Abstract
Neurodevelopmental abnormalities of temporal–limbic structures may underlie both adult psychiatric syndromes and increased addiction vulnerability, leading to high frequencies of “dual diagnosis” disorders. Although the amygdala is implicated in various mental disorders and drug addiction, no studies have explored the impact of early developmental damage to the amygdala on phenotypes relating to mental illness and addictions as co-occurring processes. We tested rats with neonatal amygdala lesions (NAML) vs. SHAM-operated controls in a battery of tests—novel field activity, elevated plus maze (EPM), and social interaction (SI) at baseline and after odor and restraint stress—followed by measures of cocaine sensitization (15 mg/kg vs. saline × 5 days + challenge session 2 weeks later) and remeasurement of SI. NAMLs showed increased novelty-related locomotion, less fear responding in the EPM, and resistance to predator-odor- but not to restraint-induced suppression of SI. NAMLs also had elevated cocaine sensitization profiles, and cocaine history differentially affected subsequent SI in NAMLs compared with SHAMs. NAMLs may provide models for understanding a shared neurobiological basis for and complex interactions among psychiatric symptoms, drug exposure history, and addiction vulnerability.
Keywords: amygdala, cocaine, neurodevelopment, dual diagnosis, social interaction
Substance use disorder comorbidity in mental illness spans differential psychiatric diagnoses and drugs of abuse (Kessler, 2004; Regier et al., 1990). In many treatment settings, these “dual diagnosis” presentations are the majority of cases, associated with increased medical and psychiatric morbidity and mortality, financial destitution, and criminal incarceration (Dickey, Normand, Weiss, Drake, & Azeni, 2002; Dixon, 1999; RachBeisel, Scott, & Dixon, 1999). Emerging clinical and basic research suggests that varieties of mental illness and the addiction process involve integrated neurocircuits leading to increased addiction vulnerability regardless of or despite psychoactive consequences of drug use (Chambers, Krystal, & Self, 2001; Volkow, 2004). Functional and/or developmental abnormalities of the hippocampal formation and other temporal–limbic circuits are associated with a range of major neuropsychiatric and personality disorders that are frequently dually diagnosed (Blumberg et al., 2003; Bremner et al., 2000, 1995; Driessen et al., 2000; Weinberger, 1999). Meanwhile, these temporal–limbic regions are also directly connected with and modulate functional and neuroadaptative processes of frontal cortical–striatal motivational circuits impacted by addictive drugs (Chambers et al., 2001; Goto & O’Donnell, 2004; Kelley & Domesick, 1982; O’Donnell, Lewis, Weinberger, & Lipska, 2002; Pennartz, Groenewegen, & Lopez da Silva, 1994).
Animal modeling work shows that adult-onset or developmental disruptions to temporal–limbic structures produce not only clusters of behavioral abnormalities that model affective or schizophrenic syndromes but also multiple behavioral markers of enhanced addiction vulnerability. For example, adult-onset olfactory bulbectomy in rats produces distributed neural systems abnormalities involving components of the amygdala, hippocampal formation, and bed nucleus of the stria terminalis and a behavioral syndrome encompassing features of affective disorders, heightened behavioral sensitization to cocaine, and self-administration of amphetamine (Chambers, Sheehan & Taylor, 2004; Holmes et al., 2002; Kelly, Wrynn, & Leonard, 1997). Rats with neonatal ventral hippocampal lesions (NVHL) show a developmental behavioral and neurobiological marker syndrome consistent with features of schizophrenia (Flores et al., 2005; Lipska & Weinberger, 2000), increased cocaine self-administration behavior across several stages of the addiction process (Chambers & Self, 2002), impulsivity in natural-reward learning worsened by cocaine history (Chambers, Jones, Brown, & Taylor, 2005), and enhanced long-term sensitization to cocaine and ethanol (Chambers & Taylor, 2004; Conroy, Rodd, & Chambers, 2007).
The amygdala is another key brain region implicated in multiple neuropsychiatric disorders spanning psychotic, mood, anxiety, personality, and pervasive developmental disorder classifications (Anand & Shekhar, 2003; Hull, 2002; Joyal et al., 2003; Rusch et al., 2003; Shekhar, Truitt, Rainnie, & Sajdyk, 2005; Sheline, Sanghavi, Mintun, & Gado, 1999; Sweeten, Posey, Shekhar, & McDougle, 2002). It is also involved in reinforcement learning and drug taking (Baxter & Murray, 2002; Carelli, Williams, & Hollander, 2003; Everitt, Cardinal, Parkinson, & Robbins, 2003; Kilts, 2001), and its basolateral regions have direct connectivity with frontal cortical–striatal circuits implicated in addiction (Baxter & Murray, 2002; Ishikawa & Nakamura, 2003; Kita & Kitai, 1990; Sah, Faber, Lopez De Armenta, & Power, 2003).
Although a growing literature has examined the role of neonatal amygdala lesions (NAML) in developmental aspects of autism, schizophrenia, and other disorders of abnormal social and affective behavior (Daenen, Van der Heyden, Kruse, Wolterink, & Van Ree, 2001; Diergaarde, Gerrits, Stuy, Spruijt, & Van Ree, 2004), no researchers have investigated the impact of NAML from an addiction-model perspective. We examined the effects of repeated cocaine injections in shaping the psychomotor behavior of NAML rats as a possible indirect measure of addiction vulnerability. For characterizing the impact of NAML and drug history on psychiatrically relevant behavioral phenotypes, the sensitization paradigm was embedded within a larger series of tests measuring aspects of novelty responsivity, anxiety, and social interaction (SI). This study design attempted to model complex dual diagnosis phenomena in which psychiatric syndromes and heightened drug responsivity may not only emerge in parallel from the same neurobiological abnormalities but also in which drug history might differentially influence neuropsychiatric phenotypes.
Method
Subjects and Surgery
Pregnant Wistar rats (Harlan, Indianapolis, IN) arrived in the laboratory at 14–15 days’ gestation and were individually housed on a 12-hr light–dark cycle (lights on 7 a.m.) and given free access to food and water. Seven days after birth (Postnatal Day [PD] 7), male pups weighing 15–18 g were removed for 1–2 hr for surgery based on the protocol developed by Lipska, Jaskiw, and Weinberger (1993) and modified for NAML by Daenen et al (2001). Pups were randomly assigned SHAM (sham-operated) or NAML status, with balancing of lesion assignments within litters. For surgery, subjects were anesthetized by hypothermia on ice for 15–20 min. A midline anterior–posterior incision was made on the head’s dorsal surface, and the pups were secured to a stereotaxic platform with tape, which also held the wound open. A 26s-gauge Hamilton needle was lowered bilaterally into the amygdala (anterior–posterior, −1.0 mm; medial–lateral, ± 3.8 mm; and dorsal–ventral, −6.0 mm; angled 4°). The lesion group received bilateral infusions of 0.3 μL (10 μg/μL) ibotenic acid (Sigma, St. Louis, MO) dissolved in artificial cerebrospinal fluid (aCSF), while the SHAM group received only 0.3 μL of aCSF. Infusions were delivered over 135 s, after which the needle was left in place for 3 min to prevent backflow. The wound was closed with Vetbond tissue adhesive (3M, St. Paul, MN), and the pups were warmed on a heating pad before being returned to their mothers. The pups and mothers were left undisturbed until weaning at PD 21. From PD 35 to PD 49, rats were housed in pairs and subsequently were singly housed. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1986) and Ethical Principles of Psychologists and Code of Conduct (American Psychological Association, 2002) and were approved by the Indiana University Institutional Animal Care and Use Committee.
