Abstract
The Gln27Glu polymorphism but not the Arg16Gly polymorphism of the beta2-adrenergic receptor (ADRB2) gene appears to be associated with a broad range of aging-associated phenotypes, including cancers at different sites, myocardial infarction (MI), intermittent claudication (IC), and overall/healthy longevity in the Framingham Heart Study Offspring cohort. The Gln27Gln genotype increases risks of cancer, MI and IC, whereas the Glu27 allele or, equivalently, the Gly16Glu27 haplotype tends to be protective against these diseases. Genetic associations with longevity are of opposite nature at young-old and oldest-old ages highlighting the phenomenon of antagonistic pleiotropy. The mechanism of antagonistic pleiotropy is associated with an evolutionary-driven advantage of carriers of a derived Gln27 allele at younger ages and their survival disadvantage at older ages as a result of increased risks of cancer, MI and IC. The ADRB2 gene can play an important systemic role in healthy aging in evolutionary context that warrants exploration in other populations.
Keywords: Healthy aging, ADRB2 polymorphisms, longevity, Framingham Heart Study
1. Introduction
The etiology of complex phenotypes entails epistatic and gene-environment interactions that makes relevant gene association studies a challenging task. Polymorphisms in the beta2-adrenergic receptor (ADRB2) gene have long attracted the attention of health researchers for their possible multiple physiological and health effects, particularly those involving vascular responses and airway function (Buscher et al., 1999; Contopoulos-Ioannidis et al., 2005; Hindorff et al., 2005; Snyder et al., 2005). ADRB2 is a member of the receptors family that mediates the physiological effects of the hormone epinephrine and the neurotransmitter norepinephrine. Adrenergic stimulation of the ADRB2 influences, for instance, cardiovascular function by regulating vasomotor tone (Guimaraes and Moura, 2001). This function makes ADRB2 an important target in cardiovascular disease therapy. The two most common functional single nucleotide polymorphisms of the ADRB2 gene are rs1042713 (46GA) and rs1042714 (79CG), which result in changes of amino acids at codon 16, Arg to Gly, and at codon 27, Gln to Glu, respectively (George, 2008). Various studies have shown that the role of these polymorphisms is of a broad nature because of their involvement not only in vascular responses but also in pulmonary, endocrine, and central nervous systems functioning (Brodde, 2008a; b). Specifically, the roles of Arg16Gly and Gln27Glu polymorphisms in the pathophysiology of diseases of the heart (Heckbert et al., 2003), hypertension (Puddu et al., 2007), obesity (Jalba et al., 2008), diabetes (Pinelli et al., 2006), asthma (Contopoulos-Ioannidis et al., 2005), Alzheimer disease (Yu et al., 2008), COPD (Matheson et al., 2006), and cancer (Huang et al., 2001) have been studied in different populations.
The pleiotropy of the effects of these genetic variants makes them promising candidates for studies of genetic predisposition to healthy aging (Melzer et al., 2007), which is often defined as life with preserved health and physical, social and mental wellness, independence, and quality of life (Peel et al., 2004). Although a common goal is to determine genetic pathway(s) which could directly modulate senescence (Johnson, 2005), a more realistic strategy is to understand mechanisms involved in the process of development of aging-associated disorders to improve the health of an increasing elderly population in contemporary societies (Hadley and Rossi, 2005; Melzer et al., 2007; Olshansky et al., 2007; Sierra et al., 2008).
Currently, a large number of genetic studies focus on aging per se in model organisms and humans (Vijg and Suh, 2005). Likewise, a large body of studies focus on a single particular disease phenotype (e.g., Brodde, 2008b). Integration of these diverse initiatives in a systematic way could greatly advance studies of the genetics of healthy aging. The problem is that an integrative approach requires rich data on health and longevity, which are typically collected in longitudinal studies. A majority of genetic studies of disease phenotypes address particular illnesses, as mandated by the current health care paradigm. These studies often use case-control designs, which prevent rigorous investigation of pleiotropic genetic effects because of the limited number of measured phenotypes. The other problem facing studies of pleiotropic effects is that they can be more susceptible to the effects of other genes (epistasis) or non-genetic factors (gene-environment interaction) – which can partly explain disagreements in replications of the results.
In this study, we focus on the effects of common polymorphisms of the ADRB2 gene, Arg16Gly and Gln27Glu, on the probability of staying free of cancer and cardiovascular diseases, as well as on association of these polymorphisms with overall and healthy longevity and survival. Phenotypic and genotypic information are assessed for participants of the longitudinal Framingham Heart Study Offspring (FHSO) cohort that has been followed up for about 36 years.
2. Data and Methods
The FHSO phenotypic data
The FHSO cohort of respondents aged 5–70 years residing in Framingham, Massachusetts was launched in 1971–1975 with a focus on biological descendants (N=3514), their spouses (N=1576), and on adopted offspring (N=34) of the participants of the original Framingham Heart Study (FHS) cohort (Dawber, 1980; Gail and Johnson, 1989) resulting in a total sample of N=5,124 subjects; 52% women (Govindaraju et al., 2008; Kannel et al., 1979). The publicly-released limited-access FHSO data available for this study have phenotypic information assessed at six FHSO examinations performed in 1971–1975, 1979–1982, 1984–1987, 1987–1990, 1991–1995, and 1996–1997. The study participants have been followed for the occurrence of certain aging-associated diseases, with emphasis on cardiovascular diseases (CVDs) and cancer, and death through 2007. Onsets of CVDs and cancers were assessed at regular examinations at the FHS clinic and from medical records from outside clinics and hospitalization.
Aging-associated phenotypes and risk factors
We selected CVDs and cancers as the most common aging-associated diseases which were studied on their associations with common variants of the ADRB2 gene. Skin cancers were disregarded in this study. An overall longevity phenotype was defined as reaching a certain old age at the end of the follow up period (i.e., in 2007) or at date of death. For the purpose of the study (see Section “Genetic effects on longevity”), cut-offs between long and short life were left flexible ranging from the youngest possible age (when too few individuals died before the cut-off age) to the oldest possible age (when there are too few individuals reaching the cut-off age). Individuals who survived cut-off ages were considered as long living (LL) individuals compared to those who died before or on the cut-off age; called as short lived (SL) individuals. A healthy longevity phenotype was considered if LL individuals did not develop cancer and/or CVDs.
