Abstract
Purpose
To test the effect of valacyclovir alone and with aspirin on the asymptomatic shedding of HSV-1 DNA in tears and saliva of healthy individuals.
Method
The subjects (n = 45) were randomized into three groups without regard to age, sex, or race. Group 1 (n = 14) received the placebo, group 2 (n = 15) received a dose of 500 mg valacyclovir once daily, and group 3 (n = 16) received a dose of 500 mg valacyclovir once daily and 350 mg aspirin twice daily for 30 days. Ocular and oral swabs were collected twice daily for 30 days. DNA was extracted from all swabs and HSV-1 DNA copy numbers were determined. Statistical analysis was performed to compare the DNA copy numbers of the three groups.
Results
There was no significant difference in the HSV-1 DNA copy numbers in the tears or saliva among any of the three treatment groups. The mean copy numbers ± SE of mean (SEM) of HSV-1 DNA in tears were 340 ± 35, 1074 ± 320, and 630 ± 51 for groups 1, 2, and 3, and in saliva were 238 ± 35, 963 ± 462, and 493 ± 25, respectively, for groups 1, 2, and 3.
Conclusions
No correlation was found between HSV-1 shedding and valacyclovir and valacyclovir with aspirin treatment. The HSV-1 DNA copy number was not reduced by treatment with 500 mg of valacyclovir daily or with a combination of daily valacyclovir (500 mg) plus twice-daily doses of aspirin (350 mg) over 30 days.
Herpes simplex virus (HSV) is a common human pathogen that infects most individuals at an early age in life, causing a range of diseases, including ocular keratitis and herpes labialis.1,2 After primary infection, HSV enters sensory nerves, moves retrograde by the axon, and reaches the trigeminal ganglia (TG) to establish a life-long latent infection.3–5 After primary oral or orofacial infection, HSV-1 can become latent in the TG and can reactivate periodically.4,5 Most individuals with latent virus continuously shed HSV-1 DNA throughout their lives; this asymptomatic shedding could be a major source of transmission of HSV-1 infection.5 HSV is easily transmitted by direct contact with a lesion or the body fluid of a latently infected individual.3,6 The two common strains of HSV, HSV-1, and HSV-2, are known for infections worldwide. HSV-1 is usually transmitted during childhood via nonsexual contact and can cause herpetic stromal keratitis, whereas HSV-2 is the cause of most forms of genital herpes and is almost always sexually transmitted.3,5 Recent data show declines in HSV-2 seroprevalence, indicating that the trajectory of increasing HSV-2 seroprevalence in the United States has been reversed.7,8 The seroprevalence of HSV-1 has decreased but the incidence of genital herpes caused by HSV-1 may be increasing.7,8 Despite the prevalence of HSV infections, only a small number of latently infected humans experience symptomatic disease; only 1% to 6% of primary infections are clinically recognized.1 Consequently, asymptomatic shedding of HSV is considered the major form of transmission.4,9,10
Asymptomatic shedding of HSV-1 DNA from subjects harboring latent virus is a documented phenomenon9–11 that could be triggered by stress, trauma, or a surgical procedure, as well as many other stimuli.5,10 Kaufman et al.12 first reported the presence of infectious HSV-1 in tears of normal human volunteers. Since then, several reports of HSV-1 shedding in tears and saliva have been published and the number of individuals identified as positive increased after the advent of the more sensitive polymerase chain reaction procedures.9,13,14 For example, Miller and Danaher10 reviewed 22 reports on HSV shedding and found at least 70% of the population shed HSV-1 DNA asymptomatically from the oral cavity. Recently, 72% (13/18) of HSV-1 seropositive individuals who had oral swabs taken four times daily for 60 days were found to be positive for HSV-1 DNA.15 We reported that 98% (49/50) of normal individuals asymptomatically shed HSV-1 DNA through tears and saliva.9 We have also shown that the presence of HSV-1 DNA in the TG of 131 (90%) of 147 subjects was not a function of age or sex.16 These data suggest that most of the individuals that have latent virus are intermittently shedding HSV-1 asymptomatically throughout their lives,10,17 strongly indicating that asymptomatic HSV-1 shedding is a major mode of transmission of the virus. Therefore, suppression of HSV-1 shedding through antiviral treatment offers the possibility of reducing preclinical transmission.
Antivirals, including acyclovir, valacyclovir, famciclovir, cidofovir, foscarnet, and penciclovir, have been used to reduce HSV DNA shedding in humans and animal models.2,18–28 Acyclovir, famciclovir, and valacyclovir have been reported to decrease HSV-2 shedding in normal and immunocompromised individuals.20,29–32 Valacyclovir, a prodrug of acyclovir, has better oral bioavailability and has a clinical effect comparable to that of acyclovir.29 In fact, very few reports have shown the clinical efficacies of these drugs in individuals without any history of genital herpes.28 Rather, they have focused on individuals who were afflicted with active genital herpes.21,32 To our knowledge, no reports exist so far that show antiviral drug treatment in the reduction of HSV-1 (infectious virus and/or DNA) shedding in tears of human subjects.
