Abstract
Structural brain change and concomitant cognitive decline are the seemingly unavoidable escorts of aging. Despite accumulating studies detailing the effects of age on the brain and cognition, the relationship between white matter features and cognitive function in aging have only recently received attention and remain incompletely understood. White matter microstructure can be measured with diffusion tensor imaging (DTI), but whether DTI can provide unique information on brain aging that is not explained by white matter volume is not known. In the current study, the relationship between white matter microstructure, age and neuropsychological function was assessed using DTI in a statistical framework that employed white matter volume as a voxel-wise covariate in a sample of 120 healthy adults across a broad age range (18–83). Memory function and executive function were modestly correlated with the DTI measures while processing speed showed the greatest extent of correlation. The results suggest that age-related white matter alterations underlie age-related declines in cognitive function. Mean diffusivity and fractional anisotropy in several white matter brain regions exhibited a non-linear relationship with age, while white matter volume showed a primarily linear relationship with age. The complex relationships between cognition, white matter microstructure, and white matter volume still require further investigation.
Introduction
The structural brain changes and accompanying cognitive changes that occur with aging are incompletely understood. Though early cell count studies in neocortex suggested that normal aging was accompanied by extensive neuronal loss (H. Brody, 1955; H. Brody, 1970; Devaney & Johnson, 1980; Henderson, Tomlinson, & Gibson, 1980), recent evidence suggests the loss is significantly less dramatic than previously thought (Pakkenberg & Gundersen, 1997; Terry, DeTeresa, & Hansen, 1987). Age related volume decline appears to be a consequence of additional factors that include changes in synaptic density (Masliah, Mallory, Hansen, DeTeresa, & Terry, 1993), shrinkage of neurons (Terry et al., 1987), and white matter decline (Tang, Nyengaard, Pakkenberg, & Gundersen, 1997). Several recent studies using magnetic resonance imaging support the notion that age-related white matter change is especially related to age-associated cognitive change — including volumetric studies (Brickman et al., 2006; Paul et al., 2007), studies of white matter hyperintensities, evident as signal change on T2 weighted imaging (Au et al., 2006; Baum, Schulte, Girke, Reischies, & Felix, 1996; de Groot et al., 2000; Gunning-Dixon & Raz, 2003; Raz, Rodrigue, Kennedy, & Acker, 2007), and studies employing diffusion tensor imaging (DTI) to assess age-related microstructural changes in white matter function (Charlton et al., 2006; Deary et al., 2006; Grieve, Williams, Paul, Clark, & Gordon, 2007; O'Sullivan et al., 2001; Persson et al., 2006; Shenkin et al., 2005).
Diffusion tensor imaging (DTI) is sensitive to the random, diffusion-driven displacements of molecules, providing the capability to investigate tissue structure at a microscopic scale (Basser, 1995; Basser & Pierpaoli, 1996). Two typically reported indices derived from diffusion weighted imaging are mean diffusivity (MD), a measure of the overall displacement of water molecules during a specific time interval, and fractional anisotropy (FA), a measure of the directionality of diffusion. With aging, white matter MD tends to increase, and FA tends to decrease (Abe et al., 2002; Engelter, Provenzale, Petrella, DeLong, & MacFall, 2000; Pfefferbaum et al., 2000; Salat et al., 2005). This pattern has been reported in multiple brain areas, with several studies supporting an anterior to posterior gradient of change in aging (Head et al., 2004; Pfefferbaum, Adalsteinsson, & Sullivan, 2005; Sullivan, Adalsteinsson, & Pfefferbaum, 2006; Yoon, Shim, Lee, Shon, & Yang, 2007).
A typical method of DTI analysis uses a region of interest (ROI) approach where specific brain structures are traced and diffusion values are extracted. While ROI methods have the advantage of data sampling from white matter tracts in the native space of the individual, the effects of age are diffuse and it may be beneficial to evaluate all white matter tracts simultaneously. Voxel-wise analyses of brains in a common stereotactic space provide the opportunity to assess the relationship between cognition and white matter microstructure over the entire brain, without restricting analyses to predefined ROIs. This is particularly appealing in studies of white matter because there are fewer discrete boundaries or landmarks, and thus some (though certainly not all) white matter ROIs can be somewhat arbitrary. For example, using voxel-wise analysis of fractional anisotropy maps as opposed to ROI based methods, Grieve et al. (Grieve et al., 2007) found a relationship between executive function and FA in frontal white matter, but also found unexpected relationships with parietal white matter and fibers projecting to the thalamus.
Although DTI measures are purported to reflect the microstructural properties of tissue, brain volume may confound DTI results and make it difficult to interpret diffusion changes as representing a measure above and beyond the effects of atrophy. One solution to this problem is to employ an integrated approach that statistically accounts for multiple imaging modalities in addition to cognitive and demographic variables (Casanova et al., 2007; Oakes et al., 2007). An integrated approach allows testing of more sophisticated models of brain aging, and better characterization of the relative contribution of differing imaging modalities to brain and cognitive aging.
In the current study, the relationship between white matter microstructure and function in aging was evaluated using a cross-sectional design in a large group of adults spanning in age from early to late adulthood. We employed an integrated approach that allowed us to examine microstructural white matter alterations that were independent of white matter volume loss on a voxel by voxel basis using the Biological Parametric (BPM) toolbox (Casanova et al., 2007). We hypothesized that tests that are typically considered measures of executive function would be related to the integrity of frontal white matter tracts. Tests of memory, we hypothesized, would be related to temporal white matter microstructure. However, because the effects of age are diffuse, a voxel-wise approach was adopted to examine the relationship between age, cognition, and white matter microstructure over the entire brain.
Methods
Participants
Three hundred and nine healthy non-patient participants were recruited and received neuropsychological testing and MRI brain scanning as part of ongoing studies of aging, traumatic brain injury, and Alzheimer’s disease (AD). In order to limit our analyses to cognitively normal participants, 32 individuals were excluded based on one or more scores that fell one standard deviation below the mean or more on a test of memory and/or executive function, reducing our sample to 277 participants. Our lab recruits and attracts a high number of participants between the ages of 40 and 65 with parental family history of Alzheimer’s disease (a risk factor for AD). In order to ensure that our sample reflected a normally aging population, we attempted to match the prevalence of family history and presence of ApoE ε4 in these participants to the prevalence of these risk factors in the general population. Currently, there are no published prevalence estimates for parental family history of AD; so family history prevalence in our sample was matched to reported prevalence rates of AD in individuals 65 years of age and older (Evans et al., 1989), approximately 10% of the population. Participants with reported and or confirmed family history of AD were removed from the sample using the RAND function in Microsoft Excel until only 10% of the participants had a confirmed or reported positive family history. The allele frequency of ApoE ε4 differs drastically depending on the population studied (Corbo & Scacchi, 1999); based on those participants for whom genetic data was available, our sample contained a 17% allelic frequency of ApoE ε4 following adjustment for family history of AD. This prevalence of ε4 is similar to that found in Northern countries of Western Europe (Lucotte, Loirat, & Hazout, 1997).
Removing people with a positive family history reduced our sample from 277 to 223 participants. These two-hundred and twenty-three participants [89 males/134 females, (206 Caucasian, 8 African American, 5 Asian, 2 Hispanic, 2 Other), mean age = 49.62 years, (SD = 15.12); age by decade distribution: under 20 yrs = 6, 20–29 yrs = 36, 30–39 yrs = 7, 40–49 yrs = 30, 50–59 yrs = 85, 60–69 yrs = 54, 70–79 yrs = 4, over 80 yrs = 1] all had useable T1-weighted images and were included in analyses of brain volume. Of this larger group of participants, a subset of one-hundred and twenty people [49 males, 71 females, (107 Caucasian, 7 African American, 2 Asian, 1 Hispanic, 1 Other), mean age 52.35 (SD = 14.68), age by decade distribution: under 20 yrs = 5, 20–29 yrs = 12, 30–39 yrs = 6, 40–49 yrs = 10, 50–59 yrs = 49, 60–69 yrs = 33, 70–79 yrs = 4, over 80 yrs = 1] possessed both useable T1-weighted images and diffusion tensor images and were used for the behavioral and voxel-wise analysis of cognitive function. A summary of the demographics for the larger group with T1-weighted images and the subset of people with both T1-weighted images and diffusion tensor images are presented in Table 1.