ADRB2 polymorphisms in the FHSO
DNA was collected for living participants of the FHSO in the late 1980s and through 1990s (Cupples et al., 2007). Genotyping of about 1,900 offspring (mainly unrelated) subjects was performed under the Cardio-Genomics program (http://cardiogenomics.med.harvard.edu/projects/p5/assoc-results) that focused on selected candidate genetic markers of cardiovascular development (a review of genotyped resources in the FHS and FHSO can be found in (Govindaraju et al., 2008; Levy et al., 2006)). Two common Arg16Gly and the Gln27Glu polymorphisms of the ADRB2 gene were selected for this study because of their documented multiple physiological and health effects (see Introduction) that warrants exploration of their systemic effect. The released data have information on the Arg16Gly and the Gln27Glu polymorphisms for 1565 (784 women) subjects. All genotyped members (but one) of the FHSO cohort participated in the 1st (1971–1974) and the 6th (1996–1997) examinations. There were 147 deaths (93 deaths occurred among men) in this sample that occurred after the 6th examination.
Table 1 shows mean age for samples of men and women stratified by the selected polymorphisms, as well as the number of individuals with a given compound genotype. Men with Glu27Glu genotype are somewhat younger (significantly with conventional tests and non-significantly with adjustment for multiple comparisons) than men with the Gln27Gln genotype. There are no other significant differences between mean ages. The Arg16Gly and Gln27Glu polymorphisms are in modest linkage disequilibrium (LD) with r2=0.41 (evaluated using Haploview, v. 4.1 (Barrett et al., 2005)). As a result, the Arg16 allele is tightly linked to the Gln27 allele (but not vice versa) and the Glu27 allele is tightly linked to the Gly16 allele (but again not vice versa) (Table 1). Both polymorphisms are in Hardy-Weinberg equilibrium with p=0.78 (Arg16Gly) and p=0.86 (Gln27Glu).
Table 1.
Mean age (MA) and standard deviation (SD) for carriers of each genotype of the ADRB2 Gln27Glu or Arg16Gly polymorphism at first FHSO examination, and number of individuals carrying given compound genotypes of the Gln27Glu and Arg16Gly polymorphisms in the sample of the genotyped FHSO participants.
| Men |
Women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MA yrs | SD yrs | Gln27Gln | Gln27Glu | Glu27Glu | MA yrs | SD yrs | Gln27Gln | Gln27Glu | Glu27Glu | |
| MA, yrs | 37.2 | 36.4 | 35.0 | 35.5 | 36.3 | 36.4 | ||||
| SD, yrs | 9.3 | 10.2 | 9.5 | 8.9 | 9.7 | 10.1 | ||||
| Arg16Arg | 36.9 | 8.9 | 103 | 0 | 0 | 36.5 | 8.7 | 101 | 2 | 0 |
| Arg16Gly | 36.8 | 10.1 | 113 | 235 | 2 | 35.3 | 9.4 | 125 | 236 | 0 |
| Gly16Gly | 35.7 | 9.8 | 33 | 151 | 144 | 36.6 | 9.9 | 32 | 140 | 148 |
Analyses
The Cox proportional hazards regression model was used to evaluate the relative risks (RRs) of death and incidence of the selected diseases as well as probability of staying free of a given disease (“survival patterns”) within the follow up period through 2007 for carriers of the Arg16Gly and Gln27Glu polymorphisms. Associations of these polymorphisms with longevity-related phenotypes were evaluated using the logistic regression.
The analyses were first performed with adjustment for sex (when necessary) and age. Then the models were adjusted for such potential effect-mediators as systolic (SBP) and diastolic (DBP) blood pressures (mm Hg), smoking (ever smoked), diabetes, body-mass index (BMI; kg/m2), total cholesterol (TC) and high-density lipoprotein (HDL) cholesterol (mg/100 ml) (Jalba et al., 2008; Petrone et al., 2006; Pinelli et al., 2006; Puddu et al., 2007). The absolute values of SBP, DBP, TC, and HDL were used in the regression models with increments of 10 mm Hg for SBP and DBP, and 10 mg/100 ml for TC and HDL cholesterol. BMI was categorized using the U.S. federal guidelines (Flegal et al., 2005) as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obesity (>30 kg/m2). Diabetes (present or absent) was defined if the study participant was under treatment with insulin/oral hypoglycemics or the level of fasting blood glucose >= 140 mg/dL (i.e., according to the WHO-1985 guidelines). Both types of analyses provided qualitatively similar results with slightly better estimates in the analyses with full adjustment.
Our analyses show that the Gln27Glu polymorphism is more relevant to diseases than the Arg16Gly polymorphism. Therefore, we present the results for the Gln27Glu polymorphism and show potential mediating/modulating role of the Arg16Gly polymorphism by evaluating the effect of six common compound genotypes of these two polymorphisms (i.e., excluding rare Arg16Gly/Glu27Glu and Arg16Arg/Gln27Glu genotypes; see Table 1).
Given prior evidences on the associations of the Gln27Glu and Arg16Gly polymorphisms with various aging-related phenotypes including those studied in this paper (see Introduction) and very limited number of independent tests, Type I sampling error is unlikely to be an issue for our analyses. Consequently, correction for multiple comparisons was not carried out. We note that robustness of our findings is further justified by the pleiotropic effect of the Gln27Glu polymorphism (revealed in this study) as well as by a hypothesized role of the ADRB2 polymorphisms in cardiovascular development (see Section “ADRB2 polymorphisms in the FHSO”) and by the candidate-gene-focused genotyping technique that offsets potential problems relevant to genome-wide technologies (Ziegler et al., 2008).
3. Results
The Gln27Glu polymorphism and health risks
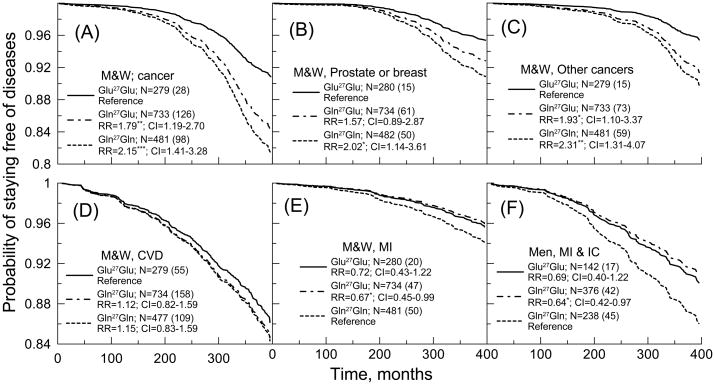
Figure 1A shows the highly significant protective role of the Glu27Glu homozygous genotype against cancer (all sites but skin) for men and women combined. To ascertain that this effect is not an artifact of biased attrition of the FHSO cohort before the blood drawing (see “ADRB2 polymorphisms” section), we evaluated survival patterns and the relative risks focusing on the 4th, 5th, and 6th examinations which were performed on or after the blood drawing. In all three cases, the results resemble those in Figure 1A (e.g., the relative risk [RR] for the Gln27Gln homozygote was 2.03, 95% Confidence interval [CI]=1.29–3.21 compared to the Glu27Glu homozygote for the 5th FHSO examination). This protective effect holds for each sex, e.g., cancer risks are significantly higher for the Gln27Gln homozygote (RR=2.14; CI=1.23–3.72 for men and RR=2.02; CI=1.05–3.86 for women) compared to the Glu27Glu homozygote. Furthermore, the protective effect appears to be not specific to a cancer type; similar relationships were observed for breast and prostate cancers (Figure 1B) as well as for the remaining cancer sites (Figure 1C).
