Abstract
Perinatal (gestation/lactation) lead exposure modifies the reinforcement efficacy of various psychoactive drugs (e.g., cocaine, opiates) across the phases of initial selection, use, and abuse (Nation et al., 2003; 2004; Rocha et al., 2005). However, changes in sensitivity to methamphetamine across the phases of drug abuse have not been examined in animals perinatally exposed to lead. Because the mainstream popularity of methamphetamine in the United States is increasing and lead exposure continues to be widespread, an examination of this drug and how it may be modified by perinatal exposure to lead is warranted. The studies reported here examined the effects of perinatal lead exposure on adult self-administration of intravenous (i.v.) methamphetamine across the maintenance phase of drug addiction. Experiment 1 examined dose-effect patterns in control and lead-exposed animals. Experiment 2 evaluated control and lead-exposed animals in a progressive ratio task. Female rats were administered a 16-mg lead or a control solution for 30 days prior to breeding with non-exposed males. Exposure continued through pregnancy and lactation and was discontinued at weaning (postnatal day [PND] 21). Animals born to control or lead-exposed dams received indwelling jugular catheters as adults (PND 70) and subsequently were randomly assigned to one of the two studies, using only one male rat per litter for each study. The data showed a general attenuation of the reinforcement efficacy of methamphetamine in animals perinatally exposed to lead, as compared to control animals.
Keywords: dose-effect testing, methamphetamine, progressive ratio, self-administration
Lead toxicity continues to be a major public health concern. Especially in the inner cities and among minorities an alarmingly high percentage of children register blood lead levels that exceed the allowable limits set forth by the Centers for Disease Control and Prevention (Kemp et al., 2007; Mielke, 1999; Pirkle et al., 1998). Developmental lead exposure throughout gestation and lactation (perinatal exposure) reliably has been shown to alter sensitivity to various psychoactive drugs when animals are tested as adults long after the lead-exposure regimen has been discontinued. For instance, perinatal lead exposure has been shown to enhance the rate of acquisition of the self-administration of cocaine (Rocha et al., 2005), produce a shift to the left in the dose effect curve (Nation et al., 2004), and increase vulnerability to reinstatement (Nation et al., 2003). By contrast, developmental lead exposure attenuates the psychoactive effects of heroin (Rocha et al., 2004).
It would be worthwhile to document the interactive relation between early lead exposure and methamphetamine seeking and use. There has been a pronounced increase in methamphetamine use in the United States just in the last few years (Cho and Melega, 2002; Roth and Carroll, 2004). Contributing to growing addictions, hospitalization, etc., is the ease with which the drug is manufactured, and the fact it is relatively cheap to produce.
Environmental exposure vectors that may contribute to the vulnerability to use methamphetamine should be explored. As noted, developmental lead exposure results in behavioral changes across different psychoactive drugs. Because methamphetamine operates in part to interfere with the dopamine transporter and therein enhance dopamine transmission (Ranaldi and Poeggel, 2002), and because lead has a lasting impact at this site (Pokora et al., 1996), it would seem reasonable to examine lead/methamphetamine interactions. Accordingly, the purpose of the present project was to inform on the effects of perinatal lead exposure on methamphetamine dose-effect performance (Experiment 1), and to determine potential differences in progressive ratio responding which is believed to provide an index of motivation to seek the drug (Experiment 2).
EXPERIMENT 1
In Experiment 1, littermates of lead-exposed animals and control animals were tested on a fixed-ratio 2 (FR-2) schedule of reinforcement using a range of methamphetamine doses (.01, .02, .04, .08 mg/kg) to characterize the dose-effect curve (Roth & Carroll, 2004).
Methods
Animals
Animal maintenance and research were conducted in accordance with the guidelines provided by the Texas A&M University Laboratory Animal Care Committee, and the Public Health Service Policy outlined in the publication of the Guide for the Care and Use of Laboratory Animals (1996).
Lead Exposure Regimen
For 30 consecutive days, adult female Sprague-Dawley rats (Harlan; Houston, TX) were exposed once a day to 0-mg lead (sodium acetate) or 16-mg lead (as lead acetate) daily using a 18 ga gavage needle to administer the respective solutions in a volume of 1.0 ml deionized water. This procedure has been used in previous developmental lead studies to ensure stable blood/tissue levels (cf. Nation et al., 2000; 2003; 2004; Rocha et al., 2004). The present lead concentration was selected based on previous studies that found it produces differential behavioral effects while not altering dam body weights or the locomotor ability of pups (see Miller et al., 2000). Following this 30-day toxicant exposure period, females were bred with non-exposed males. Once females tested positive for copulatory plugs, the males were removed from the home cage. Females continued to receive their daily doses of the control solution or lead acetate solution throughout the gestation and lactation periods. Standard rat chow (Teklad; Madison, WI) and tap water were available ad libitum for dams in the home cage. Although some investigators prefer more defined diets during the lead exposure regimen, at the levels of lead used in this study, this has not been an issue. Litters were culled to eight pups on PND 1, and only one pup from each litter was used in the experiment in order to avoid confounds that are sometimes evident in studies involving toxic exposure (Holson and Pearce, 1992).