Acyclovir, valacyclovir, and other nucleoside analogues are effective in the treatment of HSV-1 keratitis in animal models.33–35 We have also shown that cyclooxygenase (COX) inhibitors, such as aspirin, have the ability to reduce HSV-1 shedding in animals after thermal stress.36,37 However, most of these investigations were based on conclusions of the less sensitive methods of detection—that is, keratitis by the presence of herpetic lesions using slit-lamp examination38 and quantification of infectious HSV-1 virus by plaque assay.39 The detection of the presence of HSV-1 has been significantly improved by the use of the more sensitive real-time PCR for detecting HSV-1 DNA.14,40 Real-time PCR application has improved the understanding of HSV-1 DNA shedding.40,41
The objective of this study was to determine whether oral valacyclovir alone or in combination with aspirin has the ability to influence the shedding of herpes simplex virus DNA in the tears and saliva of volunteers with no symptomatic evidence of ocular herpes infection. The secretion of infectious virus and/or DNA in tears and saliva is a potential source of susceptibility to virus infection by individuals who harbor this virus, if their immune system weakens. Alternatively, such shedding of HSV-1 DNA could transmit infection. Thus, a direct antiviral effect such as reduction in HSV-1 DNA shedding could be significant in suppressing infection in persons who become susceptible. The effect of an antiviral on shedding could interfere with HSV-1 transmission.
Materials and Methods
Subjects
All volunteer subjects provided written informed consent in accordance with a protocol approved by the LSU Health Sciences Center Institutional Review Board and in agreement with the tenets of the Declaration of Helsinki. Men and women of different races participated; all were older than 21 years. Subjects were excluded if they had had active ocular herpetic lesions or an orofacial lesion in the past 30 days; were taking systemic or oral antiviral drugs or had taken them in the past 30 days; were taking aspirin or non-steroidal anti-inflammatory drugs; had dry eyes; had hypersensitivity to acyclovir or valacyclovir; had hypersensitivity or contraindication to the use of aspirin; had a gastrointestinal ulcer or bleeding disorder; had kidney impairment; had undergone any organ transplantation; were pregnant or nursing; or had participated in any clinical trial in the past 30 days.
Before the study began, a history of viral infections and of the use of ocular and/or systemic medication was obtained, both eyes were examined, and a blood sample was collected from each individual for HSV-1 antibody analysis. Ten (22%) of 45 subjects enrolled had a history of skin lesions. Only two (4%) subjects had had ocular and one (2%) had had genital herpes at least 6 months before enrollment in the study. None of the subjects had any herpes infection at the beginning or during the 30 days of the study. The history of herpes infection and antibody titer were not criteria for the distribution of subjects in groups.
Study Design
The study was randomized, double blind, and placebo controlled. Every subject was examined before treatment, after 15 days of treatment, and after all ocular and oral sample collections were completed. All the subjects were normal with no signs of herpetic lesions during the study period.
The subjects were randomized into three groups. Each individual in the first group received six lactose (placebo) caplets per day; in the second group, each individual received one 500-mg valacyclovir caplet and five lactose caplets per day; and each individual in the third group received one 500-mg valacyclovir caplet, two 350-mg aspirin caplets, and three placebo caplets per day. Each subject received coded medication and was instructed to take two caplets each morning, two caplets at noon, and two caplets in the evening. Subjects were in structed to collect 120 tear and saliva samples by swabbing. These collections comprised one ocular swab (tears) and one oral swab (saliva) each morning and one of each in the evening for 30 consecutive days.
Sample Collection
Blood was collected at the initial examination of each subject, to determine HSV antibody titer. Tears were obtained from one eye only, and the same eye was swabbed for the entire study. Tears and saliva were taken twice daily, ~12 hours apart. Subjects were asked to collect tear specimens by touching the inner surface of their lower eyelid with an individually wrapped, sterile cotton swab, and to place the swab immediately into a labeled sterile tube. Saliva specimens were obtained by vigorously swabbing the oral mucosa. Immediately after sample collection, the tubes containing the swabs were to be stored in the subject’s home freezer. Tear and saliva samples were collected daily for 30 consecutive days and were brought to the investigator at the time of the scheduled eye examinations after every 15 days. Swabs were stored by the investigator at – 20°C until processed.
Determination of Serotype of Subjects
Blood was collected in tubes (Vacutainer; BD Biosciences, Franklin Lakes, NJ), centrifuged less than 1 hour after collection, and the serum was stored at –80°C until analysis. HSV-1 antibody levels were determined by enzyme-linked immunosorbent assay (ELISA) (HerpeSelect 1 ELISA IgG kit; Focus Diagnostics, Cypress, CA) according to the manufacturer’s instructions. Subjects having an ELISA index of more than 1.1 at 1:100 dilutions were considered seropositive for HSV-1. The antiviral neutralizing titers of the previously clarified serum samples were assayed as previously described.9 Serum samples that neutralized HSV-1 (McKrae strain) at ≥1:80 dilution were considered positive for HSV-1 antibodies.
Determination of HSV-1 DNA Copy Numbers
DNA Elution
All swabs obtained from the subjects were stored at –20°C before DNA extraction. The DNA was eluted according to the manufacturer’s instructions (Gentra Puregene DNA elution kit; Qiagen Sciences, Germantown, MD). The DNA samples were stored in DNA hydration buffer (provided with the kit) at 4°C and processed for real-time PCR. Sterile unused swabs were processed as a negative control and other swabs spiked with HSV-1 (McKrae strain) were processed as a positive control for DNA extraction using the same method.