Behavioral Procedures
Subjects underwent three phases of behavioral testing (Figure 1) beginning in early adulthood (PD 59). In Phase 1, psychiatric-related phenotypes were measured in terms of novel environment responses, social interaction, and anxiety-related behavior. Phase 2 examined addiction-related phenotypes in terms of short-term sensitization to cocaine versus saline injections, followed 2 weeks later by a cocaine challenge to all the rats. Phase 3 examined the impact of differential cocaine histories (repeated vs. single injections from Phase 2) on social interaction. Under all conditions, the experimenters were blind to lesion status.
Figure 1.
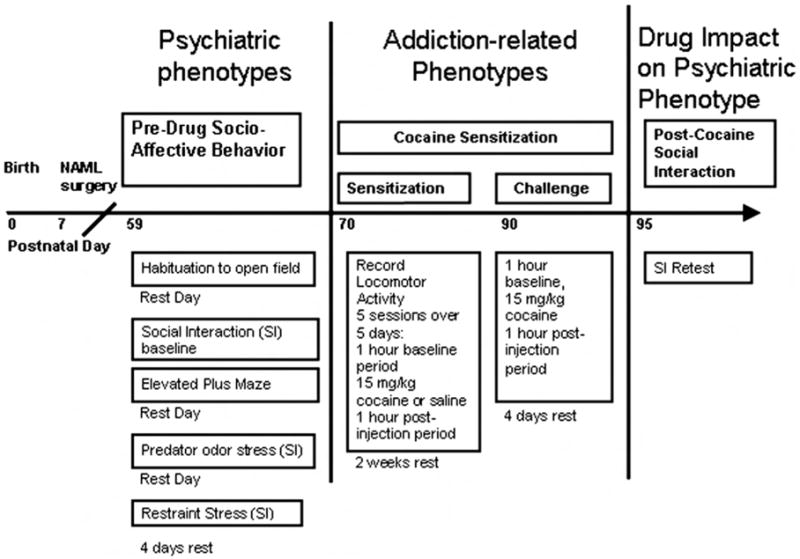
Experimental time line with respect to rat age. NAML = neonatal amygdala lesion; SI = social interaction.
Phase 1: Socio–Affective Behavior
Open field habituation and social interaction (SI)
All sessions occurred between 8 a.m. and 1 p.m. in a 91 cm × 91 cm × 30 cm SI box and were videorecorded from above under low red light (40 W). On the first day of testing (PD 59), novel open field activity was measured during a 5-min habituation session in the SI box. Activity was scored after dividing the box into nine 30 cm × 30 cm zones and counting entries into corner and center zones. SI sessions were conducted based on the protocol devised by File (1980). During each 5-min session, the experimental rat was placed into the box with a healthy partner rat with which it had been matched by sex, weight, and housing conditions and with which it had had no previous contact. Total time interacting was scored as the duration of physical contacts initiated by the experimental rat, including nonaggressive interactions such as sniffing, grooming, and playing. SI was measured for each rat in three independent sessions: as a baseline measure (second day after initial novel field assessment) and immediately after exposure to predator odor and restraint stressors (beginning second day after elevated plus maze assessment with a rest day between odor and restraint sessions). In the predator odor condition, animals were placed for 5 min into an airtight Plexiglass (Rohm & Haas, Philadelphia, PA) chamber (30 cm × 30 cm × 50 cm) in which a cat-odor-impregnated zeolite charcoal air filter (12 cm × 20 cm × 1.5 cm) had been taped to the inside of the lid. The filter had been previously placed in an enclosed litter box (Van Ness Plastics, Clifton, NJ; model CP-7) for 90 days of continuous use by a domesticated cat and then fixed to the odor chamber for 1 hr prior to rat entry. In the restraint stress condition, rats were placed in a disposable DecapiCone (Braintree Scientific, Braintree, MA) for 30 min.
Elevated plus maze (EPM)
The EPM, standing 50 cm above the ground, consisted of two open arms (50 cm × 10 cm) and two enclosed arms (50 cm × 10 cm × 30 cm) with an open roof. The open and closed arms extended perpendicularly from a common center. Rats were placed in the center and exploration of the maze was videorecorded from above for 5 min. Time spent in each set of arms was scored from videotape review.
Phase 2: Cocaine Sensitization
Locomotor activity was measured under red light in 43.2 cm × 43.2 cm × 30.5 cm arenas that were made of clear plastic and equipped with three sets of infrared beam transmitters and detectors (Med Associates, St. Albans, VT). Horizontal motion (x, y dimensions) and rearing (z dimension) beam arrays were located 5 cm and 17.5 cm above the chamber floor, respectively. Each array consisted of 16 beams located 2.5 cm apart. Data were collected using Activity Monitor Version 5.0 software (Med Associates, St. Albans, VT) configured to separate stereotypic movements from gross horizontal locomotion. The dependent measure of stereotypic activity counted beam breaks occurring when the animal was not demonstrating forward horizontal locomotion, whereas the dependent measure of distance traveled was derived from beam breaks associated with displacement of the rat’s estimated center of mass approximately 5 cm in horizontal locomotion. During the sensitization paradigm, activity was assessed over five 2-hr sessions (one session/day), each of which consisted of a 60-min pre-injection phase and a 60-min post-injection phase. After the pre-injection phase, rats received either 15 mg/kg cocaine (15 mg cocaine HCl/1.0 ml saline) or (1.0 ml/kg) saline according to initial random assignments. Two weeks later, in another 2-hr session, all rats received a challenge dose of 15 mg/kg cocaine after the pre-injection hour.
Phase 3: Impact of Cocaine History on Social Interaction (SI)
Four days after all rats had received a cocaine injection in the challenge session and 18 days after the initial injection series, the rats were again tested in the SI paradigm with a novel partner as described above.
Lesion Verification
After behavioral testing, animals were decapitated under isoflurane anesthesia. Brains were removed and immediately frozen in isopentane. Coronal sections (20 μm) were cut on a cryostat at 300-μm intervals from approximately −0.11 mm to −5.25 mm relative to bregma (Swanson, 2004), corresponding to a range that included the entire amygdala, beginning just before the medial crossing of the anterior commissure and ending in the caudal hippocampus. Sections were placed on slides, dehydrated, stained with thionin, and coverslipped. Subjects were included in the behavioral analysis if, upon microscopic examination, they showed evidence of bilateral damage to the greater amygdala in at least two consecutive sections. Subjects with notable damage to basal ganglia, piriform cortex, or hippocampal formation were excluded.