Tissue Analyses
For control and lead-exposed dams, 100–150 µl of tail-blood was drawn at breeding, parturition (PND 1), and weaning (PND 21). In addition, at the point of termination of the experiment, blood, brain, kidney, liver, and bone (tibia) were harvested from test animals for lead concentration analyses. Littermates of test animals were sacrificed on PND 1 and PND 21, and blood samples were collected for subsequent analyses.
After animals recovered from patency verification, control (Group 0-mg) and lead-exposed (Group 16-mg) test animals were anesthetized with sodium pentobarbital (50.00 mg/kg i.p.). Following collection of blood and tissue samples, lead residues were measured by inductively coupled plasma - mass spectroscopy on a Perkin Elmer DRC 2 instrument. The 208Pb isotope and 209Bi were used as internal standards. Weighted linear calibration was performed with a blank and three external standards (0.05, 20, and 200 parts per billion) and was verified by analyzing NIST SRM 1640 (trace elements in water). Data were acquired in peak hopping mode, using the autolens feature and three replicate reads per determination. Verification of the calibration and baseline were performed after every group of 10 samples and at the end of the analytical run.
Rate of pregnancy did not differ between groups (p>0.05). On PND 21, pups used for testing were weaned and housed individually. All animals were maintained on a 12-hour light/dark cycle. Testing commenced at approximately 10:00 hrs, two hrs into the 12-hr light cycle.
Surgical Procedures
Surgeries were performed at PND 60 which is a point demonstrated to be well within the adult timeframe of behavioral change produced by developmental lead exposure. Using a backplate technique, implantation of chronic indwelling jugular catheters was performed using sterile techniques (Nation et al., 2003; 2004). Rats were anesthetized using a combination of 50 mg/kg ketamine and 20 mg/kg xylazine. A catheter consisting of 0.25-mm ID Silastic tubing (Dow Corning; Midland, MI) was inserted into the right jugular vein and sutured to muscle tissue in the area of the vein. Using an 11-ga stainless steel tube as a guide, the catheter was passed subcutaneously through the body of the animal and exited the back between the scapulae. A backplate consisting of two stainless steel ovals separated by polypropylene mesh (Ethicon; Somerville, NJ) was sutured to muscle tissue below the skin. The backplate accommodated a spring leash, through which the catheter was threaded. Connecting to the backplate at one end, the other end of the leash was connected to a single fluid channel swivel. The swivel design permitted an interlock with separate connecting arms located in the home cage and operant test chambers. The movable arm allowed for free movement and delivery of appropriate solutions in either the home cage or test chamber. A 0.51-mm ID catheter continued from the top of the swivel to an infusion pump that controlled solution delivery. The rats were allowed 7 days to recover from surgery before commencing methamphetamine self-administration testing. During this recovery period, each rat received in the home cage automated hourly i.v. (200 µl) infusions of a sterile saline solution containing heparin (1.25 U/ml). Once methamphetamine self-administration testing commenced, the cannulae were flushed with .20 mls of the heparinized saline solution prior to and following daily test sessions. Each animal continued to receive hourly infusions in the home cage of heparinized saline throughout testing. Catheter patency was checked an equal number of times for each animal during testing, and at the completion of the study, by administering an intravenous infusion of 7.5 mg/kg sodium pentobarbital.
Apparatus
Twelve operant conditioning chambers (Model E-10-10, Coulbourn; Allentown, PA) in sound attenuating cubicles served as the test apparatus. Each chamber had two levers and a stimulus light located above each lever. Infusion pumps (Razel Scientific Instruments; Stamford, CT) controlled drug delivery to each of the boxes. A 20-ml syringe delivered i.v. infusion (160 µl) over a 6.00 sec time frame. The system was interfaced with 2 IBM computers, each controlling drug delivery and recording data from 6 chambers.
Procedure
All control (n=7) and lead-exposed (n=8) test animals were shaped to lever press for an infusion of .04 mg/kg methamphetamine on a FR-1 schedule where each depression of the right (active) lever activated the 20-ml syringe infusion pump and resulted in an infusion of methamphetamine and simultaneous illumination of the stimulus light above the lever. Shaping under conditions of methamphetamine reinforcement continued for 5 days. Lever responses on the left (inactive) lever were recorded but had no programmed consequences. All sessions were 2 hrs in duration and squads were run 7 days a week.