Real-Time PCR
HSV-1 copy numbers from the DNA samples were determined by calculating the number of DNA polymerase genes in the sample. The sequence of forward and reverse primers were 5′-AGA GGG ACA TCC AGG ACT TTG T-3′ and 5′-CAG GCG CTT GTT GGGT GTA C-3′, respectively (IDT, Coralville, IA). The sequence of the probe was 5′6-FAM/ACC GCC GAA CTG AGC A/3′ BHQ-1 (IDT). All reactions were performed in a total volume of 20 μL. The 20 μL of reaction mixture contained 1× master mix (TaqMan Universal; Applied Biosystems, Inc., Foster City, CA), 100 nM of primers and probe, and 5 μL of DNA sample. All reactions were performed in 96-well plates (Bio-Rad, Hercules, CA), which were centrifuged for less than 1 minute at 1000g and room temperature in a swing-out rotor (CRU 5000 centrifuge; Damon/IEC, Needham, MA) to remove any air bubbles. The reaction conditions were as follows: 95°C for denaturation for 10 seconds, 55°C for annealing for 30 seconds, and 72°C extension for 10 seconds in a real-time PCR (iCycler iQ; Bio-Rad) system for 45 cycle repeats. All samples were analyzed in triplicate. Each reaction plate contained both positive and negative controls, as described. The cosmid containing the HSV-1 DNA polymerase gene was obtained from David Bloom (University of Florida, Gainesville, FL) and used as a standard for this study. The cosmid contained a copy of a 4.8-kb restriction fragment (HindIIIA) encompassing the HSV-1 DNA polymerase gene from the HSV-1 strain 17Syn+. A standard curve was generated from 10- and 2-fold serial dilutions of the pHindIIIA cosmid.
Statistical Analysis
The longitudinal series of observations were analyzed by using a design that took within-subject correlation into account before testing for the effect of treatments. The data were analyzed in a repeated-measures design incorporating the analysis of variance (ANOVA).42 Our main concern in this analysis was to determine the significance of an effect of treatments on the level of viral DNA secretion. Viral DNA secretion levels varied over time within each subject; thus, there may be a statistically significant effect of samples over time, or a significant difference in the time course for the different treatments (time by treatment interaction). However, these effects are of less interest, even if statistically significant, if the treatments are not found to be statistically significant. P values given are those from the F test of treatment main effects from the repeated measures ANOVAs.
Results
Demographics of Subjects
A total of 45 subjects were entered in the study. The subjects were randomly distributed according to their race, age, sex, and previous history of herpes infection (Table 1). Most of the subjects were Caucasian (56%). Although none of the subjects had herpetic infection at the time of admission into the study, some had had ocular, skin, or genital herpes infections more than 6 months previously. There were more women than men in the study, and all were of similar age. The demographics of subjects were similar in all the groups. There is no significant difference in the demographics between the groups (Table 1).
Table 1.
Distribution of Subjects According to Race, History of Infection, Sex, Average Age, and Range of Age in the Three Treatment Groups
| Total (n = 45) | Placebo (n = 14) | VCV (n = 15) | VCV+Aspirin (n = 16) | |
|---|---|---|---|---|
| Race, n (%) | ||||
| Caucasian | 25/45 (56) | 7/14 (50) | 9/15 (60) | 9/16 (56) |
| African American | 17/45 (38) | 7/14 (50) | 4/15 (27) | 6/16 (38) |
| Asian | 3/45 (7) | 0/14 (0) | 2/15 (13) | 1/16 (6) |
| *History of infection, n (%) | ||||
| Ocular herpes | 2/45 (4) | 0/14 (0) | 0/15 (0) | 2/16 (12) |
| Skin herpes | 10/45 (22) | 5/14 (36) | 3/15 (20) | 2/16 (12) |
| Genital herpes | 1/45 (2) | 0/14 (0) | 0/15 (0) | 1/16 (6) |
| Sex, n (%) | ||||
| Male | 15/45 (33) | 4/14 (29) | 6/15 (40) | 5/16 (31) |
| Female | 30/45 (67) | 10/14 (71) | 9/15 (60) | 11/16 (69) |
| Average age, y (range) | ||||
| Male | 47.5 (22–72) | 49.23 (28–72) | 45.73 (22–64) | 47.72 (27–70) |
| Female | 47.11 (25–70) | 43.40 (32–57) | 46.00 (25–64) | 52.4 (27–70) |
None of the subjects was infected at any time during the duration of the study.
HSV-1 Antibody Titer
The majority, that is, 35 (77%) of 45, of the subjects was positive by ELISA and 32 (71%) of 45 were positive by neutralization assay. Only 6 (13%) of the 45 subjects were negative by both of these methods. One of these six subjects received the placebo, two received valacyclovir (VCV) alone, and the remaining three received VCV plus aspirin. Of the six negative subjects, four shed HSV-1 DNA at least once in 30 days. Of those four, two each were from the VCV-alone group and VCV plus aspirin group.
Effect of Antiviral Treatment
Group 1: Placebo
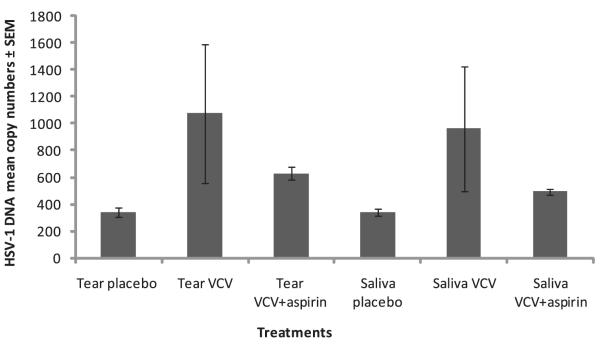
We found that the mean copy numbers of HSV-1 DNA in the tears and saliva of the subjects receiving the placebo were 340 ± 35 and 238 ± 35, respectively (Fig. 1). Specifically, 408 (49%) of 840 of the tear samples from subjects receiving the placebo were positive, and 357 (42%) of 840 of the saliva samples were positive in this group (Fig. 1). In this placebo-treatment group, 12 (86%) of 14 of the subjects had either tears or saliva positive for HSV-1 DNA at least once (Table 2, Supplementary Fig. S1, online at http://www.iovs.org/cgi/content/full/50/12/5601/DC1).