Table 1.
Demographics
| Group | N | Data Analyzed | Age Mean (SD) Range |
Sex Male/Female |
Education Mean (SD) Range |
|---|---|---|---|---|---|
| Large Sample | 223 | T1 | 49.62 (15.12), 18–83 yrs | 89/134 | 16.15 (2.4), 12–20 yrs |
| Subset of Large Sample | 120 | T-1, DTI, and Behavioral | 52.35 (14.68), 18–83 yrs. | 49/71 | 16.08 (2.39), 12–20 yrs. |
Neuropsychological Testing
On the day of the scan, participants received comprehensive neuropsychological testing. We report the results of analyses on nine of the neuropsychological test scores here, including: BVMT (Brief Visuospatial Memory Test, Revised), total raw score, and delayed recall (Benedict, 1997) to assess visuospatial learning and memory; COWAT (Controlled Oral Word Association Test; Benton, Hamsher, & Sivan, 1983), raw score to assess verbal fluency; Trail Making Test A & B, seconds to complete, (Reitan & Wolfson, 1993) to assess motor speed, sequencing, and vigilance and in the case of Trails B, the additional functions of rapid set shifting, serial retention and integration, verbal problem solving, and planning; Digit Span, raw score (Wechsler, 1997) to assess the ability to hold information in working memory; RAVLT (Rey Auditory Verbal Learning test) total over the five learning trials and 20 minute delayed recall raw scores (Rey, 1964) to assess immediate and delayed verbal memory; and WRAT-III (Wide Range Achievement Test, (Jastak Associates, 1993) reading subtest (WRAT-IIIR) to assess verbal intellectual ability. Due to unforeseen time constraints in the neuropsychological testing session, RAVLT and BVMT scores were unavailable for some participants included in the study. Solutions for dealing with missing test scores are limited, particularly in brain analyses, so participants were only included in an analysis if a score was available; the total number of participants included in the analysis of each specific test are shown in Table 2.
Table 2.
Neuropsychological Test Performance: Correlations with Age
| N | AGE | AGE (Controlling for Education) | |
|---|---|---|---|
| WRATIII-R | 120 | r = .23 (p =.010)** | r = −.18 (p = .099) |
| Digit Span | 120 | r = −.15 (p =.096) | r = −.28 (p = .008)** |
| Trails A | 120 | r = .22 (p =.016)* | r = .35 (p = .001)** |
| Trails B | 120 | r = .18 (p = .053)* | r = .18 (p = .087) |
| COWAT | 120 | r = .08 (p = .411) | r = −.02 (p =.843) |
| RAVLT total | 93 | r = −.28 (p = .006)** | r = −.22 (p = .042)* |
| RAVLT delayed | 93 | r = −.27 (p = .009)** | r = −.20 (p = .06) |
| BVMT total | 118 | r = −.37 (p < .001)** | r = −.29 (p = .007)** |
| BVMT delayed | 118 | r = −.32 (p < .001)** | r = −.27 (p = .010)** |
Significant at the .05 level (2-tailed)
Significant at the .01 level (2-tailed)
Subsets of participants also received additional neuropsychological testing, including CVLT (California Verbal Learning Test, 2nd Edition) Digit Symbol (from WAIS-III), CPT (Continuous Performance Task); and WCST (Wisconsin Card Sorting Test). Scores on these tests were only available for small subsets of the participants and because of the small Ns were not used in the regression analyses of the DTI measures.
Magnetic Resonance Imaging
All participants underwent magnetic resonance on a General Electric 3.0 Tesla SIGNA (Waukesha, WI) MRI system with a quadrature birdcage head coil. Sequences included diffusion-weighted imaging, and high resolution T1-weighted imaging. Many participants also received various fMRI tasks as part of other studies; the fMRI results are reported elsewhere (Johnson et al., 2004; Johnson et al., 2006; Ries et al., 2006; Schmitz, Kawahara, & Johnson, 2004; Trivedi et al., 2007).
Diffusion Tensor Imaging
The diffusion tensor imaging parameters have been published previously (Bendlin et al., 2008). Briefly, DTI was performed using a cardiac-gated, diffusion-weighted, spin-echo, single-shot, EPI pulse sequence, in twelve encoding directions, with 3 averages. The cerebrum was covered using 39 contiguous 3-mm thick axial slices. The acquired voxel size of 2 × 2 × 3 mm was interpolated to 0.9375 mm isotropic dimensions (256 × 256 in plane image matrix). High order shimming was performed prior to the DTI acquisition to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions. Residual spatial distortions from B0 inhomogeneities were corrected using a B0 field map, which was obtained using a pair of non-EPI gradient echo images at two echo times.
T1-weighted Imaging
A 3D T1-weighted image was obtained using an inversion recovery prepared fast gradient echo pulse sequence. The whole brain was imaged in the axial plane with the following parameters: TI = 600 ms; TR = 9 ms; TE = 1.8 ms; flip angle = 20°; acquisition matrix = 256 × 192× 124, interpolated to 256 × 256 × 124; FOV = 240 mm; slice thickness = 1.2 mm (124 slices); receiver bandwidth = ± 16 kHz; acquisition time ~7.5 min.
Diffusion tensor image processing
Image distortions in the DTI data caused by eddy currents were corrected using a 2D affine coregistration function, align linear, in the Automated Image Registration (AIR) software package (http://www.bishopw.loni.ucla.edu/AIR5/). Non-linear image distortion from static field (B0) inhomogeneities was corrected using the acquired field map and the methods of Jezzard and Balaban (Jezzard & Balaban, 1995) implemented in the Prelude (Phase Region Expanding Labeler for Unwrapping Discrete Estimates) and Fugue (FMRIB's Utility for Geometrically Unwarping EPIs) tools from the FSL software suite (S. M. Smith et al., 2004). After distortion corrections, three-dimensional maps of the diffusion tensor and derived measures, mean diffusivity (MD) and fractional anisotropy (FA), were calculated. Participant’s FA maps were normalized to a custom FA template comprised of an average of 121 FA maps acquired from non-patient participants who overlapped with (and matched the demographic composition of) the current study sample. Normalization parameters were derived from the normalization of each individual’s FA image to the FA template (via 12-parameter affine transformation and nonlinear deformation with a 25 mm cut-off and 16 iterations), and then applied to both their FA and MD maps. The normalized images were smoothed using an 8-mm isotropic Gaussian kernel. The white matter mask generated during the cross-sectional analysis of the T1-weighted images was used to restrict the DTI analysis to white matter only.
Voxel-based morphometry (VBM)
Processing of the T1-weighted images for the cross-sectional analysis was performed using Statistical Parametric Mapping software http://www.fil.ion.ucl.ac.uk/spm (SPM5). Segmentation in SPM5 employs a unified approach, combining: segmentation of the original anatomical images into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) images; normalization of the segmented images to the Montreal Neurological Institute template (specifically, a 12-parameter affine transformation and non-linear deformation with a warp frequency cutoff of 25); and bias correction, in one iterative process. A modulation step was also employed, which scales the final WM and GM images by the amount of contraction required to warp the images to the template. The resulting images were GM and WM volume maps for each participant, where original (native space) GM and WM volume is preserved even after warping. The normalized maps were smoothed using an 8-mm isotropic Gaussian kernel to optimize signal to noise, facilitate comparison across participants, and match the smoothing of the DTI maps. Analysis of white matter in the cross-sectional analysis of age employed an absolute threshold masking of 0.1 to minimize the inclusion of gray matter voxels in the white matter analysis. The final results were displayed using a cluster size threshold of 20 voxels.