Statistical Analysis
Differences between NAML and SHAM groups in the unitary measure pre-drug behavioral assays (novel SI field habituation, EPM, and pre-drug locomotor activity from Sensitization Day 1) were analyzed using independent samples t tests. The pre-drug SI series was tested using a repeated measures analysis of variance (ANOVA) (between: lesion status; within: pre-test condition). Subsequent testing examined independent factors of lesion status and drug exposure with two-way ANOVAs for unitary measures or repeated measures ANOVAs in which serial dependent measures were assessed. As indicated by significant main effects or interactions, post hoc testing was conducted with t tests (between: lesion) or least significant difference (LSD; between: lesion and drug history). Statistical significance was assumed at p < .05. Informative significant and nonsignificant (ns; p > .05) statistics are presented throughout. Data are presented as means ± standard errors of the means.
Results
Lesion Analysis
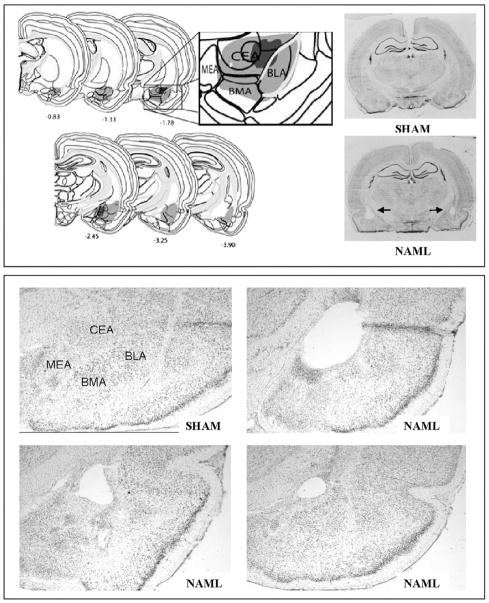
NAMLs were attempted on a total of 38 rats, of which 18 were assigned to receive saline and 20, cocaine during sensitization. From these groups, 3 saline- and 10 cocaine-assigned rats received inappropriate lesions, leaving 15 saline-assigned (NAML-SAL) and 10 cocaine-assigned (NAML-COC) rats for behavioral analysis. Appropriate amygdala damage was scored upon evidence in serial sections of cellular disarray, gliosis, atrophy, or absence of one or more major amygdalar nuclei (central, basolateral, basomedial, medial, anterior, intercalated), with or without extension or hypertrophy of the lateral ventricle into the tissue-poor amygdalar region. Figure 2 shows the minimal and maximal extents of amygdalar damage in the included set. In nearly all cases, damage encompassed both the central and basolateral nuclei; in no cases were one or the other of these nuclei undamaged on both sides of the brain. Damage to the dorsal or ventral endopiriform nuclei was deemed acceptable as long as it occurred at their boundaries with the amygdala proper within which the bulk of damage had occurred. The 13 subjects excluded from analysis showed highly heterogeneous patterns of inappropriate damage. The 3 excluded saline-assigned rats showed mutually exclusive unilateral extension of damage on the right side into the lateral hypothalamus versus hippocampus versus globus pallidus/substantia innominata. Among the 10 excluded cocaine-assigned rats, 2 showed insufficient damage to the amygdala bilaterally; 1, unilateral amygdalar damage only; 1, extension into right ventral hippocampus; 2, extension into right globus pallidus/substantia innominata; 1 bilateral extension into globus pallidus/substantia innominata; 2, extension into right lateral hypothalamus; and 1, extension into left lateral hypothalamus. Due to the pronounced heterogeneity of these inappropriate lesions, these rats were excluded from further analysis. None of the 24 SHAM rats showed evidence of excitotoxic damage.
Figure 2.
Histological verification mapping (upper panel, left) showed minimal (dark gray) and maximal (light gray) extents of lesions in brains of subjects included in the study: 25 rats with neonatal amygdala lesions (NAML) vs. 24 SHAM-operated controls. Coordinates shown are relative to bregma based on work by Swanson, 2004. Damage was usually centered at the boundary of the central (CEA) and basolateral (BLA) nuclei but often extended into the basomedial (BMA) and medial (MEA) nuclei. Exemplary whole coronal sections from a SHAM brain and a NAML brain with bilateral CEA/BLA damage (upper panel, right) is denoted by arrows. Exemplary sections from a SHAM and 3 different NAML brains featured at 25× magnification (lower panel) show variation in lesion damage with differential extents of cellular change and frank vacuolization in the amygdala. All sections were taken from approximately the same level relative to bregma (e.g. −2.5).
Behavioral Analysis
Phase 1: Socio-Affective Behavior
Habituation to novel social interaction (SI) arena
Behavioral testing began for all animals in early adulthood (PD 59), beginning with this assessment of activity in the novel SI arena. During the initial 5-min exposure to the SI arena, NAML rats (N = 25) produced 48.2 ± 2.1 line crossings compared with 41.1 ± 1.4 produced by SHAM rats (N = 24). These differences were significant in two-way t testing, t(47)=−2.8, p < .01, indicating greater novel-field-induced locomotor activation in NAMLs.
Elevated plus maze (EPM)
Over the 5-min period, NAML rats spent 16.3% ± 2.3% of their time in the open arms of the EPM compared with 11.6% ± 1.6% for SHAM rats. These differences were indicative of a trend analogous to group differences in locomotor activity in the novel SI arena. Given an a priori hypothesis of impaired stimulus-induced fear responding associated with amygdala lesions (Kalin, Shelton, & Davidson, 2004; Takahashi, Hubbard, Lee, Dar, & Sipes, 2007), one-tailed t testing revealed significant group differences, t(47) = −1.7, p < .05.
Social interaction (SI) after stressors
During the 5-min baseline test, NAML and SHAM rats showed similar times in SI (23.7 ± 3.0 s and 23.1 ± 1.8 s, respectively). Repetition of the SI test with pre-conditioned stimuli of predator odor and restraint stress produced overall decreases in SI, with pre-condition, F(2, 94) = 53.9, p < .001, interacting with lesion status—PreCondition × Lesion Status, F(2, 94) = 3.7, p < .05 (Figure 3). Post hoc analysis revealed that SI was significantly suppressed after exposure to the predator odor to a greater extent in SHAM (12.1 ± 1.1 s) compared with NAML rats (19.6 ± 2.2 s), t (−3.1) = p < .01, but SI was further suppressed after restraint stress to similar levels in SHAM (8.0 ± 1.0 s) and NAML (8.8 ± 1.3 s) rats.
Figure 3.