Figure 1.
The HSV-1 DNA mean copy number from the swabs obtained from tears and saliva of the subjects. VCV 500 mg was given once daily and 350 mg aspirin was given twice daily. The number of subjects in the placebo, VCV, and VCV+aspirin groups was 14, 15, and 16, respectively.
Table 2.
Distribution of Positive HSV-1 DNA Shedding in the Three Treatment Groups
| Body Fluid | Total (n = 45) | Placebo (n = 14) | VCV (n = 15) | VCV+Aspirin (n = 16) |
|---|---|---|---|---|
| Tears | 42/45 (93) | 12/14 (86) | 15/15 (100) | 15/16 (94) |
| Saliva | 41/45 (91) | 11/14 (79) | 15/15 (100) | 15/16 (94) |
| Overall | 43/45 (96) | 13/14 (86) | 15/15 (100) | 15/16 (94) |
Data are the number (%). Group 1 received placebo, group 2 was treated with 500 mg of valacyclovir once daily, and group 3 was treated with 500 mg of valacyclovir once and with 350 mg of aspirin twice daily. Any subject who shed ≥10 copies/μL at least once was considered positive.
Group 2: VCV
A total of 15 (33%) of 45 subjects in this study received VCV (500 mg) once daily for 30 days. The mean copy numbers of HSV-1 DNA in tears and saliva for the antiviral VCV treatment group were 1074 ± 320 and 963 ± 462, respectively (Fig. 1). Specifically, 482 (54%) of 900 of the tear samples and 465 (52%) of 900 of the saliva samples obtained from the individuals from this group were positive for HSV-1 DNA (Fig. 1, Table 3). All 15 subjects (100%) in this group shed HSV-1 DNA through tears and saliva at least once over the 30-day study period (Table 2, Supplementary Fig. S1).
Table 3.
Distribution of Tears and Saliva Containing Different Ranges of HSV-1 DNA Copy Numbers in the Three Treatment Groups
| HSV-1 DNA Copy Number Range |
||||
|---|---|---|---|---|
| Groups | 101–102 (%) | 102–103 (%) | 103–104 (%) | 104–105 (%) |
| Tears Placebo (n = 14) | 259/840 (30.8) | 144/840 (17.1) | 5/840 (0.6) | 0/840 (0.0) |
| Tears VCV (n = 15) | 187/900 (20.8) | 287/900 (31.9) | 3/900 (0.3) | 5/900 (0.6) |
| Tears VCV aspirin (n = 16) | 293/962 (30.4) | 417/962 (43.3) | 4/962 (0.4) | 0/962 (0.0) |
| Saliva placebo (n = 14) | 278/820 (33.9) | 77/820 (9.4) | 2/820 (0.2) | 0/820 (0.0) |
| Saliva VCV (n = 15) | 230/900 (25.6) | 227/900 (25.2) | 5/900 (0.6) | 3/900 (0.3) |
| Saliva VCV aspirin (n = 16) | 339/961 (35.3) | 359/961 (37.4) | 3/961 (0.3) | 0/961 (0.0) |
Data are the number (%). Group 1 received placebo, group 2 was treated with 500 mg of valacyclovir once daily, and group 3 was treated with 500 mg of valacyclovir once and with 350 mg of aspirin twice daily.
Group 3: VCV with Aspirin
Sixteen (36%) of 45 subjects in this study were administered a combination treatment of VCV (500 mg once daily) and aspirin (350 mg twice daily). The mean copy number of HSV-1 DNA for the individuals in this treatment group were 630 ± 51 and 493 ± 25 in tears and saliva, respectively, which is not significantly different from the placebo and VCV alone treatment groups (Fig. 1). The majority of the tear samples (714/962, 74%) and saliva samples (701/961, 73%) obtained from subjects in this group were positive for HSV-1 DNA (Fig. 1). One individual (1/16, 6%) was negative for HSV-1 DNA in both tears and saliva, yielding a total of 15/16 (94%) individuals from this group who were positive for HSV-1 DNA at least once in the 30-day period (Table 2, Supplementary Fig. S1).
Overall, there is no significant difference in the HSV-1 DNA copy numbers among the three groups regardless of treatment (placebo, VCV alone, or VCV in combination with aspirin).