Hypotheses and Statistical Analysis
In order to investigate the relationship between white matter microstructure and age, and microstructure and cognitive function, we performed multiple regression analyses where the DTI measure (either FA or MD) was the dependent variable and either age or cognitive function (as reflected by one of the nine test scores) was the independent variable. In order to examine white matter microstructure independently of white matter volume, we statistically controlled for volume on a voxel by voxel basis using the Biological Parametric Mapping (BPM) Toolbox implemented in SPM5. The BPM tool allows secondary imaging modalities to be used as regressors in the analysis of a primary imaging modality of interest (in this case, either FA or MD) in a massively univariate fashion. This is accomplished by solving a general linear model with a different design matrix in each voxel—in this case, for every brain voxel of FA data derived from the DTI scan, there was a matching voxel of volumetric white matter data derived from the T1 weighted scan. Education, gender, and total intracranial volume (TIV) were included as covariates for all of the analyses; age was included as an additional covariate for the analyses of cognitive data. Although TIV is not typically used as a covariate for DTI data, it is commonly used to control for the confounding effect of head size in volume studies. Since white matter volume was included in our model, TIV was employed as a covariate as well. Based on the results of previous studies, we hypothesized that older age would be associated with higher MD and lower FA, particularly in frontal tracts and tracts that serve as relays between frontal and temporal brain areas such as the uncinate fasciculus and superior longitudinal fasciculus. Furthermore, we hypothesized that cognitive function would be related to the DTI measures, where better performance was associated with higher FA and lower MD. Specifically, we expected that tests of frontal function would be related to frontal white matter, while tests of memory function would be related to temporal white matter. With regard to tests of memory, we further hypothesized that verbal memory tests would be related to left frontal white matter, while visual memory tests would be related to white matter in visual processing areas such as the inferior longitudinal fasciculus connecting the occipital and temporal lobes.
In order to examine the relationship between regional white matter volume and age, we used linear and non-linear regression in a voxel-wise fashion. The relationship between total white matter volume and age was assessed by performing linear and non-linear correlation and employing the volume estimates derived from the results of the SPM5 segmentation (i.e. the white matter probability maps), where white matter volume (in mm3) was determined by summing the voxels in the modulated, spatially normalized images and multiplying by the voxel volume. In addition to the imaging analyses, the relationship between the neuropsychological test scores and age were analyzed using SPSS version 15.0 (SPSS, Inc., Chicago IL).
Results
Demographic and Behavioral Relationships
In this sample, age was positively correlated with education, r = .34 (118), p < .001. Age was also positively correlated with performance on Trails A, r = .22 (120), p < .05, and Trails B, r = .18 (120), p <.05), with older adults performing more slowly. Scores on tests of memory were negatively correlated with age, including RAVLT total, r = −.28 (94), p <.01, RAVLT DR, r = −.27(94), p<.01, BVMT total, r = −.32 (118), p < .001, and BVMT DR, r = −.32 (118), p < .001. The WRAT-III Reading score (a measure of crystallized intelligence that is not expected to decline with age) was positively correlated with age, r = .23 (120), p < .01. Due to the strong correlation between age and education, the relationships between the neuropsychological test scores and age were also examined using partial correlation, controlling for education. When controlling for education, age was still positively correlated with Trails A, r = .35 (120), p < .001, but not Trails B (p =.09). RAVLT total remained negatively correlated with age r = −.22 (94), p <.01, RAVLT delayed recall only had a marginally significant correlation with age, r = −.20 (94), p<.06, and BVMT total remained significantly correlated with age, r = −.29 (87), p < .01, as did BVMT delayed recall, r = −.27 (112), p < .01. Digit span, which initially only showed a small negative correlation with age, became significant after controlling for education, r = −28 (120), p < .01. When education was controlled, WRAT was no longer correlated with age. Table 2 provides a summary of the correlation between test scores and age, both prior to, and when, controlling for education.
Total white matter volume; Age, Education and Gender
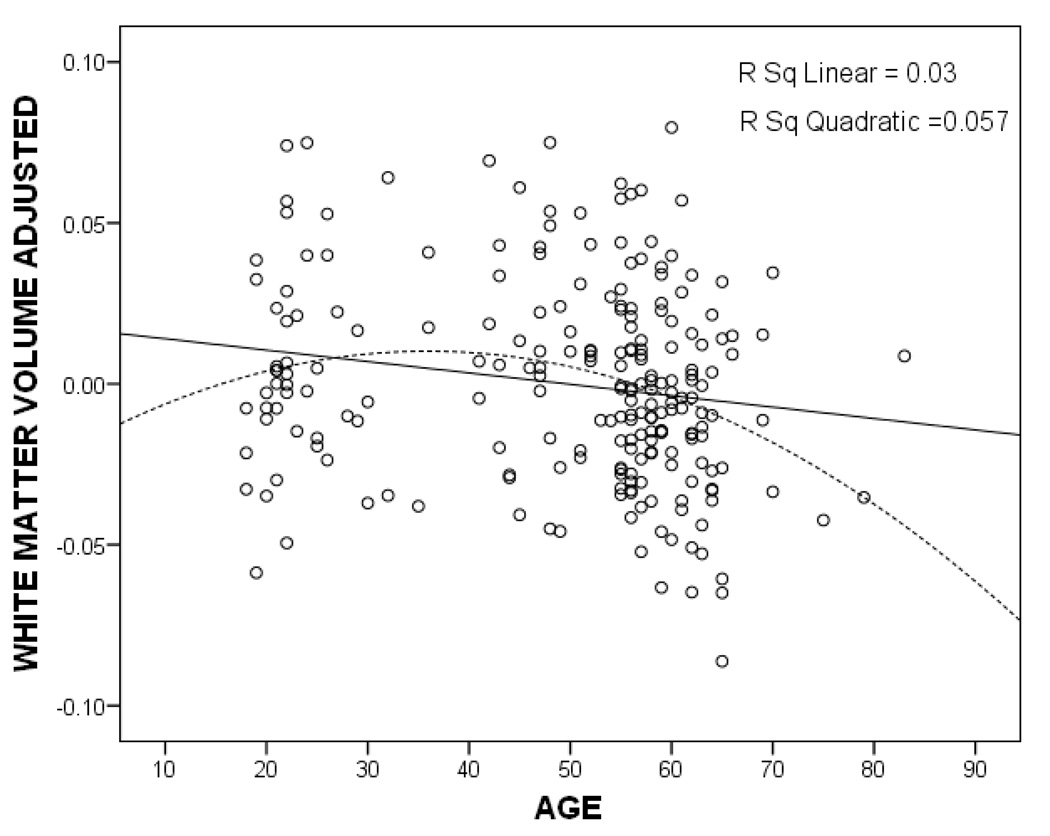
We predicted that white matter volume would show an age related decline. Using a partial correlation that controlled for gender, education and TIV, we found a significant negative correlation between age and white matter volume, r = −.18 (218), p < .01. We also tested a non-linear relationship, Figure 1 shows a plot of the data, where the quadratic fit, r = −.24, appears to account for more variability that the linear fit, though formal testing between the two correlations indicated that they were not statistically different. Previous research indicates that men and women differ significantly in total white matter volume and patterns of age-related decline (Allen, Damasio, Grabowski, Bruss, & Zhang, 2003; Good et al., 2001). We replicated these findings in our sample, with men showing significantly greater white matter volume (M =.49L, SD = .05) compared to women (M = .44L, SD = .04), p < .001. Women also showed a significant non-linear (quadratic) relationship between white matter volume and age, rs = −.24 (132), p < .01; men did not show a significant non-linear relationship, rs = −.16 (87), p = .13.
