2 belongs to the growing family of disorganized proteins (Mukhopadhyay and Hoh, 2001), such a study would require in addition the computer-assisted dynamic modeling of the MAP2 structure for establishing the putative conformational modifications of MAP2 suggested by this report.
ACKNOWLEDGEMENTS
This work was made possible by a joint fellowship INSERM-SJPS between Dr. Leterrier and Prof. Tashiro for travelling and stays, and by a collaboration program between Dr. Leterrier and Prof. Janmey (University of Pennsylvania).
The kind help of P. Legras and J. Roux (animal house of the University of Angers, Faculty of Medicine, Fr) is gratefully acknowledged.
Footnotes
Abbreviations: MAPs: microtubule-associated proteins. MT: microtubules. SDS-PAGE: sodium dodecylsulfate polyacrylamide gels. PC: phosphocellulose. GTP: guanosine triphosphate. ATP: adenosine triphosphate. cAMP; cyclic adenosine monophosphate. PKA: protein kinase A. EPAC: exchange protein directly activated by cAMP. AKAP:A kinase anchoring protein. RII: regulatory subunit II of PKA. C: catalytic subunit of PKA.
REFERENCES
- Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol. 2002;157:1187–1196. doi: 10.1083/jcb.200201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger D, Farah CA, Nguyen MD, Lauzon M, Cornibert S, Leclerc N. The projection domain of MAP2b regulates microtubule protrusion and process formation in Sf9 cells. J. Cell Sci. 2002;115:1523–1539. doi: 10.1242/jcs.115.7.1523. [DOI] [PubMed] [Google Scholar]
- Black MM. Comparison of the effects of microtubule-associated protein 2 and tau on the packing density of in vitro assembled microtubules. Proc. Natl. Acad. Sci. USA. 1987;84:7783–7787. doi: 10.1073/pnas.84.21.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G, Gupta M, Magiera MM, Rundell CJ, Fuld S, Yarwood SJ. Microtubule-associated protein 1B-light chain 1 enhances activation of Rap1 by exchange protein activated by cyclic AMP but not intracellular targeting. Mol. Pharmacol. 2006;69:374–384. doi: 10.1124/mol.105.016337. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nature reviews; Molecular Cell Biology. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Brandt R, Lee G. Functional organization of microtubule-associated protein tau. Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J. Biol. Chem. 1993;268:3414–3419. [PubMed] [Google Scholar]
- Brown PA, Berlin RD. Packing volume of sedimented microtubules: regulation and potential relationship to an intracellular matrix. J. Cell Biol. 1985;101:1492–1500. doi: 10.1083/jcb.101.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RG, Islam K, Chapman R. The multiple phosphorylation of the microtubule-associated protein MAP2 controls the MAP2-tubulin interaction. Eur. J. Biochem. 1984;141:609–615. doi: 10.1111/j.1432-1033.1984.tb08236.x. [DOI] [PubMed] [Google Scholar]
- Buxbaum RE, Dennerll T, Weiss S, Heidemann SR. F-actin and microtubule suspensions as indeterminated fluids. Science. 1987;235:1511–1514. doi: 10.1126/science.2881354. [DOI] [PubMed] [Google Scholar]
- Castoldi M, Popov AV. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr Purif. 2003;32:83–88. doi: 10.1016/S1046-5928(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule associated proteins. Dev Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Chau MF, Radeke MJ, de Inés C, Barasoain I, Kohlstaedt LA, Feinstein SC. The microtubule-associated protein tau cross-links to two distinct sites on each alpha and beta tubulin monomer via separate domains. Biochemistry. 1998;37:17692–17703. doi: 10.1021/bi9812118. [DOI] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Leclerc N, Flanagan LA, Lu M, Janmey PA, Kosik KS. Microtubule-associated protein 2c reorganizes both microtubules and microfilaments into distinct cytological structures in an actin-binding protein-280-deficient melanoma cell line. J Cell Biol. 1997;136:845–57. doi: 10.1083/jcb.136.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6(1):204. doi: 10.1186/gb-2004-6-1-204. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J Cell Sci. 2001;114:1431–1437. doi: 10.1242/jcs.114.8.1431. [DOI] [PubMed] [Google Scholar]