Subanalysis of Tear and Saliva Samples
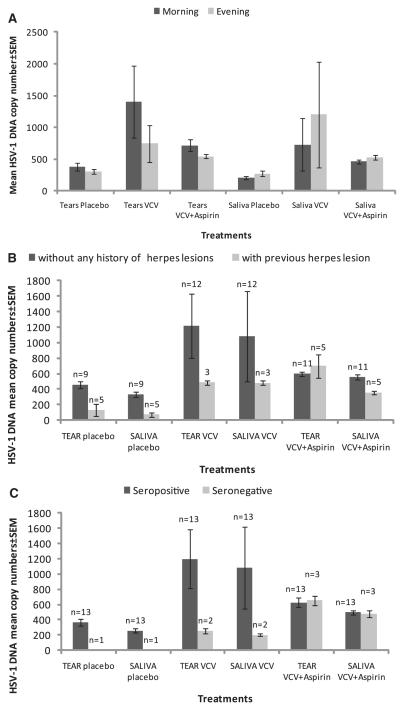
To evaluate the effects of VCV and VCV plus aspirin over time, we compared the HSV-1 DNA shedding in the morning tears and saliva to the shedding in the evening tears and saliva (Fig. 2). The mean copy number of HSV-1 DNA is not significantly different in the evening tear samples compared with the morning tear samples in all three groups. In the VCV alone group, we found an average of 747 ± 299 copies of HSV-1 DNA in the evening tear samples and 1410 ± 599 in the morning samples. The mean copy number in the placebo group was 381 ± 68 in the morning tear samples and 300 ± 41 in the evening samples. The mean HSV-1 DNA copy number in the VCV plus aspirin group was 716 ± 94 in the morning tear samples and 544 ± 31 in the evening samples (Fig. 1).
Figure 2.
Subanalysis of the HSV-1 DNA mean copy number in three groups by (A) morning and evening swabs from tears and saliva of subjects from all three groups. (B) Subject with or without history of herpes lesions, and (C) subjects seropositive or seronegative by ELISA and neutralization assay. VCV 500 mg was given once daily and 350 mg aspirin was given twice daily. The number of subjects in the placebo, VCV, and VCV+aspirin groups was 14, 15, and 16, respectively.
In the case of saliva, there was no change in the overall mean HSV-1 DNA copy number in all treatment groups. Specifically, the mean copy number of HSV-1 DNA in the morning saliva samples was 491 ± 138 and 649 ± 276 in the evening samples. The mean copy number in the morning saliva samples for the placebo and the VCV groups was 205 ± 21 and 726 ± 414, respectively, and in the evening samples was 270 ± 46 and 1200 ± 833, respectively. There was no difference in mean copy number in either the morning or evening saliva samples in the VCV plus aspirin group, where we found 522 ± 38 and 463 ± 32 copies, respectively (Fig. 2A).
We compared the HSV-1 DNA copy number in all the samples from the subjects with or without a history of herpetic lesion. There was no significant effect on HSV-1 DNA copy number in the three treatment groups (Fig. 2B). Similarly, we also compared HSV-1 DNA copy number in the subjects according to their seropositivity against HSV-1. This subanalysis shows no significant effect of treatment in either seropositive or negative subjects (Fig. 2C). In the placebo group, only one subject was seronegative and this subject was also negative for HSV-1 DNA throughout the period of study. The VCV group had two seronegative subjects and VCV+aspirin group had three seronegative subjects.
Overall HSV-1 DNA Shedding
HSV-1 DNA Shedding in Tears
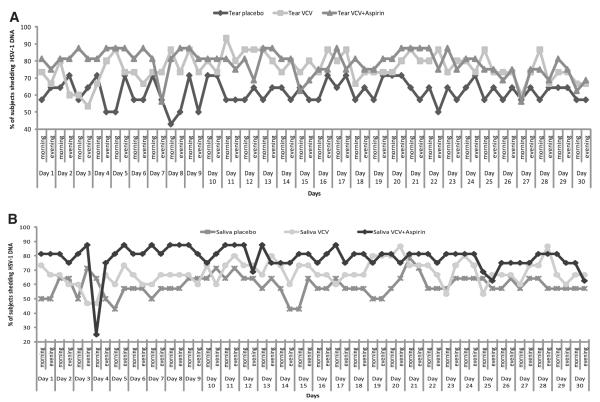
A majority of the subjects (42/45, 93%) shed HSV-1 DNA at least once in 30 days. There was no reduction in the percentage of subjects from day 1 to day 30 in all three groups (Fig. 3). A total of 1604 (59%) of 2702 tears swabs were positive (Fig. 2). The mean copy number of HSV-1 DNA in tears was 690 ± 110. The HSV-1 DNA copy numbers varied from 10 to 105, but there were only 5 samples in which the HSV-1 DNA copy number was more than 104 and only 12 swabs had between 103 and 104 copies per microliter of HSV-1 DNA (Table 3).
Figure 3.
Day-by-day evaluation of the percentage of HSV-1 DNA shedding in the subjects in all three groups in (A) tears and (B) saliva. The numbers of subjects in the placebo, VCV, and VCV+aspirin groups was 14, 15, and 16, respectively.
HSV-1 DNA Shedding in Saliva
Four (9%) of 45 subjects were negative for HSV-1 DNA in saliva. A total of 1523 (57%) of 2680 saliva swabs were positive for HSV-1 DNA. The mean copy number for HSV-1 DNA in saliva was 570 ± 183. The percentage of subjects who shed HSV-1 DNA was almost the same throughout the study in all three groups (Fig. 3). The HSV-1 DNA copy number varied from 10 to 105 but there were only 3 swabs containing >104 and only 10 swabs containing between 103 and 104 copies per microliter of DNA (Table 3).
A total of 43 (96%) of 45 subjects were positive for HSV-1 DNA at least once, either in tears or saliva (Table 2, Supplementary Fig. S1). Although the subjects were randomized, we observed HSV-1-negative subjects only in the placebo and VCV plus aspirin groups, whereas there were no negative subjects in the VCV-alone treatment group (Table 2, Supplementary Fig. S1).