Figure 1.
Relationship between total white matter volume (adjusted for total intracranial volume, gender, and education) and age. The cross-sectional pattern of white matter volume across the age span from eighteen years of age to eighty-three appears to follow a non-linear course. The linear fit is shown with a solid line and the quadratic fit is shown with a hatched line.
Voxel-based morphometry: Regional relationship between age and white matter
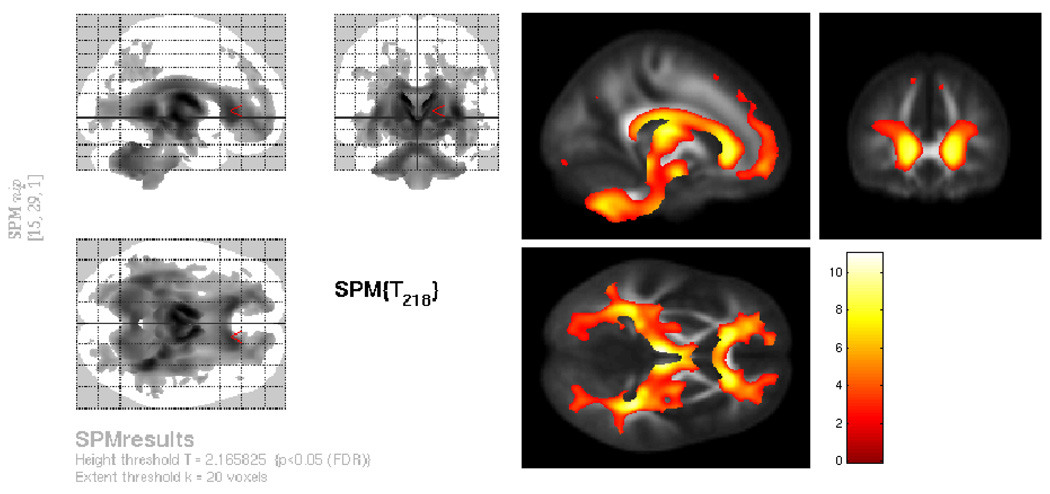
Based on previous studies, we expected that white matter volume would decline with age, with frontal areas demonstrating steeper declines, and posterior regions such as occipital areas showing less decline. As can be seen in Figure 2, we found extensive white matter decline, in frontal, temporal and parietal white matter. White matter volume was also negatively correlated with age in posterior occipital regions, brain stem, corpus callosum, and the cerebellum, p < .05, FDR, corrected. Because the analysis of total white matter volume suggested that white matter decline may be explained by a non-linear function, we performed a quadratic voxel-wise analysis where age-squared was entered as a variable in the regression analysis. Non-linear white matter decline was found in all of the same major regions as were found in the linear regression, with a slightly increased extent of change compared to the linear regression. In order to determine whether the quadratic function was significantly different from the linear function, we performed a voxel-wise test between the two slopes. Brain regions where the quadratic function explained significantly greater variance than the linear function included a small portion of anterior corpus callosum, white matter adjacent to right anterior limb of the internal capsule, a small portion of superior longitudinal fasciculus, bilateral superior corona radiata, left posterior limb of the internal capsule, and bilateral posterior corona radiata (p<.001 unc). Having determined that volume was highly related to age both linearly and non-linearly, our next question was: do the DTI measures show relationships with age that are above and beyond the effect of volume?
Figure 2.
Relationship between regional white matter volume and age, as assessed using voxel-wise analysis. Linear regression was used in a cross-sectional sample to examine the relationship between white matter volume and age. Total intracranial volume, gender, and education, were included as covariates of no interest in the regression analysis. As can be seen on the glass brain on the left, frontal, parietal, temporal, and occipital white matter volume showed a negative relationship with age. In addition, brain stem, corpus callosum, and cerebellar white matter volume showed a negative relationship with age. Colors are representative of a T-score, shown by the color bar on the bottom right.
Diffusion Tensor Imaging: Relationship Between Age and White Matter Microstructure
Fractional Anisotropy and Age
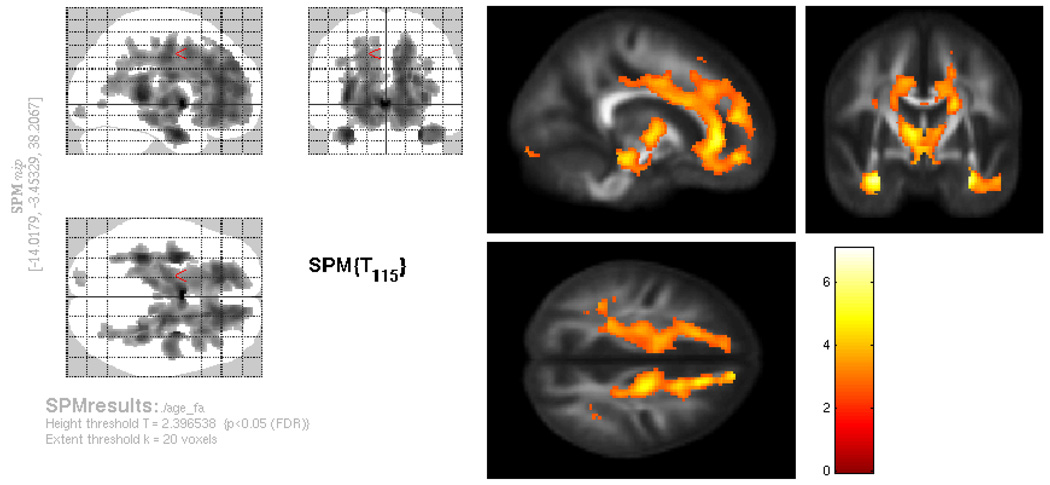
We predicted that FA would be negatively correlated with age, even when controlling for volume. As shown in Figure 3, FA in several major white matter tracts did show a negative correlation with age. Although the majority of affected regions were frontal, age effects extended to parietal, temporal and occipital white matter. Specific brain regions and tracts affected included the genu and body of the corpus callosum, sagittal stratum (inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and posterior thalamic radiations), superior longitudinal fasciculus, internal capsule, anterior, superior, and posterior corona radiata, and portions of the cingulum, p < .05, FDR corrected. In order to evaluate whether the relationship between white matter microstructure and age was stronger in frontal regions compared to posterior regions, we extracted the correlation coefficients in areas where FA was significantly related to age. Values from peak voxels in medial frontal white matter (16, 50, 12), anterior corpus callosum (−2, 18, 0), anterior temporal white matter (−34, −1, −26), posterior temporal white matter (−34, −33, 6) and occipital white matter (−69, 19, −71) were extracted and compared. In addition, since FA may differ intrinsically in different white matter tracts independently from age, we also assessed multiple points along one tract—the corpus callosum—specifically genu (8, 33, 0), body (8, 2, 28), and splenium (8, −34, 15). Negative correlations between age and FA were strongest in anterior temporal white matter, r = −.47 (120), p< .001, posterior temporal white matter, r = −.47 (120), p< .001, and frontal white matter, r = −.45 (12), p <.001, with a slightly smaller though still significant correlation between age and FA in occipital white matter, r = −.25 (120), p < .01. Mean FA was lowest in occipital white matter (m = 2.73), anterior temporal white matter (m = 2.77), and frontal white matter (2.90), and highest in posterior temporal white matter (m = 4.15). Along the corpus callosum, the genu showed the strongest negative correlation with age r = −.40 (120), p < .001, followed by the body of the corpus callosum, r = −22 (120), p < .05, and finally the splenium, which did not show a significant correlation between age and FA, r = −.08 (120), p = .39. Mean FA was lowest in the body of the corpus callosum (m = 4.77), followed by the genu (m = 4.99), and splenium (m = 5.86).
Figure 3.