For 7 days after the shaping phase, all animals were trained on a FR-2 reinforcement schedule under which delivery of .04 mg/kg of methamphetamine served as the baseline-training dose. Dose–effect testing began the day immediately following the final day of baseline testing.
During the period of dose–effect testing, drug was available for two successive days at each dose in descending order (.08, .04, .02, .01, saline). A descending pattern of drug doses was selected for testing in order to minimize the chances of extinction of the self-administration response. The findings showed that during consecutive 2-hour daily baseline sessions FR-2 schedule responding for .04 mg/kg methamphetamine varied less than 20% for animals in each group, across sessions.
Results
Body Weights
Body weights for animals during dose-effect testing did not differ between groups (mean body weights= 357g±5.58 and 364g±7.49 for Groups 0-mg and 16-mg, respectively; p< .05). Weekly fluctuations did occur but the pattern of change remained uniform across Groups.
Methamphetamine Self-administration
Baseline stability
Table 1 presents the mean right (active) lever responses and SEM values (in parenthesis) over the respective 2-day baseline training sessions that preceded each testing dose for control (Group 0-mg) and lead-exposed (Group 16-mg) animals. It is apparent that significant shifts in baseline responding did not occur over the course of dose–effect testing for either group, and that self-administration responding at the baseline dose of .04 mg/kg was essentially the same for Group 0-mg and Group 16-mg (average range). The finding of a nonsignificant Group main effect provided statistical confirmation to this effect (p>.05).
Table 1.
Mean active (right) lever responses and (SEM) values over the respective 2-day baseline training sessions that preceded each testing dose for control (Group 0-mg) and lead-exposed (Group 16-mg) animals (Experiment 1).
| Saline | .01 | .02 | .04 | .08 | |
|---|---|---|---|---|---|
| Group | 61.80 | 76.02 | 66.75 | 71.30 | 66.67 |
| 0-mg | (6.62) | (11.12) | (4.89) | (6.46) | (5.98) |
| Group | 52.01 | 74.83 | 65.98 | 63.92 | 65.83 |
| 16-mg | (6.10) | (6.98) | (4.63) | (4.36) | (4.93) |
Dose-effect Data
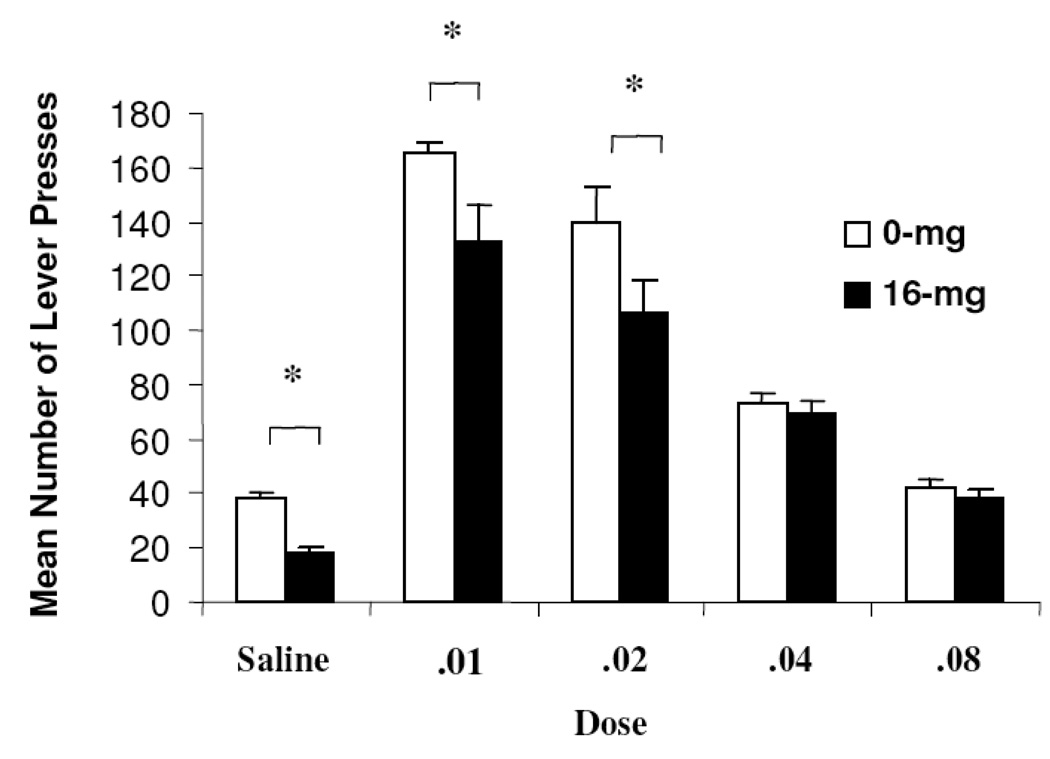
Active methamphetamine self-administration responding is depicted graphically in Figure 1. The statistical unit was the average number of lever responses for each animal across the two test sessions at each dose of methamphetamine. At all methamphetamine doses over the course of dose-effect testing, Group 16-mg animals responded less frequently than Group 0-mg animals. The findings from a Group × Dose repeated-measures ANOVA performed on active lever responding revealed significant main effects for Group (F (1, 13)=13.03, p<0.05) and Dose (F (4, 52)=97.89, p<0.05). The interaction test failed to reach significance (F (4, 52)=1.93, p>0.05). As is visually apparent from Figure 1 the main effect differences were due to lower self-administration rates in Group 16-mg animals relative to Group 0-mg animals, and higher rates of responding at the .01 mg/kg dose and the .02 mg/kg dose relative to other doses. Confirming results were provided by planned simple effects analyses where group differences were significant at saline (F (1, 14)=38.91, p<0.05), .01 mg/kg (F (1, 14)=4.88, p<0.05), and .02 mg/kg (F (1,14)=3.67, p<0.05) doses.