Figure 1.
Probability of staying free of selected geriatric diseases for carriers of different genotypes of the ADRB2 Gln27Glu polymorphism in the FHSO sample (see insets). M&W denotes combined sample of men and women. MI=myocardial infarction. IC=intermittent claudication. The letter “N” in the inset denotes sample size for a given genotype with non-missing information on all covariates. The numbers in parentheses are counts of individuals with onsets of respective condition within the follow-up period (months). The RR (CI) denotes relative risks (confidence intervals) calculated using the Cox regression model adjusted for age, sex (when necessary), systolic and diastolic blood pressures, total and high density lipoprotein cholesterol, body mass index, smoking, and diabetes. *0.01<p<=0.05; **0.0005<p<=0.01; ***p<=0.0005.
No effect of the Gln27Glu polymorphism on aggregate CVD phenotype was found (Figure 1D). Nevertheless, a significant effect was observed for more homogeneous phenotype of myocardial infarction (MI) (Figure 1E) that is in line with prior studies (Heckbert et al., 2003). We also hypothesized that MI could share common genetic background with intermittent claudication (IC) because both conditions could be linked to the development of systemic atherosclerosis. Indeed, IC is a symptom of peripheral arterial disease (PAD) and the latter is often a sign of widespread accumulation of fatty deposits in arteries, i.e., atherosclerosis. This condition may reduce blood flow not only to legs but also to heart and brain. Atherosclerosis may thus be a common risk factor for MI and PAD/IC (Murabito et al., 2002). As expected, the detrimental role of the Gln27Gln homozygote attains better significance for the compound phenotype of MI and IC, especially in men (Figure 1F).
Our analyses did not reveal significant association of the Gln27Glu polymorphism with mortality risk. For both sexes combined the RRs were RR=1.20 (CI=0.72–2.00) for the Gln27Gln homozygote and RR=1.25 (CI=0.78–2.03) for the Gln27Glu heterozygote compared to the Glu27Glu homozygote.
The Arg16Gly and Gln27Glu compound genotypes and health risks
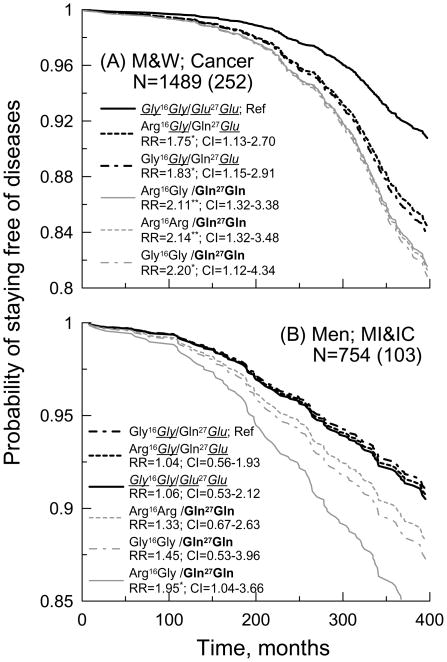
Table 1 shows that the Glu27Glu homozygote is tightly linked to the Gly16Gly homozygote. Consequently, the Gly16Gly/Glu27Glu compound genotype resembles the effect of the Glu27Glu genotype shown in Figure 1. Because the LD between the Gln27Glu and Arg16Gly polymorphisms is modest, carriers of the other two genotypes of the Gln27Glu polymorphism can carry different genotypes of the Arg16Gly polymorphism, e.g., the Gln27Glu genotype can be linked either to the Arg16Gly or Gly16Gly genotype (Table 1). Consequently, the effects of the Gln27Gln and Gln27Glu genotypes can theoretically be mediated or modulated by the Arg16Gly polymorphism.
Figure 2 shows that the effects on cancer of the Arg16Gly/Gln27Glu and Gly16Gly/Gln27Glu compound genotypes (i.e., carrying the Gln27Glu genotype) as well as the Arg16Arg/Gln27Gln, Arg16Gly/Gln27Gln, and Gly16Gly/Gln27Gln compound genotypes (i.e., carrying the Gln27Gln genotype) are the same (Figure 2A), i.e., the Arg16Gly polymorphism neither modulates nor mediates the effect of the Gln27Glu polymorphism on cancer. Although the effect of the Gln27Glu polymorphism on compound phenotype of MI and IC can be more sensitive to the Arg16Gly polymorphism than in the case of cancer, this sensitivity is not significant as well (Figure 2B). Therefore, these results provide no evidence on functional relationship between the Arg16Gly polymorphism and these aging-associated phenotypes. This result means that a protective effect is attributed to the Glu27 allele (that is equivalent to the Gly16Glu27 haplotype), whereas a detrimental effect is largely associated with the Gln27 allele of the Gln27Glu polymorphism.
Figure 2.
Probability of staying free of (A) cancer and (B) compound phenotype of myocardial infarction (MI) and intermittent claudication (IC) for (A) men and women and (B) men carrying compound genotypes of the ADRB2 Arg16Gly and Gln27Glu polymorphisms (patterns of probability are ordered according to increasing relative risks; see insets). Protective Gly16Glu27 haplotype is italicized and underlined (given that the Arg16Glu27 haplotype is virtually lacking, see Table 1, haplotypes are uniquely determined from the respective genotypes). The letter “N” in the inset denotes sample size with non-missing information on all covariates. The numbers in parentheses are counts of individuals with onsets of respective condition within the follow-up period (months). The RR (CI) denotes relative risks (confidence intervals) calculated using the Cox regression model adjusted for age, sex (when necessary), systolic and diastolic blood pressures, total and high density lipoprotein cholesterol, body mass index, smoking, and diabetes. *0.01<p<=0.05; **p<=0.01.
Genetic effects on longevity
Because the Gln27Glu polymorphism is associated with risks of cancers and MI, and because these diseases are among the leading causes of death in developed societies, this polymorphism might be involved in characterization of overall and healthy longevity (see definition of the longevity phenotypes in section “Aging-associated phenotypes and risk factors”). Given that incidence rate of these diseases begin to notably increase in adulthood (at about 50 years), cut-off for longevity was left flexible conditional on the available sample size (see next paragraph). This technique helps also offset the chance nature of findings provided that the revealed associations exhibit consistent (but not stochastic) pattern across age.