Relationship between regional fractional anisotropy (FA) and age, assessed using voxel-wise analysis, controlling for white matter volume. There was a negative relationship between FA and age in several brain white matter tracts. As can be seen in the glass brain on the left, frontal white matter was particularly affected, but age effects on FA could be seen throughout the brain, including extensive portions of the corpus callosum, inferior fronto-occipital fasciculus, superior and inferior longitudinal fasciculus, internal capsule, anterior, superior, and posterior corona radiata, portions of the cingulum and posterior thalamic radiations.
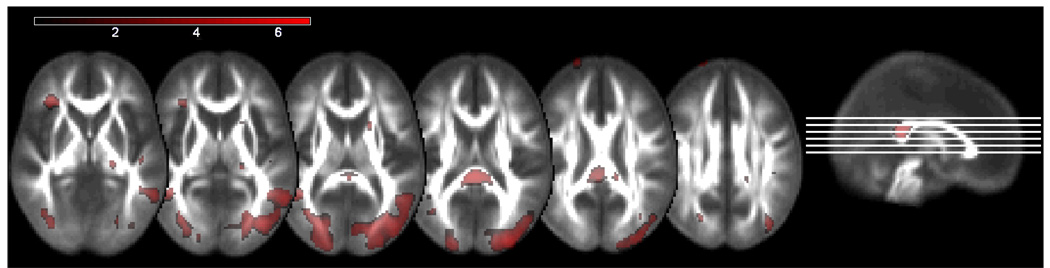
Owing to the non-linear changes found in the white matter analyses, a non-linear analysis was performed on the DTI data. Entering age-squared into the regression analysis resulted in virtually the same white matter tracts showing an effect of age. A difference map of the two images indicated that the non-linear fit extended slightly beyond the linear fit map, including more white matter. A voxel-wise statistical comparison of the two slopes revealed that the non-linear fit accounted for significantly more variability in FA than the linear fit in primarily posterior brain regions (p<.001 unc). As seen in Figure 4, these regions included the splenium of the corpus callosum, large bilateral regions of occipital white matter, bilateral cerebellar white matter, right inferior fronto-occipital fasciculus, right posterior corona radiata, left anterior thalamic radiation, left superior corona radiata, and right anterior limb of the internal capsule.
Figure 4.
Voxel-wise comparison: linear and non-linear relationship between FA and age. Fractional anisotropy demonstrated a significantly greater non-linear relationship with age compared to a linear relationship, primarily in posterior brain regions such as the splenium of the corpus callosum, large bilateral regions of occipital white matter, bilateral cerebellar white matter, right inferior fronto-occipital fasciculus, and right posterior corona radiata. Small anterior regions also showed a significant difference between the two slopes, including left anterior thalamic radiation, left superior corona radiata, and the right anterior limb of the internal capsule. Colors are representative of a T-score, shown by the color bar in the top left
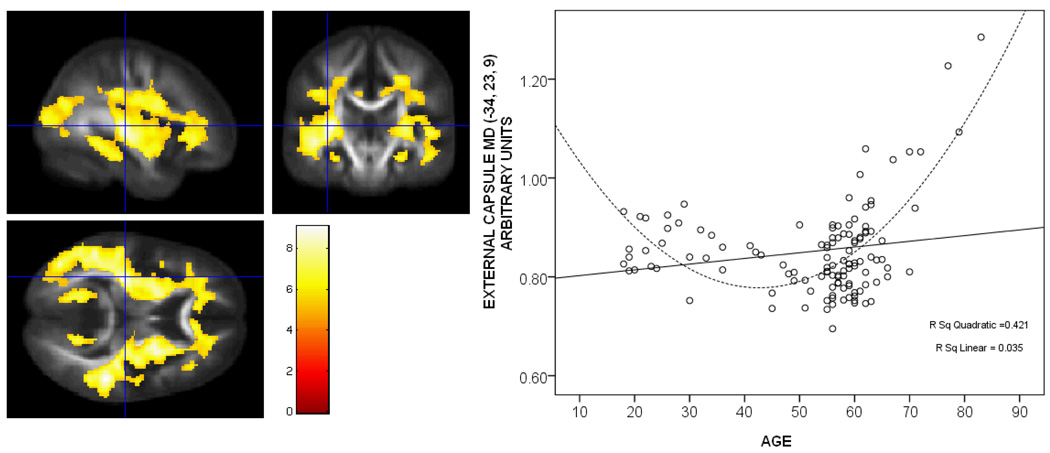
Mean Diffusivity and Age
In contrast to FA, where higher values typically reflect more intact white matter tissue, higher MD values tend to reflect a downturn in the health or integrity of the tissue in which it is measured. Similar to previously published studies of aging, white matter MD in the current sample was positively correlated with age. The extent of white matter affected was less than that found in the FA analysis. Regions showing a significant positive relationship with age were clustered anteriorly, including areas of prefrontal association fibers, superior fronto-occipital fasciculus, fibers of the cingulum and fornix, and the genu of the corpus callosum. In addition, MD in the cerebral peduncles and the thalamus was positively correlated with age, p < .05, FDR corrected. A non-linear relationship between MD and age was tested by entering age-squared into the regression analysis. MD showed a positive non-linear relationship with age in the same tracts that showed a linear age effect. In addition, several regions that did not show a linear effect did in fact show a non-linear effect. In particular, additional portions of the cingulum bundle ventral to the thalamus, fornix, stria terminalis, and fibers of the anterior thalamic radiation. A greater extent of involvement in the non-linear analysis was also observed in the anterior corona radiata, the genu of the corpus callosum, the retrolenticular and anterior limb of the internal capsule, and the uncinate fasciculus. In order to determine where the non-linear fit explained more variance than a linear fit, we performed a voxel-wise comparison of the linear and quadratic slopes. Interestingly, the non-linear fit was stronger than the linear fit in several major white matter fiber tracts including corpus callosum, cingulum, uncinate, superior and inferior longitudinal fasciculi, superior and inferior fronto-occipital fasciculi, internal and external capsule, anterior and posterior thalamic radiations, and short association fibers in frontal, temporal, parietal, and occipital white matter. Shown in Figure 5 is a plot of mean diffusivity against age in the external capsule, as well as brain images that demonstrate the widespread nature of the non-linear pattern. This differs from the FA results, where the non-linear fit accounted for more variance in primarily posterior regions. Having determined that white matter microstructure was related to age, we next examined whether white matter integrity was related to cognitive function.
Figure 5.
Voxel-wise comparison: linear and non-linear relationship between MD and age. Shown on the left are the white matter regions where mean diffusivity demonstrated a significantly greater non-linear relationship with age compared to a linear relationship, including the corpus callosum, cingulum, uncinate, superior and inferior longitudinal fasciculi, superior and inferior fronto-occipital fasciculi, internal and external capsule, anterior and posterior thalamic radiations, and short association fibers in frontal, temporal, parietal, and occipital white matter. Colors represent T-scores indicated in the color bar. On the right is a plot of mean diffusivity against age in the external capsule. The linear fit is shown with a solid line and the quadratic fit is shown with a hatched line.
Diffusion Tensor Imaging : Relationship between Cognition and White Matter Microstructure
We hypothesized that white matter microstructure would be related to cognitive function. We reasoned that using white matter volume as a covariate on a voxel by voxel basis would allow us to measure the relationships with cognition not attributable to volume differences alone. Indeed, we found that Trails A, Trials B, BVMT, Digit Span and WRAT all showed a relationship with at least one of the DTI measures. Age was included as a covariate in all of the analyses. All results were thresholded at p < .001 unc.