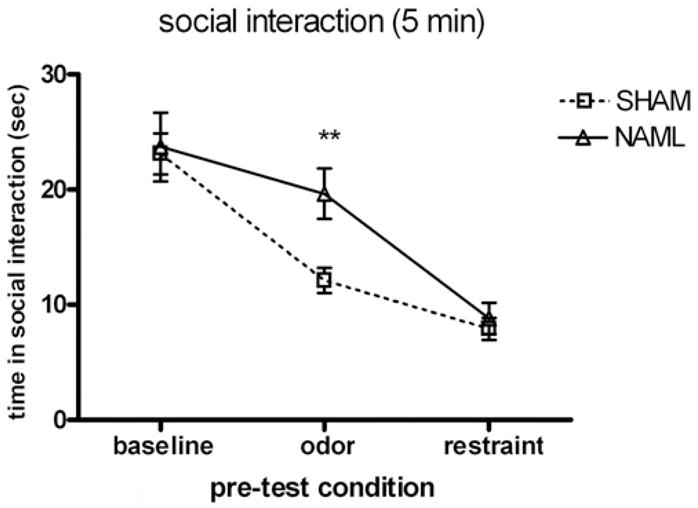
Social interaction times of experimental rats over 5-min encounters with novel healthy partners at baseline and immediately after exposure to predator odor and restraint stress. Repeated measures analysis of variance showed significant interactions between pre-test condition and lesion (p < .05) that was carried by significant differences between 25 rats with neonatal amygdala lesions (NAML) versus 24 sham-operated controls (SHAM) after the predator odor pre-condition (p < .01, post hoc t testing). Error bars indicate ±1 SEM.
Phase 2: Cocaine Sensitization
Pre-injection activation
During the pre-injection hour on the first day of cocaine sensitization regimen, in the novel context, NAML rats (N = 25) again showed significantly greater locomotor activity compared with SHAM rats (N = 24): 10,250 ± 3,290 cm versus 8,029.9 ± 391 cm, respectively, t(47) = −2.9, p < .01. Pre-injection activity of NAML versus SHAM rats subgrouped by drug assignment—SHAM-SAL (N = 11), NAML-SAL (N = 15), SHAM-COC (N = 13), and NAML-COC (N = 10)—over all the injection sessions is shown in Table 1. Analysis of pre-injection activity over the initial 5 days of cocaine sensitization revealed significant daily declines in activity—days: F(4, 180) = 35.4, p < .001—but no interactions between days and lesion, F(4, 180) = 0.83, ns, or drug status, F(4, 180) = 1.1, ns, consistent with similarly progressive activity declines across all groups. However, a significant main effect of lesion, F(1, 45) = 4.5, p < .05, but no main drug effect, F(1, 45) = 1.2, ns, or Drug × Lesion interaction, F(1, 45) = 0.02, ns, indicated that regardless of drug status, NAML rats showed relative elevations in daily pre-injection activity across the 5 days compared with SHAM rats. Subsequent analyses of drug-induced activation were therefore measured in terms of injection-induced change in activity (post-injection activity minus pre-injection activity) as a means to correct for baseline differences in pre-injection activity.
Table 1.
Daily Pre-Injection Locomotion (cm/hr) in Rats by Experimental Group
| Sensitization session (Day) |
||||||
|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | Challenge |
| SHAM-SAL | 7664 ± 449 | 6797 ± 292 | 6259 ± 304 | 4920 ± 469 | 4920 ± 625 | 7676 ± 521 |
| NAML-SAL | 9961 ± 631 | 7772 ± 806 | 7272 ± 713 | 5799 ± 542 | 5720 ± 542 | 9540 ± 637 |
| SHAM-COC | 8339 ± 617 | 7794 ± 614 | 6374 ± 656 | 6200 ± 753 | 5233 ± 612 | 7964 ± 562 |
| NAML-COC | 10683 ± 1387 | 9777 ± 1924 | 8444 ± 1190 | 8065 ± 1194 | 7138 ± 1151 | 10009 ± 934 |
Note. SHAM-SAL = sham-operated saline-assigned rats; NAML-SAL = neonatal-amygdala-lesioned saline-assigned rats; SHAM-COC = sham-operated cocaine-assigned rats; NAML-COC = neonatal-amygdala-lesioned cocaine-assigned rats.
Short-term sensitization
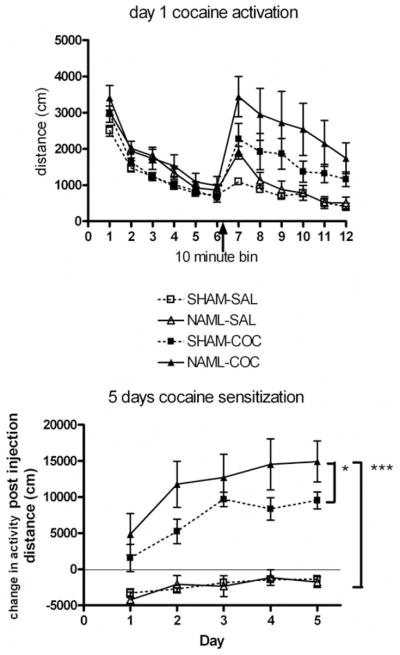
Daily cocaine injections produced locomotor activation in NAML (N = 10) compared with SHAM (N = 13) and saline-receiving NAML (N = 15) and SHAM (N = 11) rats on the first day as exemplified in Figure 4. Over the 5 days of injections, drug-induced sensitization was confirmed in both NAML and SHAM groups as indicated by significant interactions of Day × Drug, F(4, 180) = 6.1, p < .001, but not Day × Lesion interactions, F(4, 180) = 1.0, ns. Significant main effects of drug, F(1, 45) = 88.0, p < .001, and Lesion × Drug interactions, F(1, 45) = 5.3, p < .05, but not of lesion, F(1, 45) = 2.1, ns, indicated that NAML rats demonstrated significant overall elevations in cocaine sensitization patterns. This was confirmed by post hoc testing (LSD) in which both NAML-COC and SHAM-COC rats showed greater activity than saline-receiving NAML or SHAM rats (p < .001 for all comparisons), while NAML-COC rats also activated to a greater extent than SHAM-COC rats (p < .05). NAML-SAL and SHAM-SAL groups were not significantly different.
Figure 4.
Activity patterns of rats in the entire first injection session taken as locomotor distance per 10-min bin over 2 hr (upper panel). Arrow indicates time of cocaine/saline injections. Differences among sham-operated saline-assigned control (SHAM-SAL, N = 11), neonatal-amygdala-lesioned saline-assigned (NAML-SAL; N = 15), SHAM cocaine-assigned (SHAM-COC, N = 13), and NAML cocaine-assigned (NAML-COC, N = 10) groups evident on this first day exemplified patterns on subsequent days. Over 5 days of cocaine sensitization (lower panel), changes in activity post-injection (post-injection hour activity minus pre-injection hour activity) demonstrated elevated cocaine sensitization patterns in NAML rats (repeated measures analysis of variance: Drug × Lesion interaction, p < .05), resulting in greater overall activation in NAML-COC compared with SHAM-COC (*p < .05) and in NAML-COC compared with both saline groups (***p < .001) by post hoc least significant difference testing. Saline groups were consistently below 0 because activity levels in the initial hour before injection were always greater than the hour post injection. Error bars indicate ±1 SEM.
Post-injection stereotypic movement (defined as beam breaks not counted as components of horizontal locomotion) over the 5 injection days is shown in Figure 5. Cocaine produced progressive daily—Day × Drug: F(4, 180) = 10.7, p < .001—and overall—drug: F(1, 45) = 172, p < .001—increases in stereotypic movement; however, there were no significant main effects of lesion, F(1, 45) = 1.1, ns, or Lesion × Drug interactions, F(1, 45) = 1.0, ns, indicating that stereotypic movement was not differentially sensitized by cocaine according to lesion status.