Figure 1.
Mean (SEM) active lever responses for Group 0-mg (n=7) and 16-mg (n=8) at each dose (mg/kg) of methamphetamine during dose-effect testing (Experiment 1. The asterisk indicates that control and lead-exposed animals were significantly different (p< 0.05).
In terms of the analysis of inactive lever responding, neither the main effect for Group nor the Group × Dose interaction was found to be significant. Moreover, active lever responding was greater than inactive lever responding at every test dose (p<0.05).
As noted, at the end of Experiment 1 each animal in both exposure conditions received an i.v. infusion of 7.50 mg/kg sodium pentobarbital. Catheter patency was verified by rapid onset of brief anesthesia. Each of the animals included in this report had open catheters throughout the study.
Tissue Analyses
Table 2 presents the mean (SEM) blood lead concentrations, a conventional marker of lead toxicity, for non-exposed (Group 0-mg) and metal-exposed (Group 16-mg) dams at breeding, 10 days of gestation, parturition, and weaning. Blood lead residue values are shown for littermates at PND 1 and PND 21, as well as for test animals at the termination of the experiment. Also, lead concentrations in tissue (i.e. brain, kidney, liver, and tibia) are indicated for dams sacrificed at weaning.
Table 2.
Mean (SEM) blood and tissue lead concentration values for dams, littermates and test animals (Experiment 1).
| Blood Lead Concentration (µg/dl) | ||
|---|---|---|
| Group 0-mg | Group 16-mg | |
| Dams | ||
| Breeding | 2.0 (.002) | 30.7 (.023) * |
| Gestation Day 10 | 1.6 (.004) | 40.5 (.082)* |
| Parturition (PND 1) | 1.6 (.002) | 48.7 (.054)* |
| Weaning (PND 21) | .003 (.001) | 24.4 (.038) * |
| Littermates | ||
| PND 1 | 1.7 (.004) | 70.4 (.058) * |
| PND 21 | 1.1 (.004) | 14.6 (.019) * |
| Test Animals | ||
| Termination | < .5 | < .5 |
| Tissue Concentrations of Dams at Weaning (µg/g) | ||
| Group Lead-0 | Group Lead-16 | |
| Brain | .016 (.001) | .386 (.051) * |
| Kidney | .021 (.006) | 8.11 (.90) * |
| Liver | .130 (.001) | .504 (.09) * |
| Tibia | .012 (.034) | 97.22 (7.57) * |
The symbol indicates that control and lead-exposed animals were significantly different (p<.05).
By the completion of dose-effect testing, significant traces of lead in metal-exposed test animals were observable only in tibia (p< .05). Blood lead levels of lead-exposed animals had returned to control levels at the termination of testing, i.e., in both groups lead levels were below detectable limits (<.5 µg/dl).
EXPERIMENT 2
The findings from Experiment 1 reflect a general decrease in the self-administration of methamphetamine among animals developmentally exposed to lead, at least at lower doses of the drug. In other animal studies of hedonic drug use, such a behavioral pattern is characteristic of diminished receptor activity and may derive from the decreased reinforcement potency of the drug (Caine and Koob, 1994). It is unclear from Experiment 1 what caused the group separation that was evident at the lower doses of methamphetamine to disappear when higher doses of methamphetamine were tested. Higher doses of methamphetamine that fall on the descending limb of the dose-effect curve may have surmounted lead effects that were responsible for the decreased responding in lead-exposed animals at lower, and perhaps less reinforcing, doses of the drug. Also, higher doses of methamphetamine may have produced satiation in both control and lead-exposed animals at a similar rate. Finally, it must be considered that low-rate FR schedules of reinforcement (e.g., FR-2) may not offer more than a general index of rate of drug intake in a dose-effect context.