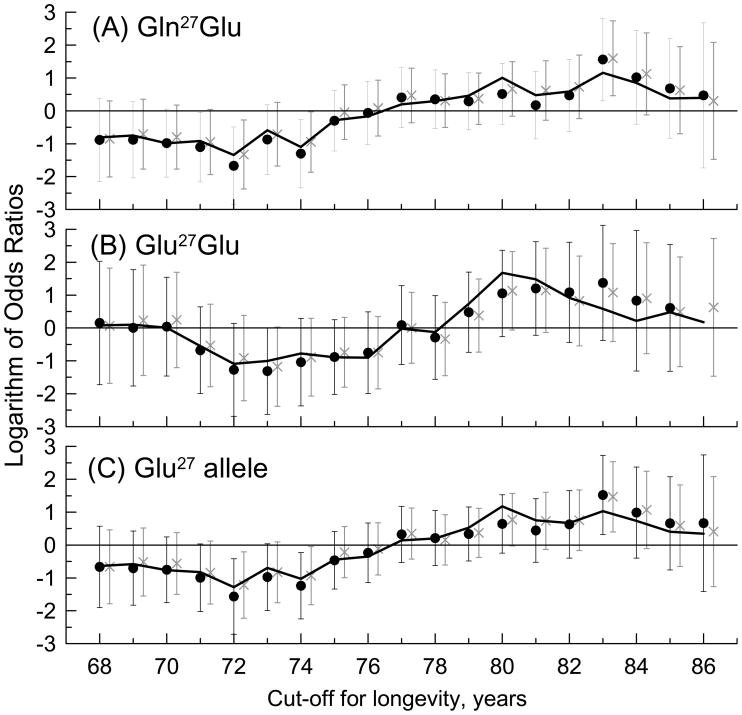
Figure 3 shows the logarithm of odds ratios of reaching old ages for men and women carrying the Gln27Glu (Figure 3A) and Glu27Glu (Figure 3B) genotypes and the Glu27 allele (Figure 3C). These analyses include a limited subset of the genotyped sample. This subset is maximal for cut-offs at young-old ages (e.g., N=773 for cut-off = 68 years). The number of SL individuals is, however, the smallest for these cut-offs due to a small number of deaths at those ages (e.g., 33 deaths occurred on or before the cut-off age = 68 years). When the cut-off age increases, the number of SL individuals gradually increases. However, the number of LL subjects (or, equivalently, the total number of individuals in the analysis) decreases because living individuals who are of cut-off ages or younger have to be censored out due to unknown life span. This results in a sample of N=135 SL subjects and N=30 LL subjects at cut-off = 86 years. These constraints limit flexibility of the cut-off ages.
Figure 3.
Patterns of chances to live long life for carriers of the (A) Gln27Glu and (B) Glu27Glu genotypes and (C) Glu27 allele compared to the Gln27Gln genotype (horizontal line). The x-axis shows varying cut-off for longevity (see Section “Aging-associated phenotypes and risk factors”). The logarithm of odds ratios is shown for models with adjustment for (i) age and sex (crosses); (ii) age, sex, systolic and diastolic blood pressures, total and high density lipoprotein cholesterol, body mass index, smoking, and diabetes (filled dots), and (iii) life span without cancer (solid line). Bars show 95% confidence interval.
Carriers of the Gln27Glu and Glu27Glu genotypes and the Glu27 allele tend to systematically live shorter lives when longevity is defined as reaching young-old ages (e.g., cut-off = 72 years) and longer lives when longevity is defined as reaching oldest-old ages (e.g., cut-off = 83 years) compared to carriers of the Gln27Gln genotype. These patterns are consistent and essentially the same for overall longevity and healthy life span (solid line in Figure 3 shows representative pattern for longevity without cancer). They also remain the same irrespective of adjustment for health-related risk factors. Estimates of the associations of compound genotypes with longevity are not reliable due to small sample sizes.
4. Discussion and conclusions
In this work, we investigated associations of genetic variants of two common polymorphisms of the ADRB2 gene, Arg16Gly and Gln27Glu, with the risks of cancer, CVDs, mortality, and longevity. Our analyses show that the Gln27Glu polymorphism, but not the Arg16Gly polymorphism is associated with selected aging-associated phenotypes. The analyses suggest two insights on: (i) the systemic role of the ADRB2 gene in healthy aging and (ii) mechanisms linking systemic genetic health effects with overall and healthy longevity in evolutionary context.
Systemic role of the ADRB2 gene in healthy aging
Our results show that the ADRB2 Gln27Glu polymorphism might be involved in regulation of a broad range of complex aging-associated phenotypes including cancer, MI, IC, and longevity (but not survival). This finding suggests that the role of this polymorphism in healthy aging can be of a systemic origin that might provide a genetic rationale for accumulated epidemiological evidence on favorable healthy aging phenotypes of long-lived individuals who have smaller prevalence of cancer, CVD, and better physiological phenotypes (Barzilai et al., 2003; Willcox et al., 2008a; Willcox et al., 2008b). Importantly, the effect of the Gln27Glu polymorphism on the risk of cancer might be sex and cancer-site insensitive (see discussion of Figure 1)—which provides further support for its systemic role. Further evidence follows from the facts that: (i) the effect of the Gln27Glu polymorphism on these aging-associated diseases (Figure 1) and longevity (Figure 3) was not altered by common physiological and behavioral risk factors including blood pressure, total and high-density lipoprotein cholesterol, diabetes, BMI, and smoking and (ii) the effect of the Gln27Glu polymorphism holds for overall and healthy longevity (Figure 3).
The systemic role of the Gln27Glu polymorphism is further augmented by the consistently unfavorable role of the derived Gln27 allele in etiology of cancer, MI and IC. The ancestral Glu27 allele seems to be playing the protective role for these phenotypes. The mode of inheritance for these alleles is different for cancer, MI, and IC. Specifically, Figures 1A–C show the recessive effect of the Glu27 allele on cancer phenotype whereas Figures 1E and 1F show its dominant effect on MI and on compound phenotype of MI and IC.
The ADBR2 receptor is expressed on cells of various tissues (e.g., immune, vascular, pulmonary, nervous (Brodde, 2008a; b)). Some studies suggest that a common ADRB2 signaling pathway (involving ADRB2 mediated catecholamine, e.g., epinephrine, induced activation of adenylate cyclase through the action of G proteins) interacts with multiple proteins from various cell molecular pathways (Benovic, 2002). This might provide a biological rationale for pleiotropic physiological effects of the ADRB2 gene (Brodde, 2008a; Leineweber et al., 2004).