Figure 5.
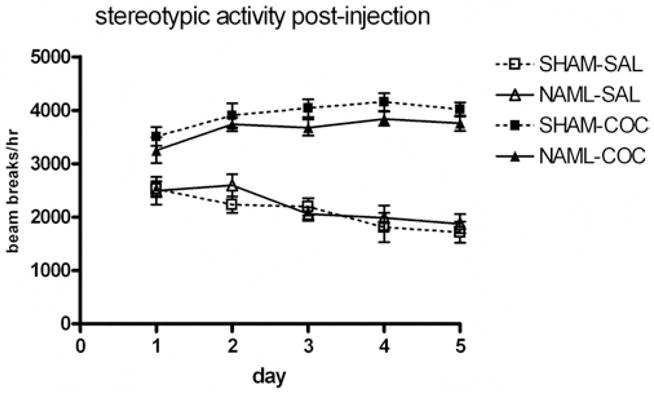
Stereotypic behavior of rats in the hour post-injection over the 5 days of sensitization revealed cocaine-induced increases and sensitization of stereotypic behavior (repeated measures analysis of variance for main effect of drug, p < .001, and Drug × Day interaction, p < .001), but no differences occurred as a result of neonatal-amygdala-lesioned (NAML) rats. SHAM-SAL = sham-operated saline-assigned controls; NAML-SAL = neonatal-amygdala-lesioned saline-assigned rats; SHAM-COC = sham-operated cocaine-assigned rats; NAML-COC = neonatal-amygdala-lesioned cocaine-assigned rats. Error bars indicate ±1 SEM.
Long-term sensitization
Cocaine injections (15 mg/kg) were delivered to all rats 2 weeks after the initial short-term series of injections (15 mg/kg cocaine vs. saline × 5 days) (Figure 6). A two way ANOVA revealed a marginally nonsignificant main effect of drug history, F(1, 45) = 3.7, p < .059, on injection-induced change in activity. However, NAML rats showed significant increases in cocaine-induced change in activity—lesion: F(1, 45) = 4.6, p < .05—in which NAML rats with cocaine history showed significantly greater activation than SHAM rats with cocaine history and the NAML and SHAM rats with saline histories (p < .01 for all comparisons by LSD post hoc).
Figure 6.
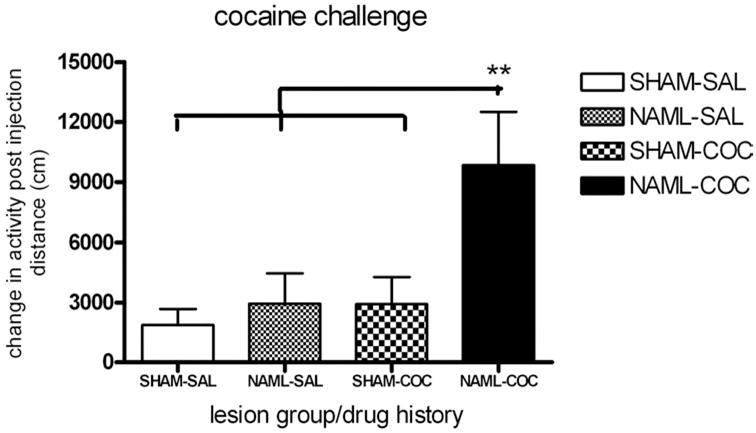
Two weeks after the last injection of the initial series (15 mg/kg cocaine vs. saline × 5 days), sham-operated saline-assigned control (SHAM-SAL, N = 11), neonatal-amygdala-lesioned saline-assigned (NAML-SAL; N = 15), SHAM cocaine-assigned (SHAM-COC, N = 13), and NAML cocaine-assigned (NAML-COC, N = 10) groups of rats received a single cocaine injection (15 mg/kg). Change in activity due to cocaine injection was increased in NAML rats (analysis of variance for main effect of lesion, p < .05, with a marginal effect of drug history, p = .06), and NAML rats with cocaine history activated more than all other rat groups (**p < .01, least significant difference post hoc testing). Error bars indicate ±1 SEM.
Phase 3: Impact of Cocaine History on Social Interaction (SI)
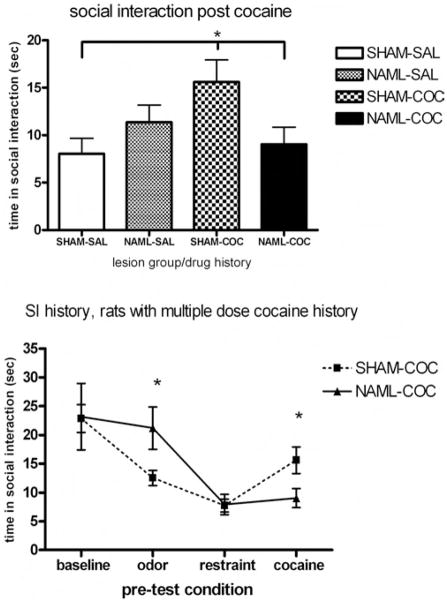
Four days after the cocaine challenge session, NAML versus SHAM rats showed differential levels of SI, based on history of repeated cocaine versus saline injections in the short-term sensitization series (Figure 7, upper panel). While neither the main effects of lesion status, F(1, 45) = 0.6, ns, nor history of repeated cocaine injections, F(1, 45) = 1.7, ns, affected SI time, Lesion × Drug History interactions, F(1, 45) = 6.4, p < .5, were significant. Post hoc analysis (LSD) revealed that SHAM rats with histories of repeated cocaine injections showed greater SI time compared with SHAM rats with histories of only single cocaine injections or NAML rats with histories of repeated cocaine injections, p < .05; all other comparisons were not significant. A secondary analysis of the entire series of SI tests occurring both pre- and post-cocaine sensitization for only those rats receiving repeated cocaine injections (Figure 7, lower panel), showed general effects of precondition, F(3, 63)= 14.9, p < .001, that were differentially expressed based on lesion status: Pre-Condition × Lesion, F(3, 63) = 3.4, p < .05. While the main effect of lesion alone did not alter overall social behavior across these tests, F(1, 21) = 0.07, ns, post hoc testing (t tests) specified that NAML rats were refractory to odor-induced suppression of SI before serial cocaine injections (p < .05) and refractory to cocaine history–related elevation in SI after cocaine sensitization seen in SHAM rats (p < .05).
Figure 7.
Four days after the cocaine challenge, differential effects of repeated-cocaine- versus saline-dosing history emerged in social interaction (SI) among rats depending on lesion status (upper panel; analysis of variance [ANOVA] interaction between drug history and lesion, p < .05). Sham-operated (SHAM) rats with histories of multiple cocaine doses (SHAM-COC, N = 13) showed greater SI times than did SHAM rats with only saline histories prior to cocaine challenge (SHAM-SAL, N = 11) and NAML rats with histories of multiple cocaine doses (NAML-COC, N = 10; p < .05, least significant difference post hoc testing). In rats with multiple-cocaine-dose histories (lower panel), SI was differentially influenced by lesion status and pre-condition (ANOVA interaction, p < .05), with SI of NAML rats differing from that of SHAM rats immediately after predator odor exposure and 4 days after cocaine injections (*p < .05, post hoc t testing). Error bars represent ±1 SEM.