Over the course of the study, we analyzed 5383 tear and saliva samples from 45 subjects and of this total, 3127 (58%) were found to be positive for HSV-1 DNA. Any tear or saliva sample containing less than 10 copies per microliter of DNA extracted was considered to be negative, to reduce the number of false positives. The copy number of HSV-1 DNA varied randomly among the subjects. Most of the tears and saliva swabs that were positive ranged from 102 to 103 copies of HSV-1 DNA. Very few tears and saliva samples had >103 HSV-1 DNA copies (Table 3).
Discussion
This is the first study to our knowledge in normal individuals in which the effect of VCV alone and in combination with aspirin has been evaluated on the shedding of HSV-1 DNA in tears and saliva. VCV is a treatment choice for many viral infections, such as genital herpes and cold sores, and has been reported to reduce HSV-2 DNA shedding in genital herpes.18,20,21,32 In this investigation, we assessed the effect of VCV, a frequently used antiviral for HSV infection, at a dose of 500 mg daily (alone) or in combination with aspirin, a COX inhibitor, where the aspirin was administered at 350 mg twice daily. We used the combination therapy of VCV with aspirin based on previous reports in the literature indicating that aspirin reduced the clinical signs of herpes infection in a trial of patients with herpes labialis or genital herpes.43,44 Furthermore, we have reported the suppression of HSV-1 reactivation by the COX inhibitors in mice latently infected with HSV-1.36,37
The VCV dose was selected according to a previous published report32 and the U.S. pharmacopoeia at the time this study started, stating that the effective minimum dose used was 500 mg. A previous study with 69 immunocompetent participants with genital HSV-2 showed a significant reduction, but not complete suppression, in HSV-2 DNA shedding with 500 mg VCV when given twice daily.21 Similarly, a once-daily dose of 500 mg VCV significantly reduced the risk of transmission of genital herpes.32 Other studies using higher doses of VCV have shown reductions in HSV-2 shedding, but none of these studies demonstrated complete suppression of shedding with VCV or any other antiviral.18–21,28 Although once-daily doses of more than 500 mg VCV have been used in some cases,20,21 our goal was to evaluate the effect of a baseline dosage of VCV on ocular and oral shedding of HSV-1 DNA, either alone or in combination with aspirin.
We found no reduction in the HSV-1 DNA copy number with a once-daily dose of 500 mg VCV. The mean copy number and number of positive tear and saliva swabs in the VCV-treated group were not significantly different from the placebotreated group. Apart from VCV alone, we tested it in combination with aspirin and found no significant reduction in HSV-1 DNA shedding with 500 mg VCV once daily together with twice-daily 350 mg aspirin.
Our results are related in part to the sensitivity of using real-time PCR for detecting the presence of HSV-1 DNA in comparison to many of the previously published reports, in which the number of HSV-1-positive subjects varied significantly.26,39 Kameyama et al.39 found only 4.5% of the normal individuals were positive for infectious HSV-1, but these results were based on the conventional culture technique of virus on specific cell lines. A recent report by Miller and Danaher,10 who reviewed existing data from 22 published reports concerning HSV shedding, concluded that at least 70% of the population shed HSV-1 asymptomatically at least once a month in saliva.10 The HSV shedding was analyzed by culture technique in 10 of 22 of these reports, whereas in 12 other studies PCR was used.10 HSV-1 DNA quantification by PCR is sensitive, precise, and easy, and it is routinely used for HSV detection in saliva, tears,9 cornea,45 cerebrospinal fluid,46 and blood samples.46 PCR-quantified HSV-1 copy numbers have not been correlated to antibody titer in the subjects analyzed. We further tested the antibody titer against HSV-1 by ELISA and neutralization assay and found six subjects to be seronegative, although four of them shed HSV-1 DNA. Only two subjects (subjects 42 and 43), both negative for HSV-1 DNA in saliva and tears, were negative for HSV antibodies by both methods of analysis. In the present study, 35 (78%) of our 45 subjects were seropositive by ELISA, 32 (71%) were seropositive by neutralization assay, and 6 (14%) were negative by both methods. These results are similar to those obtained in our previous study in which 74% of the population was positive by ELISA and 20% were seronegative by both methods.9 These seropositive and -negative subjects were distributed randomly in all the treatment groups.
Asymptomatic shedding of HSV-1 DNA is a very common phenomenon and probably the major source of transmission of HSV-1. A normal individual continuously sheds HSV-1 without any sign of herpetic infection. The immune status of an individual, reactivation properties of virus (low or high phenotypic reactivator), and environmental factors (stress, trauma) are important in asymptomatic shedding of infectious virus; a slight change in these factors could lead to an infectious herpes outbreak. Thus, this study, in which we sought to evaluate the effect of VCV alone and in combination therapy to potentially suppress HSV-1 shedding, is an important contributory step toward reducing the risk of herpes infection. Other researchers have shown that giving VCV at varied doses and for varied periods in diseased or immunocompromised individuals reduces the frequency of HSV-2 DNA shedding and may be a means of reducing sexual transmission of genital herpes.21,32 However, our study showed no effect on suppression of HSV-1 DNA shedding with a 500-mg single dose of VCV, either alone or with aspirin at a 350-mg dose twice daily, over the 30-day period in normal individuals. These data may help explain why antivirals have had much less prophylactic efficacy against HSV-1 lesions, including ocular herpes, than against genital herpes. Other outcomes of this study are that HSV-1 DNA shedding from tears and saliva was not related to the anti body titer against HSV-1, race, sex, or age. Our options for building on this study include increasing the dosage of VCVand also assessing treatment regimens in conjunction with behavioral patterns and body fluids (blood, tears, or saliva) that provide the most susceptibility to herpes infection.