In an effort to clarify the interactive effects between perinatal lead exposure and methamphetamine self-administration during the adult cycle, Experiment 2 employed a progressive ratio (PR) task in which a more challenging schedule of reinforcement was required to obtain i.v. methamphetamine deliveries. The PR schedule of reinforcement has been cited as offering a more direct measure of the reinforcement efficacy of a given dose of a drug, than is possible with FR schedules that require low rates of responding for each presentation of the reinforcer (Arnold and Roberts, 1997).
Methods
Animals
As in Experiment 1, female rats were gavaged daily with 0-mg or 16-mg lead for 30 days prior to breeding with non-exposed males. Metal administration continued through gestation and lactation and was discontinued at weaning (PND 21).
Tissue was collected as described in Experiment 1.
Surgical Procedures
Because of limited equipment access, surgeries for Experiment 2 were performed at PND 60 using an indwelling jugular catheter that was anchored on the skull of the rat, rather than the back as in Experiment 1. As was the case for the backplate method employed in Experiment 1, the headplate surgical procedures for Experiment 2 were performed under aseptic conditions using a combination of intraperitoneal (i.p.) injections of ketamine (60 mg/kg) and xylazine (20 mg/kg) as the anesthetic. Immediately after surgery rats were administered an intramuscular (i.m.) injection of penicillin g potassium (250,000 U/ml). Following implantation of the catheter into the jugular vein, the tubing was passed subcutaneously (s.c.) to the skull of the rat. Using a stereotaxic instrument to secure the animal, a pedestal was constructed of dental acrylic fixing the assembly to the skull. The rat was allowed 7 days recovery from surgery before testing began. During this recovery period, each rat received daily i.v. infusions (0.1 ml) of sterile saline solution containing heparin (1.25 U/ml). Cannulae were flushed daily with heparin saline both prior to and following each testing session.
Apparatus
Sixteen operant conditioning chambers (Med Associates, ENV-001; St. Albans, VT) in sound-attenuating cubicles served as the test apparatus. Each chamber had two levers and a stimulus light located above each lever. Infusion pumps (model A with 1-rpm motors, Razel Scientific Instruments; Stamford, CT) controlled drug delivery to each of the boxes. A 20-ml syringe delivered i.v. infusions (100 µl) over a 12.0-s time frame.
All animals received free access to food and water for 7 days while recovering from surgery. Uncontaminated water was available ad libitum throughout the study. Animals were weighed daily prior to testing. Food was placed in home cages following the end of each daily testing session.
Procedure
Shaping procedures and baseline training followed the format of Experiment 1, i.e., methamphetamine (.04 mg/kg) was used to shape group 0mg (n=7) and group 16-mg (n=7) animals for 5 days on a FR-1, at which point all animals were switched to a FR-2 schedule. After stability was obtained (responding varied less than 20% across sessions), animals were shifted to the PR schedule for a two-day testing session at each dose. Prior to each PR test at each dose, animals were returned to two consecutive days of baseline responding on a FR-2 for methamphetamine (i.e., .04 mg/kg/infusion) in order to assess changes in tolerance that may have occurred following chronic administration of the psychostimulant. As with Experiment 1, baseline responding varied less than 20% for each animal in each exposure condition.
The PR schedule followed closely the procedures outlined by Duvauchelle et al (1998), as well as those summarized in a previous report by Hubner and Koob (1990). The PR schedule used in this investigation involved an exponential equation in which the reinforcement number is a natural logarithmic function of the ratio value: ratio =5 × exp (reinforcer number × 0.2) –5. For example, for 16 reinforcements, the ratio progresses as follows: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118.
The PR session ended according to which occurred first; 3 hrs or when the animal failed to complete the ratio for a particular reinforcer (dose) within 1 hr from the delivery of the previous reinforcer. For example, an animal completing a final ratio of 5 received a total of 5 drug infusions, completed 9 lever presses within an hour, and pressed a total of 22 times during the PR session for that day. For both group 0-mg and group 16-mg animals, a within-subjects assessment procedure was administered with animals receiving doses of vehicle (i.e., heparinized saline), .01, .02, .04, .08 mg/kg per infusion methamphetamine in a descending order, for 2 consecutive days. As indicated, baseline testing (i.e., 2-hrs, on a FR-2 for .04 mg/kg methamphetamine) for 2 consecutive days was used to measure criterion stability between PR testing sessions. The mean total number of lever presses executed during each 2-day PR testing session was used as the measure of drug efficacy for each dose. Catheter patency was assessed daily by flushing the catheter with 0.1 ml of a sterile saline solution containing heparin (1.25 U/ml). The mean total number of lever presses for each 2-day testing session at each dose (saline, .01, .02, .04, .08) of methamphetamine, were analyzed using analysis of variance (ANOVA) tests.