Processing Speed
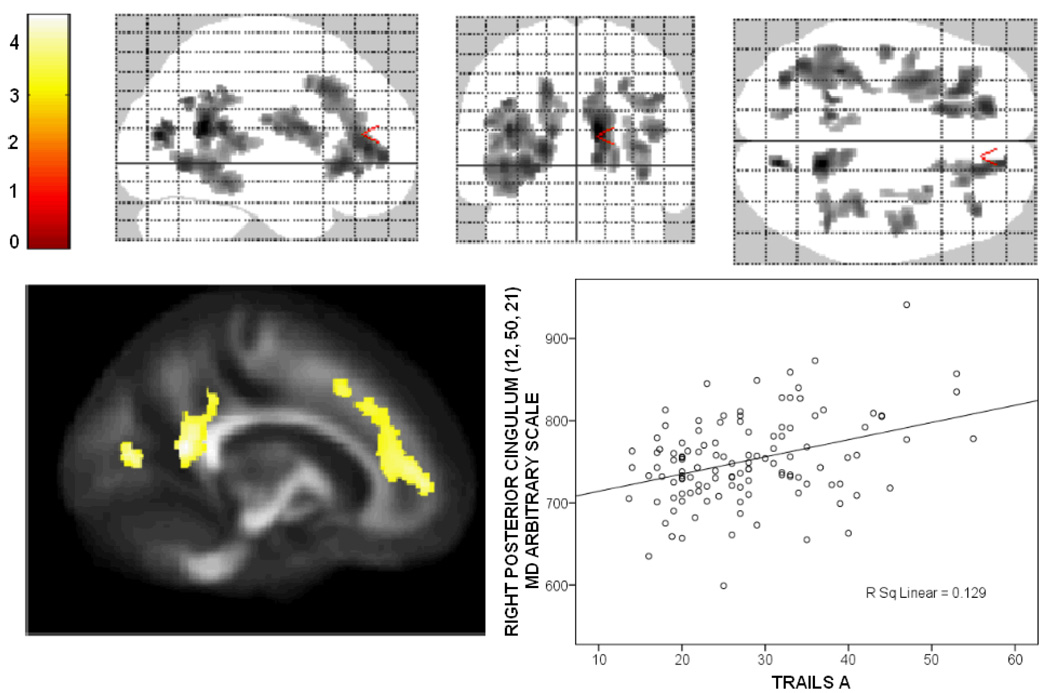
General processing (and/or motor) speed measured by Trails A was related to both FA and MD. Mean diffusivity was extensively related to Trails A. As shown in Figure 6, MD was positively correlated with Trails A in bilateral anterior corona radiata, bilateral superior fronto-occipital fasciculus, bilateral superior longitudinal fasciculus, bilateral internal capsule, posterior cingulum, right inferior fronto-occipital fasciculus, and small portions of bilateral posterior corona radiata. A plot of the positive relationship between scores on Trials A (measured in seconds) and MD in the cingulum is also shown in Figure 6. FA was negatively correlated with Trails A in a small region of right sagittal stratum, a small region in the left frontal short association fibers, and a small portion of stria terminalis adjacent to the right lateral ventricle.
Figure 6.
Relationship between white matter microstructure and processing speed. Of the cognitive domains, processing speed showed the greatest extent of relationship to the diffusion tensor imaging measures; the extent can be seen in the glass brain on the top. Regions where mean diffusivity was positively correlated with performance on Trails A included bilateral anterior corona radiata, bilateral superior fronto-occipital fasciculus, bilateral superior longitudinal fasciculus, bilateral internal capsule, posterior cingulum, right inferior fronto-occipital fasciculus, and small portions of bilateral posterior corona radiata. The colors in the sagittal section on the left represent T-scores, indicated by the color bar on the top left.
Working memory and episodic memory
Simple working memory, as measured by digit span, was positively correlated with FA in regions that were clustered in fronto-parieto-temporal white matter—portions of the superior corona radiata and short association fibers adjacent to left corpus callosum. Visual memory as measured by BVMT showed a relationship with both FA and MD. The BVMT delayed recall score was positively correlated with FA in bilateral posterior corona radiata, bilateral anterior thalamus, a small region in left middle frontal white matter, and a small region in left rostral mid-brain. Delayed recall was negatively correlated with MD in bilateral anterior thalamus, and three regions of right sided white matter: superior frontal, middle frontal, and a frontoparietal–temporal region. The BVMT total score was positively correlated with white matter FA in bilateral anterior thalamus, right internal capsule, left anterior corona radiata, bilateral posterior corona radiata, right superior corona radiata, and right external capsule. BVMT total score was not related to MD. Verbal learning and memory as measured by the RAVLT total and delayed scores was not related to the DTI measures.
Scores on tests of executive function
Complex attention and rapid set shifting as measured by Trails B was related to FA, but not MD. Higher Trails B scores (which reflect poorer performance) were negatively correlated with FA in bilateral superior corona radiata, and right superior longitudinal fasciculus. The Controlled Oral Word Association Test, thought to represent frontal function, did not show a significant relationship with FA or MD in frontal white matter or any other brain regions.
General Verbal Achievement
Verbal achievement as measured by WRAT-IIIR was positively correlated with FA in right fornix. WRAT-IIIR also showed a negative relationship with FA, where higher WRAT-IIIR scores were associated with lower FA scores in right frontal uncinate fasciculus, two small areas of bilateral frontal association fibers and a small cluster in left fronto-parietal white matter. There was no significant relationship between WRAT-IIIR and MD.
Discussion
The current study assessed the relationship between cognitive function as measured by specific neuropsychological test scores and white matter microstructure as measured by DTI. In order to assess the relationship between microstructure and cognition independently of gross volume loss, we controlled for white matter volume on a voxel by voxel basis using the Biological Parametric Mapping toolbox. With the exception of one recent study (Hugenschmidt et al., 2008), previous DTI studies have not controlled for the effect of volume on the DTI indices in a voxel-wise fashion.
As predicted, several of the neuropsychological tests were related to white matter microstructure as measured with DTI. In terms of extent, Trails A showed the greatest relationship with white matter microstructure in its positive relationship with MD. White matter plays a critical role in processing speed, so it is not surprising that performance on this test was related to white matter integrity. Trails B was also related to white matter microstructure, specifically with FA, in bilateral superior corona radiata, and right superior longitudinal fasciculus. Trails B is typically considered a test of frontal function, and has been associated with functional activation in dorsolateral, and medial frontal regions, but also left middle and superior temporal gyrus (Zakzanis, Mraz, & Graham, 2005). Since the superior longitudinal fasciculus forms an anatomical connection between frontal and temporal regions, it is possible that microstructural changes in this fiber tract account for variability in the performance of this task. O’Sullivan et al. (2001) also found a relationship between Trails Making Test and diffusion, specifically a positive correlation between anterior white matter diffusivity and Trails B minus Trails A.
Both working and visual memory were related to age. This is not surprising, as memory change is a chief complaint of older age. The relationship between white matter microstructure and working memory as measured by digit span was lateralized to the left hemisphere. Performance on the digit span task likely involves multiple strategies, including verbal rehearsal that invokes left cerebral structures (especially in the case of digit span forward) and visual imagery (especially in the case of digit span backward), which has been shown to involve both left and right cerebral structures. Damage to the regions where we found a relationship with digit span, is associated with disruptions to visual processing (Regan, Giaschi, Sharpe, & Hong, 1992). Parietal gray matter near the tracts we observed are typically active during working memory tasks (Jonides et al., 1998).
Visuospatial memory as measured by BVMT was associated with both FA and MD measures in the anterior thalamus. Isolated lesions to the thalamus in humans are rare; however, animal studies suggest that anterior thalamic lesions produce impairments in spatial memory tasks (Aggleton, Hunt, Nagle, & Neave, 1996; Sziklas & Petrides, 1999). The BVMT was also related to FA in the left anterior frontal lobe, a region implicated in several studies of non-verbal memory (Courtney, Petit, Maisog, Ungerleider, & Haxby, 1998; Klingberg, O'Sullivan, & Roland, 1997; Moritz, Johnson, McMillan, Haughton, & Meyerand, 2004; Ranganath, Johnson, & D'Esposito, 2000).