Supplementary Material
Acknowledgments
Supported in part by National Institutes of Health Grants EY002622 (HEK), EY006311 (JMH), and EY02377; an LSU Eye Center Core Grant for Vision Research; an unrestricted research grant from LSU Health Sciences Center; a Research to Prevent Blindness Senior Scientific Investigator Award (JMH); the Louisiana Vaccine Center and the South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents (JMH); an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness, New York, New York; and funding from the Louisiana Lions Eye Foundation, New Orleans.
Footnotes
Disclosure: M. Kumar, None; J.M. Hill, None; C. Clement, None; E.D. Varnell, None; H.W. Thompson, None; H.E. Kaufman, None
References
- 1.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20(1):1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Whitley RJ, Roizman B. Herpes simplex viruses. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical Virology. ASM Press; Washington, DC: 2009. pp. 409–436. [Google Scholar]
- 3.Kaufman HE, Rayfield MA, Gebhardt BM. Herpes simplex infections. In: Kaufman HE, Barron BA, McDonald MB, editors. The Cornea. Butterworth-Heinemann; Boston: 1998. pp. 247–277. [Google Scholar]
- 4.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed Lippincott Williams & Wilkins; Baltimore, MD: 2007. pp. 2503–2602. [Google Scholar]
- 5.Toma HS, Murina AT, Areaux RG, Jr, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23(4):249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 6.Sacks SL, Griffiths PD, Corey L, et al. HSV shedding. Antiviral Res. 2004;63(suppl 1):S19–S26. doi: 10.1016/j.antiviral.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Xu F, Lee FK, Morrow RA, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J Pediatr. 2007;151(4):374–377. doi: 10.1016/j.jpeds.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 8.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CS, Danaher RJ. Asymptomatic shedding of herpes simplex virus (HSV) in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(1):43–50. doi: 10.1016/j.tripleo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Hyland PL, Coulter WA, Abu-Ruman I, et al. Asymptomatic shedding of HSV-1 in patients undergoing oral surgical procedures and attending for noninvasive treatment. Oral Dis. 2007;13(4):414–418. doi: 10.1111/j.1601-0825.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman HE, Brown DC, Ellison EM. Recurrent herpes in the rabbit and man. Science. 1967;156(3782):1628–1629. doi: 10.1126/science.156.3782.1628. [DOI] [PubMed] [Google Scholar]
- 13.Abiko Y, Ikeda M, Hondo R. Secretion and dynamics of herpes simplex virus in tears and saliva of patients with Bell’s palsy. Otol Neurotol. 2002;23(5):779–783. doi: 10.1097/00129492-200209000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda M, Deai T, Hibino T, Higaki S, Hayashi K, Shimomura Y. Quantitative analysis of herpes simplex virus genome in tears from patients with herpetic keratitis. Cornea. 2003;22(7 suppl):S55–S60. doi: 10.1097/00003226-200310001-00008. [DOI] [PubMed] [Google Scholar]
- 15.Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198(8):1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JM, Ball MJ, Neumann DM, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82(16):8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kriesel JD, Pisani PL, McKeough MB, Baringer JR, Spruance SL. Correlation between detection of herpes simplex virus in oral secretions by PCR and susceptibility to experimental UV radiation-induced herpes labialis. J Clin Microbiol. 1994;32:3088–3090. doi: 10.1128/jcm.32.12.3088-3090.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora A, Mendoza N, Brantley J, Yates B, Dix L, Tyring S. Double blind study comparing 2 dosages of valacyclovir hydrochloride for the treatment of uncomplicated herpes zoster in immunocompromised patients 18 years of age and I. J Infect Dis. 2008;197(9):1289–1295. doi: 10.1086/586903. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett BL, Tyring SK, Fife K, et al. Famciclovir treatment options for patients with frequent outbreaks of recurrent genital herpes: The RELIEF trial. J Clin Virol. 2008;43(2):190–195. doi: 10.1016/j.jcv.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Bavaro JB, Drolette L, Koelle DM, et al. One-day regimen of valacyclovir for treatment of recurrent genital herpes simplex virus 2 infection. Sex Transm Dis. 2008;35(4):383–386. doi: 10.1097/OLQ.0b013e31815e4190. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190(8):1374–1381. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman HE, Varnell ED, Thompson HW. Cidofovir and experimental herpetic stromal disease. Arch Ophthalmol. 1999;117(7):925–928. doi: 10.1001/archopht.117.7.925. [DOI] [PubMed] [Google Scholar]