Results
Body Weights
Body weights for animals during progressive ratio testing did not differ between groups (mean body weights= 364g±7.12 and 373g±8.42 for Groups 0-mg and 16-mg, respectively; p> .05). Weekly fluctuation did occur but the pattern of change remained stable across groups.
Progressive Ratio Data
Baseline stability
Table 3 presents the mean and SEM baseline values of right (active) lever responses during the 2-hr baseline session that preceded each PR testing session at each dose. As was the case for dose-effect testing in Experiment 1, baseline performance remained stable for each exposure condition throughout the period of testing and the groups were not significantly different (p>.05).
Table 3.
Mean and (SEM) values for average number of right (active) lever responses during the 2-day baseline session that preceded each respective PR testing session at each dose (Experiment 2).
| Saline | .01 | .02 | .04 | .08 | |
|---|---|---|---|---|---|
| Group 0- mg |
46.18 (9.09) |
46.67 (5.19) |
43.86 (4.48) |
47.19 (7.38) |
46.88 (5.02) |
| Group 16- mg |
49.50 (8.63) |
34.02 (6.20) |
44.33 (5.76) |
41.30 (3.20) |
49.16 (7.31) |
Total Number of Lever Presses
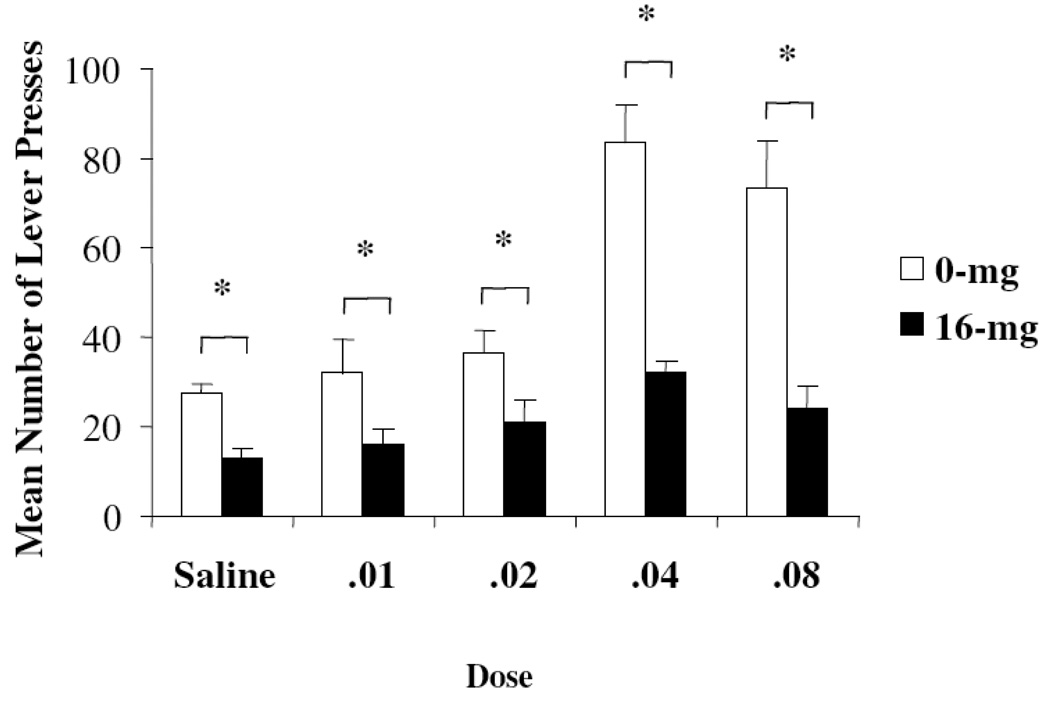
Figure 2 shows the respective total mean number of lever presses on the right (active) lever for Groups 0-mg [n=7] (±) and 16-mg [n=7] (±) at the different doses of methamphetamine reinforcement. The results of the Group × Dose repeated-measures ANOVA on total number of lever presses revealed a significant main effect for Group (F (1,12)=34.01, p<0.05) as well as a significant main effect for Dose (F (4, 48) =21.49, p<0.05). In addition, a significant Group × Dose interaction reached an acceptable level for statistical significance (F(4,48)=7.31, p<0.05). Individual comparisons indicated the main effect for Group was the result of lower self-administration rates in Group 16-mg animals, and the Dose effect was due to increased responding with higher doses of methamphetamine. The interaction was produced by greater separation between Group 0-mg animals and Group 16-mg animals at the two highest doses of the drug relative to other comparisons (all p values <0.05). As in Experiment 1, virtually no responses were committed to the inactive lever and catheter patency was confirmed for all animals at the end of the experiment.