Discussion
Young adult NAML rats show persistent elevations in locomotor behavior to novel conditions, reduced fear-related behavior suggested by EPM testing, and resistance to suppression of SI produced by pre-exposure to predator odor. Although generally consistent with deficits in stimuli-related discrimination and behavioral disinhibition long attributed to temporal-limbic and/or amygdalar damage (S. Brown & Schafer, 1887; Kluver & Bucy, 1937; Weiskrantz, 1956), this syndrome also encompassed elevated short- and long-term sensitization patterns with repeated cocaine injections. Finally, history of repeated versus single cocaine injections differentially impacted subsequent SI in NAML compared with SHAM rats.
Altered responses to novel objects or settings have been characterized as typical of bilateral amygdala damage in rats and monkeys whether induced neonatally or in adulthood (Burns, Annett, Kelley, Everitt, & Robbins, 1996; Daenen et al., 2001; Diergaarde et al., 2004; Kalin et al., 2004; Nachman & Ashe, 1974; Prather et al., 2001). Since normal upper ranges of exploratory behavior may be bounded by threat-awareness (e.g., novelty neophobia), these findings may reflect a fundamental deficit of amygdala-mediated cautionary behavior normally provoked by ambiguous contexts or stimuli (Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004a; Kalin et al., 2004; Kalin & Shelton, 2003). In this study, locomotor reactivity to novel contexts was tested twice: first, at the beginning of experimental series in the SI arena, and second, during the initial pre-injection hour of the cocaine sensitization series. Both of these tests identified elevated activation to novel environments in NAML rats, suggesting the robustness and persistence of this trait. Consistent with distributed roles of temporal–limbic structures (e.g., both amygdala and ventral hippocampus) in modulating novelty reactivity, increased responsiveness to contextual novelty has also been identified in adult rats with neonatal ventral hippocampus lesions (NVHLs; Lipska et al., 1993). However, this effect is often minimal or inconsistent in NVHLs (Chambers & Taylor, 2004; Conroy et al., 2007), while NAMLs more powerfully augment novel open field activity when directly compared with NVHLs (Daenen et al., 2001).
That NAMLs produce reductions in fear-related behaviors was also suggested by elevations in time spent in the anxiogenic open arm of the EPM and failure of predator odor pre-exposure to suppress SI to the extent observed in SHAMs. In contrast, adult-onset lesions to the central nucleus of the amygdala do not appear to produce differential EPM responding in rats with such lesions compared with controls at baseline, although pre-exposure to restraint stress suppresses open arm entries in control but not adult-lesioned rats (McHugh, Deacon, Rawlins, & Bannerman, 2004; Moller, Wiklund, Sommer, Thorsell, & Heilig, 1997). The SI series demonstrated that neither gross deficits in SI per se nor generalized impairments in the capacity of all pre-test stressors to suppress SI can account for the failure of predator odor to suppress SI in NAML rats. Differential forms and severities of psychological stress produce differential patterns and extremes of neural activation within the amygdala and other stress-responsive circuits (Crane, French, & Buller, 2005; Takahashi, Nakashima, Hong, & Watanabe, 2005). Our data suggest that NAMLs produce deficits in the generation of fear-responses that are most pronounced under mild to moderately stressful conditions and/or are encoded to some extent by olfactory processing channels. Although the present study did not rule out whether NAMLs produce anosmia, olfactory sensation and some capacity for olfactory-based discrimination and learning are maintained with both neonatal (Sullivan & Wil-son, 1993) and adult-onset amygdala lesions (Shoenbaum, Setlow, Nugent, Saddoris, & Gallagher, 2003). In general, our results are in agreement with work showing that unconditioned fear responding to predator odor is reduced in rats with adult-onset ibotenic acid lesions to the basolateral amygdala (Takahashi et al., 2007).
Elevated short-term sensitization profiles to repeated cocaine injections in NAML rats suggests persistence of hypersensitivity to activating effects of cocaine superimposed on an intact sensitization process across injection days. Although NAML rats showed elevated daily pre-injection activity compared with SHAMs, this activity could not have accounted for elevations in post–cocaine injection activity, given our analysis of daily change in activity post-injection (i.e., post-injection activity minus pre-injection activity), which rendered the injection-induced activation curves of saline-receiving NAML and SHAM rats identical. Also, lesion-based differences in nonhorizontal behavior (i.e., stereotypic behavior) could not have accounted for differences in locomotor sensitization by interfering with locomotion, because cocaine-receiving NAML and SHAM rats showed similar patterns of stereotypic behavior.
Effects of single or repeated injections of cocaine or other psychostimulants in NAML rats have not been previously reported, although increased locomotor responses to single injections of phencyclidine (Daenen, Wolterink, & Van Ree, 2003) have been identified. In contrast with the present results, rats with adult-onset amygdala lesions given a single amphetamine or cocaine injection show increased stereotypic activity without increased horizontal locomotion (E. E. Brown & Fibiger, 1993; Schaub, Schnelzeis, & Mittleman, 1997; Wolf, Dahlin, Hu, Xue, & White, 1995), and sensitization of horizontal locomotion to repeated cocaine injections is actually abolished (Wolf et al., 1995). These apparent differences in effects between neonatal and adult-onset amygdala lesions are consistent with other studies suggesting that the age at which an amygdala lesion occurs can modulate phenotypic outcome (Daenen, Wolterink, Gerrits, & Van Ree, 2002a, 2002b). However, future studies involving differential age testing of sensitization or head-to-head comparisons between subjects with neonatal and subjects with adult-onset amygdala lesions are needed to accurately characterize developmentally specific effects of NAMLs on cocaine sensitization.
In the cocaine challenge session, NAML rats with prior cocaine history showed the greatest activation to a single injection of cocaine compared with their cocaine-history SHAM and saline-history NAML counterparts, demonstrating that NAMLs augment long-term cocaine sensitization. Sensitization has been demonstrated in rodents using a variety of drugs (cocaine, amphetamine, nicotine, opiates, alcohol) that are pharmacologically and psycho-actively divergent but share common capacities to produce dopamine efflux into the ventral striatum and ultimately, addictive behavior (Di Chiara & Imperato, 1988; Wise & Bozarth, 1987). Behavioral sensitization may be analogous to processes of motivational sensitization as a core feature of addictive disease (Robinson & Berridge, 1993), and neuroplastic changes underlying behavioral sensitization occur in cortical–striatal circuits also implicated in reward-motivated operant behavior (Vanderschuren & Kalivas, 2000). Given previous modeling of dual diagnosis syndromes involving temporal–limbic disruptions via the NVHL or olfactory bulbectomy, in which enhancement of novelty reactivity, drug sensitization and self-administration have all been identified (Chambers & Self, 2002; Chambers et al., 2004; Chambers & Taylor, 2004; Holmes et al., 2002), the present results are suggestive of NAML-enhancement of addiction vulnerability that requires confirmation in self-administration studies.