- Eyer J, McLean WG, Leterrier JF. Effect of a single dose of ββ'-iminodipropionitrile in vivo on the properties of neurofilaments in vitro: comparison with the effect of iminodipropionitrile added directly to neurofilaments in vitro. J. Neurochem. 1989;52:1759–1765. doi: 10.1111/j.1471-4159.1989.tb07254.x. [DOI] [PubMed] [Google Scholar]
- Farah CA, Liazoghli D, Perreault S, Desjardins M, Guimont A, Anton A, Lauzon M, Kreibich G, Paiement J, Leclerc N. Interaction of microtubule-associated protein-2 and p63: a new link between microtubules and rough endoplasmic reticulum membranes in neurons. J Biol Chem. 2005;280:9439–9449. doi: 10.1074/jbc.M412304200. [DOI] [PubMed] [Google Scholar]
- Fellous A, Francon J, Lennon AM, Nunez J. Microtubule assembly in vitro. Purification of assembly-promoting factors. Eur. J. Biochem. 1977;78:167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- Friden B, Nordh J, Wallin M, Deinum J, Norden B. Effetcs of proteolysis of the extending parts of the high-molecular-weight microtubule-associated proteins on interactions between microtubules. Biochim. Biophys. Acta. 1988;955:135–142. doi: 10.1016/0167-4838(88)90187-2. [DOI] [PubMed] [Google Scholar]
- Gupta M, Yarwood SJ. MAP1A light chain 2 interacts with exchange protein activated by cyclic AMP 1 (EPAC1) to enhance RAP1 GTPase activity and cell adhesion. J. Biol. Chem. 2005;280:8109–8116. doi: 10.1074/jbc.M413697200. [DOI] [PubMed] [Google Scholar]
- Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33:9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7:224. doi: 10.1186/gb-2006-7-6-224. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendritic elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MA, Avila J, Andreu JM. Physicochemical characterization of the heat-stable microtubule-associated protein MAP2. Eur. J. Biochem. 1986;154:41–48. doi: 10.1111/j.1432-1033.1986.tb09356.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Hisanaga S, Shiomura Y. MAP2 is a component of crossbridges between microtubules ad neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etching immunoelectron microscopy and reconstitution studies. J. Neurosci. 1988;8:2769–2779. doi: 10.1523/JNEUROSCI.08-08-02769.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida J, Itoh TJ, Hotani H, Nishiyama KI, Murofushi H, Bulinski JC, Hisanaga S. The projection domain of MAP4 suppresses the microtubule-bundling activity of the microtubule-binding domain. J. Mol. Biol. 2002;320:97–106. doi: 10.1016/S0022-2836(02)00402-3. [DOI] [PubMed] [Google Scholar]
- Itoh TJ, Hisanaga S, Hosoi T, Kishimoto T, Hotani H. Phosphorylation states of microtubule-associated protein 2 (MAP2) determine the regulatory role of MAP2 in microtubule dynamics. Biochemistry. 1997;36:12574–12582. doi: 10.1021/bi962606z. [DOI] [PubMed] [Google Scholar]
- Jameson L, Frey T, Zeeberg B, Dalldorf F, Caplow M. Inhibition of microtubule assembly by phosphorylation of microtubule-associated proteins. Biochemistry. 1980;19:2472–2479. doi: 10.1021/bi00552a027. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Hvidt S, Käs J, Lerche D, Maggs A, Sackmann E, Schliwa M, Stossel TP. The mechanical properties of actin gels. Elastic modulus and filament motions. J Biol Chem. 1994;269:32503–32513. [PubMed] [Google Scholar]
- Janmey PA, McCormick ME, Rammensee S, Leight JL, Georges PC, MacKintosh FC. Negative normal stress in semiflexible biopolymer gels. Nat Mater. 2007;6:48–51. doi: 10.1038/nmat1810. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Chen J, Hirokawa N. Microtubule bundling by tau proteins in vivo: analysis of functional domains. The EMBO J. 1992;11:3953–3961. doi: 10.1002/j.1460-2075.1992.tb05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap 1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kindler S, Schulz B, Goedert M, Garner CC. Molecular structure of microtubule-associated protein 2b and 2c from rat brain. J. Biol. Chem. 1990;265:19679–19684. [PubMed] [Google Scholar]