In vascular tissue, for instance, the ADBR2 receptor regulates vasodilation and in healthy myocardium it regulates chronotropic and inotropic responses to endogenous and exogenous adrenergic agents (Brodde and Michel, 1999). The Arg16Gly and Gln27Glu polymorphisms are both associated with vasodilator responsiveness (Brodde, 2008b) and thus can be connected with cardiovascular events. Prior studies, however, are not in agreement on which specific variants of these polymorphisms are associated with enhanced vasodilation (Brodde, 2008b). Our findings are in line with earlier epidemiologic studies that support the protective effect of the Glu27 allele on risks of MI, as documented both in longitudinal population-based studies (e.g., the Cardiovascular Health Study (Heckbert et al., 2003)) and in case-control studies (e.g., Sala et al., 2001).
As for ADRB2 and cancer, ADRB2 has been shown to stimulate both fibroblast and endothelial cells proliferation (Iaccarino et al., 2005) and has been linked to inhibiting cellular immunity in cancer (Uotila, 1996). Recent studies also suggest a novel molecular pathway involving ADRB2 in the process of malignant transformation. This pathway is associated with repression of the ADRB2 gene expression by a Polycomb group protein (EZH2) that promotes prostate cells invasion (Thompson, 2007; Yu et al., 2007). Dysregulation of EZH2 has previously been linked to a number of cancers, including melanoma, lymphoma, breast and prostate cancers (Bracken et al., 2003; Varambally et al., 2005; Visser et al., 2001; Yu et al., 2007). Recent studies have also demonstrated a potential mechanism connecting behavioral stresses and certain types of cancer that includes ADRB2 mediated activation of the tumor cell cAMP-protein kinase A signaling pathway (Thaker et al., 2007). Epidemiological case-control studies have shown that carriers of the ADRB2 Glu27 allele could be protected against breast cancer (Huang et al., 2001) and that the Gly16 allele could be protective against lung cancer (Wang et al., 2006), which is broadly in line with our finding of favorable effect of the Gly16Glu27 haplotype in relation to cancer.
Mechanisms linking systemic genetic health effects with longevity in evolutionary context
The association patterns of the Gln27Glu polymorphism with longevity (Figure 3) exhibit consistent inverse relationships at younger and older ages supporting antagonistic pleiotropy theory on a change of the functional role of genetic variants with aging (Williams and Day, 2003). The effect of the Glu27 allele tends to be of dominant nature in these associations. This allele, which is protective against the aging-associated diseases, appears to be unfavorable early in life but becomes favorable for longevity at older ages. Figure 4 helps understand why this role changes with aging.
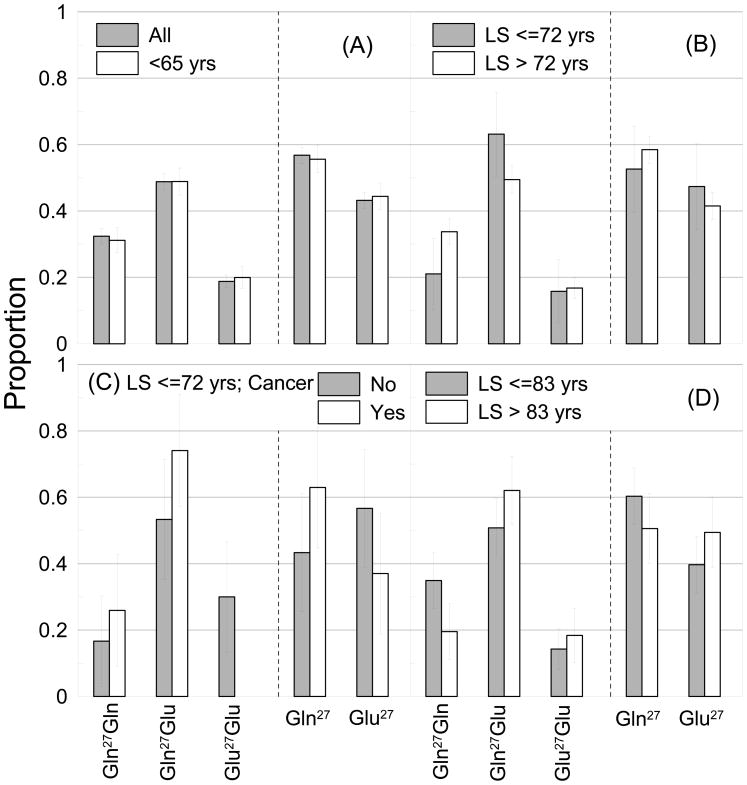
Figure 4.
Proportions of carriers of the Gln27Gln, Gln27Glu, and Glu27Glu genotypes in different samples. (A) The entire sample (All) and a subsample of young-old individuals (aged <65 years at the end of follow up in the year 2007. (B) Samples of short-lived (LS ≤ 72 years) and long-living (LS>72 years) individuals. LS denotes life span. (C) Samples of short-lived individuals as in (B) but with history of cancer (Yes) and without it (No). (D) The same as in (B) but for cut-off at 83 years. Bars show 95% confidence interval.
Figure 4A shows that the Gln27 allele is significantly more frequent than the Glu27 allele in the young-old population (e.g., who reached ages 65 years, about 40% of the sample, at the end of follow up in 2007) resembling the pattern for the entire sample. This implies that evolutionary selection likely worked against ancestral Glu27 allele and favored derived Gln27 allele because mortality selection at young ages could not explain the observed proportions given small death rates of adults (e.g., http://www.cdc.gov/nchs/deaths.htm) and infants (Singh and Yu, 1995) in the contemporary U.S.
Figure 4B shows that frequencies of the Gln27 and Glu27 alleles are the same in the sample of individuals who died relatively early in life (LS ≤ 72 years) whereas the Gln27 allele is significantly more frequent than the Glu27 allele in the LL group (who are predominantly survivors; LS>72 years) resembling patterns in Figure 1A. Furthermore, the frequency of the Gln27 allele in the sample of individuals who died later in life (Figure 4D, LS ≤ 83 years) becomes significantly larger than that of the Glu27 allele, whereas frequencies of these alleles become the same in the LL group (Figure 4D, LS>83 years). This implies that the frequency of the Gln27 allele increases in the group of deceased individuals and declines in the LL group who are predominantly survivors (equivalently, the frequency of the Glu27 allele exhibits opposite behavior). This dynamics is explained by survival disadvantage of carriers of the Gln27 allele attributable to increased risks of cancer, MI, and IC as shown in Figure 4C for the case of cancer (similar patterns hold when deaths among MI and IC patients are added). Note that because of the recessive effect of the Glu27 allele on cancer phenotype (Figure 1A) this protective role is more pronounced for the Glu27Glu homozygote.