By placing the cocaine injection series between two phases of SI testing, the present study allowed assessment of the contribution of drug history toward altering social behavior as a general component of psychiatric illness. SHAMs with repeated cocaine injection histories showed increased SI compared with their single-cocaine-injection SHAM counterparts, while repeated cocaine injection histories nonsignificantly reduced SI in NAML rats compared with their single injection counterparts. These results suggest that recent cocaine sensitization history can have differential effects on subsequent social behavior, based on underlying neurodevelopmental amygdalar damage. In unlesioned rats, recent cocaine history can impair SI (Costall et al., 1990) and alter levels of stress-related neuropeptide neurotransmitters within the amygdala (Richter & Weiss, 1999; Zhou, Spangler, Ho, & Kreek, 2003). Whether our results reflect NAML-based alterations in neural mechanisms mediating the expression of cocaine withdrawal, tolerance, or sensitization within the domain of social behavior requires further investigation. Examination of the entire sequence of SI tests in only those rats that received repeated cocaine injections does suggest that across pre-conditions that alter social behavior, NAML rats were relatively refractory to pre-conditions that decrease (odor pre-condition) or increase (repeated cocaine injection history) SI in SHAM rats. This pattern is consistent with the putative role of the intact amygdala in flexibly configuring social and other forms of motivated behavior, according to current affective states or learned (e.g. sensitized) affective associations.
Further work is needed to better characterize how NAMLs model aspects of one or more human psychiatric disorders. Currently, only a few studies have examined the NAML syndrome from behavioral (e.g., Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004b; Daenen et al., 2001; Diergaarde et al., 2004) or neurobiological perspectives (Bouwmeester, Snapper, Ronken, Kruse, & Van Ree, 2003; Gerrits, Wolterink, & Van Ree, 2006; Van den Buuse, Garner, & Koch, 2003) with many investigators suggesting NAMLs model social developmental deficits of schizophrenia and/or autism (Daenen et al., 2002b). However, our results and other lines of evidence suggest the NAML syndrome could more directly relate to forms of developmental psychopathology marked most prominently by impulsive behavior rather than by the social deficits and stereotypic behaviors associated with autism. For instance, although our procedural methods likely lacked sensitivity for detecting relatively fine or qualitative differences between groups, we observed neither gross deficits in baseline social behavior and interest nor abnormalities in saline- or psychostimulant-induced rates of stereotypic behavior in NAMLs. Similarly, in NAML monkeys that grow up in groups, social interest and ranges of social behavioral repertoires are not altered per se, but the timing of particular social behaviors are disordered such that either affiliative–approach behavior or fear-related social withdrawal occur at inappropriate junctures (Bauman et al., 2004b). Humans with progressive bilateral amygdalar degeneration can also show a full range of social behaviors and interest but demonstrate alterations in socio-affective perception, poor social decision making, and susceptibility to predatory or unstable relationships (Adolphs, Tranel, & Damasio, 1998; Adolphs, Tranel, Damasio, & Damasio, 1995). Given the similarity of these traits with features of the borderline personality syndrome and recent neuroimaging evidence identifying amygdalar abnormalities in patients with borderline personality syndrome (Rusch et al., 2003; Tebartz van Elst et al., 2003), NAMLs may also model aspects of certain personality disorders, which, like schizophrenia, entail high rates of dual diagnosis.
Regardless of the psychiatric diagnostic specificity of the NAML syndrome, we speculate that it entails mechanisms of addiction vulnerability similar to those implicated in the NVHL model of schizophrenia. Like the ventral hippocampus, the basolateral amygdala sends direct glutamatergic fibers into both the prefrontal cortex and the nucleus accumbens (Baxter & Murray, 2002; Ishikawa & Nakamura, 2003; Kita & Kitai, 1990; Sah et al., 2003). The basolateral nucleus also has reciprocal connectivity with the ventral hippocampus (Pitkanen, Pikkarainen, Nurminen, & Ylinen, 2000), and neural recordings suggest the hippocampus and the amygdala work interactively to modulate frontal–cortical–ventral striatal information processing and learning (Finch, 1996). On the basis of this functional anatomy and the fact that NVHLs produce developmental alterations in frontal–cortical–striatal cellular markers and physiology (O’Donnell et al., 2002), further studies should test whether NAMLs also introduce analogous cortical–striatal alterations leading to enhanced response profiles to addictive drugs.
In summary, these findings contribute to growing data that developmental disturbance of temporal–limbic regions—that may instantiate a neurobiological basis component of several major adult psychiatric syndromes—also confers increased short- and long-term responsiveness to addictive drugs and differential effects on psychiatric-related phenotypes after drug exposure. Further studies using the NAML may be useful for understanding addiction vulnerability in the mentally ill or interpreting clinical data suggesting that history of chronic substance use alters neuropsychiatric illness trajectories and treatment responsiveness (Le Fauve et al., 2004).
Acknowledgments
This work was supported by the U.S. Department of Health and Human Services Public Health Service Grants K08 DA019850-01 (to R. Andrew Chambers), K01 MH01869 (to Tammy J. Sajdyk), and R01 MH65702 (to Anantha Shekhar), and by an internal grant provided by Indiana University (to Joan E. Lafuze). There are no conflicts of interest, financial or otherwise, related directly or indirectly to this article for any of the authors.