When controlling for age and education, verbal memory as measured by RAVLT did not show a significant relationship with age, and did not show a relationship with the DTI measures. Rose et al. (S E Rose et al., 2006) have found a relationship between RAVLT and diffusion indices, although they included participants with MCI in their sample. Our sample was limited to cognitively normal participants, which may explain why we did not find a relationship.
Verbal fluency, as measured with COWAT, was not related to age or the DTI measures. We have previously found COWAT to be related to left lateral inferior and middle frontal gyri (Newman, 2007); however, that study employed a larger sample and investigated gray matter only. O’Sullivan et al. (2001) have previously found a relationship between verbal fluency and FA, though they employed an ROI approach which may account for this discrepancy.
The results of the DTI and age analyses replicate previous findings indicating that FA is negatively correlated with age and MD is positively correlated with age. Wide-spread age effects were observed in frontal white matter, though temporal and parietal, and occipital fibers also showed a pattern of lower FA with higher age. The results are complementary to the findings of Hugenschmidt et al., who also examined FA and age while controlling for white matter volume (Hugenschmidt et al., 2008)—though the extent of the relationship with age found in the current study were somewhat greater than the extent reported by Hugenschmidt et al. (2008). This may be due a variety of methodological differences including higher MRI field strength and approximately double the number of subjects in the present report.
Previous research indicates that the effect of age on white matter microstructure appears to follow an anterior to posterior gradient (Head et al., 2004; Salat et al., 2005; Sullivan et al., 2001; Sullivan et al., 2006). In general, our results replicate previous findings. Extracting the correlation coefficient from peak voxels in the corpus callosum indicated that the correlation between age and fractional anisotropy was greatest in the genu, followed by the body of the corpus callosum, and not significant in the splenium. Similarly, values extracted from medial frontal white matter showed a stronger correlation between age and FA than values extracted from the corpus callosum or occipital white matter. The exception to an anterior to posterior gradient was the correlation between age and fractional anisotropy in temporal white matter, with both anterior and posterior temporal white matter demonstrating a comparable correlation to frontal white matter. Alterations to the medial temporal lobe have been attributed to preclinical Alzheimer’s pathology in high risk populations such as MCI (Fellgiebel et al., 2004; Huang, Friedland, & Auchus, 2007; S. E. Rose et al., 2006). It is unlikely that Alzheimer’s disease risk was a strong determinant of the pattern found in this study, as we took care to ensure that our sample was cognitively normal and not heavy on participants with increased risk for Alzheimer’s disease by way of family history or presence of ApoE ε4, suggesting that medial temporal lobe alterations are indeed a component of normal aging.
The relationship between mean diffusivity and age was not as widespread as the relationship found with FA, but still present even when controlling for white matter volume. Whereas FA affected large fiber bundles such as the corpus callosum and superior longitudinal fasciculus, age effects on MD were noted in relatively smaller regions such as clusters in prefrontal short association fibers, the fornix, and thalamic white matter, and cerebral peduncles. Interestingly, the relationship between age and mean diffusivity appears to be more non-linear than linear: a voxel-wise comparison between the two fits revealed that a non-linear slope was stronger than a liner slope across a wide expanse of brain white matter. These results complement previous findings suggesting that white matter decline follows a non-linear course over the lifespan (Bartzokis et al., 2001; Courchesne et al., 2000; Jernigan et al., 2001; Raz et al., 2005). It is interesting to note, however, that in the case of white matter volume—a significantly better non-linear fit was only found in small portions of the major white matter tracts. In comparison, the non-linear diffusivity pattern covered a much greater extent of the brain than the non-linear volume pattern.
Significance testing between the linear and non-linear fits describing fractional anisotropy and age indicated that a non-linear fit was superior primarily in posterior brain regions. These data suggest that posterior brain regions, like frontal brain regions, undergo significant microstructural changes across the life span but may follow a different pattern than do frontal regions. That the FA results differed from the MD results suggest that different age-related processes underlie the two measures.
Until recently, white matter integrity has been relatively understudied in relation to cognition and aging in normal populations. Although volume loss has been well documented in aging, it is possible that more subtle brain changes underlie age-related cognitive change, even before loss of volume. Diffusion tensor imaging has the distinct advantage of measuring structural alterations on a microscopic scale, allowing it to capture more information than gross measurements of volume. Much of what is known about age-related structural white matter change comes from studies of non-human primates (Peters, 2002); changes include splitting of the myelin sheath, the formation of enclosures of dense cytoplasm, and ballooning of the myelin in the form of round cavities that can include fluid filled spheres (Feldman & Peters, 1998). It is possible that changes to myelin integrity could result in disturbances to rapid nerve conduction, suggested by the relationship between mean diffusivity and processing speed in the present study.
Although this study controlled for volume within a statistical framework, we can not rule out the possibility that changes in MD and FA resulted in part from volumetric effects. Studies of human brain tissue have indicated that there is a loss of white matter with increasing age (Tang et al., 1997). In older brains, the total volume of white matter is lower by 15%, and there is an age-related decline in small diameter myelinated fibers. In turn, evidence from diseases such as multiple sclerosis suggests that the loss of white matter fibers can be accompanied by decreased FA, (Ciccarelli et al., 2003; Kealey, Kim, & Provenzale, 2004; Schmierer et al., 2007). Some of the age effects (though not all) were found in proximity to the ventricles. It is possible that older subjects with atrophy did not normalize as well as younger subjects into the common stereological space, a common problem with studies of aging. All of the normalized maps were inspected for accuracy, but subtle variation in normalization across the life-span can not be ruled out.
A few additional limitations should be noted. First, although this study adds to the growing number of studies that combine imaging techniques, it is still limited by the inclusion of only 2 imaging modalities. Inclusion of T2 weighted images in the analyses would have greatly assisted in controlling for the possibility of white matter hyperintensities in older adults, particularly since diffusion measures are likely to be correlated with white matter hyperintensities as identified on T2 weighted images (Kochunov et al., 2007). Another limitation of the present study is its cross sectional nature. Total intracranial volume and education were controlled, which can mitigate some of the concerns regarding cross-sectional designs, since these factors have been shown to vary between younger and older adults and can contribute to a cohort effect (Reynolds, Johnston, Dodge, DeKosky, & Ganguli, 1999; T. W. Smith, 1993). Still, in order to assess true age-related change, longitudinal studies are required. In our sample, education was positively correlated with age. This suggests that the older adults in the current study represent a cohort that is experiencing optimal aging, making the results somewhat less generalizable to a wider population. Furthermore, our sample was largely Caucasian (91%), which may also limit the conclusions that can be made in regard to normal aging in the general population. Finally, in order to ensure a clean sample, a number of participants were excluded due to poor or missing cognitive scores or risk for Alzheimer’s disease. These restrictions limited the number of participants, particularly in the 30–50 year age range, which in turn may have limited the generalizability of the correlational analyses, or possibly affected the slope of the correlations. Restricting the sample to cognitively normal participants may also have limited the range and variability of our cognitive scores and undoubtedly impacted our power to detect relationships with the DTI measures.
Despite the loss of participants, avoiding an over-representation of AD risk factors is important. Though many studies control for ApoE ε4 genotype, few if any imaging studies of normal aging control for parental family history of AD. This clearly needs to be addressed in future studies of aging, since family history can modulate brain measures even in cognitively normal, healthy participants (Bassett et al., 2006; Johnson et al., 2007; Johnson et al., 2006; Mosconi et al., 2007).
Conclusion
The present study found a relationship between cognitive function and white matter microstructure while controlling for white matter brain volume on a voxel by voxel basis. The results lend further support to accumulating evidence that cognitive changes in aging are related to the integrity of white matter microstructure. The results also suggest that DTI is sensitive to microstructural brain changes that are independent from volumetric changes. The relationships between memory and executive function and white matter microstructure were less extensive than the relationship seen between age and white matter microstructure. Of the cognitive domains, processing speed showed the largest extent of relationship with white matter microstructure. Future studies will benefit from longitudinal designs, in addition to employing additional imaging modalities to further refine structure-cognition relationships in aging.