Figure 2.
Mean (SEM) total number of active lever responses for Groups 0-mg (n=7) and 16-mg (n=7) at each methamphetamine dose (mg/kg) tested during the progressive-ratio procedure (Experiment 2). The asterisk indicates that control and lead-exposed animals were significantly different (p<0.05).
Tissue Analyses
The relevant dam and littermate tissue data for animals in Experiment 2 are the same as presented in Experiment 1 (see table 2). Blood lead levels of lead-exposed animals had returned to control levels by the completion of testing, i.e., in both groups lead levels were below detectable limits (<.5 µg/dl). As in Experiment 1, by the completion of progressive-ratio testing, significant traces of lead in metal-exposed test animals were observable only in tibia (p< .05).
Discussion
The results of Experiment 1 showed an inverted U-shaped function for both group 0-mg animals and group 16-mg animals with respect to response rates at different doses of methamphetamine. That is, for both conditions animals first increased self-administration as methamphetamine doses increased and then with further increases in dose responding systematically declined. There was evidence, however, that response rates were lower among lead-exposed animals relative to controls at lower drug doses, and even at the saline infusion level. In Experiment 2, it was observed that group 16-mg animals exhibited dramatically lower completed ratios than group 0-mg animals at all test doses where methamphetamine served as the reinforcement outcome within a progressive ratio preparation. These data are suggestive of an inverse interactive relation between methamphetamine and developmental lead exposure. Note that lead-based impairment of motor function was not likely a determining feature of the present findings since in Experiment 2 lever pressing did not differ across groups at the higher doses of a methamphetamine prime. The clinical relevance of these findings derive from recent reports that show adult minorities and their children living in urban areas have elevated blood lead levels that fall in the range of levels reported here for dams and pups at PND 21 (Kemp et al., 2007; Pirkle et al., 1998).
The findings in both experiments of lower self-administration of saline in group 16-mg animals is not altogether surprising given this test was conducted last. It is quite possible that the greater efficacy of methamphetamine reinforcement in group 0-mg animals at prior doses of the drug during dose-effect testing translated into greater conditioned (contextual) responding for saline in these animals relative to lead-exposed animals. The rationale is that feed-forward (Pavlovian) mechanisms (conditioned stimuli) operated to create a greater drug context for group 0-mg animals, and thus the conditioned cues for saline self-administration were stronger for these animals.
It is noteworthy that the patterns apparent in Experiments 1 and 2 are consistent with findings reported in an earlier study completed in this laboratory. Rocha et al. (2008) found that rats exposed developmentally to the identical lead treatment employed here were slower to acquire self-administration responding for a .02 mg/kg methamphetamine infusion than their control counterparts. Moreover, similarly exposed animals were less likely than controls to reinstate responding in an extinction context when administered a drug (methamphetamine [i.p.]) prime. So, converging evidence underscores the notion of a profile of developmental lead-induced attenuation of methamphetamine reinforcement, and in this regard the data agree with earlier published data showing attenuation of intravenous self-administration of heroin by developmental lead exposure (Rocha et al., 2004). However, it is noted that an increase in sensitivity to the reinforcing properties of cocaine, i.e., a displacement to the left in the dose-effect curve has been found (Nation et al., 2004) and increased reinstatement occurs among lead-exposed animals relative to controls (Nation et al., 2003). At this juncture it is not possible to explain why such differences across drugs occur, but whatever the causes the patterns appear to be very reliable. Obviously, metal/drug interactions appear to involve complex neurologic dynamics that are peculiar to the particular parameters of the investigation. In an effort to elucidate why such reliable phenomenological differences would occur, mechanistic studies are currently underway in our laboratory.
Along these lines, it may be instructive to briefly speculate on possible mechanisms involved in the present experiments. Numerous studies have shown that noncontingent (experimenter delivered) injections of methamphetamine decrease dopamine transporter (DAT) levels (e.g., McCann et al., 1998; Volkow et al., 2001). More directly relevant to the present investigation, recently it has been observed that methamphetamine self-administration resulted in an increase in tyrosine hydroxylase in the mesolimbic dopamine system (Shepard et al., 2006). Because these changes are associated with the reinforcement properties of methamphetamine, and inasmuch as lead exposure is known to antagonize mesolimbic dopamine systems (cf. Cory-Slechta, 1995; Pokora et al., 1996), it is reasonable to assert that the attenuation of methamphetamine reinforcement in the present studies derived at least partly from the effects of developmental lead exposure on dopamine function. Importantly, it would appear that such effects are long-lasting and potentially permanent (cf. Miller et al., 2000). What remains at issue, however, is why such disturbances in dopamine activity would not occasion similar attenuation of cocaine sensitivity (see above), which also is modulated by dopamine.