Thus, the derived Gln27 allele was selected during evolution because of its favorable role for certain (unknown) conditions beneficial for the early life. However, this advantageous effect appears to be not relevant for the oldest-old ages—which follows from the change of the favorable role of the Gln27 allele to an unfavorable one (Figure 3). This unfavorable role is the result of a survival disadvantage for carriers of the derived Gln27 allele and, respectively, a survival advantage for carriers of the ancestral Glu27 allele associated with the late-life health conditions. Given unknown forces driving the evolutionary advantage of the Gln27 allele (that deserves further exploration), the entire biochemical mechanism relevant to the observed opposite genetic effects at different ages is unclear. It is clear, however, that in part it should be related to cancer- and CVD-specific pathways (discussed in the previous section). It is worth noting that these finding are in line with an apparently antagonistic effect of genetic variants of the ADRB2 gene on hypertension (Bao et al., 2005) and cognition (Bochdanovits et al., 2009) in early and late life with some indications on a putative role of the white matter integrity in the splenium (Penke et al., 2010).
Our results also add to the discussion on replication of genetic associations in independent samples. Replication is sometimes viewed as a “gold standard” in the era of genome-wide association studies given relevant statistical and technological issues (McCarthy et al., 2008; Ziegler et al., 2008). Only a few candidate longevity-associated genes, among many others, have been replicated thus far in two or more human studies, including APOE (see, e.g., (Christensen et al., 2006; Salvioli et al., 2006)), CETP (Barzilai et al., 2003; Koropatnick et al., 2008), and FOXO3A (Flachsbart et al., 2009; Willcox et al., 2008a). The question is if lack of replication is a definitive signature of a false positive or not?
Replication in independent samples implies that the effects of the respective genetic variants can: (i) have smaller chances of being an artifact of a particular procedure (e.g., technology, genotyping, sampling, etc.), (ii) be weakly sensitive to population heterogeneity and, consequently, have a stronger systemic component, and (iii) have strong but the same-type sensitivity to population heterogeneity in all studied samples. Therefore, lack of replication might be informative, because this could indicate differences in sensitivity to population heterogeneity in different samples, i.e., provide clues on factors affecting possible discordance.
Our study provides arguments that lack of replication can be evolutionary-driven. For instance, because evolution has worked differently in whites, blacks, and Asians, the frequency of the derived Gln27 allele appears to be markedly different in these ethnic groups, i.e., 0.54–0.65 in whites, 0.73–0.80 in blacks, and 0.80–0.9 in Asians (Brodde, 2008a). Therefore, an unfavorable role of this allele for the late-life phenotypes (that implies the case of antagonistic pleiotropy) revealed in the FHSO sample of whites might not necessarily be replicated in a sample of blacks or Asians because these late-life phenotypes may not have driven evolutionary selection of the Gln27 allele. Lack of replication, however, might reveal race-specific factors contributing to the late-life phenotypes. This is in line with increasingly recognized sensitivity of the effect of common polymorphisms to local environment or/and genetic heterogeneity (Zintzaras et al., 2008). The latter factors can even selectively alter the effect of specific polymorphisms not affecting the others even if they are in the same gene and are in modestly-strong LD (Kulminski et al., 2010)).
Broadly, evolutionary selection did not directly work against genetic variants which could be harmful for inherently non-Mendelian late-life phenotypes that was suggested in prior works (Medawar, 1952) and evidently seen in our study. The human genome was established to provide better robustness under an adverse environment, which is markedly different from modern conditions, especially in developed societies (Kuningas et al., 2008). Evolutionary forces (e.g., genetic drift) can be responsible for entirely stochastic change in allele frequencies (Zimmer, 2001). These arguments along with others suggest that even a truly positive effect can be not necessarily replicated in other populations because of the inherent complexity of a net of causation of aging-associated phenotypes, which is not a result of a global genetic program similar to, for instance, the program of development (Thomas and Clayton, 2004; Vijg and Suh, 2005). As a consequence, replication studies should be accompanied by strategies for revealing potential effect modulators.
Our analyses and current knowledge about molecular-biological mechanisms of ADRB2 mediating health effects support a systemic role of the ADRB2 gene in healthy aging. We also show that the same allele of the Gln27Glu polymorphism of this gene could be detrimental early in life but its role may change to a favorable one in late life implying the phenomenon of antagonistic pleiotropy. These important findings warrant further exploration in other populations.
Major limitation of our study is a small sample of the oldest-old individuals that prevents generalization of the case of antagonistic pleiotropy to the very old ages. Given concerns on definitive validity of strategy on replication of genetic associations in different setting (see above), the best strategy would be to replicate the results on antagonistic pleiotropy in the same cohort but followed for longer time period. This is a reasonable strategy given the ongoing nature of the FHS.
Acknowledgments
The research reported in this paper was supported by the National Institute on Aging (NIA) grants R01 AG-028259, R01-AG-027019, and R01-AG-030612. The data were provided by Boston University and the National Heart, Lung, and Blood Institute (NHLBI) under data and material distribution agreement for NIA grant R01AG030612. The Framingham Heart Study (FHS) is conducted and supported by the NHLBI in collaboration with Boston University (Contract No. N01-HC-25195). This manuscript was not prepared in collaboration with investigators of the FHS and does not necessarily reflect the opinions or views of the Framingham Heart Study, Boston University, or NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao X, Mills PJ, Rana BK, Dimsdale JE, Schork NJ, Smith DW, Rao F, Milic M, O’Connor DT, Ziegler MG. Interactive effects of common beta2-adrenoceptor haplotypes and age on susceptibility to hypertension and receptor function. Hypertension. 2005;46:301–307. doi: 10.1161/01.HYP.0000175842.19266.95. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Benovic JL. Novel beta2-adrenergic receptor signaling pathways. J Allergy Clin Immunol. 2002;110:S229–235. doi: 10.1067/mai.2002.129370. [DOI] [PubMed] [Google Scholar]