- Kurachi M, Kikumoto M, Tashiro H, Komiya Y, Tashiro T. Real-time observation of the disassembly of stable neuritic microtubules induced by laser transection: possible mechanisms of microtubule stabilization in neurites. Cell Motil Cytoskeleton. 1999;42:87–100. doi: 10.1002/(SICI)1097-0169(1999)42:2<87::AID-CM1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclerc N, Baas PW, Garner CC, Kosik KS. Juvenile and mature MAP2 isoforms induce distinct patterns of process outgrowth. Mol. Biol. Cell. 1996;7:443–455. doi: 10.1091/mbc.7.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Timasheff SN. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 1975;14:5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- Leterrier JF, Liem RK, Shelanski ML. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J. Cell Biol. 1982;95:982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier JF, Linden M, Nelson BD. How do microtubules interact in vitro with purified subcellular organelles? Biochem. J. (Letter) 1990;269:556–558. doi: 10.1042/bj2690556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier JF, Käs J, Hartwig J, Vegners R, Janmey PA. Mechanical effects of neurofilament crossbridges: modulation by phosphorylation, lipids, and interactions with F-actin. J. Biol. Chem. 1996;271:15687–15694. doi: 10.1074/jbc.271.26.15687. [DOI] [PubMed] [Google Scholar]
- Linden M, Nelson BD, Leterrier JF. The specific binding of the microtubule-associated protein 2 (MAP2) to the outer membrane of rat brain mitochondria. Biochem. J. 1989;261:167–173. doi: 10.1042/bj2610167. (Err. Biochem. J. 262, 1002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litersky JM, Johnson GV, Jakes R, Goedert M, Lee M, Seubert P. Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent kinase II within its microtubule-binding domains at Ser-262 and Ser-356. Biochem. J. 1996;316:655–660. doi: 10.1042/bj3160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement using the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luo Z, Shafit-Zagardo B, Erlichman J. Identification of the MAP2- and P75-binding domain in the regulatory subunit (RIIb) of type II cAMP-dependent protein kinase. J. Biol. Chem. 1990;265:21804–21810. [PubMed] [Google Scholar]
- MacLean-Fletcher SD, Pollard TD. Viscosimetric analysis of the gelation of Acanthamoeba extracts and purification of two gelation factors. J. Cell Biol. 1980;85:414–428. doi: 10.1083/jcb.85.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiera MM, Gupta M, Rundell CJ, Satish N, Ernens I, Yarwood SJ. Exchange protein directly activated by cAMP (EPAC) interacts with the light chain (LC) 2 of MAP1A. Biochem. J. 2004;382:803–810. doi: 10.1042/BJ20040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Hoh JH. AFM force measurements on microtubule-associated proteins: the projection domain exerts a long-range repulsive force. FEBS Letters. 2001;505:374–378. doi: 10.1016/s0014-5793(01)02844-7. [DOI] [PubMed] [Google Scholar]
- Murthy AS, Flavin M. Microtubule assembly using the microtubule-associated protein MAP2 prepared in defined states of phosphorylation with protein kinase and phosphatase. Eur. J. Biochem. 1983;137:37–46. doi: 10.1111/j.1432-1033.1983.tb07792.x. [DOI] [PubMed] [Google Scholar]
- Newlon MG, Roy M, Morikis D, Carr DW, Westphal R, Scott JD, Jennings PA. A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. The EMBO J. 2001;20:1651–1662. doi: 10.1093/emboj/20.7.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar RA, Dingus J, Bayley H, Vallee RB. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron. 1989;3:639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Rammensee S, Janmey PA, Bausch AR. Mechanical and structural properties of in vitro neurofilament hydrogels. Eur Biophys J. 2007;36:661–668. doi: 10.1007/s00249-007-0141-7. [DOI] [PubMed] [Google Scholar]