References
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Ethical principles of psychologists and code of conduct. Washington, DC: Author; 2002. [Google Scholar]
- Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: Special emphasis on the amygdala. Annals of the New York Academy of Sciences. 2003;985:370–388. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004a;24(3):711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in Rhesus monkeys. Journal of Cognitive Neuroscience. 2004b;16(8):1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore J, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Snapper J, Ronken E, Kruse CG, Van Ree JM. Effects of neonatal amygdala lesions of [125I] neurotensin binding in specific brain areas of adult rat. European Journal of Neuroscience. 2003;17:1319–1322. doi: 10.1046/j.1460-9568.2003.02606.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. American Journal of Psychiatry. 2000;157(1):115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. American Journal of Psychiatry. 1995;152(7):973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology. 1993;113:123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Brown S, Schafer EA. An investigation into the function of the occipital and temporal lobes of the monkey’s brain. Proceedings in Biological Science/Royal Society of London. 1887;43:26. [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: Implications for limbic–striatal interactions. Behavioral Neuroscience. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine associated cues. Journal of Neuroscience. 2003;23(22):8204–8211. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology. 2005;179(2):470–478. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JK, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: An animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27(6):889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Sheehan T, Taylor JR. Locomotor sensitization to cocaine in rats with olfactory bulbectomy. Synapse. 2004;52:167–175. doi: 10.1002/syn.20017. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: Sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biological Psychiatry. 2004;56(5):308–316. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacology Biochemistry and Behavior. 2007;86:386–394. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Onavi ES, Tyers MB. Ondansetron inhibits a behavioral consequence of withdrawing from drugs of abuse. Pharmacology Biochemistry and Behavior. 1990;36(2):339–344. doi: 10.1016/0091-3057(90)90414-d. [DOI] [PubMed] [Google Scholar]
- Crane JW, French KR, Buller KM. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restrain stress. Stress. 2005;8(3):199–211. doi: 10.1080/10253890500333817. [DOI] [PubMed] [Google Scholar]
- Daenen EWPM, Van der Heyden JA, Kruse CG, Wolterink G, Van Ree JM. Adaptation and habituation to an open field and responses to various stressful events in animals with neonatal lesions in the amygdala or ventral hippocampus. Brain Research. 2001;918:153–165. doi: 10.1016/s0006-8993(01)02987-0. [DOI] [PubMed] [Google Scholar]
- Daenen EWPM, Wolterink G, Gerrits MAFM, Van Ree JM. Amygdala or ventral hippocampal lesions at two early stages of life differentially affect open field behavior later in life: An animal model of neurodevelopmental psychopathological disorders. Behavioral Brain Research. 2002a;131(1–2):67–78. doi: 10.1016/s0166-4328(01)00350-3. [DOI] [PubMed] [Google Scholar]
- Daenen EWPM, Wolterink G, Gerrits MAFM, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behavior later in life. Behavioral Brain Research. 2002b;136(2):571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- Daenen EWPM, Wolterink G, Van Ree JM. Hyperresponsiveness to phencyclidine in animals lesioned in the amygdala on day 7 of life. European Neuropsychopharmacology. 2003;13(4):273–279. doi: 10.1016/s0924-977x(03)00029-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey B, Normand ST, Weiss RD, Drake RE, Azeni H. Medical morbidity, mental illness, and substance use disorders. Psychiatric Services. 2002;53(7):861–867. doi: 10.1176/appi.ps.53.7.861. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Gerrits MAFM, Stuy A, Spruijt BM, Van Ree JM. Neonatal amygdala lesions and juvenile isolation in the rat: Differential effects on locomotor and social behavior later in life. Behavioral Neuroscience. 2004;118(2):298–305. doi: 10.1037/0735-7044.118.2.298. [DOI] [PubMed] [Google Scholar]
- Dixon L. Dual diagnosis of substance abuse in schizophrenia: Prevalence and impact on outcomes. Schizophrenia Research. 1999;35(Suppl 1):S93–S100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Science. 2003;985:233–250. [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. Journal of Neuroscience Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudat/putamen and nucleus accumbens. Hippocampus. 1996;6:495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, et al. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Gerrits MAFM, Wolterink G, Van Ree JM. Cerebral metabolic consequences in the adult brain after neonatal excitotoxic lesions of the amygdala in rats. European Neuropsychopharmacology. 2006;16(5):358–365. doi: 10.1016/j.euroneuro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Prefrontal lesion reverses abnormal mesoaccumbens response in an animal model of schizophrenia. Biological Psychiatry. 2004;55(2):172–176. doi: 10.1016/s0006-3223(03)00783-2. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Masini CV, Primeaux SD, Garrett JL, Zellner A, Stogner KS, et al. Intravenous self-administration of amphetamine in a rat model of depression. Synapse. 2002;46(4):4–10. doi: 10.1002/syn.10105. [DOI] [PubMed] [Google Scholar]
- Hull AM. Neuroimaging findings in post-traumatic stress disorder. Systematic review. British Journal of Psychiatry. 2002;181:102–110. [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex of the rat. Journal of Neuroscience. 2003;23(31):9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal CC, Laasko MP, Tiihonen J, Syvalahti E, Vilkman H, Laakso A, et al. The amygdala and schizophrenia: A volumetric magnetic resonance imaging study in first-episode, neuroleptic naive patients. Biological Psychiatry. 2003;54(11):1302–1304. doi: 10.1016/s0006-3223(03)00597-3. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton S, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Annals of the New York Academy of Science. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anterograde and retrograde-horseradish peroxidase study. Neuroscience. 1982;7(10):2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: An update. Pharmacology & Therapeutics. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The epidemiology of dual diagnosis. Biological Psychiatry. 2004;56:730–737. doi: 10.1016/j.biopsych.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Kilts CD. Imagining the roles of the amygdala in drug addiction. Psychopharmacology Bulletin. 2001;35(1):84–94. [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. Journal of Comparative Neurology. 1990;298(1):40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. American Journal of Physiology. 1937;119:352–353. [Google Scholar]
- Le Fauve CE, Litten RZ, Randall CL, Moak DH, Salloun IM, Green AI. Pharmacological treatment of alcohol abuse/dependence with psychiatric comorbidity. Alcohol Clinical and Experimental Research. 2004;28:302–312. doi: 10.1097/01.alc.0000113413.37910.d7. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: Schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RMJ, Rawlins JNP, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behavioral Neuroscience. 2004;118(1):63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Research. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversion, and sodium appetite in rats. Journal of Comparative and Physiological Psychology. 1974;87:622–643. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86–23) Washington, DC: U.S. Government Printing Office; 1986. [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska B. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cerebral Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Groenewegen HJ, Lopez da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioral, electrophysiological, and anatomical data. Progress in Neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Annals of the New York Academy of Science. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106(4):653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- RachBeisel J, Scott J, Dixon L. Co-occurring severe mental illness and substance use disorders: A review of recent research. Psychiatric Services. 1999;50(11):1427–1434. doi: 10.1176/ps.50.11.1427. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drugs of abuse. Journal of the American Medical Association. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;34:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rusch N, van Elst L, Ludaescher P, Wilke M, Huppertz H, Thei T, et al. A voxel-based morphometric MRI study in female patients with borderline personality disorder. Neuroimage. 2003;20(1):385–392. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armenta M, Power J. The amygdaloid complex: Anatomy and physiology. Physiological Reviews. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Schaub CL, Schnelzeis MC, Mittleman G. The effects of limbic lesions on locomotion and stereotypy elicited by dopamine agonists in the rat. Behavioral Brain Research. 1997;84:129–143. doi: 10.1016/s0166-4328(96)00142-8. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8(4):209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of the orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning & Memory. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Role of the amygdala in early olfactory associative learning. Behavioral Neuroscience. 1993;107:254–263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: Structure of the rat brain. 3. New York: Elsevier; 2004. [Google Scholar]
- Sweeten TL, Posey DJ, Shekhar A, McDougle CJ. The amygdala and related structures in the pathophysiology of autism. Pharmacology Biochemistry and Behavior. 2002;71(3):449–455. doi: 10.1016/s0091-3057(01)00697-9. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behavioral Neuroscience. 2007;121(1):100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: A behavioral and neural analysis of predator odor-induced fear. Neuroscience and Biobehavioral Reviews. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: A volumetric magnetic resonance imaging study. Biological Psychiatry. 2003;54(2):163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, Garner B, Koch M. Neurodevelopmental animal models of schizophrenia: Effects on pre-pulse inhibition. Current Molecular Medicine. 2003;3:459–471. doi: 10.2174/1566524033479627. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, Kalivas P. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Volkow ND. The reality of comorbidity: Depression and drug abuse. Biological Psychiatry. 2004;56:714–717. doi: 10.1016/j.biopsych.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biological Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative Physiology Psychology. 1956;4:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: Comparison with N-methyl-D-aspartate antagonists. Neuroscience. 1995;69(2):417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic “binge” cocaine. Brain Research/Molecular Brain Research. 2003;114(1):73–79. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]