- Bochdanovits Z, Gosso FM, van den Berg L, Rizzu P, Polderman TJ, Pardo LM, Houlihan LM, Luciano M, Starr JM, Harris SE, Deary IJ, de Geus EJ, Boomsma DI, Heutink P, Posthuma D. A Functional polymorphism under positive evolutionary selection in ADRB2 is associated with human intelligence with opposite effects in the young and the elderly. Behav Genet. 2009;39:15–23. doi: 10.1007/s10519-008-9233-0. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodde OE. Beta1- and beta2-adrenoceptor polymorphisms and cardiovascular diseases. Fundam Clin Pharmacol. 2008a;22:107–125. doi: 10.1111/j.1472-8206.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Brodde OE. Beta-1 and beta-2 adrenoceptor polymorphisms: functional importance, impact on cardiovascular diseases and drug responses. Pharmacol Ther. 2008b;117:1–29. doi: 10.1016/j.pharmthera.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- Buscher R, Herrmann V, Ring KM, Kailasam MT, O’Connor DT, Parmer RJ, Insel PA. Variability in phenylephrine response and essential hypertension: a search for human alpha(1B)-adrenergic receptor polymorphisms. J Pharmacol Exp Ther. 1999;291:793–798. [PubMed] [Google Scholar]
- Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contopoulos-Ioannidis DG, Manoli EN, Ioannidis JP. Meta-analysis of the association of beta2-adrenergic receptor polymorphisms with asthma phenotypes. J Allergy Clin Immunol. 2005;115:963–972. doi: 10.1016/j.jaci.2004.12.1119. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Arruda HT, Benjamin EJ, D’Agostino RB, Sr, Demissie S, DeStefano AL, Dupuis J, Falls KM, Fox CS, Gottlieb DJ, Govindaraju DR, Guo CY, Heard-Costa NL, Hwang SJ, Kathiresan S, Kiel DP, Laramie JM, Larson MG, Levy D, Liu CY, Lunetta KL, Mailman MD, Manning AK, Meigs JB, Murabito JM, Newton-Cheh C, O’Connor GT, O’Donnell CJ, Pandey M, Seshadri S, Vasan RS, Wang ZY, Wilk JB, Wolf PA, Yang Q, Atwood LD. The Framingham Heart Study 100K SNP genome-wide association study resource: overview of 17 phenotype working group reports. BMC Med Genet. 2007;8(Suppl 1):S1. doi: 10.1186/1471-2350-8-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber TR. The Framingham study: the epidemiology of atherosclerotic disease. Harvard University Press; Cambridge, Mass: 1980. [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Gail MH, Johnson NL. Proceedings of the American Statistical Association: sesquicentennial invited papers session. American Statistical Association; Alexandria, VA: 1989. [Google Scholar]
- George CH. Genetic polymorphisms in beta1 and beta2 adrenergic receptors: variations without a theme? Heart Rhythm. 2008;5:822–825. doi: 10.1016/j.hrthm.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Govindaraju DR, Cupples LA, Kannel WB, O’Donnell CJ, Atwood LD, D’Agostino RB, Sr, Fox CS, Larson M, Levy D, Murabito J, Vasan RS, Splansky GL, Wolf PA, Benjamin EJ. Genetics of the Framingham Heart Study population. Adv Genet. 2008;62:33–65. doi: 10.1016/S0065-2660(08)00602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Hadley EC, Rossi WK. Exceptional survival in human populations: National Institute on Aging perspectives and programs. Mech Ageing Dev. 2005;126:231–234. doi: 10.1016/j.mad.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Heckbert SR, Hindorff LA, Edwards KL, Psaty BM, Lumley T, Siscovick DS, Tang Z, Durda JP, Kronmal RA, Tracy RP. Beta2-adrenergic receptor polymorphisms and risk of incident cardiovascular events in the elderly. Circulation. 2003;107:2021–2024. doi: 10.1161/01.CIR.0000065231.07729.92. [DOI] [PubMed] [Google Scholar]
- Hindorff LA, Heckbert SR, Psaty BM, Lumley T, Siscovick DS, Herrington DM, Edwards KL, Tracy RP. beta(2)-Adrenergic receptor polymorphisms and determinants of cardiovascular risk: the Cardiovascular Health Study. Am J Hypertens. 2005;18:392–397. doi: 10.1016/j.amjhyper.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Huang XE, Hamajima N, Saito T, Matsuo K, Mizutani M, Iwata H, Iwase T, Miura S, Mizuno T, Tokudome S, Tajima K. Possible association of beta2- and beta3-adrenergic receptor gene polymorphisms with susceptibility to breast cancer. Breast Cancer Res. 2001;3:264–269. doi: 10.1186/bcr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino G, Ciccarelli M, Sorriento D, Galasso G, Campanile A, Santulli G, Cipolletta E, Cerullo V, Cimini V, Altobelli GG, Piscione F, Priante O, Pastore L, Chiariello M, Salvatore F, Koch WJ, Trimarco B. Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circ Res. 2005;97:1182–1189. doi: 10.1161/01.RES.0000191541.06788.bb. [DOI] [PubMed] [Google Scholar]
- Jalba MS, Rhoads GG, Demissie K. Association of Codon 16 and Codon 27 beta2-Adrenergic Receptor Gene Polymorphisms with Obesity: A Meta-analysis. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.327. [DOI] [PubMed] [Google Scholar]
- Johnson TE. Genes, phenes, and dreams of immortality: the 2003 Kleemeier Award lecture. J Gerontol A Biol Sci Med Sci. 2005;60:680–687. doi: 10.1093/gerona/60.6.680. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Koropatnick TA, Kimbell J, Chen R, Grove JS, Donlon TA, Masaki KH, Rodriguez BL, Willcox BJ, Yano K, Curb JD. A prospective study of high-density lipoprotein cholesterol, cholesteryl ester transfer protein gene variants, and healthy aging in very old Japanese-american men. J Gerontol A Biol Sci Med Sci. 2008;63:1235–1240. doi: 10.1093/gerona/63.11.1235. [DOI] [PubMed] [Google Scholar]
- Kulminski AM, Culminskaya IV, Ukraintseva SV, Arbeev KG, Akushevich I, Land KG, Yashin AI. Polymorphisms in the ACE and ADRB2 genes and risks of aging-associated phenotypes: the case of myocardial infarction. Rejuvenation Research. 2010 doi: 10.1089/rej.2009.0905. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuningas M, Mooijaart SP, van Heemst D, Zwaan BJ, Slagboom PE, Westendorp RG. Genes encoding longevity: from model organisms to humans. Aging Cell. 2008;7:270–280. doi: 10.1111/j.1474-9726.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- Leineweber K, Buscher R, Bruck H, Brodde OE. Beta-adrenoceptor polymorphisms. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:1–22. doi: 10.1007/s00210-003-0824-2. [DOI] [PubMed] [Google Scholar]