- Rubino HM, Dammerman M, Shafit-Zagardo B, Erlichman J. Localization and characterization of the binding site for the regulatory subunit of type II cAMP-dependent protein kinase on MAP2. Neuron. 1989;3:631–638. doi: 10.1016/0896-6273(89)90273-0. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Progress in Neurobiology. 2000;61:133–168. doi: 10.1016/s0301-0082(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Sato M, Schwartz WH, Selden SC, Pollard TD. Mechanical properties of brain tubulin and microtubules. J. Cell Biol. 1988;106:1205–1211. doi: 10.1083/jcb.106.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- Severin FF, Shanina NA, Kuznetsov SA, Gelfand VI. MAP2-mediated binding of chromaffin granules to microtubules. FEBS Letters. 1991;282:65–68. doi: 10.1016/0014-5793(91)80445-9. [DOI] [PubMed] [Google Scholar]
- Shelanski ML, Gaskin F, Cantor CR. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- Tang JX, Ito T, Tao T, Traub P, Janmey PA. Opposite effects of electrostatics and steric exclusion on bundle formation by F-actin and other filamentous polyelectrolytes. Biochemistry. 1997;36:12600–12607. doi: 10.1021/bi9711386. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Vallee RB. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J. Biol. Chem. 1982;257:3284–3290. [PubMed] [Google Scholar]
- Tokuraku K, Katsuki M, Matui T, Kuroya T, Kotani S. Microtubule-binding property of microtubule-associated protein 2 differs from that of microtubule-associated protein 4 and tau. Eur J Biochem. 1999;264:996–1001. doi: 10.1046/j.1432-1327.1999.00710.x. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoresis transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B, Biernat J, Baumann K, Mandelkow EM, Mandelkow E. Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol Biol Cell. 1995;6:1887–1902. doi: 10.1091/mbc.6.12.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi N, Marsh P, Goold RG, Wood-Kaczmar A, Gordon-Weeks PR. Glycogen synthase kinase-3beta phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. J Cell Sci. 2005;118:993–1005. doi: 10.1242/jcs.01697. [DOI] [PubMed] [Google Scholar]
- Umeyama T, Okabe S, Kanai Y, Hirokawa N. Dynamics of microtubules bundled by microtubule-associated protein 2c (MAP2c) J. Cell Biol. 1993;120:451–465. doi: 10.1083/jcb.120.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Dibartolomeis MJ, Theurkauf WE. A protein kinase bound to the projection portion of MAP2 (microtubule-associated protein 2) J. Cell Biol. 1981;90:568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil D, Blumenthal DK, Brown S, Taylor SS, Trewhella J. Differential effects of substrate on type I and type II PKA holoenzyme dissociation. Biochemistry. 2004;43:5629–5636. doi: 10.1021/bi0499157. [DOI] [PubMed] [Google Scholar]
- Voter WA, Erikson HP. Electron microscopy of map2 (microtubule-associated protein 2) J. Ultrastr. Res. 1982;80:374–382. doi: 10.1016/s0022-5320(82)80051-8. [DOI] [PubMed] [Google Scholar]
- Wagner OI, Rammensee S, Korde N, Wen Q, Leterrier JF, Janmey PA. Softness, strength and self-repair in intermediate filament networks. Exp Cell Res. 2007;313:2228–2235. doi: 10.1016/j.yexcr.2007.04.025. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis KT, Azhar S, Rho MB, Lewis SA, Cowan NJ, Murphy DB. The mechanism of equilibrium binding of microtubule-associated protein 2 to microtubules. J. Biol. Chem. 1993;268:15158–15167. [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse S, Walsh DA. Mg X ATP2-dependent interaction of the inhibitor protein of the cAMP-dependent protein kinase with the catalytic subunit. J. Biol. Chem. 1983;258:3682–3692. [PubMed] [Google Scholar]
- Wiche G. High-Mr microtubule-associated proteins: properties and functions. Biochem. J. 1989;259:1–12. doi: 10.1042/bj2590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille H, Mandelkow E-M, Dingus J, Vallee RB, Binder LI, Mandelkow E. Domain structure and antiparallel dimers of microtubule-associated protein 2 (MAP2) J. Struct. Biol. 1992a;108:49–61. doi: 10.1016/1047-8477(92)90006-v. [DOI] [PubMed] [Google Scholar]
- Wille H, Mandelkow E-M, Mandelkow E. The juvenile microtubule-associated protein MAP2c is a rod-like molecule that forms antiparallel dimers. J. Biol. Chem. 1992b;267:10737–10742. [PubMed] [Google Scholar]