Acknowledgements
This study was supported by a Merit Review Grant from the Department of Veterans Affairs, R01 MH 65723, ROI AG021155-01 (SCJ), and by the facilities and resources at the William S. Middleton Memorial Veterans Hospital. The authors wish to thank Britta Jabbar and Gemma Gliori for their invaluable assistance with this project. In addition, we would like to acknowledge the kind support of researchers and staff at the Waisman Center, University of Wisconsin, Madison, where MR imaging took place. Finally, thank you to all of our volunteers.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, et al. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiology of Aging. 2002;23(3):433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behavioural Brain Research. 1996;81(1–2):189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18(4):880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Archives of Neurology. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Archives of General Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, et al. Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain. 2006;129(Pt 5):1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum KA, Schulte C, Girke W, Reischies FM, Felix R. Incidental white-matter foci on MRI in "healthy" subjects: evidence of subtle cognitive dysfunction. Neuroradiology. 1996;38(8):755–760. doi: 10.1007/s002340050342. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test-Revised. Lutz, FL: Psychological Assessment Resources Inc; 1997. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd ed. Iowa City: AJA Associates; 1983. [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, et al. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60(5):444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. Journal of Comparitive Neurologyl. 1955;102(2):511–516. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- Brody H. Structural changes in the aging nervous system. In: Blumenthal HT, editor. The Regulatory Role of the Nervous System in Aging, Interdisciplinary Topics in Gerontology. Vol. 7. Basel: Karger Press; 1970. pp. 9–21. [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, et al. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34(1):137–143. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Werring DJ, Barker GJ, Griffin CM, Wheeler-Kingshott CA, Miller DH, et al. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging--evidence of Wallerian degeneration. Journal of Neurology. 2003;250(3):287–292. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a 'thrifty' allele? Annals of Human Genetics. 1999;63(Pt 4):301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279(5355):1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Annals of Neurology. 2000;47(2):145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006;66(4):505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Devaney KO, Johnson HA. Neuron loss in the aging visual cortex of man. Journals of Gerontology. 1980;35(6):836–841. doi: 10.1093/geronj/35.6.836. [DOI] [PubMed] [Google Scholar]
- Engelter ST, Provenzale JM, Petrella JR, DeLong DM, MacFall JR. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. American Journal of Roentgenology. 2000;175(2):425–430. doi: 10.2214/ajr.175.2.1750425. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, et al. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. Journal of the American Medical Association. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- Feldman ML, Peters A. Ballooning of myelin sheaths in normally aged macaques. Journal of Neurocytology. 1998;27(8):605–614. doi: 10.1023/a:1006926428699. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, et al. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dementia and Geriatric Cognitive Disorders. 2004;18(1):101–108. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. American Journal of Neuroradiology. 2007;28(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14(4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Henderson G, Tomlinson BE, Gibson PH. Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. Journal of the Neurological Sciences. 1980;46(1):113–136. doi: 10.1016/0022-510x(80)90048-9. [DOI] [PubMed] [Google Scholar]
- Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. American Journal of Neuroradiology. 2007;28(10):1943–1948. doi: 10.3174/ajnr.A0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, et al. Relating imaging indices of white matter integrity and volume in healthy older adults. Cerebral Cortex. 2008;18(2):433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Wide Range Achievement Test-Third Edition. Wilmington: Wide Range Inc; 1993. Jastak Associates. [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magnetic Resonance in Medicine. 1995;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42(7):980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Ries ML, Hess TM, Carlsson CM, Gleason CE, Alexander AL, et al. Effect of Alzheimer disease risk on brain function during self-appraisal in healthy middle-aged adults. Archives of General Psychiatry. 2007;64(10):1163–1171. doi: 10.1001/archpsyc.64.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, et al. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. Journal of Neuroscience. 2006;26(22):6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, et al. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18(13):5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealey SM, Kim Y, Provenzale JM. Redefinition of multiple sclerosis plaque size using diffusion tensor MRI. American Journal of Roentgenology. 2004;183(2):497–503. doi: 10.2214/ajr.183.2.1830497. [DOI] [PubMed] [Google Scholar]
- Klingberg T, O'Sullivan BT, Roland PE. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cerebral Cortex. 1997;7(5):465–471. doi: 10.1093/cercor/7.5.465. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35(2):478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Lucotte G, Loirat F, Hazout S. Pattern of gradient of apolipoprotein E allele *4 frequencies in western Europe. Human Biology. 1997;69(2):253–262. [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43(1):192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Moritz CH, Johnson SC, McMillan KM, Haughton VM, Meyerand ME. Functional MRI neuroanatomic correlates of the Hooper Visual Organization Test. Journal of the International Neuropsychological Society. 2004;10(7):939–947. doi: 10.1017/s1355617704107042. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, et al. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(48):19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L, Trivedi M, Bendlin B, Ries M, Johnson SC. The Relationship Between Gray Matter Morphometry and Neuropsychological Performance. Brain Imaging and Behavior. 2007;1(1–2):3–10. doi: 10.1007/s11682-007-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical "disconnection" as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, Davidson RJ. Integrating VBM into the General Linear Model with voxelwise anatomical covariates. Neuroimage. 2007;34(2):500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. Journal of Comparative Neurology. 1997;384(2):312–320. [PubMed] [Google Scholar]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, et al. Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, et al. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16(7):907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. Journal of Neurocytology. 2002;31(8–9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26(3):891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magnetic Resonance in Medicine. 2000;44(2):259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. Journal of Neuroscience. 2000;20(22):RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Regan D, Giaschi D, Sharpe JA, Hong XH. Visual processing of motion-defined form: selective failure in patients with parietotemporal lesions. Journal of Neuroscience. 1992;12(6):2198–2210. doi: 10.1523/JNEUROSCI.12-06-02198.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. 2 ed. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Reynolds MD, Johnston JM, Dodge HH, DeKosky ST, Ganguli M. Small head size is related to low Mini-Mental State Examination scores in a community sample of nondemented older adults. Neurology. 1999;53(1):228–229. doi: 10.1212/wnl.53.1.228. [DOI] [PubMed] [Google Scholar]
- Ries ML, Schmitz TW, Kawahara TN, Torgerson BM, Trivedi MA, Johnson SC. Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage. 2006;29:485–492. doi: 10.1016/j.neuroimage.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE, McMahon KL, Janke AL, O'Dowd B, de Zubicaray G, Strudwick MW, et al. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(10):1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE, McMahon KL, Janke AL, O'Dowd B, de Zubicaray G, Strudwick MW, et al. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment 10.1136/jnnp.2005.074336. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(10):1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CA, Boulby PA, Scaravilli F, Altmann DR, Barker GJ, et al. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35(2):467–477. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, Macgillivray TJ, Deary IJ, Starr JM, Rivers CS, et al. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovascular Diseases. 2005;20(5):310–318. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith TW. The relationship of age to education across time. Social Science Research. 1993;22:300–311. [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16(7):1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sziklas V, Petrides M. The effects of lesions to the anterior thalamic nuclei on object-place associations in rats. European Journal of Neuroscience. 1999;11(2):559–566. doi: 10.1046/j.1460-9568.1999.00448.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of Aging. 1997;18(6):609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Annals of Neurology. 1987;21(6):530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Hess TM, Fitzgerald ME, Atwood CS, et al. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: Risk factors for Alzheimer's disease. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. New York: Psych Corp; 1997. [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW. Region-specific changes of cerebral white matter during normal aging: A diffusion-tensor analysis. Archives of Gerontology and Geriatrics. 2007 doi: 10.1016/j.archger.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43(13):1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]