Another possible neural pathway that may contribute to developmental lead/ methamphetamine interactions involves the hypothalamo-pituitary-adrenal (HPA) axis. It has been reported that yohimbine, a competitive alpha2-adrenergic receptor antagonist, reinstates methamphetamine seeking in a reinstatement model (Shepard et al., 2004), and that the corticotrophin-releasing hormone antagonist CP-154, 426 attenuates methamphetamine-induced reinstatement of self-administration responding (Moffett and Goeders, 2007). Related to these findings, it is known that stimulation of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) by the GABAB receptor agonist baclofen decreases methamphetamine self-administration (Ranaldi and Poeggel, 2002). Insofar as lead disturbs these neurochemical modulatory events (see Lasley and Gilbert, 2002), it must be considered that such perturbations may have been instrumental in producing the present pattern of results. Also, it should be understood that because gavage procedures were used in the present experiments for administration of lead, differential stress-related differences in HPA activity may have contributed to the effects observed here. However, inasmuch as both control and lead-exposed dams received equivalent gavage treatments it is not likely that differing levels of stress were chief determinants of our present or former findings.
Whatever the dynamics underlying developmental lead/methamphetamine interactions, the practical implications of the behavioral profile reported here must be considered. Attenuation of the reinforcement effects of psychoactive drugs in general may define a form of tolerance and this could alter patterns of drug use/abuse.
Acknowledgements
This research was supported by United States Public Health Grants DA13188 and MH65728. All aspects of the research reported here were conducted in accordance with the guidelines provided by the Texas A&M University Laboratory Animal Care Committee, and the Public Health Service Policy outlined in the publication of the Guide for the Care and Use of Laboratory Animals (1996).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Therap. 1994;270:209–218. [PubMed] [Google Scholar]
- Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter systems. Ann Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- Duvauchelle C, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/s0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparious species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res. 1990;508:20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- Kemp FW, Neti PV, Howell RW, Wenger P, et al. Elevated blood lead concentrations and vitamin D deficiency in winter and summer in young urban children. Environ Health Perspect. 2007;115:630–635. doi: 10.1289/ehp.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME. Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci. 2002;66:139–147. doi: 10.1093/toxsci/66.1.139. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosc. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milke HW. Lead in the inner cities. Amer Sci. 1999;87:62–73. [Google Scholar]
- Miller DK, Nation JR, Jost TE, Schell JB, Bratton GR. Differential effects of adult and perinatal lead exposure on morphine-induced locomotor activity in rats. Pharmacol Biochem Behav. 2000;67:281–290. doi: 10.1016/s0091-3057(00)00362-2. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacol. 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Nation JR, Cardon AL, Heard HM, Valles R, Bratton GR. Perinatal lead exposure and relapse to drug-seeking behavior in the rat: a cocaine reinstatement study. Psychopharmacol. 2003;168:236–243. doi: 10.1007/s00213-003-1405-2. [DOI] [PubMed] [Google Scholar]
- Nation JR, Miller DK, Bratton GR. Developmental lead exposure alters the stimulatory properties of cocaine at PND 30 and PND 90 in the rat. Neuropsychopharmacol. 2000;23:444–454. doi: 10.1016/S0893-133X(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Nation JR, Smith KR, Bratton GR. Early developmental lead exposure increases sensitivity to cocaine in a self-administration paradigm. Pharmacol Biochem Beh. 2004;77:127–135. doi: 10.1016/j.pbb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, et al. Exposure of the U.S. population to lead, 1991–1994. Environm Health Perspect. 1998;11:745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokora MJ, Richfield EK, Cory-Slechta DA. Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: time course of effects and interactions with chronic dopamine agonist treatments. J Neurochem. 1996;67:1540–1550. doi: 10.1046/j.1471-4159.1996.67041540.x. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Poeggel K. Baclofen decreases methamphetamine self-administration in rats. NeuroReport. 2002;13:1107–1110. doi: 10.1097/00001756-200207020-00007. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Bratton GR, Nation JR. Developmental lead exposure alters methamphetamine self-administration in the male rat: acquisition and reinstatement. Drug Alcoh Depend. 2008 doi: 10.1016/j.drugalcdep.2007.12.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, Nation JR. Self-administration of heroin in rats: effects of low-level lead exposure during gestation and lactation. Psychopharmacol. 2004;174:203–210. doi: 10.1007/s00213-003-1742-1. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, Nation JR. Enhanced acquisition of cocaine self-administration in rats developmentally exposed to lead. Neuropsychopharmacol. 2005;30:2058–2064. doi: 10.1038/sj.npp.1300729. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacol. 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychi. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacol. 2006;185:505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Amer J Psychi. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]