- 23.Kaye SB, Madan N, Dowd TC, Hart CA, McCarthy K, Patterson A. Ocular shedding of herpes simplex virus. Br J Ophthalmol. 1990;74(2):114–116. doi: 10.1136/bjo.74.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarus HM, Belanger R, Candoni A, Aoun M, Jurewicz R, Marks L. Intravenous penciclovir for treatment of herpes simplex infections in immunocompromised patients: results of a multicenter, acyclovir-controlled trial. The Penciclovir Immunocompromised Study Group. Antimicrob Agents Chemother. 1999;43(5):1192–1197. doi: 10.1128/aac.43.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loutsch JM, Sainz B, Jr, Marquart ME, et al. Effect of famciclovir on herpes simplex virus type 1 corneal disease and establishment of latency in rabbits. Antimicrob Agents Chemother. 2001;45(7):2044–2053. doi: 10.1128/AAC.45.7.2044-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller CS, Avdiushko SA, Kryscio RJ, Danaher RJ, Jacob RJ. Effect of prophylactic valacyclovir on the presence of human herpesvirus DNA in saliva of healthy individuals after dental treatment. J Clin Microbiol. 2005;43(5):2173–2180. doi: 10.1128/JCM.43.5.2173-2180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller CS, Cunningham LL, Lindroth JE, Avdiushko SA. The efficacy of valacyclovir in preventing recurrent herpes simplex virus infections associated with dental procedures. J Am Dent Assoc. 2004;135(9):1311–1318. doi: 10.14219/jada.archive.2004.0407. [DOI] [PubMed] [Google Scholar]
- 28.Sperling RS, Fife KH, Warren TJ, Dix LP, Brennan CA. The effect of daily valacyclovir suppression on herpes simplex virus type 2 viral shedding in HSV-2 seropositive subjects without a history of genital herpes. Sex Transm Dis. 2008;35(3):286–290. doi: 10.1097/OLQ.0b013e31815b0132. [DOI] [PubMed] [Google Scholar]
- 29.Yin MT, Brust JCM, Tieu HV, et al. Anti herpesvirus, anti-hepatitis virus, and antirespiratory virus agents. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical Virology. ASM Press; Washington, DC: 2009. pp. 409–436. [Google Scholar]
- 30.Scott LL, Hollier LM, McIntire D, Sanchez PJ, Jackson GL, Wendel GD., Jr. Acyclovir suppression to prevent clinical recurrences at delivery after first episode genital herpes in pregnancy: an openlabel trial. Infect Dis Obstet Gynecol. 2001;9(2):75–80. doi: 10.1155/S106474490100014X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996;124:8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. 1996. [DOI] [PubMed] [Google Scholar]
- 32.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 33.Anand BS, Hill JM, Dey S, et al. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, val-val-acyclovir, against HSV-1 epithelial and stromal keratitis in the rabbit eye model. Invest Ophthalmol Vis Sci. 2003;44:2529–2534. doi: 10.1167/iovs.02-1251. [DOI] [PubMed] [Google Scholar]
- 34.Majumdar S, Nashed YE, Patel K, et al. Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: in vitro and in vivo evaluations. J Ocul Pharmacol Ther. 2005;21(6):463–474. doi: 10.1089/jop.2005.21.463. [DOI] [PubMed] [Google Scholar]
- 35.Sanitato JJ, Asbell PA, Varnell ED, Kissling GE, Kaufman HE. Acyclovir in the treatment of herpetic stromal disease. Am J Ophthalmol. 1984;98(5):537–547. doi: 10.1016/0002-9394(84)90237-x. [DOI] [PubMed] [Google Scholar]
- 36.Gebhardt BM, Varnell ED, Kaufman HE. Acetylsalicylic acid reduces viral shedding induced by thermal stress. Curr Eye Res. 2004;29(2–3):119–125. doi: 10.1080/02713680490504588. [DOI] [PubMed] [Google Scholar]
- 37.Gebhardt BM, Varnell ED, Kaufman HE. Inhibition of cyclooxygenase 2 synthesis suppresses herpes simplex virus type 1 reactivation. J Ocul Pharmacol Ther. 2005;21(12):114–120. doi: 10.1089/jop.2005.21.114. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman HE, Varnell ED, Thompson HW. Trifluridine, cidofovir, and penciclovir in the treatment of experimental herpetic keratitis. Arch Ophthalmol. 1998;116(6):777–780. doi: 10.1001/archopht.116.6.777. [DOI] [PubMed] [Google Scholar]
- 39.Kameyama T, Sujaku C, Yamamoto S, Hwang CB, Shillitoe EJ. Shedding of herpes simplex virus type 1 into saliva. J Oral Pathol. 1988;17(9–10):478–481. doi: 10.1111/j.1600-0714.1988.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 40.Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002;30(6):1292–1305. doi: 10.1093/nar/30.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leigh JF, Acharya N, Cevallos V, Margolis TP. Does asymptomatic shedding of herpes simplex virus on the ocular surface lead to false-positive diagnostic PCR results? Br J Ophthalmol. 2008;92(3):435–436. doi: 10.1136/bjo.2007.122150. [DOI] [PubMed] [Google Scholar]
- 42.Cole JWL, Gizzle JE. Applications of multivariate analysis of variance to repeated measurements. Exp Biometrics. 1966;22:810–828. [Google Scholar]
- 43.Wachsman M, Aurelian L, Burnett JW. The prophylactic use of cyclooxygenase inhibitors in recurrent herpes simplex infections. Br J Dermatol. 1990;123(3):375–380. doi: 10.1111/j.1365-2133.1990.tb06298.x. [DOI] [PubMed] [Google Scholar]
- 44.Karadi I, Karpati S, Romics L. Aspirin in the management of recurrent herpes simplex virus infection. Ann Internal Med. 1998;128(8):696–697. doi: 10.7326/0003-4819-128-8-199804150-00027. [DOI] [PubMed] [Google Scholar]
- 45.Robert PY, Adenis JP, Denis F, Alain S, Ranger-Rogez S. Herpes simplex virus DNA in corneal transplants: prospective study of 38 recipients. J Med Virol. 2003;71(1):69–74. doi: 10.1002/jmv.10454. [DOI] [PubMed] [Google Scholar]
- 46.Malm G, Forsgren M. Neonatal herpes simplex virus infections: HSV DNA in cerebrospinal fluid and serum. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F24–F29. doi: 10.1136/fn.81.1.f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.