- Levy D, DePalma SR, Benjamin EJ, O’Donnell CJ, Parise H, Hirschhorn JN, Vasan RS, Izumo S, Larson MG. Phenotype-genotype association grid: a convenient method for summarizing multiple association analyses. BMC Genet. 2006;7:30. doi: 10.1186/1471-2156-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson MC, Ellis JA, Raven J, Johns DP, Walters EH, Abramson MJ. Beta2-adrenergic receptor polymorphisms are associated with asthma and COPD in adults. J Hum Genet. 2006;51:943–951. doi: 10.1007/s10038-006-0043-z. [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, Hirschhorn JN. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- Medawar PB. An Unsolved problem of biology; an inaugural lecture delivered at University College, London, 6 December, 1951. H.K. Lewis and Co; London: 1952. [Google Scholar]
- Melzer D, Hurst AJ, Frayling T. Genetic variation and human aging: progress and prospects. J Gerontol A Biol Sci Med Sci. 2007;62:301–307. doi: 10.1093/gerona/62.3.301. [DOI] [PubMed] [Google Scholar]
- Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- Peel N, Bartlett H, McClure R. Healthy ageing: how is it defined and measured? Australasian Journal on Ageing. 2004;23:115–119. [Google Scholar]
- Penke L, Maniega SM, Houlihan LM, Murray C, Gow AJ, Clayden JD, Bastin ME, Wardlaw JM, Deary IJ. White Matter Integrity in the Splenium of the Corpus Callosum is Related to Successful Cognitive Aging and Partly Mediates the Protective Effect of an Ancestral Polymorphism in ADRB2. Behav Genet. 2010 doi: 10.1007/s10519-009-9318-4. [DOI] [PubMed] [Google Scholar]
- Petrone A, Zavarella S, Iacobellis G, Zampetti S, Vania A, Di Pietro S, Galgani A, Leonetti F, Di Mario U, Buzzetti R. Association of beta2 adrenergic receptor polymorphisms and related haplotypes with triglyceride and LDL-cholesterol levels. Eur J Hum Genet. 2006;14:94–100. doi: 10.1038/sj.ejhg.5201521. [DOI] [PubMed] [Google Scholar]
- Pinelli M, Giacchetti M, Acquaviva F, Cocozza S, Donnarumma G, Lapice E, Riccardi G, Romano G, Vaccaro O, Monticelli A. Beta2-adrenergic receptor and UCP3 variants modulate the relationship between age and type 2 diabetes mellitus. BMC Med Genet. 2006;7:85. doi: 10.1186/1471-2350-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddu P, Puddu GM, Cravero E, Ferrari E, Muscari A. The genetic basis of essential hypertension. Acta Cardiol. 2007;62:281–293. doi: 10.2143/ac.62.3.2020818. [DOI] [PubMed] [Google Scholar]
- Sala G, Di Castelnuovo A, Cuomo L, Gattone M, Giannuzzi P, Iacoviello L, De Blasi A. The E27 beta2-adrenergic receptor polymorphism reduces the risk of myocardial infarction in dyslipidemic young males. Thromb Haemost. 2001;85:231–233. [PubMed] [Google Scholar]
- Salvioli S, Olivieri F, Marchegiani F, Cardelli M, Santoro A, Bellavista E, Mishto M, Invidia L, Capri M, Valensin S, Sevini F, Cevenini E, Celani L, Lescai F, Gonos E, Caruso C, Paolisso G, De Benedictis G, Monti D, Franceschi C. Genes, ageing and longevity in humans: problems, advantages and perspectives. Free Radic Res. 2006;40:1303–1323. doi: 10.1080/10715760600917136. [DOI] [PubMed] [Google Scholar]
- Sierra F, Hadley E, Suzman R, Hodes R. Prospects for Life Span Extension. Annu Rev Med. 2008 doi: 10.1146/annurev.med.60.061607.220533. [DOI] [PubMed] [Google Scholar]
- Singh GK, Yu SM. Infant mortality in the United States: trends, differentials, and projections, 1950 through 2010. Am J Public Health. 1995;85:957–964. doi: 10.2105/ajph.85.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Joyner MJ, Turner ST, Johnson BD. Blood pressure variation in healthy humans: a possible interaction with beta-2 adrenergic receptor genotype and renal epithelial sodium channels. Med Hypotheses. 2005;65:296–299. doi: 10.1016/j.mehy.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Lutgendorf SK, Sood AK. The neuroendocrine impact of chronic stress on cancer. Cell Cycle. 2007;6:430–433. doi: 10.4161/cc.6.4.3829. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Clayton DG. Betting odds and genetic associations. J Natl Cancer Inst. 2004;96:421–423. doi: 10.1093/jnci/djh094. [DOI] [PubMed] [Google Scholar]
- Thompson TC. Suppression of tumor suppressors in prostate cancer: the emergence of a novel oncogenic pathway. Cancer Cell. 2007;12:405–407. doi: 10.1016/j.ccr.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Uotila P. The role of cyclic AMP and oxygen intermediates in the inhibition of cellular immunity in cancer. Cancer Immunol Immunother. 1996;43:1–9. doi: 10.1007/BF03354243. [DOI] [PubMed] [Google Scholar]
- Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, Wei JT, Pienta KJ, Ghosh D, Rubin MA, Chinnaiyan AM. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Vijg J, Suh Y. Genetics of longevity and aging. Annu Rev Med. 2005;56:193–212. doi: 10.1146/annurev.med.56.082103.104617. [DOI] [PubMed] [Google Scholar]
- Visser HP, Gunster MJ, Kluin-Nelemans HC, Manders EM, Raaphorst FM, Meijer CJ, Willemze R, Otte AP. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma. Br J Haematol. 2001;112:950–958. doi: 10.1046/j.1365-2141.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Hao B, Chen X, Zhao N, Cheng G, Jiang Y, Liu Y, Lin C, Tan W, Lu D, Wei Q, Jin L, Lin D, He F. Beta-2 adrenergic receptor gene (ADRB2) polymorphism and risk for lung adenocarcinoma: a case-control study in a Chinese population. Cancer Lett. 2006;240:297–305. doi: 10.1016/j.canlet.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008a;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Wang NC, He Q, Rosenbaum M, Suzuki M. Life at the extreme limit: phenotypic characteristics of supercentenarians in Okinawa. J Gerontol A Biol Sci Med Sci. 2008b;63:1201–1208. doi: 10.1093/gerona/63.11.1201. [DOI] [PubMed] [Google Scholar]
- Williams PD, Day T. Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution. 2003;57:1478–1488. doi: 10.1111/j.0014-3820.2003.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Cao Q, Mehra R, Laxman B, Tomlins SA, Creighton CJ, Dhanasekaran SM, Shen R, Chen G, Morris DS, Marquez VE, Shah RB, Ghosh D, Varambally S, Chinnaiyan AM. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Yu JT, Tan L, Ou JR, Zhu JX, Liu K, Song JH, Sun YP. Polymorphisms at the beta2-adrenergic receptor gene influence Alzheimer’s disease susceptibility. Brain Res. 2008;1210:216–222. doi: 10.1016/j.brainres.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Konig IR, Thompson JR. Biostatistical aspects of genome-wide association studies. Biom J. 2008;50:8–28. doi: 10.1002/bimj.200710398. [DOI] [PubMed] [Google Scholar]
- Zimmer C. Evolution: the triumph of an idea. HarperCollins; New York: 2001. [Google Scholar]
- Zintzaras E, Raman G, Kitsios G, Lau J. Angiotensin-converting enzyme insertion/deletion gene polymorphic variant as a marker of coronary artery disease: a meta-analysis. Arch Intern Med. 2008;168:1077–1089. doi: 10.1001/archinte.168.10.1077. [DOI] [PubMed] [Google Scholar]